Tumor Immunology
Objectives
The objectives of this lecture are to know
1. Definition of tumor immunology .
2. Types of tumor antigens.
3. Effector mechanisms in cancer immunity.
4. Immunotherapy of malignancy
Tumor Immunology
Tumor immunology is the study of
1. The antigenic properties of transformed cells,
2. The host immune responses to these tumor cells,
3. The immunologic consequences to the host of the growth of malignant
cells, and
4. The means by which the immune system can be modulated to recognize
tumor cells and promote tumor eradication.
Tumor or neoplasm
— A tumor, or neoplasm , is a collection of the clonal descendants of a cell
whose growth has gone unchecked. When a tumor continues to grow and
to invade healthy tissue, it is considered to be a cancer. Malignant tumors
are distinguished from benign tumors by their progressive growth and
invasiveness. Metastasis is a characteristic of many malignant tumors
(cancers).
Immune surveillance
— The immune surveillance: cancer cells frequently arise within the body
but are normally eliminated before they multiply sufficiently to become
clinically detectable.
lect:6
Dr. Khalid Waleed
M.B.ch.B., Msc., PhD. Immunology

Or it is a physiologic function of the immune system is to recognize and
destroy clones of transformed cells before they grow into tumors. Tumors
arise only if they are able to escape immune surveillance.
Failure of Immune surveillance
Failure of immunosurveillance may be due to the fact that in the early
development of a tumor, the amount of antigen may be too small to
stimulate the immune system and, due to the rapid proliferation of
malignant cells, the immune system is quickly overwhelmed.
Some tumors may secrete immunosuppressive molecules and others may
induce suppressor cells.
Some tumors may shed their unique antigens which block antibodies and
T cells from reacting with malignant cells.
Tumor antigens
The principal biologic mechanisms that may lead to the appearance
of immunogenic tumor antigens including mutation, gene
activation, and clonal amplification
Tumor cells express antigens on their surfaces that are often the
targets of immune responses.
Many tumor antigens are cellular peptides presented by MHC
molecules that stimulate antigen-specific T-cell proliferation.
Types of tumor antigens
1. Tumor-specific antigens (TSAs).
2. Tumor-associated antigens.
3. Tissue differentiation antigens.
1. Tumor-specific antigens (TSAs)
Tumor-specific antigens are unique to tumor cells and do not occur
on other cells in the body.

TSAs are not found on normal somatic cells but result from
mutations of genes and the resulting altered proteins that are
expressed by the tumor cells.
Tumor-specific antigens have been demonstrated on tumors induced
with chemical or physical carcinogens and on some virally induced
tumors.
Identification of TSAs on naturally occurring tumors has proved
difficult, most likely because the immune response generally
eliminates cells that express TSAs at levels great enough to be
antigenic. The are two types of TSAs: virally induced and
chemically induced Tumors antigens.
Viraly induced tumor
Tumour-inducing viruses contain viral genes that, when integrated
into the cellular genome, can transform the virus-infected cell into a
tumour cell.
Several tumour-inducing viruses have been identified such as
Simian virus 40, polyoma, papilloma and adenoviruses. These DNA
viruses express the transforming DNA early after infection and their
early genes may integrate into the DNA of the infected cell. This
event can transform the cell, and expression of the early genes is
required to maintain its transformed state.
These viral gene-encoded antigens are shared by all cells
transformed by the same virus and may therefore function as
tumour-specific antigens.
In human papillomavirus-induced cervical cancer, the transforming
E6 and E7 proteins are expressed, and in mouse models these
antigens have been effectively targeted for active immunization
against cervical cancer. The RNA viruses, except for Human T

lymphotropic virus-1, have not been identified as tumorigenic in
humans.
Chemically induced tumors
There have been many chemical carcinogens identified, but their
principal mechanism of cancer induction is similar in that it appears
to be mutational.
Chemically-induced tumors are different from virally-induced
tumors in that they are extremely heterogeneous in their antigenic
characteristics.
Thus, any two tumors induced by the same chemical, rarely share
common tumor specific antigens.
2.Tumor-associated antigens : Tumor-associated antigens are not unique
to the tumor cells and instead are also expressed on normal cells under
conditions that fail to induce a state of immunologic tolerance to the
antigen. The expression of the antigen on the tumor may occur under
condition that enable the immune system to respond to the antigen. It
includes: 1. Oncofetal Tumor Antigens 2. Oncogene Proteins as Tumor
Antigens.
Oncofetal Tumor Antigens
A. Alpha-fetoprotein
The normal range of AFP concentrations in humans is 0-20 ng/ml.
This level rises considerably in patients with hepatomas and non-
seminal testicular carcinoma.
