Assis. Prof. DR.MAHA SHAKIR
In this system we will discuss the following:Hyperemia and Congestion
HemorrhageEdema
Thrombosis
Disseminated Intravascular Coagulation
Embolism
Infarction
Shock
Hypermia and Congestion
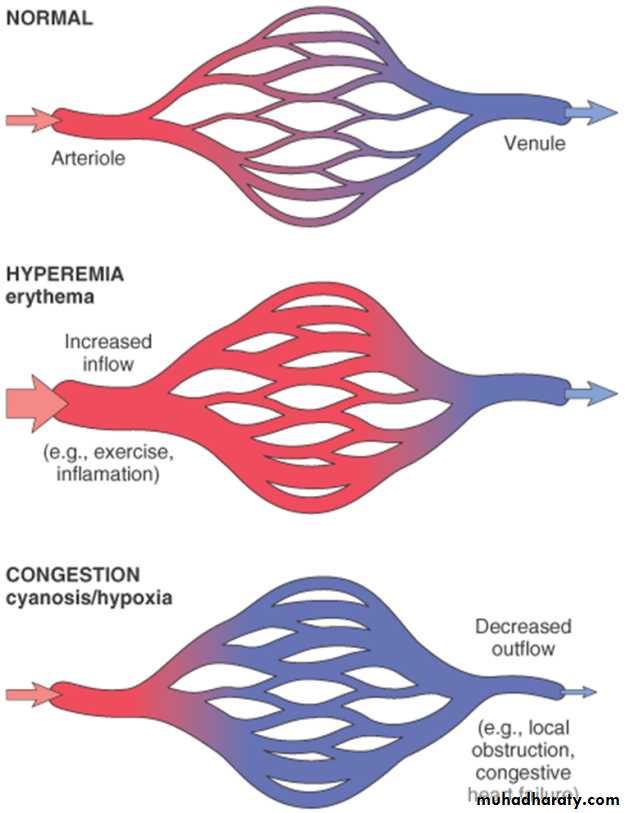
Definition:Both of them can be defined as a local increase in volume of blood in a particular tissue.
Hyperemia
It is an active process resulting from arteriolar dilation and increased blood inflow, as occurs at sites of:exercising skeletal muscle
or acute inflammation
Hyperemic tissues are redder than normal
because of engorgement with oxygenated blood.
Congestion
Congestion is a passive process resulting from impaired outflow of venous blood from a tissue. It can occur:systemically, as in cardiac failure,
or locally as a consequence of an isolated venous obstruction.
• Congested tissues have an abnormal blue-red color (cyanosis) that stems from the accumulation of deoxygenated hemoglobin in the affected area.
In long-standing chronic congestion, inadequate tissue perfusion and persistent hypoxia may lead to:
parenchymal cell death.
secondary tissue fibrosis.
the elevated intravascular pressures may cause edema or sometimes rupture capillaries forming focal hemorrhage.
MORPHOLOGY
Cut surfaces of hyperemic or congested tissuesfeel wet and
typically ooze blood.
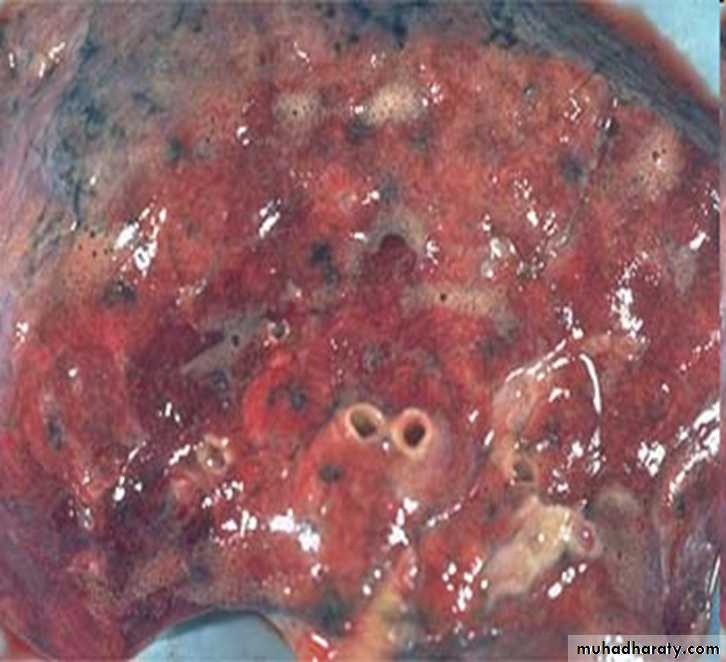
• Pulmonary congestion:
Acute
chronic
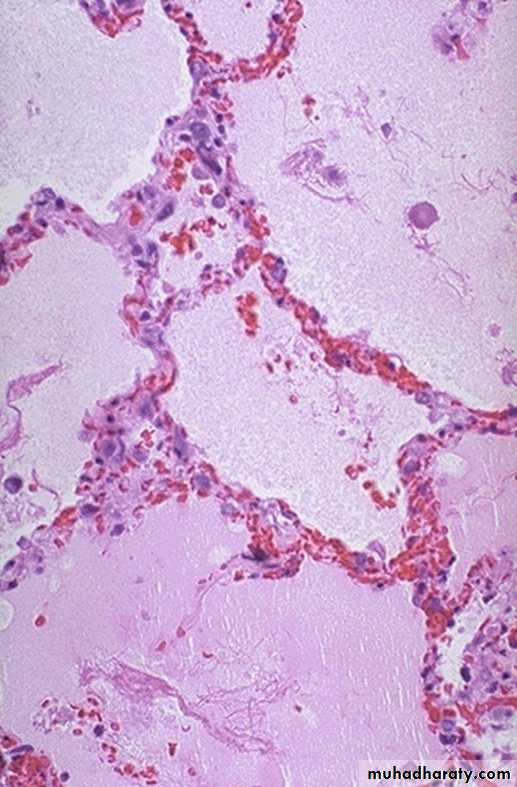
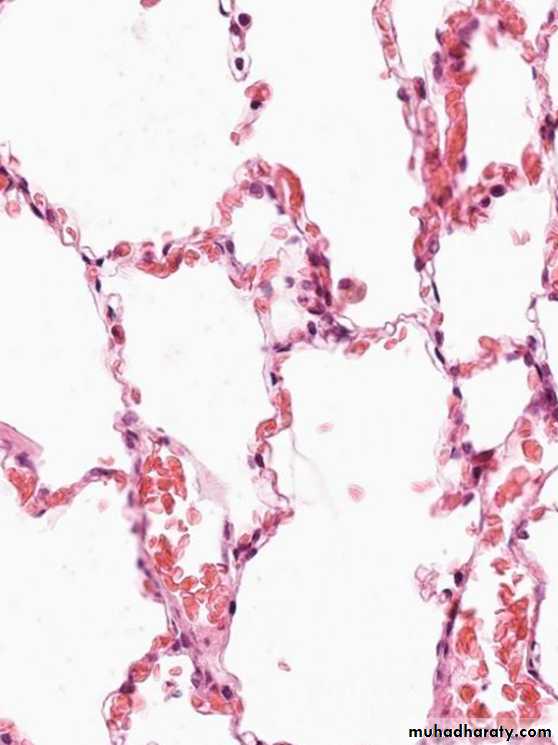
1.Acute pulmonary congestion:
Alveolar capillaries engorged with blood
variable degrees of alveolar septal edema and intraalveolar hemorrhage.
