
Amenorrhea
Amenorrhea, or the absence of menses, is a common symptom of
several pathophysiologic states. This condition traditionally has
been divided into primary amenorrhea, in which menarche (the
first menses) has not occurred, and secondary amenorrhea, in
which menses has been absent for 6 months or more. A more
functional or clinical division of menstrual disorders based on
initial history and physical examination would be as follows:
1\primary amenorrhea with sexual infantalism,
2\ primary amenorrhea with breast development and
mullerian anomalies (normal secondary sexual character),
3\amenorrhea and oligomenorrhea with breast development
and normal mullerian structures. this group includes disorders
causing primary as well as secondary amenorrhea, oligomeno-
rrhea, and the hyperandrogenic states.
Primary Amenorrhea
The diagnosis of primary amenorrhea is made when no
spontaneous uterine bleeding has occurred by the age of 16
years. The workup should be initiated earlier if there is no
evidence of breast development (thelarche) by age 14 years.
The presence of normal breast development confirms gonadal
secretion of estrogen but not necessarily the presence of ovarian
tissue. The presence of normal amounts of pubic and axillary hair
confirms gonadal or adrenal secretion of androgens as well as the
presence of functional androgen receptors.
1\PRIMARY AMENORRHEA WITH SEXUAL
INFANTILISM
Patients with primary amenorrhea and no secondary sexual
characteristics (sexual infantalism) display the absence of
gonadal hormone secretion. The differential diagnosis is based on
whether the defect is the result of a lack of gonadotropin secretion
(hypogonadotropic hypogonadism) or an inability of the
ovaries to respond to gonadotropin (hypergonadotropic
hypogonadism due to gonadal agenesis or dysgenesis). The
distinction can be made by the measurement of a basal serum
follicle-stimulating hormone (FSH).
A\Hypogonadotropic Primary Amenorrhea and Sexual
Infantilism
Patients with hypogonadotropic hypogonadism have low FSH
levels, whereas patients with hypergonadotropic hypogonadism
(e.g., gonadal dysgenesis) have elevated FSH levels in the
menopausal range (>40 mIU/L).
Hypogonadotropic hypogonadism may be caused by lesions of
the hypothalamus like craniopharyngioma or other central
nervous system tumor or by functional disorders that result in
inadequate
gonadotropin-releasing
hormone
(GnRH)
synthesis and release.,
Kallman s syndrome is a rare
condition that is characterized by a failure to start or a failure to
complete
. It is also accompanied by a lack of sense of smell
) Kallmann syndrome occurs due to a failure of the
to release
magnetic resonance imaging (MRI) or computerized tomography
(CT) of the hypothalamic-pituitary area is recommended.
]
Hypogonadotropic
hypogonadism
resulting
in
primary

amenorrhea and sexual infantilism may also be the result of
lesions of the pituitary, including prolactin-secreting adenomas,
or a general process of pituitary failure. These patients should be
screened for other pituitary hormonal deficiencies by testing for
thyroid-stimulating hormone (TSH), growth hormone, and
adrenocorticotropic hormone (ACTH).
Finally, apparent hypogonadotropic hypogonadism may actually
represent constitutionally delayed puberty. This delay in the
normal onset of puberty is generally attributed to undefined
hereditary factors because there is commonly a history of late
puberty in family members. Constitutional delay of puberty is a
diagnosis of exclusion.
B\Hypergonadotropic Primary Amenorrhea and Sexual
Infantilism
Patients with hypergonadotropic hypogonadism have some form
of failed gonadal development or premature gonadal failure and
will have elevated FSH levels.
These patients may have gonadal agenesis (the absence or early
disappearance of the normal gonad). Examples in males who may
appear to be female in some cases are pure gonadal dysgenesis,
or the testicular regression syndrome. These patients have an
apparently normal 46 XY karyotype but lack testicular develop-
ment. If fetal testicular regression occurs between 8 and 10 weeks
of gestation, they may have female external genitalia with or
without ambiguity in addition to a lack of gonads, a hypoplastic
uterus (secondary to absent secretion of antimullerian hormone),
and rudimentary genital ducts .
Other individuals with hypergonadotropic primary
amenorrhea and sexual infantilism may have gonadal

dysgenesis, the presence of an abnormally developed gonad due
to chromosomal defects. The differential diagnosis includes 45
XO (Turner syndrome) , and pure gonadal dysgenesis (46 XX and
46 XY). Although most affected patients show no signs of
secondary sexual characteristics, occasionally an individual with
mosaicism or Turner syndrome will have sufficient ovarian
follicular activity and secrete enough estrogen to cause breast
development, menstruation, ovulation, and rarely even
pregnancy.
