Coll. Medicine \3rd stage
Parasitologyprof. Dr. Amal KH. KH.
Coll. Medicine \3rd stage
Parasitologyprof. Dr. Amal KH. KH.
Lec.5
HaemoflagellatesTrypanosomes :
Trypanosomes are hemoflagellate protozoa. Two distinctly forms of genus Trypansoma occur in humans. They cause African trypanosomiasis (or African sleeping sickness) and American typanosomiasis respectively.
The complex Trypanosoma brucei have two subspecies that are morphologically indistinguishable cause distinct disease patterns in humans:
T. b. gambiense causes West African sleeping sickness .
T. b. rhodesiense causes East African sleeping sickness.
The protozoan parasite, Trypanosoma cruzi, causes American typansosmiasis (or Chagas' disease), that can be transmitted to humans by blood-sucking reduviid bugs.
Trypanosoma brucei
African trypanosomiasis – sleeping sicknessT. brucei gambiense (Gambian sleeping sickness) is seen in western and central parts of equatorial Africa and T. brucei rhodesiense (Rhodesian sleeping sickness) in east Africa . approximately 20.000 cases are reported each year. T. brucei gambiense and T. brucei rhodesiense are similar in all aspect except their geographic distribution and clinical manifestation . T. rhodesiense , which could infect man, in whom it caused an acute disease; and T. gambiense, also infective to man but producing a much more chronic disease. T. b. gambiense and T. b. rhodesiense parasites inhabit the connective tissue. In man and other vertebrate hosts, these are found in the blood stream, lymph nodes and cerebrospinal fluid.
T. brucei gambiense T. brucei rhodesiense
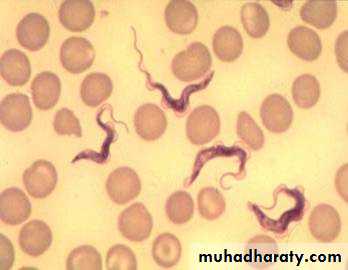
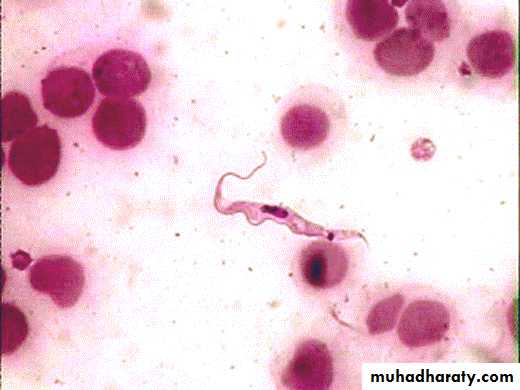
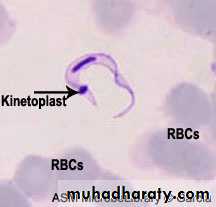
Morphology :The parasite exists in two forms , trypomastigotes and epimastigotes . trypomastigotes are seen in the vertebrates as well as in the insect host (see the next diagram). trypomastigotes show two morphological variations . one of these is a long , slender variant which is present extracellularly in blood , lymph and tissue fluids . these variants multiply by longitudinal binary fission and survive in the tissue of the vertebrate host due to their ability to change the outer variant surface glycoprotein (VSG) coat . each VSG is immunogenic but antigenically different from the preceding VSG and is changed every 8 to 10 days to evade the immune response . in the insect vector , these long ,slender , trypomastigotes multiply in the mid – gut .
These long ,slender , trypomastigotes after some multiplication cycles convert into , short , stumpy , non dividing forms . in human and other vertebrates they are present in blood, lymph and tissue fluid . these forms are infective to the insects host . the short , stumpy , non dividing trypomastogotes are also seen in the salivary of the insect hosts, where they called metacyclic trypomastigotes . these metacyclic trypomastigotes are infective to human beings and other vertebrates .
The other forms of T. brucei gambiense and T. brucei rhodesiense are the epimastigotes . epimastigotes are seen in the insect host . these forms multiply by longitudinal fission in the salivary gland and ultimately produced metacyclic trypomastigotes .