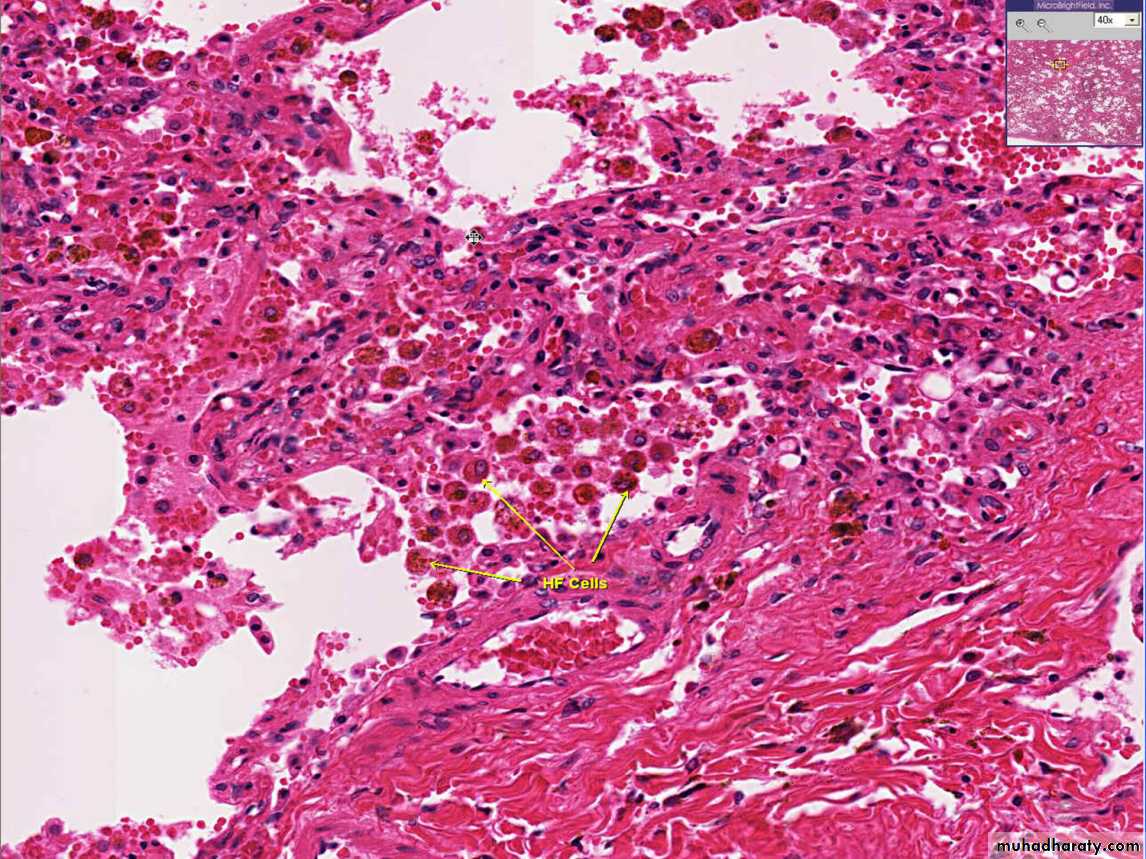
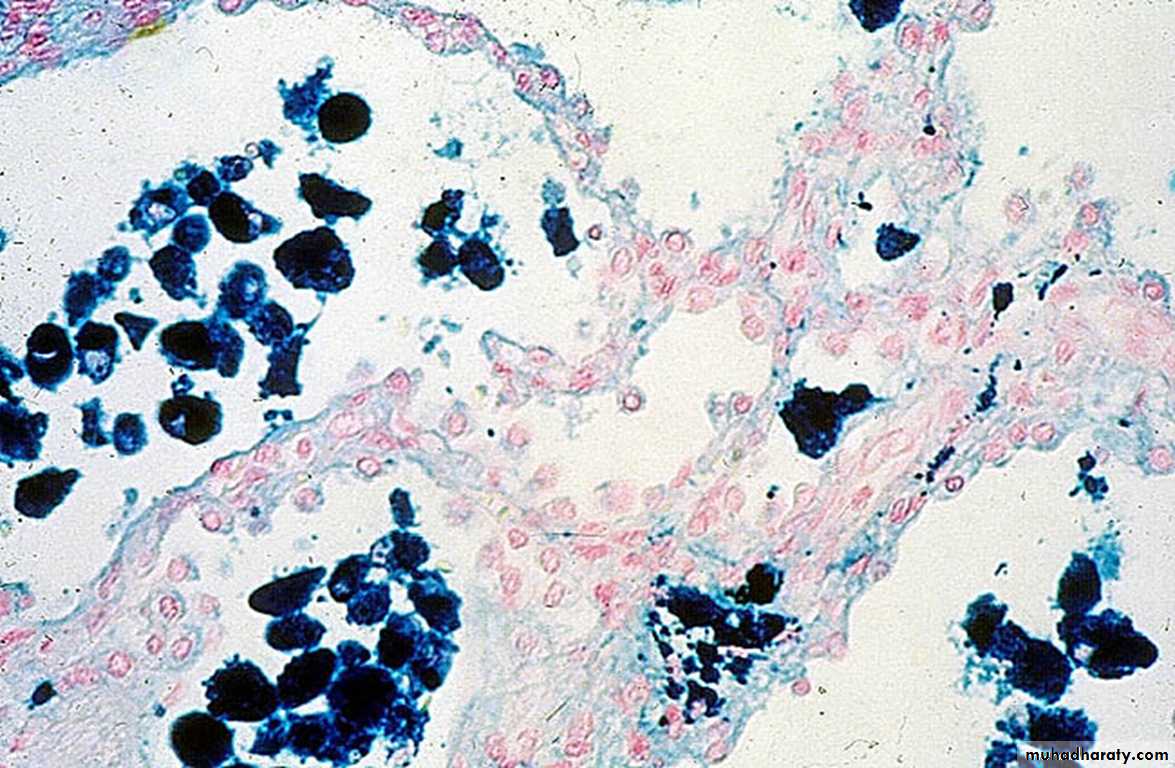
2.Chronic pulmonary congestion
Thickened & fibrotic septaAlveolar spaces contain hemosiderin-laden macrophages (“heart failure cells”) derived from phagocytosed red cells.
b) Hepatic congestion
1-Acute hepatic congestion:the central vein and sinusoids are distended with blood,
and there may even be necrosis of centrally located hepatocytes.
