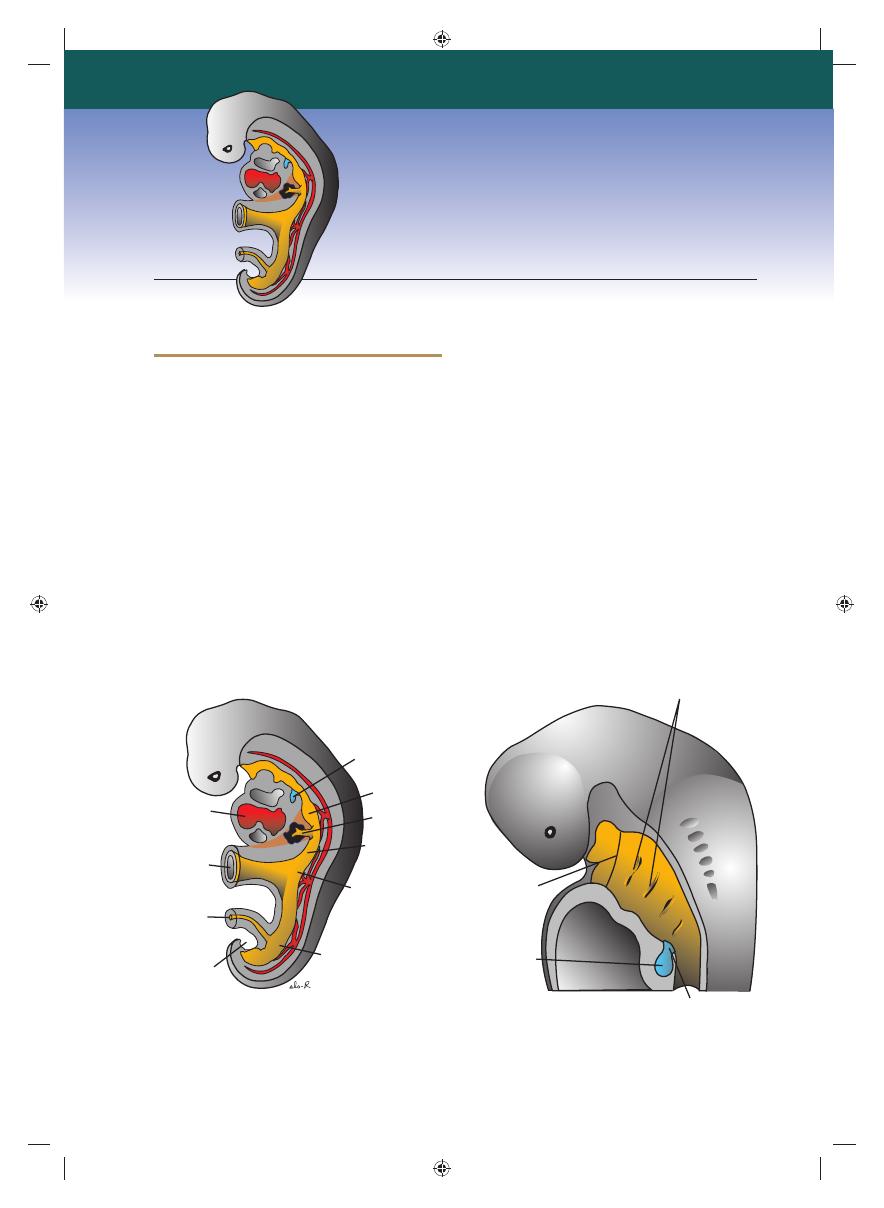
201
FORMATION OF THE LUNG BUDS
When the embryo is approximately 4 weeks old,
the respiratory diverticulum (lung bud)
appears as an outgrowth from the ventral wall
of the foregut (Fig. 14.1A). The appearance and
location of the lung bud are dependent upon an
increase in retinoic acid (RA) produced by
adjacent mesoderm. This increase in RA causes
upregulation of the transcription factor TBX4
expressed in the endoderm of the gut tube at
the site of the respiratory diverticulum. TBX4
induces formation of the bud and the continued
growth and differentiation of the lungs. Hence,
epithelium
of the internal lining of the lar-
ynx, trachea, and bronchi, as well as that of the
lungs, is entirely of endodermal origin. The
cartilaginous, muscular
, and connective tis-
sue
components of the trachea and lungs are
derived from splanchnic mesoderm surround-
ing the foregut.