In individuals with the presence of a Y chromosome, there
is a risk for developing a gonadoblastoma (a benign germ cell
tumor of the gonad) All patients with hypergonadotropic hypogo-
nadism should have a karyotype performed. Because it is
important to identify mosaicism,.
Patients with sexual infantilism may be treated to stimulate
breast development by very gradually increasing estrogen doses.
One commonly used regimen is to start with 0.3 mg conjugated
estrogen every other day and slowly increase over 3- to 6-month
intervals.
Individuals
with
persistent
hypogonadotropic
hy-
pogonadism who seek fertility require either human menopausal
gonadotropin injections or pulsatile GnRH administered by an
infusion pump. Patients with gonadal dysgenesis who have a
normal uterus and cervix can achieve pregnancy only by in vitro
fertilization using donor oocytes.
\]
Turner Syndrome
The disorder is characterized by partial or complete loss
(monosomy) of one of the X chromosomes
Individuals with Turner syndrome may benefit from growth
hormone (GH) therapy, which can help to normalize height
Estrogen and progesterone replacement therapy will generally
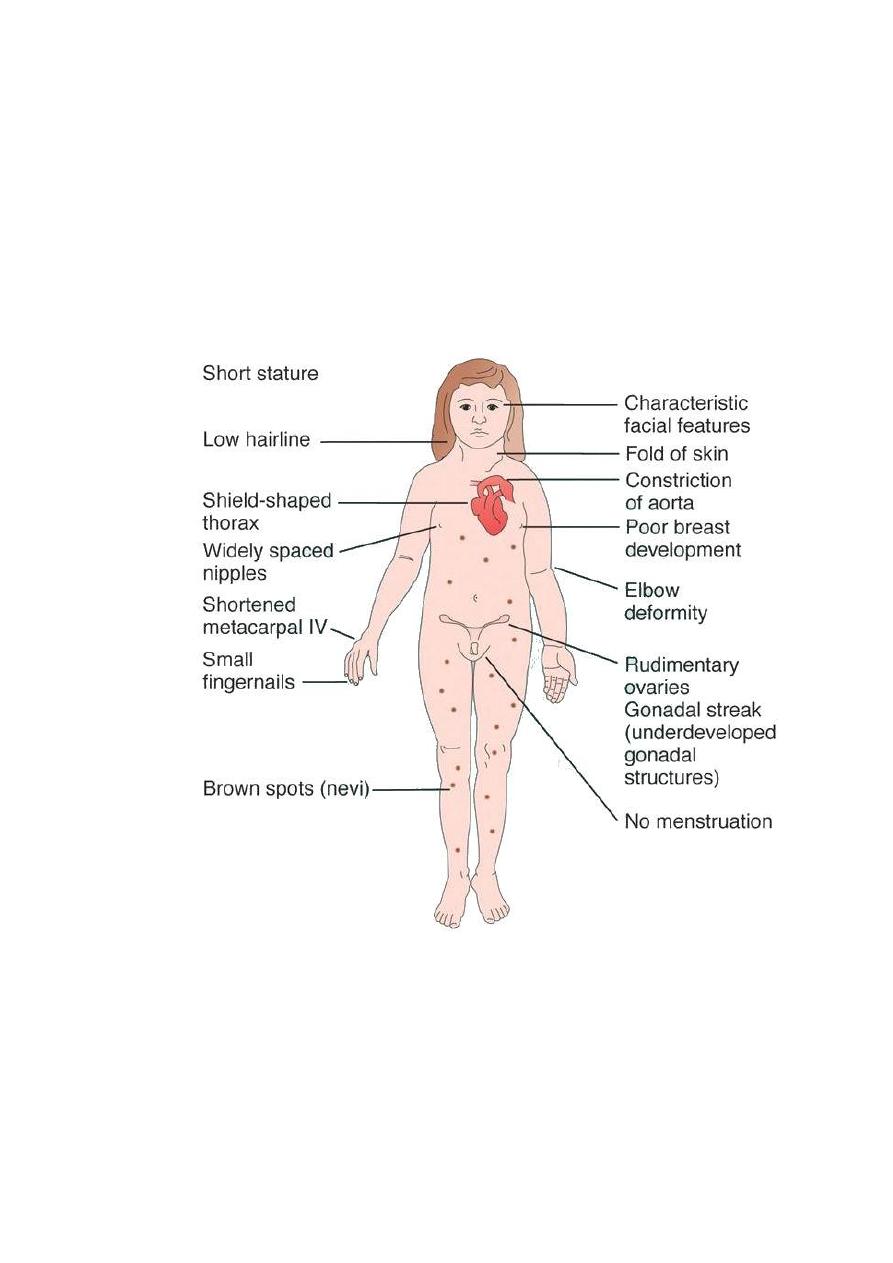
promote puberty and the development of secondary sexual
characteristics. Hormone replacement therapy is usually begun
around 12-14 years of age. Replacement therapy must be
continued until menopause.