The periportal hepatocytes, better oxygenated???WHY
because of their proximity to hepatic arterioles, experience
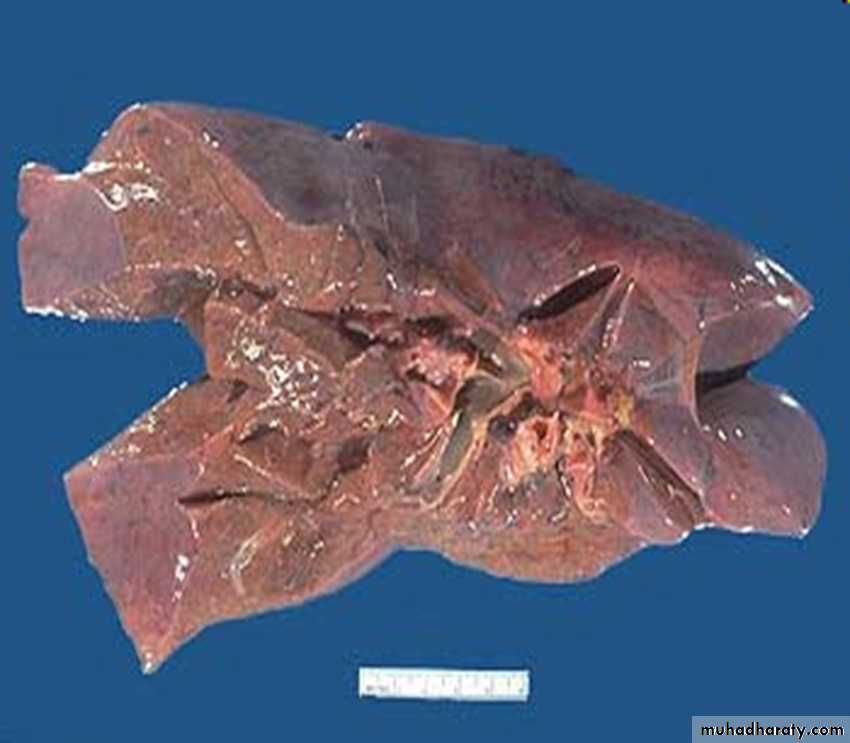
less severe hypoxia and may develop only reversible fatty change.2- Chronic passive congestion of liver:
Gross examinationthe central regions of the hepatic lobules, viewed, are red-brown and slightly depressed
the surrounding zones of uncongested tan, sometimes fatty, liver (nutmeg liver)
Microscopic findings
centrilobular hepatocyte necrosis,
hemorrhage
hemosiderin-laden macrophages.
Hemorrhage
Hemorrhage, defined as the extravasation of blood from vessels, is most often the result of:damage to blood vessels
or defective clot formation.
capillary bleeding can occur in chronically congested tissues.
Trauma,atherosclerosis,
or inflammatory or neoplastic erosion of a vessel wall also may lead to hemorrhage,
• Bleeding may be extensive if the affected vessel is a large vein or artery.
• The risk of hemorrhage is increased in a wide variety of clinical disorders collectively called:
• hemorrhagic diatheses.
• Including: inherited or acquired defects in:
vessel walls,
platelets,
or coagulation factors,
Hemorrhage may be manifested by different appearances and clinical consequences.
• Hemorrhage may be external or accumulate within a tissue as a hematoma, which ranges in significance from trivial to fatal.• Large bleeds into body cavities are described variously according to location
hemothorax,
hemopericardium,
hemoperitoneum,
or hemarthrosis (in joints).
Petechiae
are minute (1 to 2 mm in diameter) hemorrhages into skin, mucous membranes, or serosal surfaces causes include :low platelet counts (thrombocytopenia),
defective platelet function,
and loss of vascular wall support, as in vitamin C deficiency.
Purpura
are slightly larger (3 to 5 mm) hemorrhages.
Purpura can result from:
the same disorders that cause petechiae,
Trauma
vascular inflammation (vasculitis), and increased vascular fragility.
Ecchymoses
are larger (1 to 2 cm) subcutaneous hematomas (colloquially called bruises).Extravasated red cells are phagocytosed and degraded by macrophages; the characteristic color changes of a bruise result from the enzymatic conversion of hemoglobin (red-blue color) to bilirubin (blue-green color) and eventually hemosiderin (golden-brown).
The clinical significance of any particular hemorrhage depends on:
the volume of blood that is lostthe rate of bleeding..
The site of hemorrhage also is important;
• chronic or recurrent external blood loss culminates in iron deficiency anemia
• By contrast, iron is efficiently recycled from phagocytosed red cells,
• so internal bleeding (e.g., a hematoma) does not lead to iron deficiency.Edema
Definition: edema is an accumulation of interstitial fluid within tissues.
Extravascular fluid can also collect in body cavities such as:
a) Hydrothorax
b) Hydropericardium
c) Hydroperitoneum (ascites)
Anasarca
Mechanism of edema formation:
Approximately 60% of lean body weight is water,two thirds of which is intracellular.
Most of the remaining water is found in extracellular compartments in the form of interstitial fluid;
only 5% of the body’s water is in blood plasma.
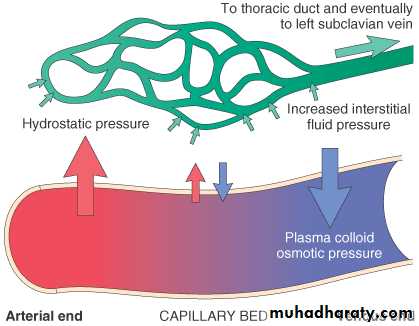
The capillary endothelium acts as a semi permeable membrane and highly permeable to water & to almost all solutes in plasma with an exception of proteins.
Normally, any outflow of fluid into the interstitium from the arteriolar end of the microcirculation is nearly balanced by inflow at the venular end. Therefore, normally, there is very little fluid in the interstitium.
Causes of Edema:
1) Increased Hydrostatic pressure2) Decrease plasma Oncotic pressure
3) Increased vascular permeability
4) Lymphatic channels obstruction
5) Sodium retention
1) Increased Hydrostatic pressure
mainly caused by disorders that impair venous return.• Local increases in intravascular pressure caused, for example, by deep venous thrombosis in the lower extremity can cause edema restricted to the distal portion of the affected leg.
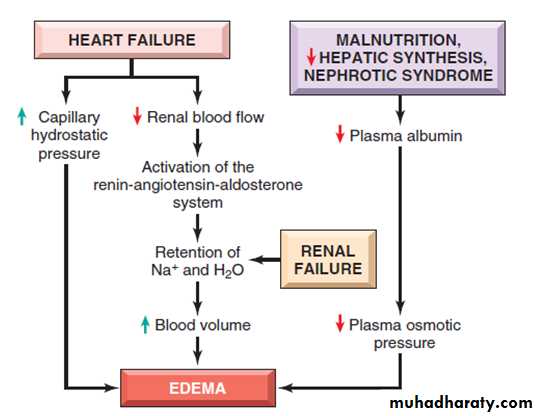
• Generalized increases in venous pressure, with resultant systemic edema, occur most commonly in congestive heart failure
Several factors increase venous hydrostatic pressure in patients with congestive heart failure
The reduced cardiac output leads to systemic venous congestion
reduction in cardiac output results in hypoperfusion of the kidneys, triggering the renin-angiotensin-aldosterone axis and inducing sodium and water retention (secondary hyperaldosteronism).
• a vicious circle of fluid retention, increase blood volume, increased venous hydrostatic pressures, and worsening edema ensues.
• Unless cardiac output is restored or renal water retention is reduced this vicious circle continues.
2) Decrease plasma Oncotic pressure
Reduction of plasma albumin concentrationsUnder normal circumstances, albumin accounts for almost half of the total plasma protein.
Common causes of reduced plasma osmotic pressure.
Albumin lost from the circulation.
Or albumin synthesized in inadequate amounts.
Nephrotic syndrome
is the most important cause of albumin loss from the blood.the glomerular capillaries become leaky, leading to the loss of albumin (and other plasma proteins) in the urine and the development of generalized edema.
Reduced albumin synthesis occurs in the setting of severe liver disease (e.g., cirrhosis) and protein malnutrition.
low albumin levels lead to edema, reduced intravascular volume, renal hypoperfusion, and secondary hyperaldosteronism.