Initially, the lung bud is in open commu-
nication with the foregut (Fig. 14.1B). When
the diverticulum expands caudally, however,
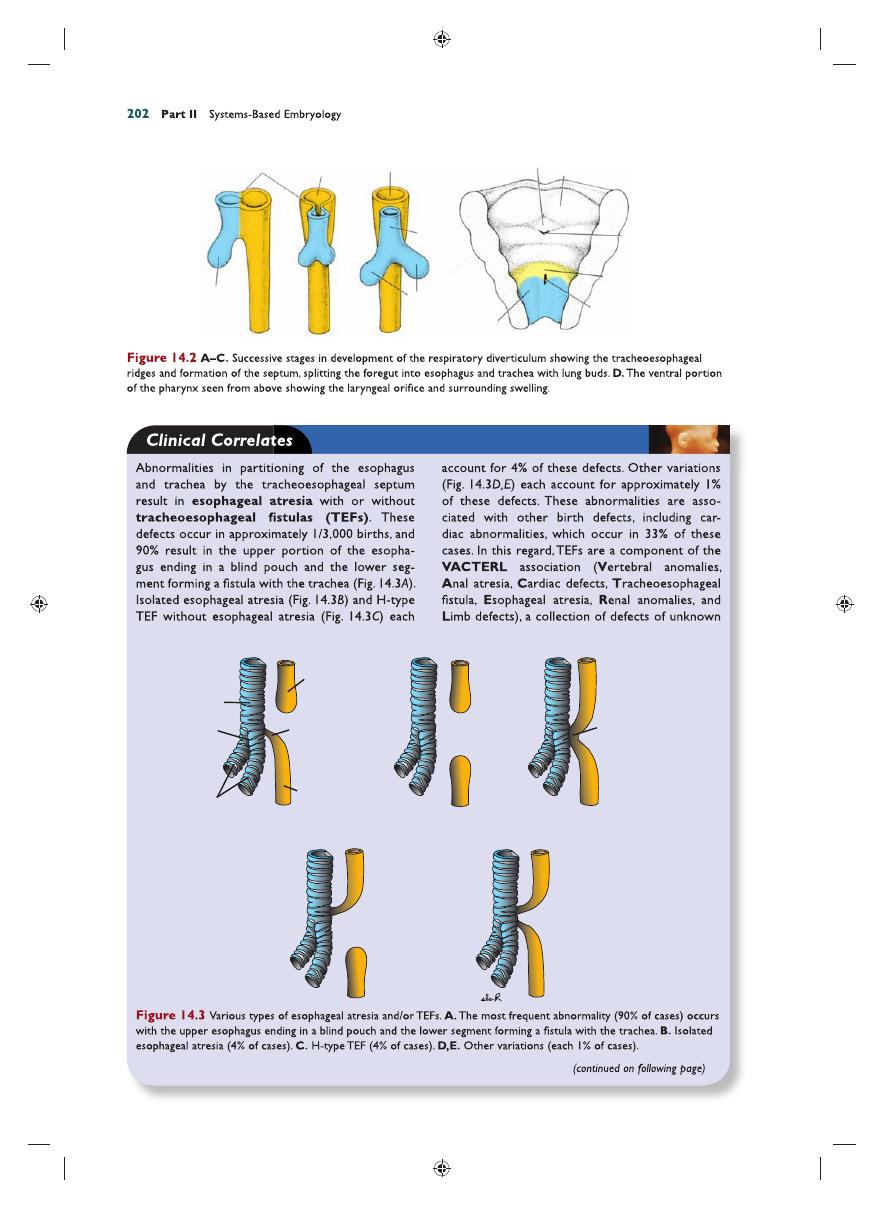
two longitudinal ridges, the tracheoesoph-
ageal ridges
, separate it from the foregut
(Fig. 14.2A). Subsequently, when these ridges
fuse to form the tracheoesophageal septum,
the foregut is divided into a dorsal portion, the
esophagus
, and a ventral portion, the trachea
and lung buds (Fig. 14.2B,C). The respiratory
primordium maintains its communication with
the pharynx through the laryngeal orifi ce
(Fig. 14.2D).