Life cycle :
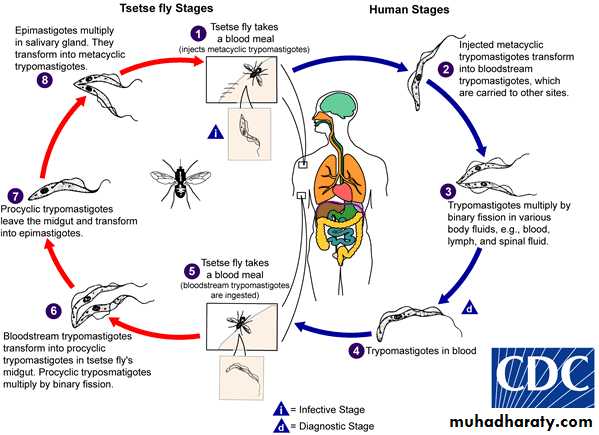
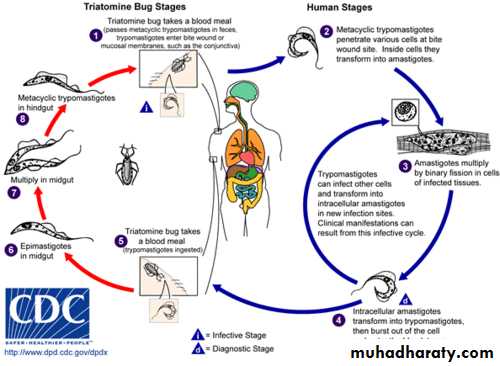
T. brucei gambiense and T. brucei rhodesiense have a complex life cycle . they required to two host to complete their life cycle . they do not have a mode of sexual reproduction . human and other vertebrates are the vertebrate host and insects are the invertebrate hosts . Human is only vertebrate host to T. brucei gambiense . human and wild mammals are the vertebrate host of T. brucei rhodesiense. Tsetse flies (Glossina sp) act as invertebrate hosts . Glossina palpalis for T. brucei gambiense , while Glossina morsitans for T. brucei rhodesiense .
The infective form for human and other vertebrates are the metacyclic trypomastigotes . they are introduced in the tissue of host by the bite of an infected tsetse flies . metacyclic trypomastigotes convert into long, slender trypomastigotes which is multiply locally as well as in blood . the short ,stumpy , non dividing trypomastigotes formed after some multiplication cycles are sucked by the tsetse flies during their blood meal . these forms reach the mid -gut and convert themselves into procyclic trypomastigotes . these procyclic trypomastigotes multiply in mid gut and reach salivary gland where they change into epimastigotes. Epimastigotes, in turn, multiply and produce metacyclic trypomastigotes . when these metacyclic trypomastigotes reach a new vertebrate host , the life cycle of T. brucei gambiense and T. brucei rhodesiense is completed (see the following diagram).
Pathogenesis and clinical manifestation :
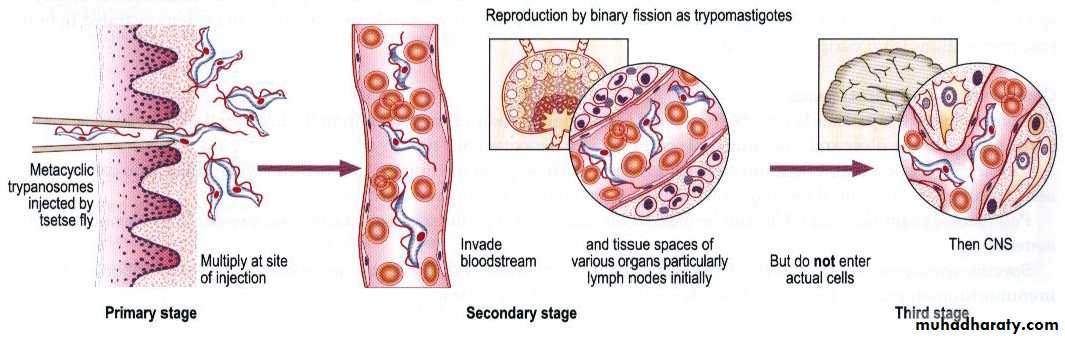
Infection occurs in 3 stages: A Trypanosomal chancre can develop on the site of inoculation. This is followed by a haematolymphatic stage with symptoms that include fever, lymphadenopathy, and pruritus. In the meningoencephalitic stage , invasion of the central nervous system can cause headaches, somnolence, abnormal behavior, and lead to loss of consciousness and coma. The course of infection is much more acute with T. b. rhodesiense than T. b. gambiense .Chancre :
Trypansomal chancre is an acute inflammatory local response seen in a week or so after the bite of infected tsetse fly. It is large, red and rubbery. It is more frequently seen in Rhodesian trypanosomiasis. It shows an intense inflammatory infiltration, vasodilatation and interstitial oedema. The chancre tissue is filled with parasites. A painful trypansomal chancre appears within a few days at the site of bite and resolves spontaneously within several weeks. It is characterized by erythema, swelling and local tenderness.Haematolymphatic stage :
In the early stage of the disease, after development of the chancre, infection of the blood and lymph system results in a more or less acute febrile illness. Infected lymph glands, especially those at back of the neck, may become very enlarged; the swollen cervical glands constitute ”Winterbottom’s sign”, a classical diagnostic indication of T. b. gambiense . Oedema, hepatosplenomegaly and tachycardia are other frequent findings.
Meningoencephalitic stage:
More serious effects results from the penetration of the parasites into the CNS, which may occur at any time from weeks (T. b. rhodesiense ) to years (T. b.gambiense ) after initial infection. Here the parasites multiply in the blood vessels, tissue fluids and cerebrospinal fluid (CSF). The outcome of the inflammatory process (meningoencephalitis) is brain damage leading to somnolence , coma and, unless treated, death in almost all cases.
