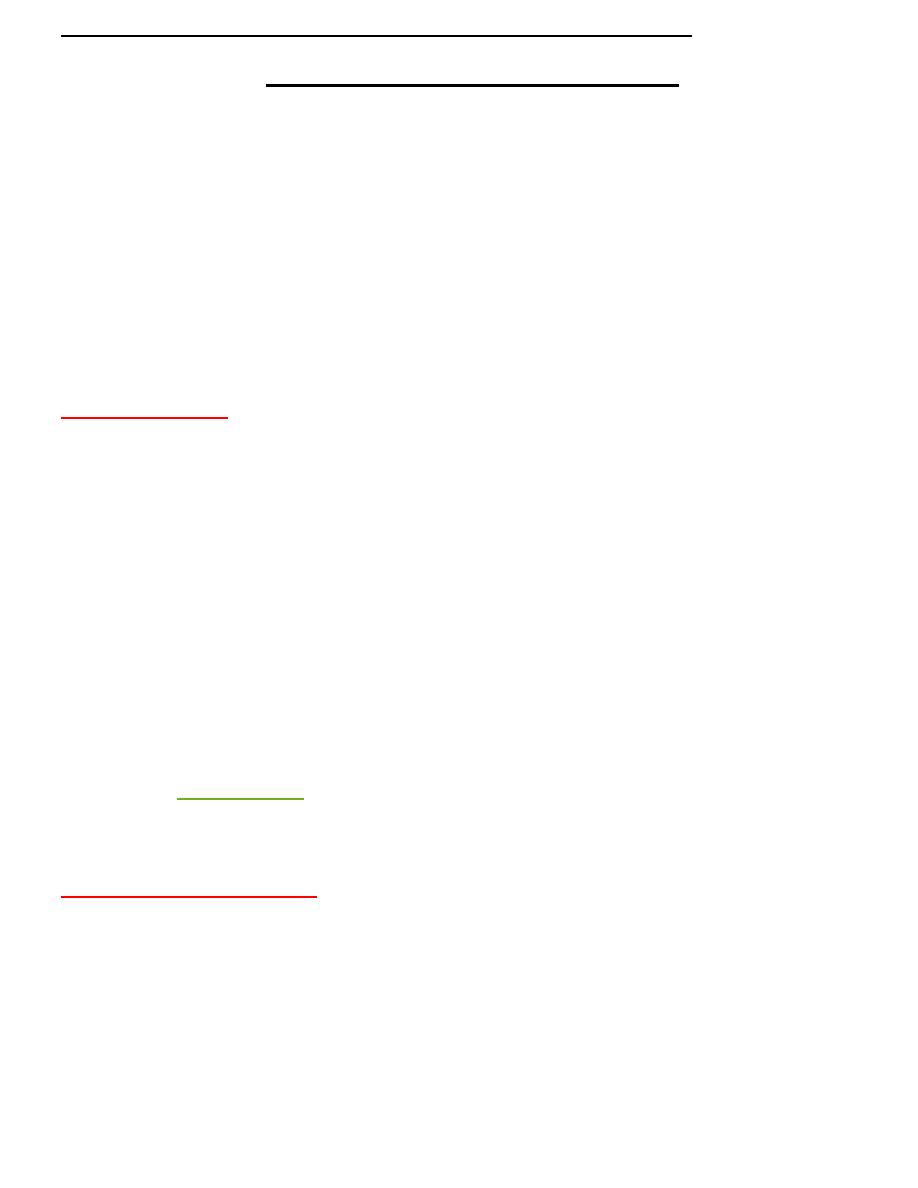
Practical medical chemistry first stage medicine college
Reducing and Non Reducing Sugar
Asst.prof: Idries.Muhson.Abeed
There are generally two types of sugar namely
reducing
and
non-reducing
sugar based on
their reducing property. All the
are reducing sugars as they have free
anomeric carbon in their structure (free aldehyde group or a free ketone group) that can
reduce cupric salt. However, in the
disaccharides
,
the monosaccharide unit is linked
together by glycosidic linkage formed between OH hydroxyl group of one and anomeric
carbon of others. Some disaccharides( sucrose ) , trisaccharide ( Raffinose) and poly
saccharides cannot reduce cupric salt due to no availability of free anomeric carbon in
their structure and hence they are termed as
non-reducing
sugars.