Chapter
14
Respiratory System
Openings of
pharyngeal pouches
Laryngotracheal
orifice
Respiratory
diverticulum
Respiratory
diverticulum
Heart
Vitelline
duct
Allantois
Cloacal
membrane
A
B
Attachment of
buccopharyngeal
membrane
Hindgut
Liver bud
Duodenum
Midgut
Stomach
Figure 14.1
A. Embryo of approximately 25 days’ gestation showing the relation of the respiratory diverticulum to the
heart, stomach, and liver. B. Sagittal section through the cephalic end of a 5-week embryo showing the openings of the
pharyngeal pouches and the laryngotracheal orifi ce.
Sadler_Chap14.indd 201
Sadler_Chap14.indd 201
8/26/2011 4:17:37 AM
8/26/2011 4:17:37 AM
Lateral lingual swelling
Respiratory
diverticulum
Foramen
cecum
Tuberculum impar
Lung
buds
Trachea
Esophagus
Tracheoesophageal
ridge
Foregut
Epiglottal
swelling
Laryngeal
orifice
I
I
II
II
IV
A
C
B
VI
Laryngeal
swellings
D
A
E
B
D
C
Trachea
Bifurcation
Bronchi
Tracheoesophageal
fistula
Distal part of
esophagus
Proximal blind-
end part of
esophagus
Communication
of esophagus
with trachea
Sadler_Chap14.indd 202
Sadler_Chap14.indd 202
8/26/2011 4:17:37 AM
8/26/2011 4:17:37 AM
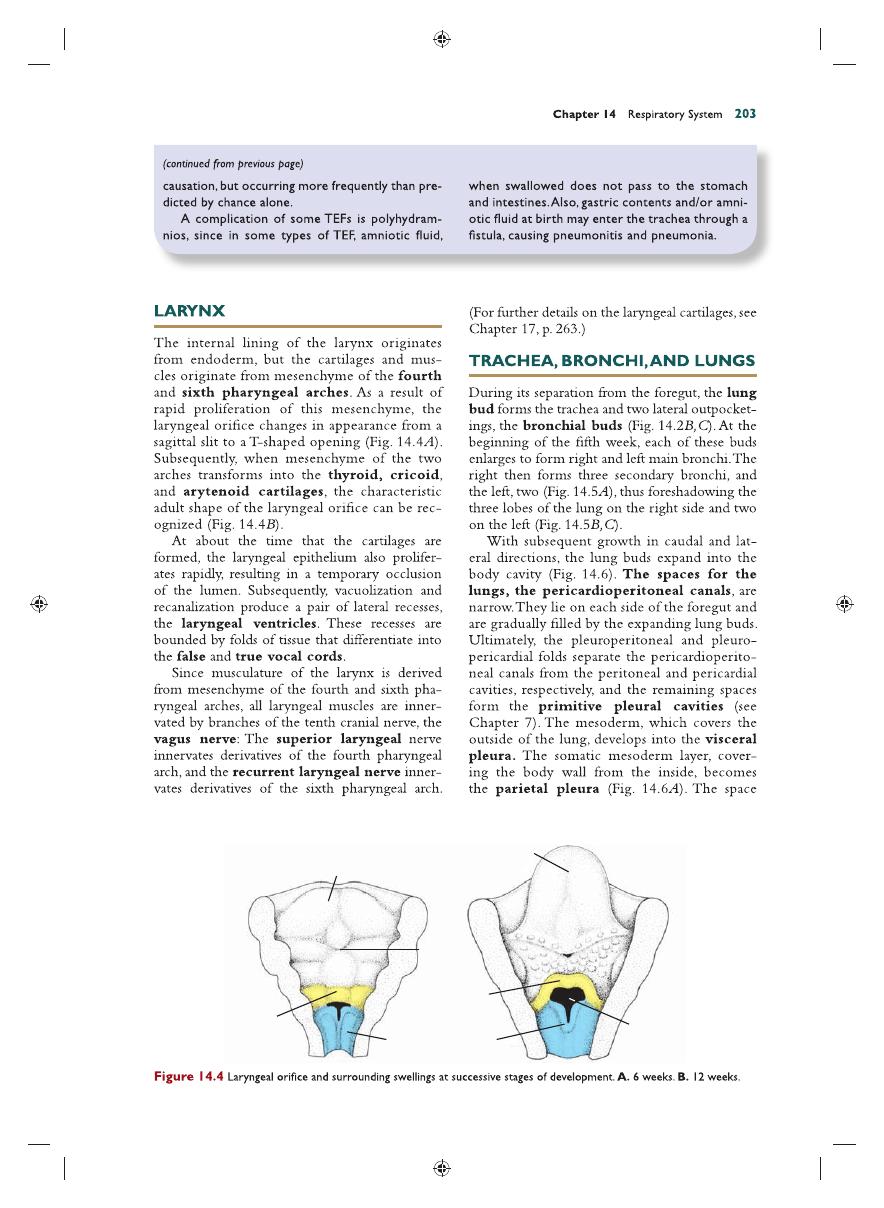
Lingual swelling
Body of tongue
Foramen
cecum
Epiglottis
Epiglottal
swelling
Arytenoid swellings
Laryngeal
orifice
l
ll
lll
lV
Vl
B
A
Sadler_Chap14.indd 203
Sadler_Chap14.indd 203
8/26/2011 4:17:40 AM
8/26/2011 4:17:40 AM
204
Part II Systems-Based Embryology