A 5-fold or higher rise in this protein is used for monitoring
hepatomas and testicular cancers.
AFP level may also be raised in some non-malignant conditions,
such as cirrhosis, in hepatitis and other forms of liver damage.

B. Carcinoembryonic antigens
CEA levels in normal people range up to 2.5 ng/ml ,
They increase significantly in certain malignancies, particularly
colo-rectal cancers.
They may also rise in some non-malignant conditions (chronic
cirrhosis, pulmonary emphysema and heavy smoking.
Levels that are 4-5 times normal have been used to predict
recurrence of colo-rectal tumors.
Oncogene Proteins as Tumor Antigens
Oncogene Proteins as Tumor Antigens (mutant cellular gene
products): A number of tumors have been shown to express tumor-
associated antigens encoded by cellular oncogenes. These antigens
are also present in normal cells encoded by the corresponding proto-
oncogene.
For example, human breast-cancer cells exhibit elevated expression
of onocogen-encoded neu protein, a growth-factor receptor, where
as normal adult cells express only trace amounts of neu protein.
Because of this difference in the neu levels, anti-neu monoclonal
antibodies can recognized and selectively eliminate breast-cancer
cells with out damaging normal cells.
4. Tissue differentiation antigens
Prostatic tumor may carry prostatic specific antigen (PSA). It is a
normal differentiation antigen for prostate. It is also released into the
serum and can be measured as a screening test for prostatic cancer.
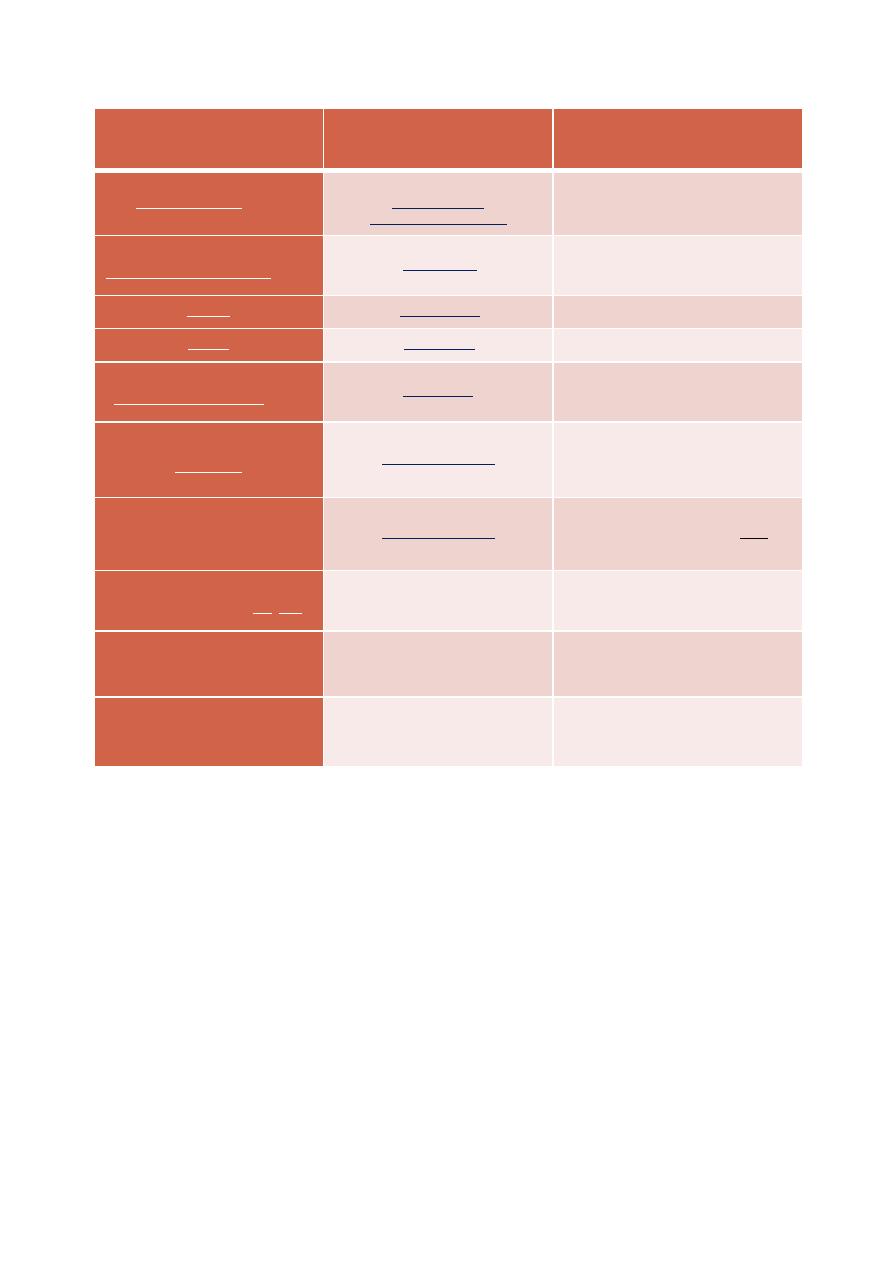
Tumor antigen
Tumor in which it is found
Remarks
Alphafetoprotein (AFP)
Carcinoembryonic antigen (CEA)
Occasional lung or breast cancer
epithelial tumor antigen (ETA)
normally present in minute quantities;
greatly elevated levels in melanoma
Melanoma-associated antigen
(MAGE)
Also normally present in the testis
Various tumors
Normal intracellular enzyme
Oncoprotein
PSA
Her-2/neu
Prostate
Breast
Ig idiotypes
lymphoma
B-cells
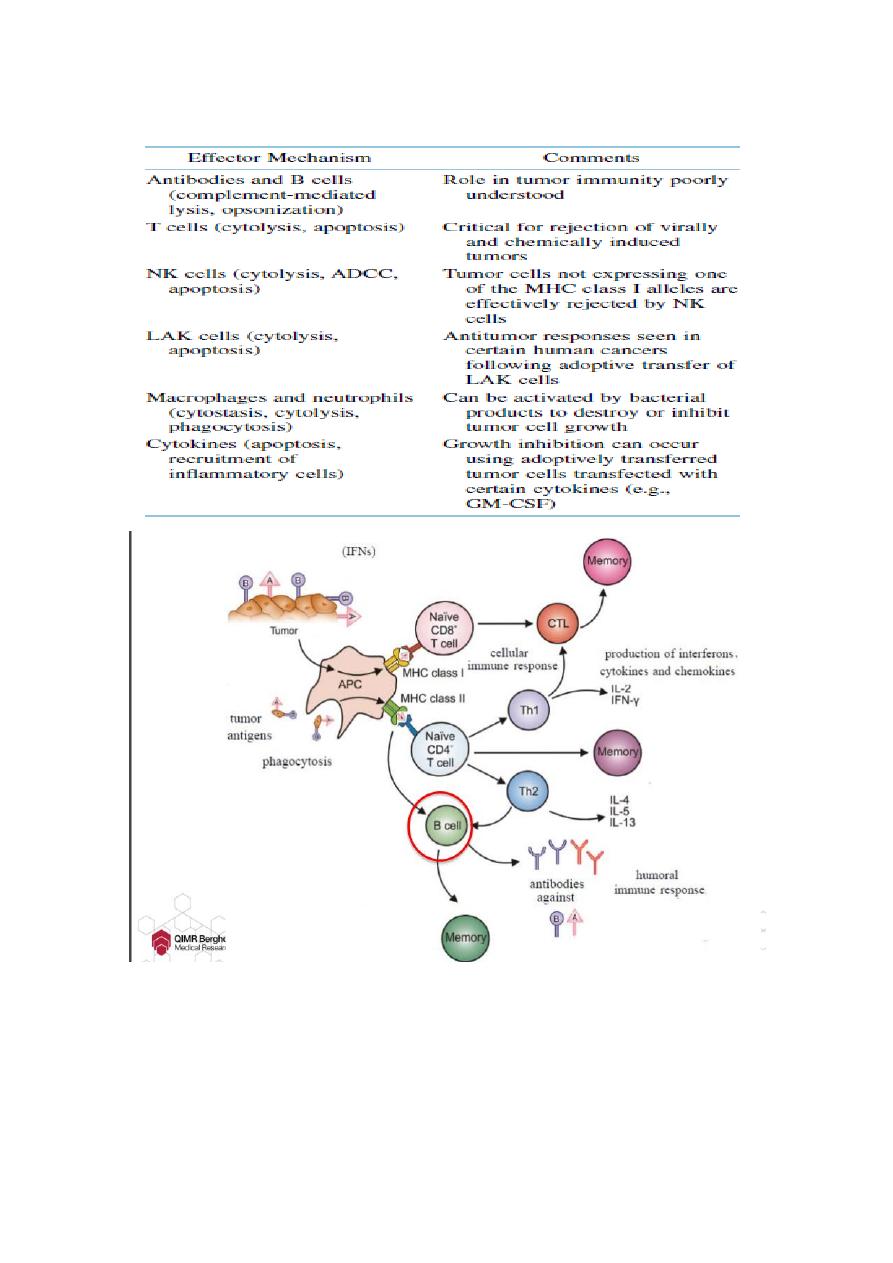
Effector Mechanisms in Cancer Immunity

Phases of immune interaction with tumor cells
— Three phases of immune interaction with tumor cells
1. Elimination: Tumor cells are detected and destroyed by the immune
system.