Most individuals with Turner syndrome remain unable to
conceive children. In vitro fertilization (IVF) with a donor egg
and an implanted pregnancy is sometimes possible.
2\PRIMARY
AMENORRHEA
WITH
BREAST
DEVELOPMENT
AND
MULLERIAN
ANOMALIES
(normal secondary sexual charecters)

Patients with primary amenorrhea, breast development, and some
defect of mullerian structures fall into two categories: those with
complete androgen insensitivity syndrome (AIS), formerly called
testicular feminization, and those with mullerian dysgenesis or
agenesis. The distinction between these two diagnoses can be
made by the measurement of a serum testosterone level and
determination of the karyotype.
A\Androgen Insensitivity Syndrome
Patients with complete androgen insensitivity syndrome have a
defect in the androgen receptor. Their karyotype is 46 XY, and
they demonstrate male levels of testosterone, their testes are
located within the abdominal wall or cavity (cryptorchic). This
location, with greater body heat, typically does not allow for
normal male hormonal secretion. Breast development (with
smaller nipples and areolae than normal genotypical females) is
caused by the testicular secretion of estrogens and by the
conversion of circulating androgen to estrogens in the liver and
elsewhere. The testicles of individuals with AIS secrete normal
male amounts of antimullerian hormone; therefore, patients have
only a vaginal dimple and no uterus. Treatment should consist of
gonadal resection to avoid neoplasia (i.e., gonadoblastomas and
dysgerminomas) once puberty is complete. The creation of a
neovagina when the patient is prepared for sexual activity is pos-
sible. Psychological counseling is an important component in the
care of these patients.
B\Mullerian Dysgenesis or Agenesis
Patients with primary amenorrhea, breast development, and a 46
XX karyotype have levels of testosterone appropriate for females.
This clinical diagnosis may be caused by mullerian defects that

cause destruction of the vaginal canal (e.g., imperforate hymen or
a transverse vaginal septum) or by the absence of a normal cervix
or uterus and normal fallopian tube'. An imperforate hymen
should be suspected in adolescents who report monthly
dysmenorrhea in the absence of vaginal bleeding. Clinical these
patients often present with a vaginal bulge and a midline cystic
mass on rectal examination. Ultrasonography confirms the
presence of a normal uterus and ovaries with a hematocolpos.
These patients should be treated with hymenectomy.
Alternatively, women may present with similar symptoms
BUT without a vaginal bulge. When ultra sonography confirms a
normal uterus and ovaries a transverse, obstructing vaginal
septum or cervical agenesis should be suspected. MRI is the
diagnostic procedure of choice in these patients. If the MRI scan
confirms a transverse septum, surgical correction is indicated.
Finally; rectal examination and ultrasonography may be a
sign of the absence of a uterus indicating mullerian agenesis or
Meyer-Rokitansky-Kuster- Hauser syndrome. This syndrome
is characterized by a failure of the mullerian ducts to fuse distally
and to form the upper genital tract. These patients may have
unilateral or bilateral rudimentary uterine tissues ,fallopian tubes,
and ovaries. It is uncommon to have functional endometrial
tissue.
Congenital anatomic abnormalities of the uterus or vagina,
or both, are often associated with renal abnormalities such as a
unilateral solitary kidney or a double renal collecting system,
among others. Therefore, these patients should have an
intravenous pyelogram or other diagnostic study to confirm a
normal urinary system.
Diagnosis of Primary Amenorrhea

A diagnostic approach to primary amenorrhea. (FSH = follicle-stimulating hormone; LH = luteinizing
hormone; TSH = thyroid-stimulating hormone.)
3\Amenorrhea
and
Oligomenorrhea
with
Breast
Development and Normal Mullerian Structures
Disorders in which the patient has breast development and

a demonstrable cervix and uterine fundus on physical
examination may cause primary as well as secondary
amenorrhea, or may present as oligomenorrhea (menstrual cycles
at greater than 35- to 45-day intervals).
