
Immunology / Immune Tolerance and Autoimmunity
Objectives
The objectives of this lecture are to know
1. Immune tolerance.
2. The difference between self and induced tolerance.
3. Autoimmunity and autoimmune diseases.
4. Treatment of autoimmune diseases.
Immune Tolerance and Autoimmunity
Definitions
Tolerance is a state of specific unresponsiveness (to a specific antigen).
Self-tolerance, a property of both B and T lymphocytes, is a state of
specific unresponsiveness to self-antigen.
It occurs when the interaction of an autoantigen with self-antigen-specific
lymphocytes fails to activate the lymphocytes. Thus, to be tolerized, a
cell must express an antigen-specific receptor, either a B-cell receptor
(BCR) or T-cell receptor (TCR). In normal hosts, tolerance protects
against autoimmune tissue injury.
Induced tolerance in which tolerance to external antigens can be created
by manipulating the immune system.
The antigen which causes the tolerance is called tolerogen.
Central and peripheral tolerance
Both B cells and T cells can be made tolerant, but it is more
important to Tolerize T cells than B cells because B cells cannot make
antibodies to most antigens without the help of T cells.
lect:3
Dr. Khalid Waleed
M.B.ch.B., Msc., PhD. Immunology

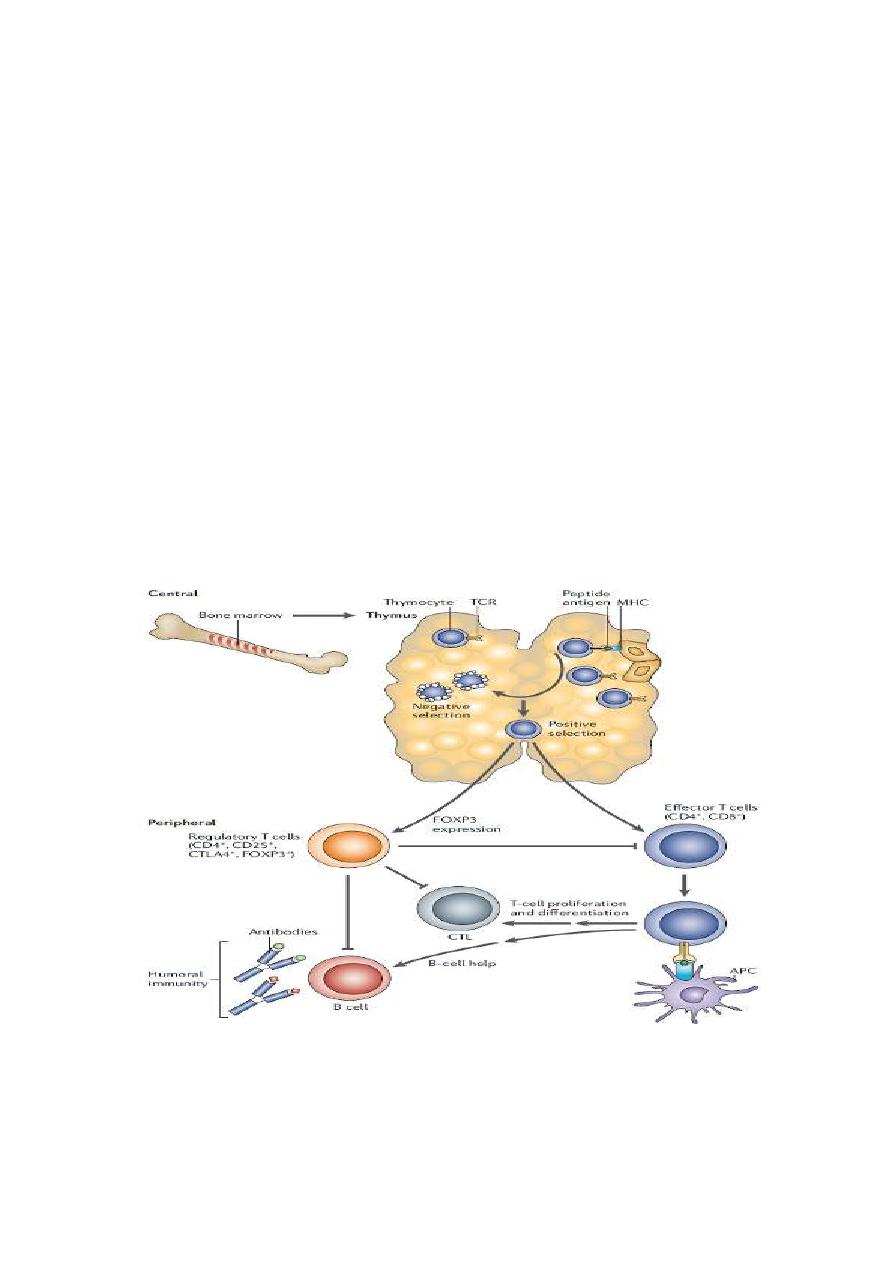
central tolerance (occurs in the primary lymphoid organs: the bone
marrow for B cells and the thymus for T cells).