between the parietal and visceral pleura is the
pleural cavity
(Fig. 14.7).
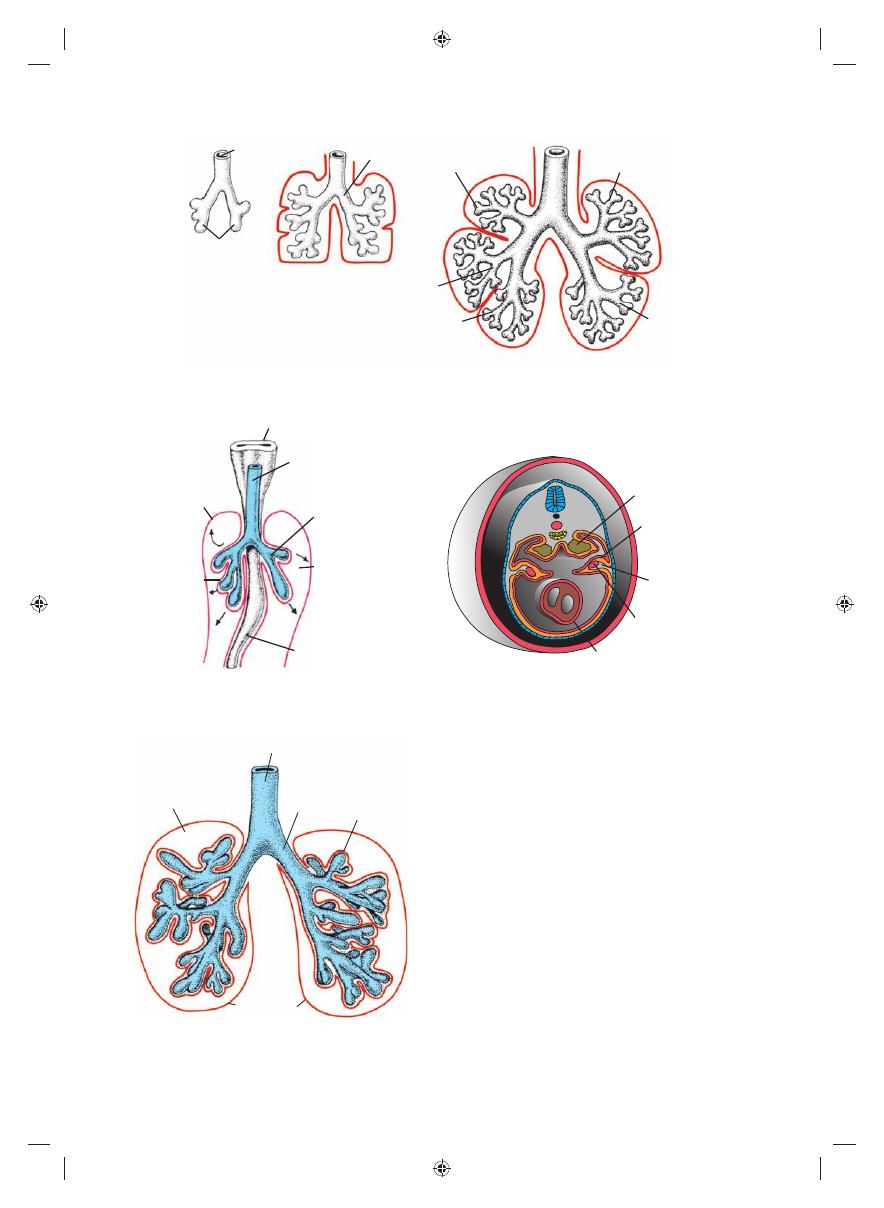
During further development, secondary
bronchi divide repeatedly in a dichotomous
fashion, forming 10 tertiary (segmental)
bronchi in the right lung and 8 in the left, cre-
ating the bronchopulmonary segments of
the adult lung. By the end of the sixth month,
approximately 17 generations of subdivisions
have formed. Before the bronchial tree reaches
its fi nal shape, however, an additional six divi-
sions form during postnatal life
. Branching
is regulated by epithelial-mesenchymal inter-
actions between the endoderm of the lung
buds and splanchnic mesoderm that surrounds
them. Signals for branching, which emit from
the mesoderm, involve members of the fi bro-
blast growth factor family. While all of these
new subdivisions are occurring and the bron-
chial tree is developing, the lungs assume a more
caudal position, so that by the time of birth, the
Lung
buds
Left bronchus
Right upper lobe
Left upper
lobe
Left
lower
lobe
Right
middle lobe
Right lower lobe
A
B
C
Trachea
Figure 14.5
Stages in development of the trachea and lungs. A. 5 weeks. B. 6 weeks. C. 8 weeks.
Lung bud
Pleuro-
pericardial
fold
Phrenic
nerve
Common
cardinal
vein
Heart
B
Pharynx
Trachea
Parietal
pleura
Visceral
pleura
Lung bud
Pericardioperitoneal
canal
Visceral peritoneum
A
Figure 14.6