• Increased salt and water retention by the kidney fails to correct the plasma volume deficit and exacerbates the edema, because the primary defect—low serum protein—persists.
3) Increased Vascular permeability:
usually occurs due to acute inflammation.
In inflammation, chemical mediators are produced. Some of these mediators cause increased vascular permeability which leads to loss of fluid & high molecular weight albumin and globulin into the interstitium.
• Inflammatory edema differs from non inflammatory edema by the following features:
a) Inflammatory edema (exudate)
Due to inflammation-induced increased permeability and leakage of plasma proteins.
Forms an exudate [protein rich]
Specific gravity > 1.012
• b) Non-inflammatory oedema (transudate)
A type of edema occurring in hemodynamic derangement (i.e. increased plasma hydrostatic pressure & decreased plasma oncotic pressure.)Formed transudate [protein poor]
Specific gravity < 1.012.
• 4) Lymphatic channels obstruction
Edema may result from lymphatic obstruction that compromises resorption of fluid from interstitial spaces. Impaired lymphatic drainage and consequent lymphedemaIt usually results from a localized obstruction caused by an inflammatory or neoplastic conditions.
For example,
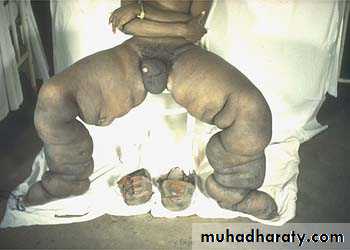
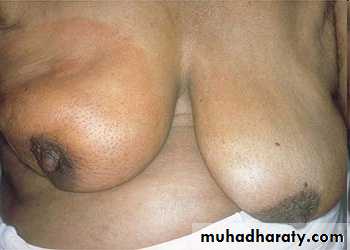
• the parasitic infection filariasis can cause massive edema of the lower extremity and external genitalia (so-called “elephantiasis”) by producing inguinal lymphatic and lymph node fibrosis.• Infiltration and obstruction of superficial lymphatics by breast cancer may cause edema of the overlying skin; the characteristic finely pitted appearance of the skin of the affected breast is called peau d’orange (orange peel).
Breast, lymphedema secondary to breast carcinoma
– Clinical presentation Breast cancer cells have blocked the lymphatic channels draining fluid from the skin of the right breast, leading to passive congestion and resulting in a peau d'orange (orange skin) appearance. Compare with the normal left breast.
• Lymphedema also may occur as a complication of therapy. in women with breast cancer who undergo axillary lymph node resection and/or irradiation, both of which can disrupt and obstruct lymphatic drainage, resulting in severe lymphedema of the arm.
• 5) Sodium and water retention:
Excessive retention of salt (and its obligate associated water) can lead to edema by increasing hydrostatic pressure (because of expansion of the intravascular volume) and reducing plasma osmotic pressure. Excessive salt and water retention are seen in a wide variety of diseases that compromise renal function, including poststreptococcal glomerulonephritis and acute renal failureCauses of sodium and water retention:
1- excessive salt intake with renal insufficiency.2- increased tubular reabsorption of sodium
3-Renal hypo perfusion
4-Increased renin- angiotensin- aldosterone secretion.
Morphology of edema
Gross examination:
clearing and separation of the extracellular matrix (ECM) elements. edema most commonly encountered in:
subcutaneous tissues,
lungs,
and brain.
Subcutaneous edema
can be diffusebut usually accumulates preferentially in parts of the body where hydrostatic pressures are highest.
• edema typically is most pronounced in the legs with standing and the sacrum with recumbency.
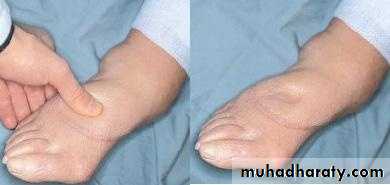
dependent edema. Finger pressure over edematous subcutaneous tissue displaces the interstitial fluid, leaving a finger-shaped depression; this appearance is called pitting edema.
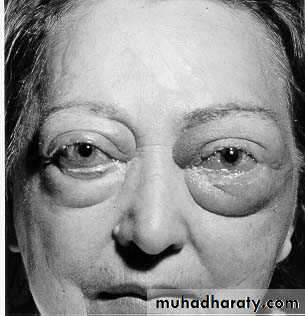
Edema resulting from renal dysfunction or nephrotic syndrome often manifests first in loose connective tissues (e.g., the eyelids, causing periorbital edema).
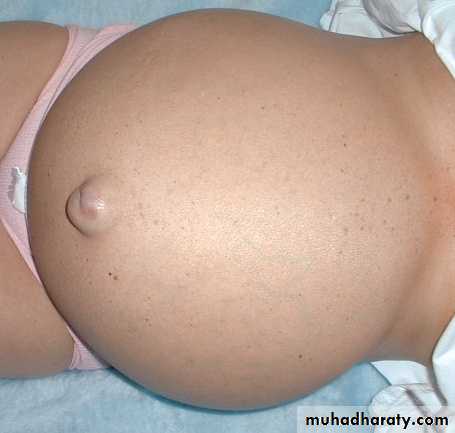
ASCITES
PERIORBITALEDEMA“Pitting” Edema
Pulmonary edema
the lungs often are two to three times their normal weight,and sectioning shows frothy, sometimes blood-tinged fluid. consisting of a mixture of:
air,
edema fluid,
and extravasated red cells.
Brain edema
• can be:localized (e.g., because of abscess or tumor)
generalized
• depending on the nature and extent of the pathologic process or injury.
• With generalized edema:
The sulci are narrowed .
The gyri swell and become flattened against the skull.