Because central tolerance is not perfect and some self-reactive
lymphocytes find their way into the periphery. Thus Tolerance induced in
mature lymphocytes is referred to as peripheral tolerance.
T-cell central tolerance
T cells develop in the thymus. As they mature, they have two
chains that make up the T-cell receptor for antigen (TCR). There is a
virtually unlimited repertoire of receptor specificities created in the
population of T cells within the thymus. In the thymus, the epitopes
recognized by these receptors consist of:
• A small molecule, usually a peptide of 6–8 amino acids derived from
body proteins; that is, "self" proteins
• A histocompatibility molecule
o Class II for CD4+ T cells.
o Class I for CD8+ T cells.
T cells whose receptors bind these epitopes so tightly that they could
attack the cell displaying them are deleted by apoptosis.
This process, called clonal deletion, involves the killing of T cells
(“negative selection”) that react against antigens (primarily self major
histocompatibility complex [MHC] proteins).
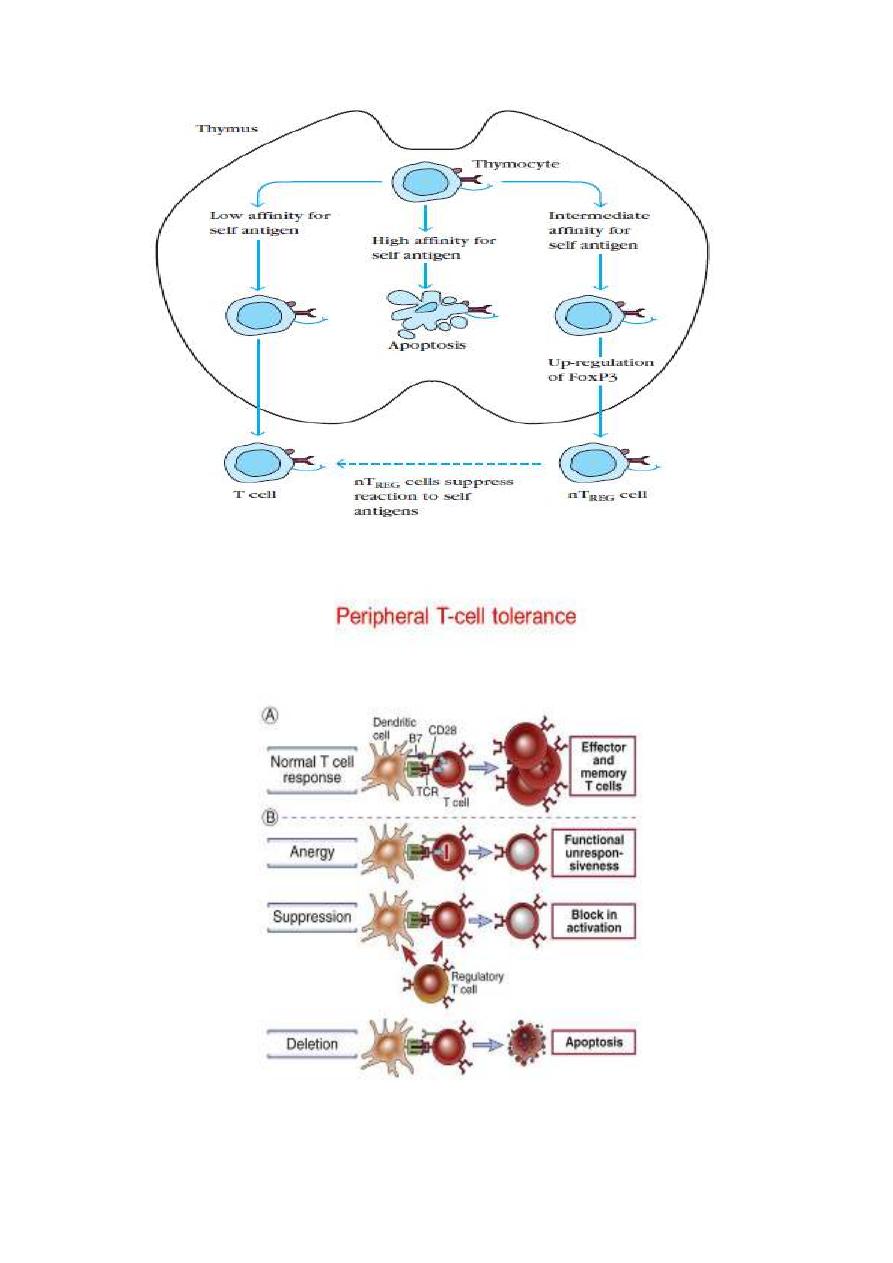
T-cell peripheral tolerance
Peripheral tolerance is necessary because some antigens are not expressed
in the thymus and therefore some self-reactive T cells are not killed in the
thymus. T cells peripheral tolerance is achieved by:
1. Clonal anergy is the term used to describe self-reactive T cells that
are not activated because proper costimulation does not occur.