Benedict’s test
Benedict’s test is a chemical test that can be used to check for the presence of reducing
sugars in a given analyte. Therefore, simple carbohydrates containing a free ketone or
aldehyde
can be identified with this test. The test is based on Benedict’s
reagent (also known as Benedict’s solution), which is a complex mixture of sodium citrate,
sodium carbonate, and the pentahydrate of copper(II) sulfate.
When exposed to reducing sugars, the reactions undergone by Benedict’s reagent result in
the formation of a brick-red precipitate, which indicates a positive Benedict’s test. An
image detailing the changes in the colour of Benedict’s reagent (from clear blue to brick-
red) that are triggered by exposure to reducing sugars is provided below.
It can be noted that Benedict’s test can also be used to check for the presence of glucose in a
urine sample. Since this test detects any aldehydes and α-hydroxy ketones and glucose is an
aldose whose open-chain forms an aldehyde group, the test yields a positive result when
glucose is present in the analyte. However, a positive reaction can also be given by the
presence of
, homogentisic acid, and other reducing substances urine.
Therefore, a positive Benedict’s test does not necessarily imply that the test subject is
diabetic.
Benedict’s Test Principle
When a reducing sugar is subjected to heat in the presence of an alkali, Therefore, , the
cupric ions (Cu
2+
) in Benedict’s reagent are reduced to cuprous ions (Cu
+
). These cuprous
ions form copper(I) oxide with the reaction mixture and precipitate out as a brick-red
coloured compound.
the solution changes its color to orange-red/ brick red precipitate. The red-colored cuprous
oxide is insoluble in water and hence, separate out from the solution. When the
concentration of the reducing sugar is high in the solution, then the color becomes more
intense (reddish) and the volume of the precipitate increases in the test tube making it
clearly visible.
Objectives of Benedict’s Test
•
To determine the presence or absence of reducing sugar in the solution.