Expansion of the lung buds into the pericardioperitoneal canals. At this stage, the canals are in communication
with the peritoneal and pericardial cavities. A. Ventral view of lung buds. B. Transverse section through the lung buds showing
the pleuropericardial folds that will divide the thoracic portion of the body cavity into the pleural and pericardial cavities.
Trachea
Bronchus Visceral
pleura
Pleural cavity
Parietal pleura
Figure 14.7
Once the pericardioperitoneal canals sepa-
rate from the pericardial and peritoneal cavities, respec-
tively, the lungs expand in the pleural cavities. Note the
visceral and parietal pleura and defi nitive pleural cavity. The
visceral pleura extends between the lobes of the lungs.
Sadler_Chap14.indd 204
Sadler_Chap14.indd 204
8/26/2011 4:17:41 AM
8/26/2011 4:17:41 AM
Chapter 14 Respiratory System
205
Respiratory
bronchiole
Lung
epithelium
Blood
capillaries
Thin
squamous
epithelium
Terminal
sacs
Flat endothelium
cell of blood
capillary
Respiratory
bronchiole
Terminal
bronchiole
A
B
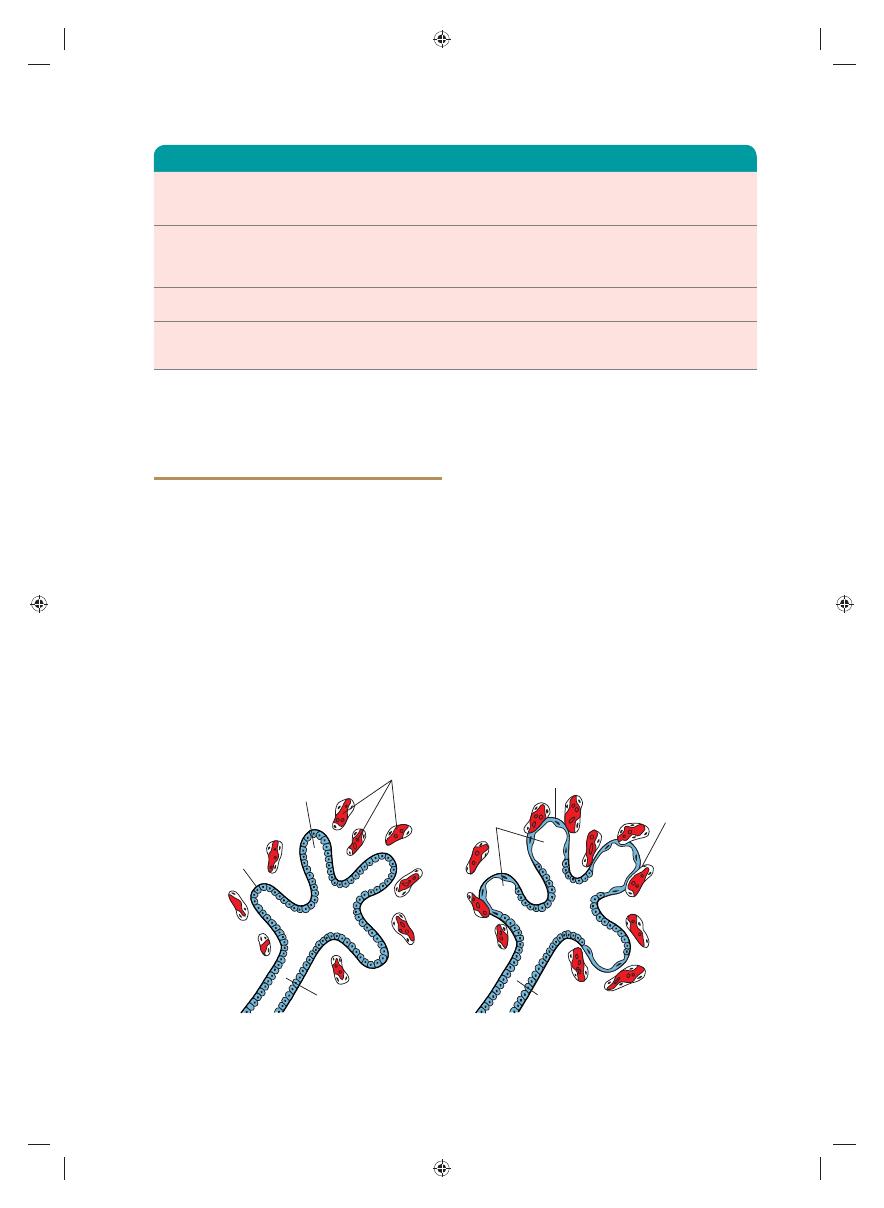
Figure 14.8
Histological and functional development of the lung. A. The canalicular period lasts from the 16th to the
26th week. Note the cuboidal cells lining the respiratory bronchioli. B. The terminal sac period begins at the end of the
sixth and beginning of the seventh prenatal month. Cuboidal cells become very thin and intimately associated with the