• HEMOSTASIS AND THROMBOSIS
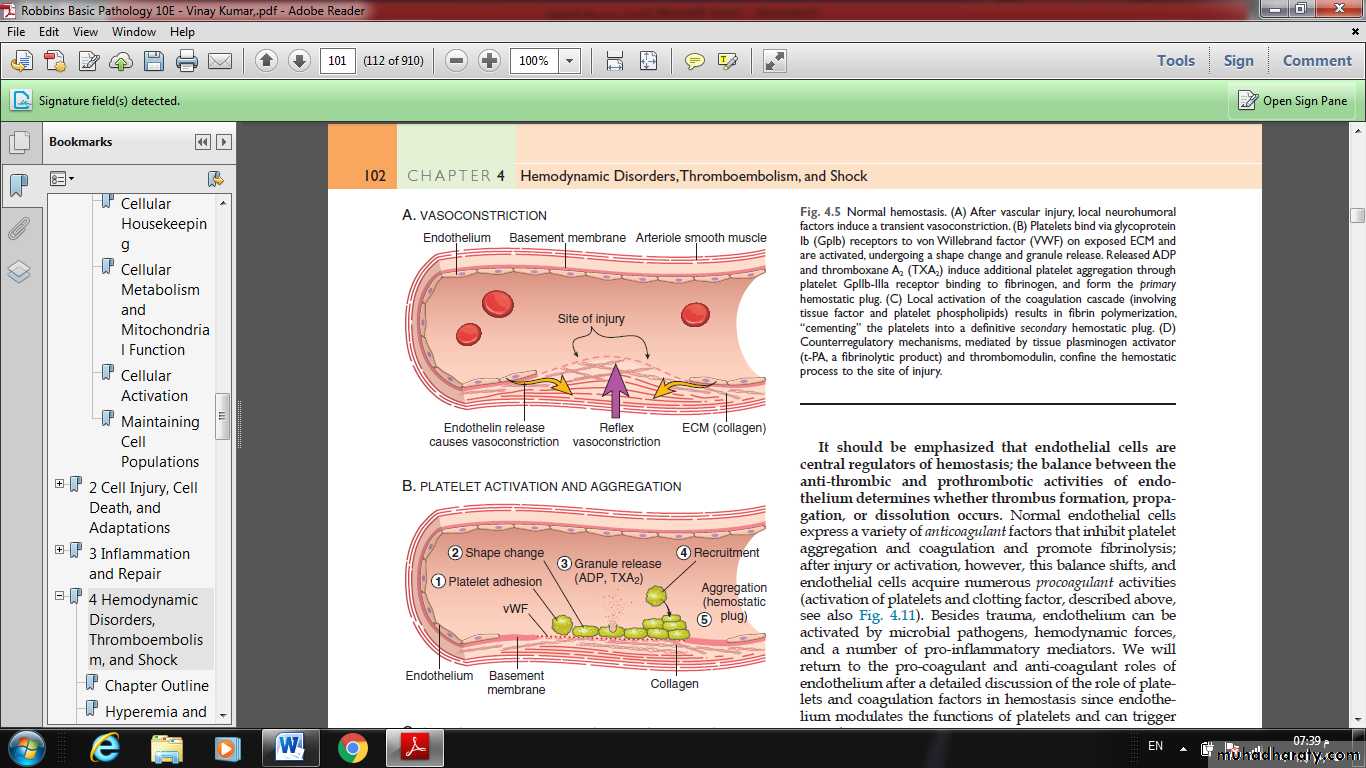
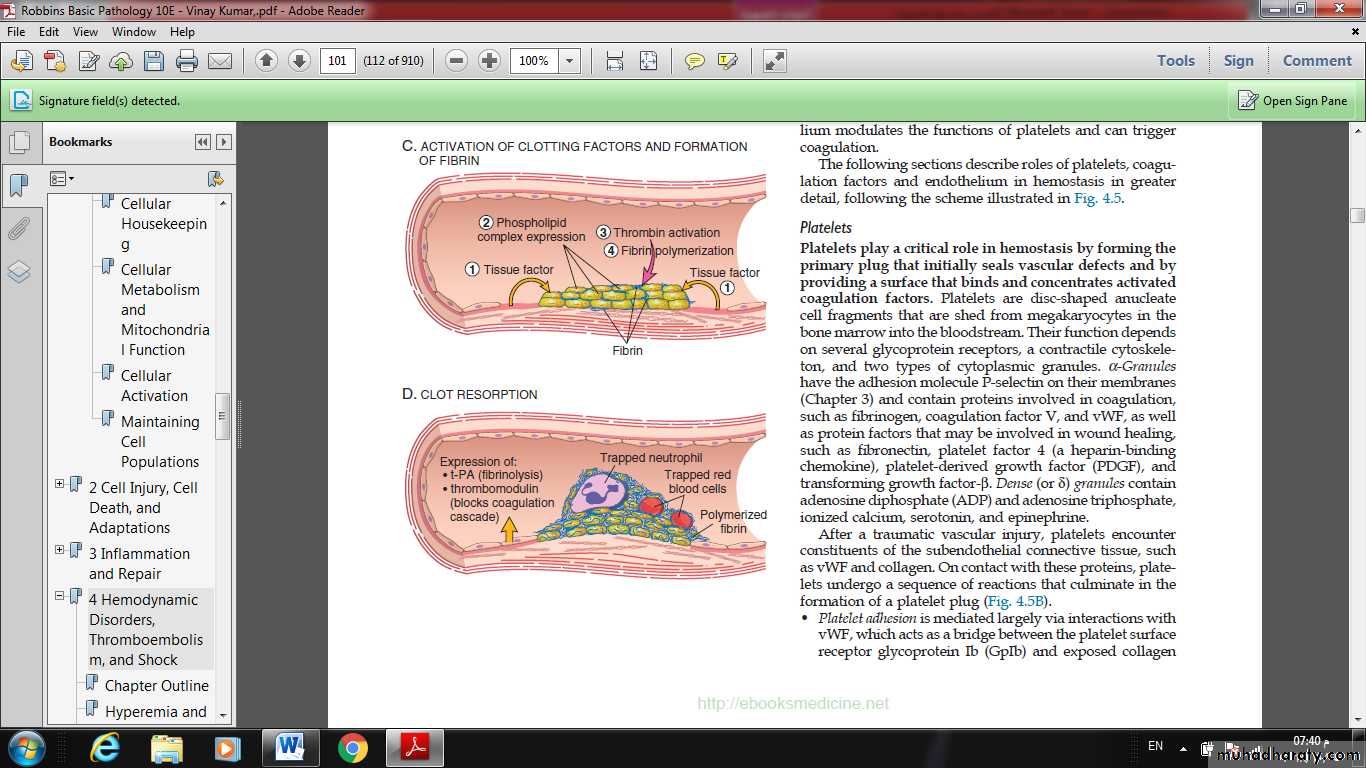
Normal hemostasiscomprises a series of regulated processes that culminate in the formation of a blood clot that limits bleeding from an injured vessel.
The pathologic counterpart of hemostasis is thrombosis, the formation of blood clot (thrombus) within non-traumatized, intact vessels.
(A) After vascular injury, local neurohumoral factors induce a transient vasoconstriction.
(B) Platelets bind via glycoprotein Ib (GpIb) receptors to von Willebrand factor (VWF) on exposed ECM and are activated, undergoing a shape change and granule release. Released ADP and thromboxane A2 (TXA2) induce additional platelet aggregation through platelet GpIIb-IIIa receptor binding to fibrinogen, and form the primary hemostatic plug.
(C) Local activation of the coagulation cascade results in fibrin polymerization, “cementing” the platelets into a definitive secondary hemostatic plug.
(D) Counterregulatory mechanisms, mediated by tissue plasminogen activator (t-PA, a fibrinolytic product) and thrombomodulin, confine the hemostatic process to the site of injury.