•
To determine the glucose concentration in the solution quantitatively.
Benedict’s solution
is a deep-blue alkaline chemical reagent used to test for the presence of
the aldehyde functional group -CHO which consists of copper sulfate pentahydrate (CuSO
4
.
5H
2
O), sodium carbonate (Na
2
CO
3
), sodium citrate (Na
3
C
6
H
5
O
7
) and distilled water.
Sodium carbonate renders alkaline conditions which are required for the redox reaction,
while sodium citrate is a complexing agent which complexes with the copper (II) ions to
avoid degradation into copper (I) ions during storage.
The procedure of Benedict’s Test
1. Pipette out 2 ml (10 drops) of Benedict’s reagent and placed it in the clean test tube
2. Approximately 1 ml of sample (urine) is added to Benedict’s reagent.
3. The test tube is placed over the boiling water bath for 3-5 minutes (can be heated
directly over flame).
4. Observe for color change in the solution of test tubes or precipitate formation.
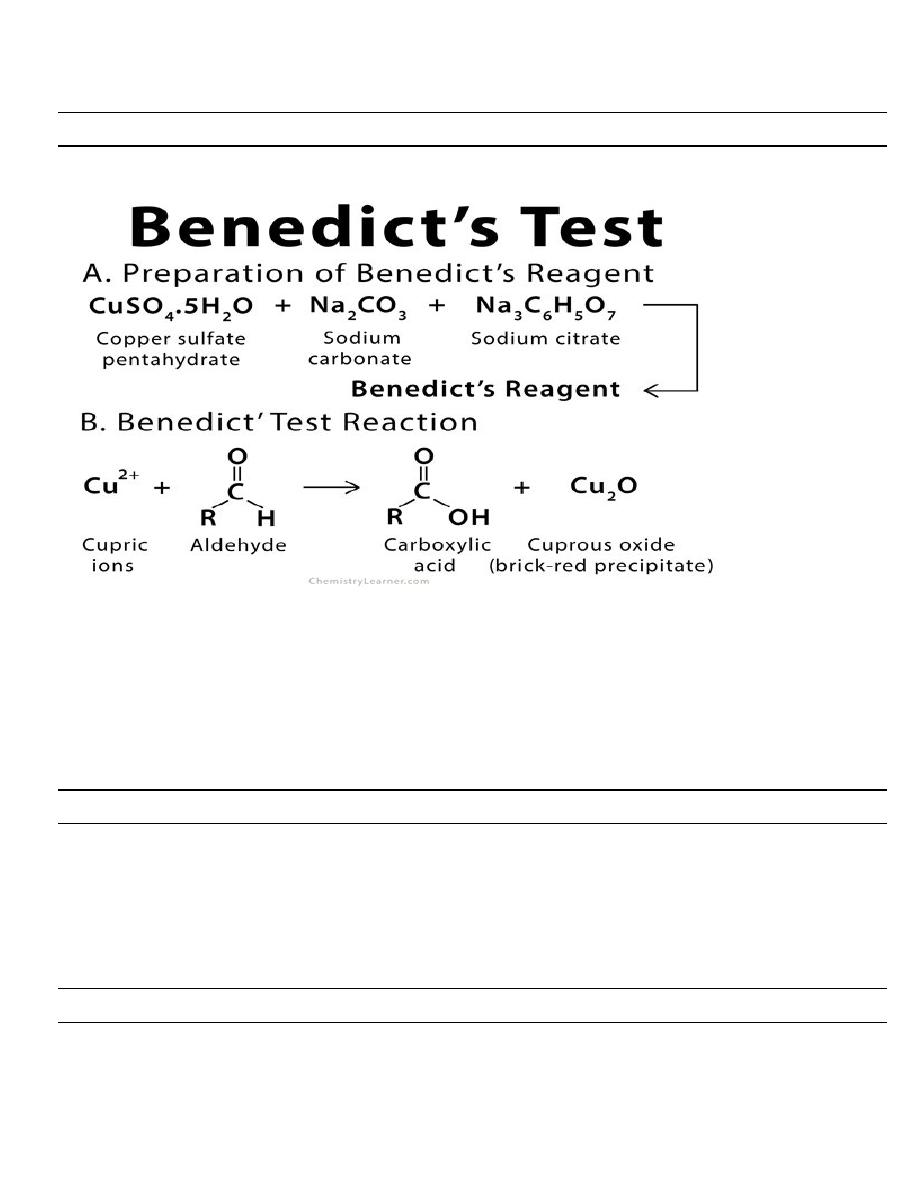
Observation (Results) of Benedict’s Test
Appearance of solution
The concentration of
reducing sugar (g%)
Interpretation
Brick red with heavy precipitate
2% or >2%
A large amount of
reducing sugar is present
Brownish orange with red precipitate
1.5%
A moderate amount of
reducing sugar is present
Yellow with precipitate
1%
A small amount of
reducing sugar is present
Greenish blue and cloudy
0.5%
Traceable amount of
reducing sugar is present
Greenish blue with yellow precipitate
0.25%
Traceable amount of
reducing sugar is present
Green with no precipitate
0.1%
Traceable amount of
reducing sugar is present
Blue color or cloudy
Nil
No reducing sugar is
present

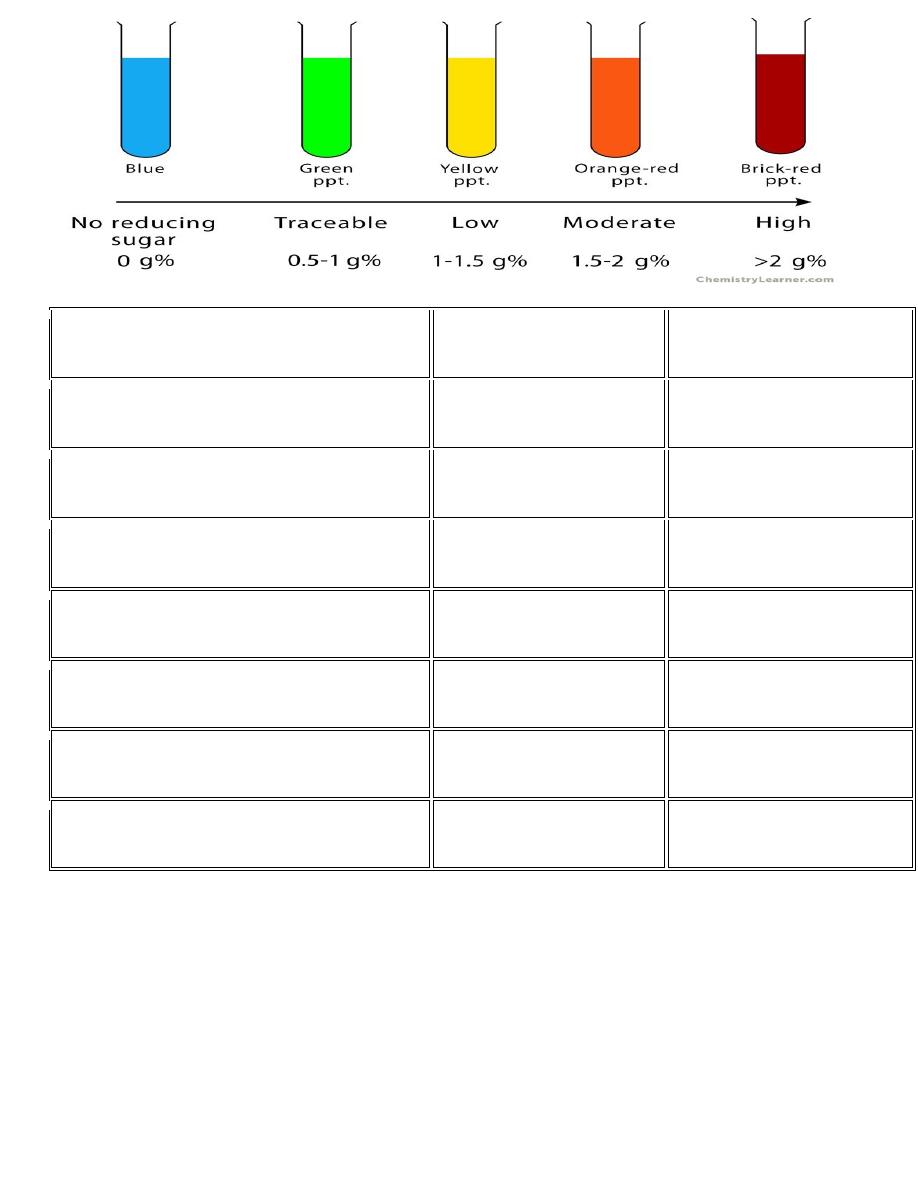
Iodine Test :
This test is specific for polysaccharides. This test is used to differentiate polysaccharides
from the rest of carbohydrates. It is given positive by starch and glycogen. It can also be
used to differentiate between glycogen, starch, and cellulose.
Principle;
The iodine test is based on the absorptive properties possessed by large polysaccharide