endothelium of blood and lymph capillaries or form terminal sacs (primitive alveoli).
bifurcation of the trachea is opposite the fourth
thoracic vertebra.
MATURATION OF THE LUNGS
Up to the seventh prenatal month, the bronchi-
oles divide continuously into more and smaller
canals (canalicular phase) and the vascular sup-
ply increases steadily (Fig. 14.8A). Terminal
bronchioles
divide to form respiratory bron-
chioles
and each of these divides into three to
six alveolar ducts (Fig. 14.8B). The ducts end in
terminal sacs (primitive alveoli)
that are sur-
rounded by fl at alveolar cells in close contact with
neighboring capillaries (Fig. 14.8B). By the end of
the seventh month, suffi cient numbers of mature
alveolar sacs and capillaries are present to guar-
antee adequate gas exchange, and the premature
infant is able to survive (Fig. 14.9) (Table 14.1).
During the last 2 months of prenatal life and
for several years thereafter, the number of termi-
nal sacs increases steadily. In addition, cells lining
the sacs, known as type I alveolar epithe-
lial cells
, become thinner, so that surrounding
capillaries protrude into the alveolar sacs
(Fig. 14.9). This intimate contact between
epithelial and endothelial cells makes up the
blood–air barrier. Mature alveoli
are not
present before birth. In addition to endothelial
cells and fl at alveolar epithelial cells, another cell
type develops at the end of the sixth month.