2. Clonal ignorance refers to self-reactive T cells that ignore self
antigens. These self-reactive T cells are either kept ignorant by
physical separation from the target antigens (e.g., the blood–brain
barrier) or ignore self antigens because the antigens are present in
such small amounts.
3. Suppressed by regulatory T cells (Treg) producing inhibitory
cytokines.
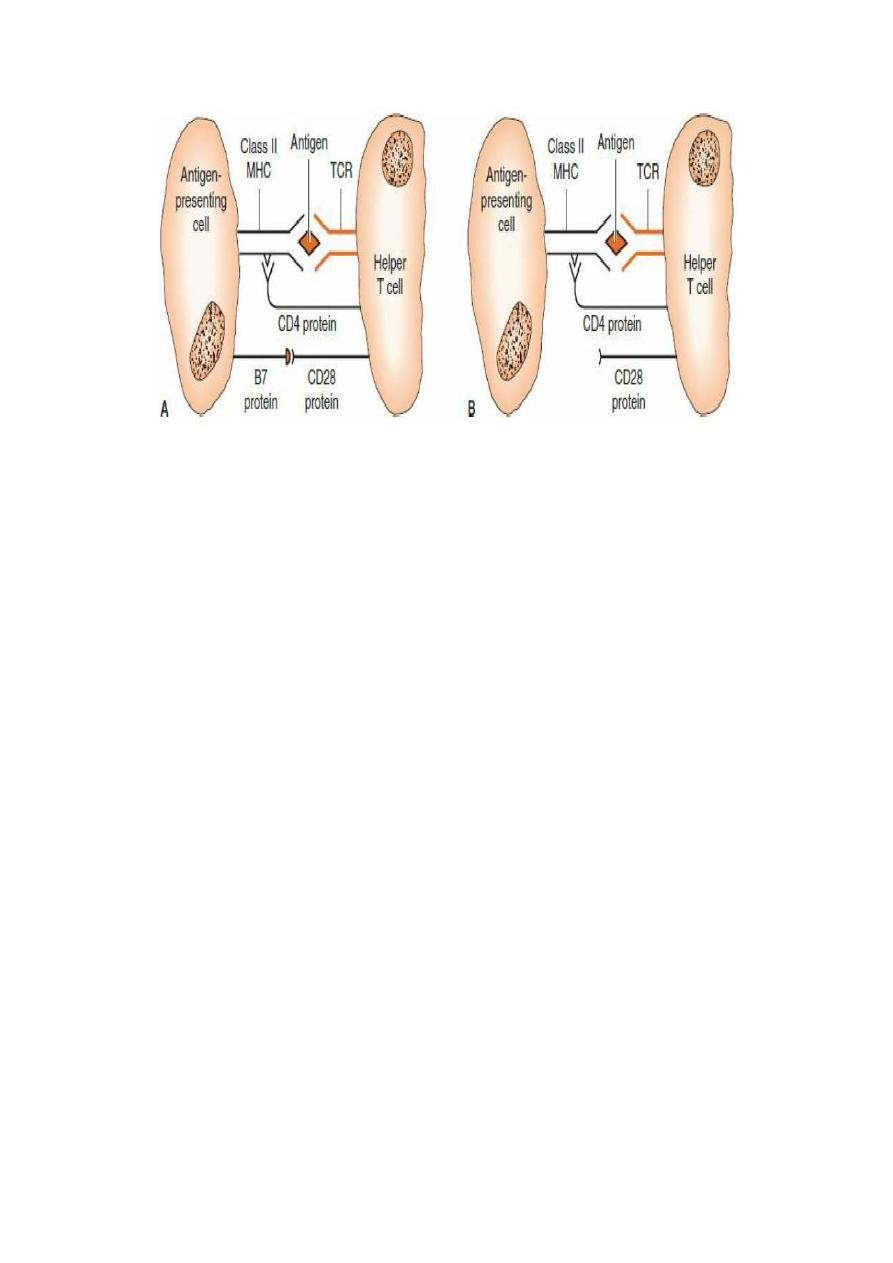
Clonal anergy outside the thymus. A: B7 protein on the antigen presenting cell
interacts with CD28 on the helper T cell, and full activation of the helper T cell
occurs. B: B7 protein on the antigen-presenting cell is not produced; therefore,
CD28 on the helper T cell does not get a costimulatory signal. Anergy of the helper T
cell occurs despite interaction of the T-cell receptor (TCR) with the antigen.