All patients with menstrual bleeding disorders should be
tested for pregnancy. Initial history taking should include
questions about the timing of thelarche, pubarche, and menarche.
The timing and development of the menstrual disorder (present
since puberty or new), significant weight change, strenuous
exercise activities, dietary habits, sexual activity, concomitant
illnesses or complaints, abnormal facial or body hair growth,
scalp hair loss, acne, and the presence or absence of hot flashes
and vaginal dryness should be noted. A comprehensive list of
medications and dietary supplements taken should be obtained.
In addition to a pregnancy test, the initial investigation of
the amenorrheic patient should include an FSH level and a
progestin challenge test. Failure of the patient to have
withdrawal bleeding after receiving a progestational agent
indicates significant hypoes- trogenism or hyperandrogenism,
a uterine defect, or pregnancy. The absence of a withdrawal
bleed after the administration of a progestational agent due to a
uterine defect can be ruled out by the presence of withdrawal
bleeding following sequential estrogen and progestin therapy.
A\UTERINE DEFECTS(normal estrogen level)
Women who do not have withdrawal bleeding after l hormonal
challenge test and who have a history of uterine instrumentation,
particularly a dilation and urettage, following vaginal delivery or
pregnancy termination may have Asherman's syndrome. This

interesting syndrome is characterized by intrauterine scarring
(synechiae), and these patients may have normal ovulatory cycles
with cyclic premenstrual symptoms. Patients with Asherman’s
syndrome should be evaluated by hysterosalpingography or
sonohysterography. the treatment of choice is hysteroscopic
treatment with excision of the
synechiae ,followed by insertion of an IUCD with estrogen
progesterone supplement for 3 months.
B\AMENORRHEA AND OLIGOMENORRHEA
ASSOCIATED WITH HYPOESTROGENISM
The differential diagnosis for patients with amenorrhea
associated with low levels of estrogen includes:
\
hypothalamic-pituitary
dysfunction
(hypothalamic
amenorrhea) . have low FSH and prolactin levels.
\\ premature ovarian failure have high FSH and normal
prolactin levels.
\\\hyperprolactinemia have high prolactin and low FSH levels.
\Hypothalamic-Pituitary Dysfunction
Patients with hypothalamic amenorrhea include women
experiencing severe weight loss, women undergoing excessive
exercise resulting in low body fat, and women experiencing
severe psychological stress. Also included are women with severe
systemic diseases such as disseminated malignancies and patients
with pituitary or central nervous system lesions. In its most severe
and life-threatening form, women may have pituitary failure or
anorexia nervosa. All patients with hypothalamic-pituitary
dysfunction should be evaluated for the status of the other
pituitary hormones.

Sheehan ’ s s yndrome
This term describes hypopituitarism that presents in the
late postpartum period, and which is caused by haemorrhage
and hypotension at the time of delivery. The hypotension
results in avascular necrosis that affects the
anterior pituitary more commonly than the posterior
pituitary. Women most frequently present with failure of
lactation and subsequent amenorrhoea, but they may
have any feature of hypopituitarism including the more
subtle features of hypoadrenalism and hypothyroidism.
\\Premature Ovarian Failure
Premature ovarian failure is defined as ovarian failure before the
age of 40 years. A karyotype is performed to exclude mosaicism
(i.e., some cells bearing a Y chromosome). If cells with a Y
chromosome are present, a gonadectomy to prevent malignant
transformation is indicated.
Other causes of premature ovarian failure include ovarian
injury from surgery, radiation, or chemotherapy; galactosemia;
carrier status of the fragile X syndrome; and autoimmunity.. It is
not unusual for patients with premature ovarian failure to have
episodes of normal ovarian and menstrual function. Patients with
premature ovarian failure require hormonal therapy (estrogen and
a progestin) to reduce the risk for osteoporosis.
\\\Amenorrhea
and
oligomenorrhea
with
hyperprolactinemia and galactorrhea:
The principal action of prolactin is to stimulate lactation.
Hypersecretion of prolactin leads to gonadal dysfunction by
interrupting the secretion of GnRH, which inhibits the release of
LH and FSH and, in turn, impairs gonadal steroidogenesis. The
primary influence on prolactin secretion is tonic inhibition of
dopamine input from the hypothalamus. Any event disrupting this
inhibition can result in a rise in prolactin levels.