molecules. The glucose chains in most of polysaccharides are organized to form helices. The
space between the turns of the helix can hold small iodine molecules. This is seen with
amylase chains found in starch. Glycogen and amylopectin can also absorb these iodine
molecules on their surface. The absorptive property of polysaccharides decreases upon
heating.
This test is only given by starch. Starch reacts with
colour solution. On
heating
the blue colour disappears and on cooling the blue colour
reappears. The chemical reaction is given below.
Note: The appearance of blue colour solution confirms the presence of starch
.
Observations and Inference:
Test
Glucose
Lactose
Sucrose
Starch
Molisch’s test
Purple ring
Purple ring
Purple ring
Purple ring
Benedict’s test
Red precipitate
Red precipitate No precipitate
No precipitate
Tollen’s test
Appearance of
silver mirror.
Appearance of
silver mirror.
No silver mirror No silver mirror
Iodine test
No reaction
No reaction
No reaction
Appearance of
blue colour
solution.
Procedure of Iodine Test
1. Take 1 ml of a given sample( starch) in a clean, dry test tube.
2. Add about 2-3 drops of
Iodine solution
to the tube and mix them in a vortex.
3. Observe the appearance of color in the test tube.
4. Heat the test tube in the water bath until the color disappears.
5. Take the test tubes out for cooling
6. Note down the appearance of color seen in the test tubes.
Result and Interpretation of Iodine Test
•
The appearance of a blue-black or purple color represents a positive test, indicating the
presence of starch.
•
If there is no change in color, the result is negative and indicates the absence of starch.
Uses of Iodine Test
•
This test is used to detect the presence of starch in various samples.
•
Similarly, this test is performed to test the process of
Limitations of Iodine Test
•
This test cannot be performed under acidic conditions as the starch hydrolyses under
such circumstances.
•
This test is a qualitative test and doesn’t signify the concentration of starch.