These cells, type II alveolar epithelial cells,
produce surfactant, a phospholipid-rich fl uid
capable of lowering surface tension at the air–
alveolar interface.
Before birth, the lungs are full of fl uid that
contains a high chloride concentration, little
protein, some mucus from the bronchial glands,
TABLE 14.1
Maturation of the Lungs
Pseudoglandular period
5–16 wk
Branching has continued to form
terminal bronchioles. No respiratory
bronchioles or alveoli are present.
Canalicular period
16–26 wk
Each terminal bronchiole divides into
two or more respiratory bronchioles,
which in turn divide into three to six
alveolar ducts.
Terminal sac period
26 wk to birth
Terminal sacs (primitive alveoli) form,
and capillaries establish close contact.
Alveolar period
8 mo to childhood
Mature alveoli have well-developed
epithelial endothelial (capillary)
contacts.
Sadler_Chap14.indd 205
Sadler_Chap14.indd 205
8/26/2011 4:17:42 AM
8/26/2011 4:17:42 AM

Thin squamous
epithelium
Blood
capillary
Lymph
capillary
Mature alveolus
Alveolar
duct
Respiratory bronchiole
Sadler_Chap14.indd 206
Sadler_Chap14.indd 206
8/26/2011 4:17:43 AM
8/26/2011 4:17:43 AM
Chapter 14 Respiratory System
207
Respiratory movements after birth bring air
into the lungs, which expand and fi ll the pleural
cavity. Although the alveoli increase somewhat in
size, growth of the lungs after birth is due pri-
marily to an increase in the number of respira-
tory bronchioles and alveoli. It is estimated that
only one-sixth of the adult number of alveoli
are present at birth. The remaining alveoli are
formed during the fi rst 10 years of postnatal life
through the continuous formation of new primi-
tive alveoli.
Summary
The respiratory system
is an outgrowth of the
ventral wall of the foregut, and the epithelium
of the larynx, trachea, bronchi, and alveoli origi-
nates in the endoderm. The cartilaginous, mus-
cular, and connective tissue components arise in
the mesoderm. In the fourth week of develop-
ment, the tracheoesophageal septum separates
the trachea from the foregut, dividing the foregut
into the lung bud anteriorly and the esophagus
posteriorly. Contact between the two is main-
tained through the larynx, which is formed by
tissue of the fourth and sixth pharyngeal arches.
The lung bud develops into two main bronchi:
the right forms three secondary bronchi and
three lobes; the left forms two secondary bronchi
and two lobes. Faulty partitioning of the foregut
by the tracheoesophageal septum causes esopha-
geal atresias and TEFs (Fig. 14.3).
After a pseudoglandular (5 to 16 weeks) and
canalicular (16 to 26 weeks) phase, cells of the
cuboidal-lined respiratory bronchioles change
into thin, fl at cells, type I alveolar epithelial
cells
, intimately associated with blood and lymph
capillaries. In the seventh month, gas exchange
between the blood and air in the primitive
alveoli
is possible. Before birth, the lungs are
fi lled with fl uid with little protein, some mucus,
and surfactant, which is produced by type II
alveolar epithelial cells
and which forms a
phospholipid coat on the alveolar membranes.
At the beginning of respiration, the lung fl uid
is resorbed except for the surfactant coat, which
prevents the collapse of the alveoli during expi-
ration by reducing the surface tension at the air–
blood capillary interface. Absent or insuffi cient
surfactant in the premature baby causes respi-
ratory distress syndrome (RDS)
because of
collapse of the primitive alveoli (hyaline mem-
brane disease)
.
Growth of the lungs after birth is primarily
due to an increase in the number of respiratory
bronchioles and alveoli and not to an increase in
the size of the alveoli. New alveoli are formed
during the fi rst 10 years of postnatal life.
Problems to Solve
1.
A prenatal ultrasound revealed polyhydram-
nios, and at birth, the baby had excessive
fl uids in its mouth. What type of birth defect
might be present, and what is its embryologi-
cal origin? Would you examine the child
carefully for other birth defects? Why?
2.
A baby born at 6 months’ gestation is having
trouble breathing. Why?
Sadler_Chap14.indd 207
Sadler_Chap14.indd 207
8/26/2011 4:17:45 AM
8/26/2011 4:17:45 AM