The consequences of hyperprolactinemia that are clinically
significant include menstrual disturbances and galactorrhea
Causes of elevated prolactin
C\Amenorrhea and oligomenorrhea with Normal Estrogen
Patients with amenorrhea or oligomenorrhea who
consistently have normal levels of estrogen have a mild form
of hypothalamic anovulation that may be caused by low
body weight and exercise issues, psychological stress,
• Pregnancy (10-fold rise from baseline)
• Excessive exercise
• Postprandial states
• Stimulation of the chest wall or nipple
• Medications
• Metoclopramide
• Phenothiazines
• Butyrophenones
• Risperidone
• Monoamine oxidase inhibitors
• Tricyclic antidepressants
• Serotonin reuptake inhibitors
• Verapamil
• Reserpine
• Methyldopa
• Estrogens
• Craniopharyngiomas
• Granulomatous infiltration of the pituitary or
hypothalamus
• Acromegaly
• Severe head trauma
• Prolactinomas
• Pituitary stalk compression
• Hypothyroidism
• Chronic renal failure
• Marijuana or narcotic use
recent pregnancy, or lactation. They may also have been
treated with Depo Provera or combined hormonal
contraceptives in the recent past. These iatrogenic causes
usually resolve spontaneously within 6 months. Some women
with amenorrhea or oligomenorrhea and normal estrogen
levels may have a subclinical androgen excess disorder, such
as a mild form of polycystic ovary syndrome (PCOS).
D\Amenorrhea
and
Oligomenorrhea
with
Hyperandrogenism
Hyperandrogenism is the clinical manifestation of elevated levels
of male hormones in women. Features may range from mild
unwanted excess hair growth and acne to alopecia (hair loss),
more extensive hirsutism, , masculinization and virilization.
Hirsutism is the presence of male-like hair growth caused by
conversion of vellus to terminal hairs in areas such as the face,
chest, abdomen, or upper thighs. Signs of masculinization
include loss of female body fat and decreased breast size.
Virilization is the addition of temporal balding, deepening of the
voice, and enlargement of the clitoris to any of the previous signs
of excess male hormone. Androgens in women are normally pro-
duced in the ovaries and the adrenal glands Hyperandrogenic
disorders may be divided into functional and neoplastic disorders
of the adrenal or ovary .

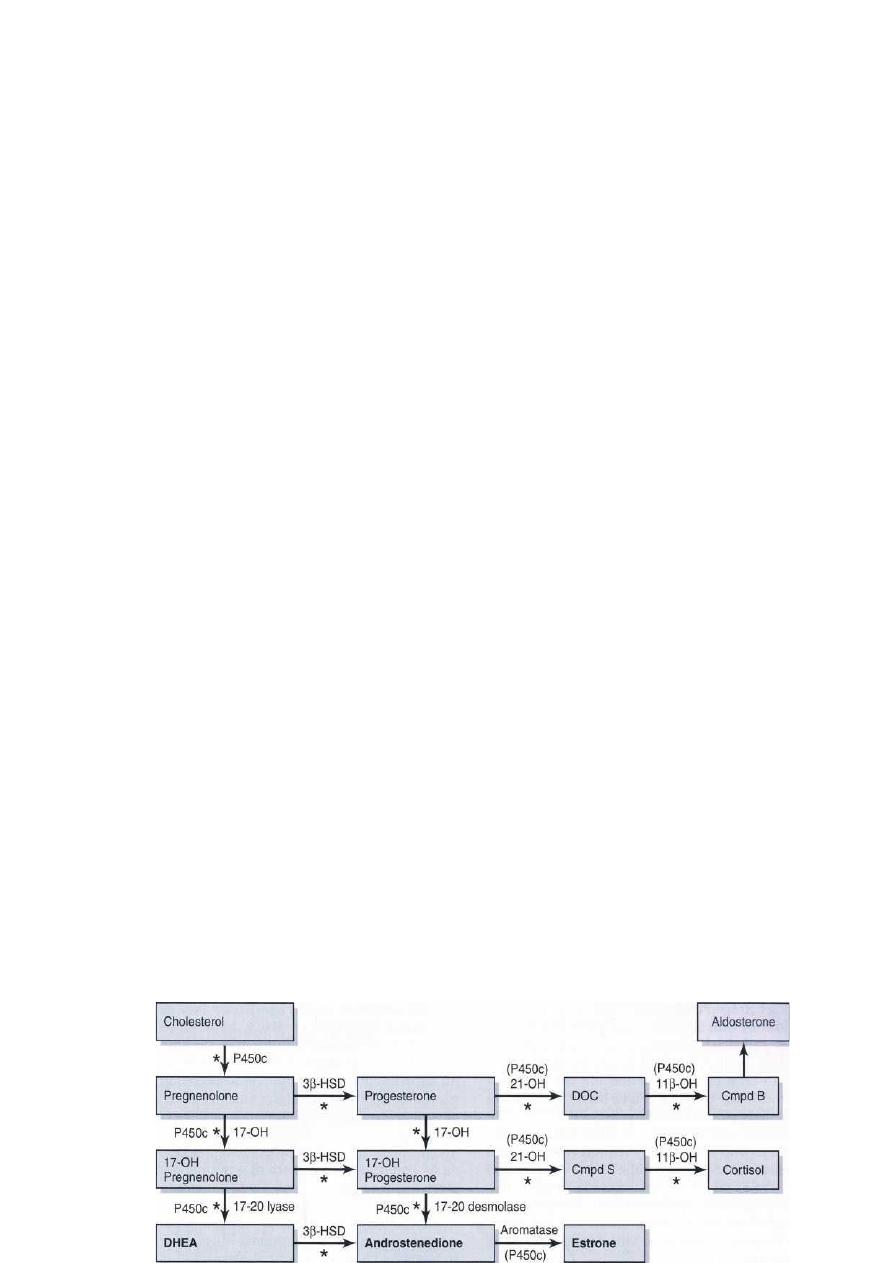
Diagrammatic representation of the steroid biosynthetic pathways.
Hyperandrogenic Disorders
In general, hyperandrogenic disorders can be attributed to
excessive secretion of androgens by the ovaries, by the adrenals,
or both.
ADRENAL DISORDERS
Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is a general term used to
describe an assortment of disorders that arise from inborn
glandular
enzyme
deficiencies
associated
with
the