2. Equilibrium: Tumor cells are not eliminated by the immune system.
3. Escape: Tumor cells escape immune control.
Mechanisms of tumor cell immune escape
1. Loss of antigen or (MHC) expression.
2. Resistance to cytotoxicity.
3. Defects in tumor antigen processing /presentation.
4. Loss of crucial genes involved in the immune response.
5. Recruitment
of
immunosuppressive
cells
to
the
tumor
microenvironment.
Immunotherapy of malignancy
Immunotherapy of malignancy employs various preparations for the
augmentation of tumor-specific as well as nonspecific immune responses.
—Approaches include
(a) active immunization;
(b) passive therapy with antibodies;
(c) antibody–drug conjugates, which is antibody joined to a
chemotherapy drug, radioactive particle, or a toxin;
(d) local application of live bacterial vaccines (BCG);
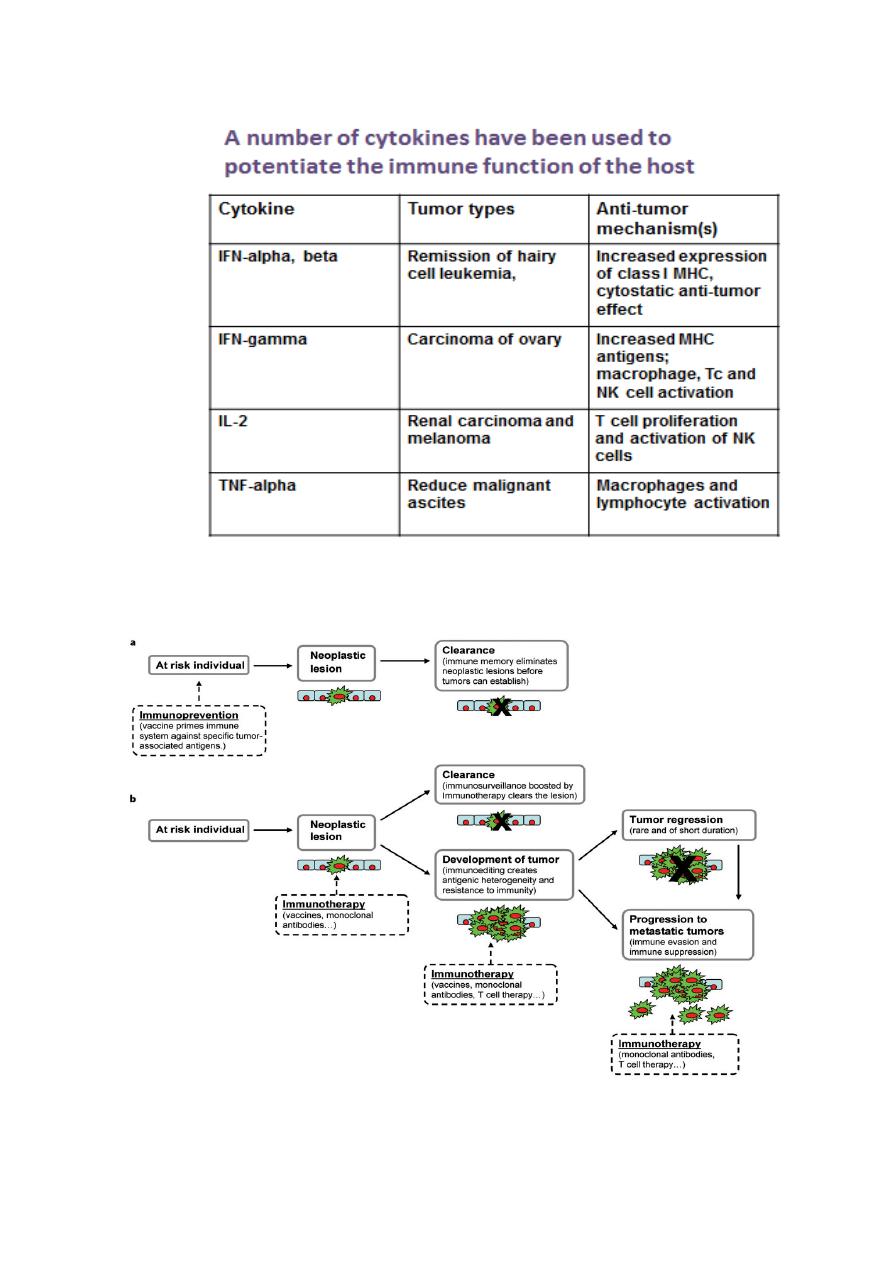
(e) use of cytokines;
(f) adoptive transfer of effector cells.