Regulatory CD4 T cells (TReg cells)
In T cells, a third mechanism for maintaining tolerance, is through the
activity of regulatory T cells (TReg cells).
The TReg can be divided into two groups: thymus-derived natural TReg
(n TReg) cells and Periphery-induced adaptive TReg cells.
Both populations express FOXP3 and suppress immune responses
through contact-dependent mechanisms and the production of soluble
factors, including the cytokines transforming growth factor (TGF)-β, IL-
10 and IL-35.
Regulatory CD4 T cells (TReg cells)

B-cell tolerance
B cells become tolerant to self by two mechanisms:
(1) clonal deletion, while the B-cell precursors are in the bone marrow.
B cells bearing an antigen receptor for a self protein can escape clonal
deletion (apoptosis) underwent a process called receptor editing. In this
process, a new, different light chain is produced that changes the
specificity of the receptor so that it no longer recognizes a self protein.
(2) clonal anergy of B cells in the periphery.
Notes
• T-cell tolerance is the most important.
• Tolerance in B cells is less complete than in T cells.
• It is estimated that as many as 50% of self-reactive B cells undergo
receptor editing.
• T cells do not undergo receptor editing.
• Breakdown of self-tolerance results in autoimmunity.
• Most autoimmune diseases are mediated by antibodies.

Autoimmunity and Autoimmune Diseases
Autoimmunity
Autoimmunity is defined as the presence of autoreactive T and / or B
lymphocytes in the periphery.
It is mainly caused by the fact that the central tolerance mechanisms,
which are responsible for counter-selection of autoreactive lymphocytes,
are not perfect.
A limited number of these autoreactive cells can mature and enter the
periphery
Autoimmune diseases
Autoimmune diseases is defined as a clinical syndrome caused by
activation of T cells or B cells, or both specific for self reactive antigens,
that upon activation lead to chronic inflammation and often irreversible
structural and functional damage.
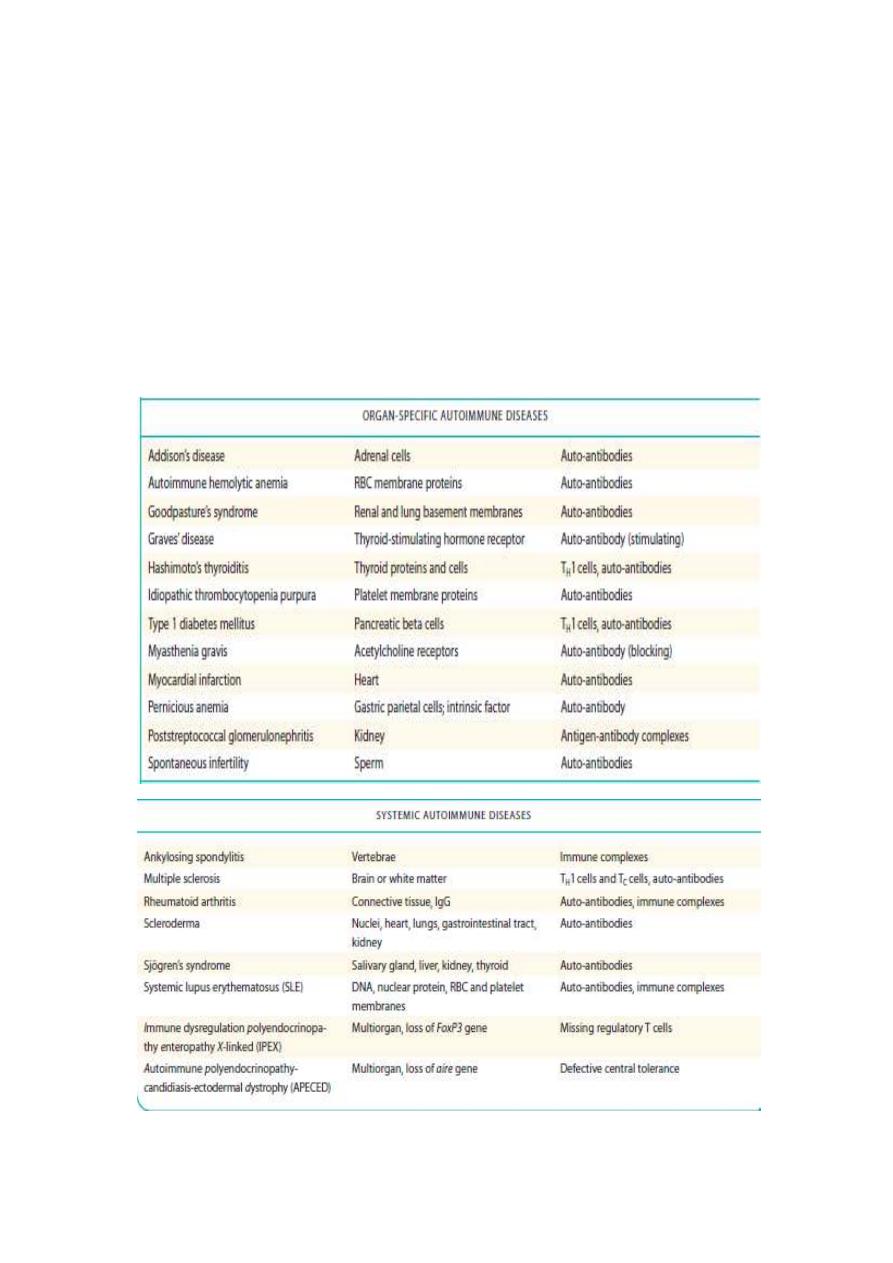
There are two types of autoimmune diseases including, organ-specific
(affect particular targets in the body), whereas systemic diseases engage
multiple organs.