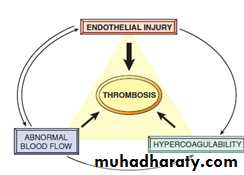
Thrombosis
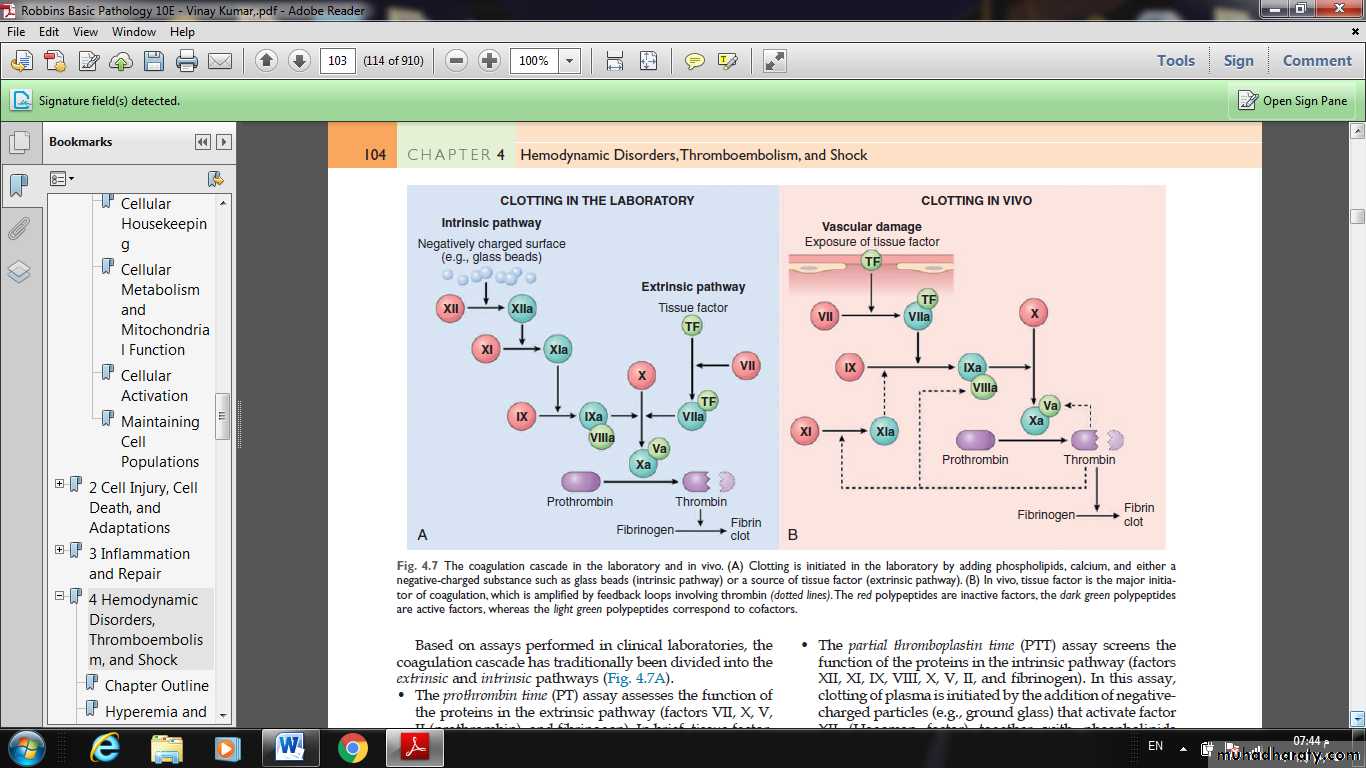
The pathologic counterpart of hemostasis is thrombosis, the formation of blood clot (thrombus) within non-traumatized, intact vessels.The primary abnormalities that lead to intravascular thrombosis are:
(1) endothelial injury,
(2) stasis or turbulent blood flow, and
(3) hypercoagulability of the blood
(the so-called “Virchow triad”).
Endothelial injury
Endothelial injury leading to platelet activation.underlies thrombus formation in the heart and the arterial circulation, where the high rates of blood flow impede clot formation.
This is the reason behind the use of aspirin and other platelet inhibitors in coronary artery disease and acute myocardial infarction.
severe endothelial injury may trigger thrombosis by exposing VWF and tissue factor.
Most common examples are:
• Endocardial injury during myocardial infarction.
Injury over ulcerated plaque in severely atherosclerotic arteries.
Abnormal Blood Flow
Turbulence (chaotic blood flow)contributes to:
arterial thrombosis.
cardiac thrombosis
by causing:
endothelial injury or dysfunction,
forming countercurrents and local pockets of stasis.
Stasis
is a major factor in the development of venous thrombi.Under conditions of normal laminar blood flow, platelets (and other blood cells) are found mainly in the center of the vessel lumen.
separated from the endothelium by a slower-moving layer of plasma.
• stasis and turbulence have the following deleterious effects:
Both promote endothelial cell activation and enhanced procoagulant activity.Stasis allows platelets and leukocytes to come into contact with the endothelium when the flow is sluggish.
Stasis also slows the washout of activated clotting factors and impedes the inflow of clotting factor inhibitors.
• Turbulent and static blood flow contributes to thrombosis in a number of clinical settings.
Ulcerated atherosclerotic plaques expose subendothelial ECM ,turbulence.
aneurysms create local stasis
Acute myocardial infarction results in focally noncontractile myocardium. Ventricular remodeling after more remote infarction can lead to aneurysm formation. cause local blood stasis.
Mitral valve stenosis (e.g., after rheumatic heart disease) results in left atrial dilation. In conjunction with atrial fibrillation, a dilated atrium also produces stasis and is a prime location for the development of thrombi.
Hyperviscosity syndromes (such as polycythemia vera,) increase resistance to flow and cause small vessel stasis;
sickle cell anemia the deformed red cells cause vascular occlusions, and the resultant stasis also predisposes to thrombosis.
Hypercoagulability
Hypercoagulability refers to an abnormally high tendency of the blood to clot, and is typically caused by alterations in coagulation factors.It is an important underlying risk factor for venous thrombosis.
can be divided into primary (genetic) and secondary (acquired) disorders.
Primary (Genetic)
Common (>1% of the Population)Factor V mutation
Prothrombin mutation
Increased levels of factor VIII, IX, or XI or fibrinogen
Rare
Anti-thrombin III deficiency
Protein C deficiency
Protein S deficiency
Secondary (Acquired)
Prolonged bed rest or immobilization
Myocardial infarction
Atrial fibrillation
Tissue injury (surgery, fracture, burn)
Cancer
Prosthetic cardiac valves
Disseminated intravascular coagulation
Heparin-induced thrombocytopenia
MORPHOLOGY
Thrombi can develop anywhere in the cardiovascular system.Arterial or cardiac thrombi typically arise at sites of endothelial injury or turbulence;
venous thrombi characteristically occur at sites of stasis.
Thrombi are focally attached to the underlying vascular surface
propagate toward the heart;arterial thrombi grow in a retrograde direction from the point of attachment,
venous thrombi extend in the direction of blood flow.
The propagating portion of a thrombus tends to be poorly attached and therefore prone to fragmentation and migration through the blood as an embolus.
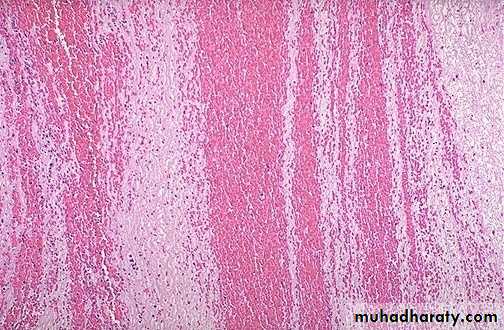
Thrombi can have grossly (and microscopically) apparent laminations called lines of Zahn;
these represent pale platelet and fibrin layers alternating with darker red cell–rich layers.
Such lines are significant in that they are only found in thrombi that form in flowing blood;
their presence can therefore usually distinguish antemortem thrombosis from the bland nonlaminated post mortum thrombi
Although thrombi formed in the “low-flow” venous system superficially resemble postmortem clots, careful evaluation generally shows ill-defined laminations.