There are differences between the clinical manifestations of East African and West African trypanosomiasis. In T. b. rhodesiense (East African trypanosomiasis), there is usually little obvious glandular involvement and Winterbottom’s sign may not be present; weight loss is rapid, and CNS is involved early. Untreated persons usually die within 9 months to a year after onset of disease. The incubation period is commonly short. In T. b. gambiense (West African trypanosomiasis) chronic CNS disease developed.
Diagnosis:
The diagnosis rests upon demonstrating trypanosomes by microscopic examination of chancre fluid, lymph node aspirates, blood, bone marrow, or, in the late stages of infection, cerebrospinal fluid. A wet preparation should be examined for the motile trypanosomes, and in addition a smear should be fixed, stained with Giemsa (or Field), and examined. Concentration techniques can be used prior to microscopic examination.Treatment :
Treatment should be started as soon as possible and is based on the infected person’s symptoms and laboratory results. The drug regimen depends on the infecting species and the stage of infection. Pentamidine isethionate and suramin are the drugs of choice to treat the hemolymphatic stage of West and East African Trypanosomiasis, respectively. Melarsoprol is the drug of choice for late disease with central nervous system involvement (infections by T.b. gambiense or T. b. rhodiense ).
Prevention :
avoiding areas harbouring tsetse flies .
using a protective clothing and insect repellants .
vaccines is not available.
Trypanosoma cruzi
Trypanosoma cruzi , causes Chagas Disease that can be transmitted to humans by blood-sucking reduviid bugs . Chagas disease (South American trypansomiasis) is commonly seen in the countries of South America , it is also occur in central part of America.Morphology :
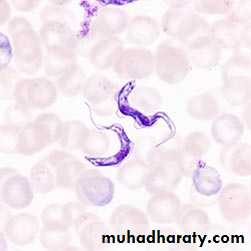
T. cruzi exist in four forms amastigotes , promastigote , epimastigotes, and trypomastigotes . Amastigote live in muscles of heart and skeletal system , nerve cell and cell of reticulo- endothelial system. This form is multiplying for promastigote , epimastigotes, and trypomastigotes. Trypomastigotes appears in peripheral blood from time to time and do not multiply in human. Trypanomastigotes are usually C-shaped and slender .
Life cycle :
T. cruzi has a complex life cycle . humans are the vertebrate hosts while reduviid bugs (Triatoma infestans) are the invertebrate host. Infective stage of the parasite to humans are the metacyclic trypomastigotes . metacyclic trypomastigotes are deposited along with the feaces of the reduviid bugs near the bite wound . these infective forms are then rubbed into the wound by the bitten person or transferred to his conjunctiva through contaminated finger . these forms enter the cells of reticulo- endothelial system and spread all over the body . in these cells metacyclic trypomastigotes are converted to amastigote . Amastigotes multiply and developed into trypomastigotes after passing through the successive stages of promastigotes and epimastigotes . trypomastigotes are released in the blood .When a reduviid bugs bites such a person , the trypomastigotes taken up along with blood meal . trypomastigotes transformed into epimastigotes that multiply and migrate to the hind gut . epimastigotes finally develop into the metacyclic trypomastigotes . the life cycle is thus completed when a new vertebrate hosts are infected with these metacyclic trypomastigotes . T. cruzi also acquired through blood transfusion and placenta .
Pathogenesis and clinical features :
Trypomastigotes may induced a local inflammatory reaction and swelling at the site of its entry . A Chagoma develops on skin while Rommana ُs sign develop as a results of unilateral oedematous swelling of eyelids .Intercellular multiplication of amastigotes damages the cells at various sites . Chagas disease can be either acute or chronic . the incubation period is generally from 1 to 2 weeks and the patient develops fever and generalized non pitting oedema in the acute form. It last for 3 to 4 weeks and may end fatally with myocardiatis or meningoencephalitis . the chronic form present with cardiological , neurological or visceral manifestation . complete heart block or brain damage may cause sudden death. pathologies of the digestive tract such as megaesophagus and megacolon; and weight loss. Chronic Chagas’ disease and its complications can be fatal. Neurons are particularly vulnerable to destruction. If the intracellular groups of parasite are concentrated in parts of gastrointestinal tract, especially in oesophagus or colon, peristalsis may be interfered with and the organ may become hugely distended. This condition is indicated by the prefix mega; for example megaoesophagus or megacolon . The unfortunate patient may be unable to swallow and die of starvation. Megacolon may become so gross as to lead to rupture of colon and death.
Laboratory diagnosis :
Microscopic examination of stained blood film show the trypomastigotes .
Xenodiagnosis , non infected reduviid bug is allowed to feed on a suspected patient then the faces of this bug are examined for trypomastigotes after 2 weeks.
Treatment and control :
Nifurtimox is the drug of choice , benznidazole is also useful. Prevention is achieved by :eradication of reduviid bug nets.
Construction of homes lacking cracks and crannies (the bug live there) .