Factors that increases the risk for auto immune diseases
• Genetic and environmental influences
• Polymorphisms associated with HLA, autoantigens, cytokines,
cytokine receptors, etc..,
• Seventy five percent of autoimmune disease occurs in females.
• Twin studies reinforce the importance of genetic contributions but
also indicate a strong environmental influence.
• Both microbial and non-microbial environmental factors are
implicated.
Mechanisms Underlying Susceptibility to Autoimmunity
1. Incomplete induction of tolerance in the thymus to peripherally
expressed autoantigens.
2. Impaired clearance and tolerance induction by apoptotic cells.
3. Defective production of regulatory T cells (FOXP3 deficiency).
4. Cytokine imbalances are often seen in autoimmune disease.
5. The development of high affinity mutated antibodies and immune
responses.

Diagnosis of autoimmune diseases
1. Complete blood counts and blood film.
2. General inflammatory indices as ESR, CRP
3. Serological techniques: detection of the autoimmune antibodies by
ELISA or Immunofluorescent and agglutination tests.
4. Immune histochemistry to stain the deposited immune complexes.
5. Measurement of complement activity and complement factors .
Treatment
Ideally, treatment for autoimmune diseases should reduce only the
autoimmune response.
The current therapies to treat autoimmune disease fall into two categories:
1. Broad spectrum immunosuppressive treatments.
2. Cell-type-specific strategies
Broad-Spectrum Therapies
Not cures but merely palliatives, reducing symptoms to provide the
patient with an acceptable quality of life.
Most general immunosuppressive treatments (e.g., corticosteroids,
azathioprine,
cyclophosphamide).
Cell-type-specific strategies
Used to treat autoimmune disorders target T cells or their products
because these cells are either directly pathogenic or provide help to
autoreactive B cells.
Monoclonal antibody (mab) against the B-cell-specific antigen CD20
(Rituximab) depletes a subset of B cells and provides short-term benefit
for RA.
Anti-TNF alpha mab(Infliximab). Anti-IL2 and anti-IL2R mabs.
Complement blocking as anti-c5 mabs.
Reference: Clinical immunology principle and practice 5
th
Edition.