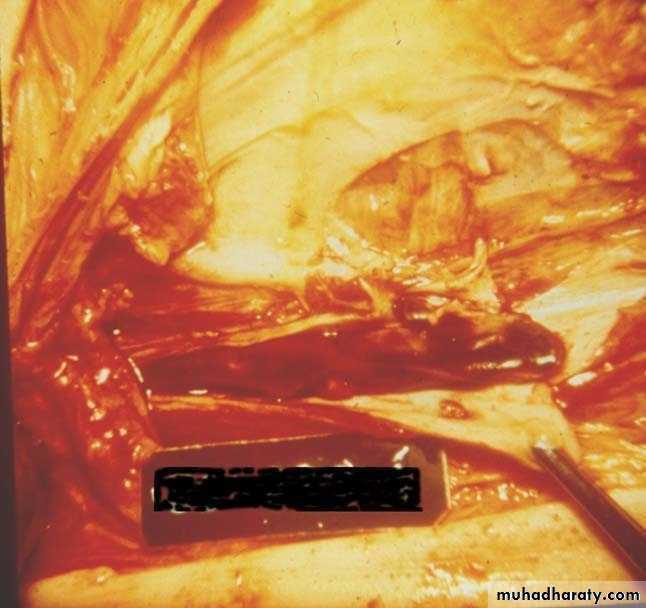
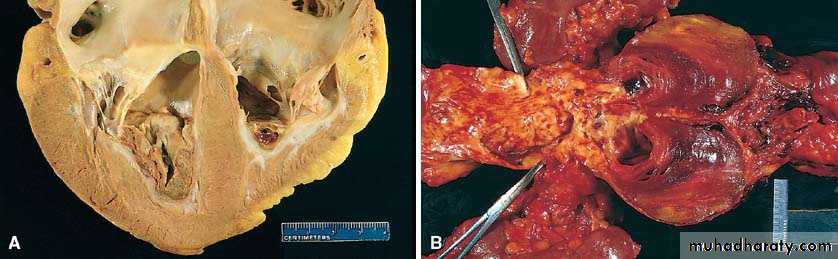
Thrombi occurring in heart chambers or in the aortic lumen are designated as mural thrombi.
Arterial thrombi
are frequently occlusive.They are typically rich in platelets, as the processes underlying their development (e.g., endothelial injury) lead to platelet activation.
Although usually superimposed on a ruptured atherosclerotic plaque, other vascular injuries (vasculitis, trauma) can also be underlying causes.
Venous thrombi (phlebothrombosis):
are almost invariably occlusive;they frequently propagate some distance toward the heart, forming a long cast within the vessel lumen that is prone to give rise to emboli.
they contain more enmeshed red cells, leading to the moniker red, or stasis, thrombi.
The veins of the lower extremities are most commonly affected
At autopsy, postmortem clots are:
gelatinousand because of red cell settling they have a dark red dependent portion and a yellow “chicken fat” upper portion;
they also are usually not attached to the underlying vessel wall.
By contrast, red thrombi typically are:
firm,
focally attached to vessel walls,
and the contain gray strands of deposited fibrin.
• Thrombi on heart valves are called vegetations.
Fate of the Thrombus
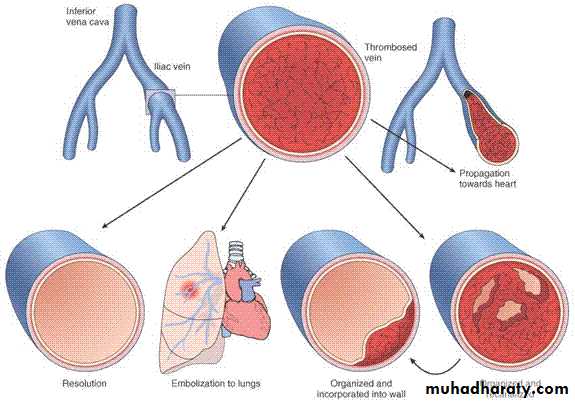
Propagation. The thrombus enlarges through the accretion of additional platelets and fibrin, increasing the odds of vascular occlusion.Embolization. Part or all of the thrombus is dislodged and transported elsewhere in the vasculature.
Dissolution. fibrinolytic factors may lead to its rapid shrinkage and complete dissolution.
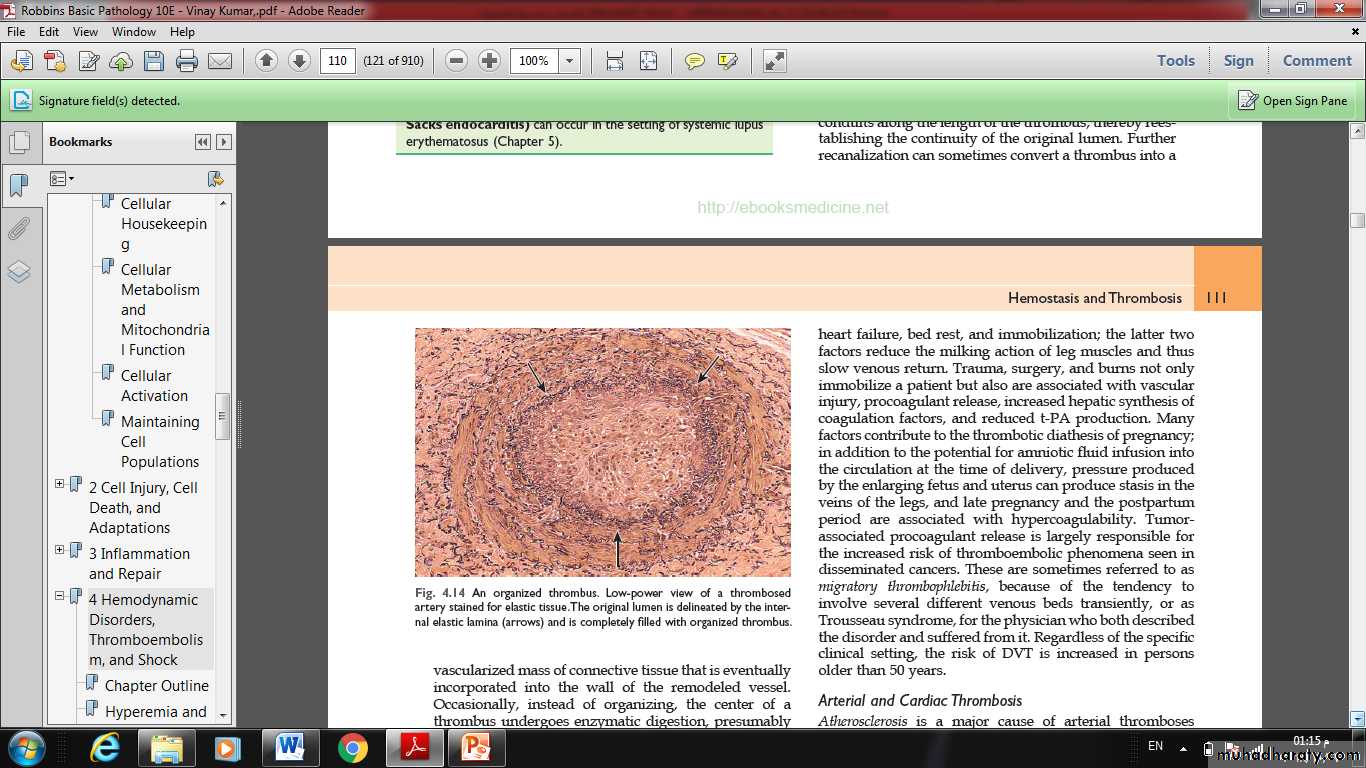
• Organization and recanalization. Older thrombi become organized by the ingrowth of endothelial cells, smooth muscle cells, and fibroblasts.
In time, capillary channels are formed reestablishing the continuity of the original lumen.
Further recanalization can sometimes convert a thrombus into a vascularized mass of connective tissue that is eventually incorporated into the wall of the remodeled vessel.
• Venous Thrombosis (Phlebothrombosis)
Most venous thrombi occur in the superficial or the deep veins of the leg.Superficial venous thrombi
usually arise in the saphenous system
particularly in the setting of varicosities
these rarely embolize
but they can be painful
and can cause local congestionand swelling from impaired venous outflow,
predisposing the overlying skin to the development of infections and varicose ulcers.
Deep venous thrombosis DVT
(DVTs) in the larger leg veins at or above the knee joint (e.g., popliteal, femoral, and iliac veins)
are more serious because they are prone to embolize.
Although such DVTs may cause local pain and edema, collateral channels often circumvent the venous obstruction. Consequently, DVTs are entirely asymptomatic in approximately 50% of patients and are recognized only after they have embolized to the lungs.
DVTs are associated with stasis and hypercoagulable states.
EMBOLISM
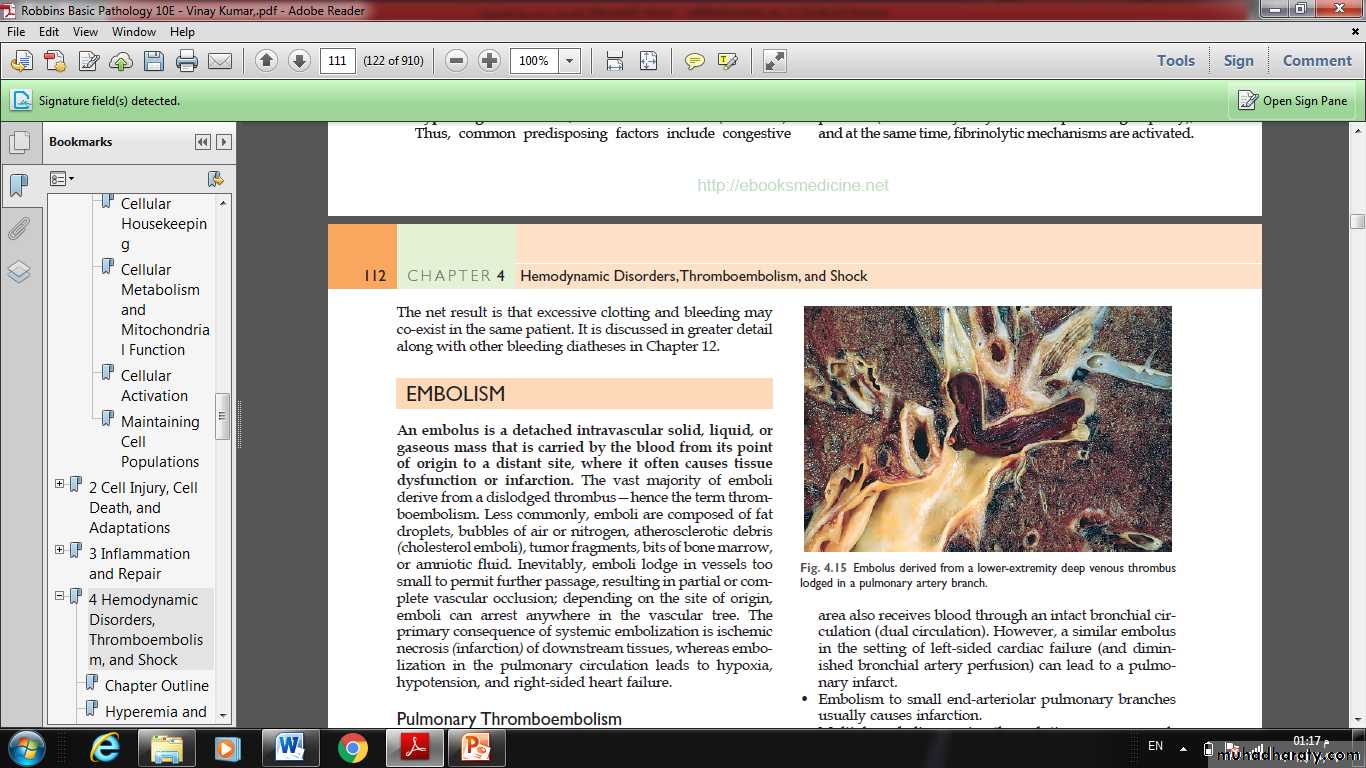
An embolus is a detached intravascular solid, liquid, or gaseous mass that is carried by the blood from its point of origin to a distant site, where it often causes tissue dysfunction or infarction.The vast majority of emboli derive from a dislodged thrombus—hence the term thromboembolism.
• Less commonly, emboli are composed of:
fat droplets.bubbles of air or nitrogen
tumor fragments,
bits of bone marrow,
or amniotic fluid.
Inevitably, emboli lodge in vessels too small to permit further passage, resulting in partial or complete vascular occlusion;
depending on the site of origin, emboli can arrest anywhere in the vascular tree.
• The primary consequence of systemic embolization
is ischemic necrosis (infarction) of downstream tissues,whereas embolization in the pulmonary circulation leads to hypoxia, hypotension, and right-sided heart failure.
Pulmonary Thromboembolism
Pulmonary emboli originate from deep venous thrombosis and are responsible for the most common form of thromboembolic disease.
In more than 95% of cases, venous emboli originate from thrombi within deep leg veins proximal to the popliteal fossa.
Fragmented thrombi from DVTs are carried through progressively larger channels and usually pass through the right side of the heart before arresting in the pulmonary vasculature.
Depending on size, a PE can occlude:
the main pulmonary artery,lodge at the bifurcation of the right and left pulmonary arteries (saddle embolus),
or pass into the smaller, branching arterioles.
the major clinical and pathologic features are the following:
Most pulmonary emboli (60%–80%) are small and clinically silent. With time, they undergo organization and become incorporated into the vascular wall.• At the other end of the spectrum, a large embolus that blocks a major pulmonary artery can cause sudden death.
• Embolic obstruction of medium-sized arteries and subsequent rupture of downstream capillaries rendered anoxic can cause pulmonary hemorrhage. Such emboli do not usually cause pulmonary infarction because the area also receives blood through an intact bronchial circulation (dual circulation).
• However, a similar embolus in the setting of left-sided cardiac failure (and diminished bronchial artery perfusion) can lead to a pulmonary hypertension and pulmonary infarct.
Embolism to small end-arteriolar pulmonary branches usually causes infarction.
Multiple emboli occurring through time can cause and right ventricular failure (cor pulmonale).Systemic thromboembolism
Systemic thromboembolism refers to emboli travelling within arterial circulation & impacting in the systemic arteries.Most systemic emboli (80%) arise from intracardiac mural thrombi.
two thirds of intracardiac mural thrombi are associated with left ventricular wall infarcts
and another quarter with dilated left atria secondary to rheumatic valvular heart disease.
The remaining (20%) of systemic emboli arise from :
thrombi on ulcerated athrosclerotic plaques,or fragmentation of valvular vegetation.
Aortic aneurysm.
Paradoxical emboli from venous side.
Unlike venous emboli, which tend to lodge primarily in one vascular bed (the lung), arterial emboli can travel to a wide variety of sites.
the lower extremities (75%)
& the brain (10%),
with the rest lodging in the intestines, kidney, & spleen.
The consequences of embolization depend on:
the caliber of the occluded vessel,
the collateral supply,
and the affected tissue’s vulnerability to anoxia;
arterial emboli often lodge in end arteries and cause infarction.