overproduction of steroids. The most common cause of CAH is
21-hydroxylase deficiency. CAH represents a spectrum of
disorders, ranging from the severe salt-wasting form (early
onset), to simple virilizing CAH,. Alternatively the late onset)
presents later in life, generally at the time of puberty or later.
These patients (late onset) do not present with genital
abnormalities, although they may develop hirsutism, acne, and
menstrual and ovulatory irregularities.
Because 21-hydroxylase is responsible for the conversion of 17-
hydroxyprogesterone to 11-deoxycortisol), a deficiency in 21-
hydroxylase results in an excessive accumulation of 17-
hydroxyprogesterone. As a result, this enzyme disorder is marked

by an elevated serum 17-hydroxyprogesterone level as well as
increases in its metabolites androstenedione and testosterone.
This disease is inherited as an autosomal recessive trait. CAH, are
treated by the administration of glucocorticoids (e.g., 0.25 mg
dexamethasone every other day at bedtime).
Cushing's Syndrome
Characteristic Cushinoid signs include truncal obesity, moon-like
faces, hypertension, easy bruisability, impaired glucose tolerance,
muscle
wasting,
osteoporosis,
abdominal
striae,
and
supraclavicular and cervical spinal fat pads. Other manifestations
include hirsutism, acne, and irregular menses. This is a rare cause
of menstrual dysfunction in women.
Adrenal Neoplasms
Adrenal tumors causing hyperandrogenism without symptoms
and signs of glucocorticoid excess are rare.
OVARIAN DISORDERS
Polycystic Ovary Syndrome
Six to 10% of women of reproductive age have some form of
PCOS. This syndrome is a chronic condition that has been defined
as anovulation or oligo-ovulation with clinical or laboratory
evidence of hyperandrogenism and without evidence of any other
underlying condition
Hyperandrogenic
Insulin
Resistance
and
Acanthosis
Nigricans Syndrome
HAIR-AN syndrome is characterized by extremely high
circulating levels of insulin (>80 mU/mL basally or >500 mU/mL
following an oral glucose challenge) due to severe insulin
resistance. Because insulin is also a mitogenic hormone, these
extremely elevated insulin levels result in hyperplasia of the basal
layers of the epidermal skin, leading to the development of
acanthosis nigricans, a velvety, hyperpigmented change of the
crease areas of the skin. In addition, because of the effect of in-
sulin on ovarian theca cells, the ovaries of many patients with the
HAIR-AN syndrome are hyperthecotic. Patients with this
disorder can be severely hyperandrogenic and even present with
virilization.
Ovarian Neoplasms
Androgen-producing ovarian tumors are extremely uncommon,
and include Sertoli-Leydig, hilus, and lipoid cell tumors
Diagnosis of Secondary Amenorrhea
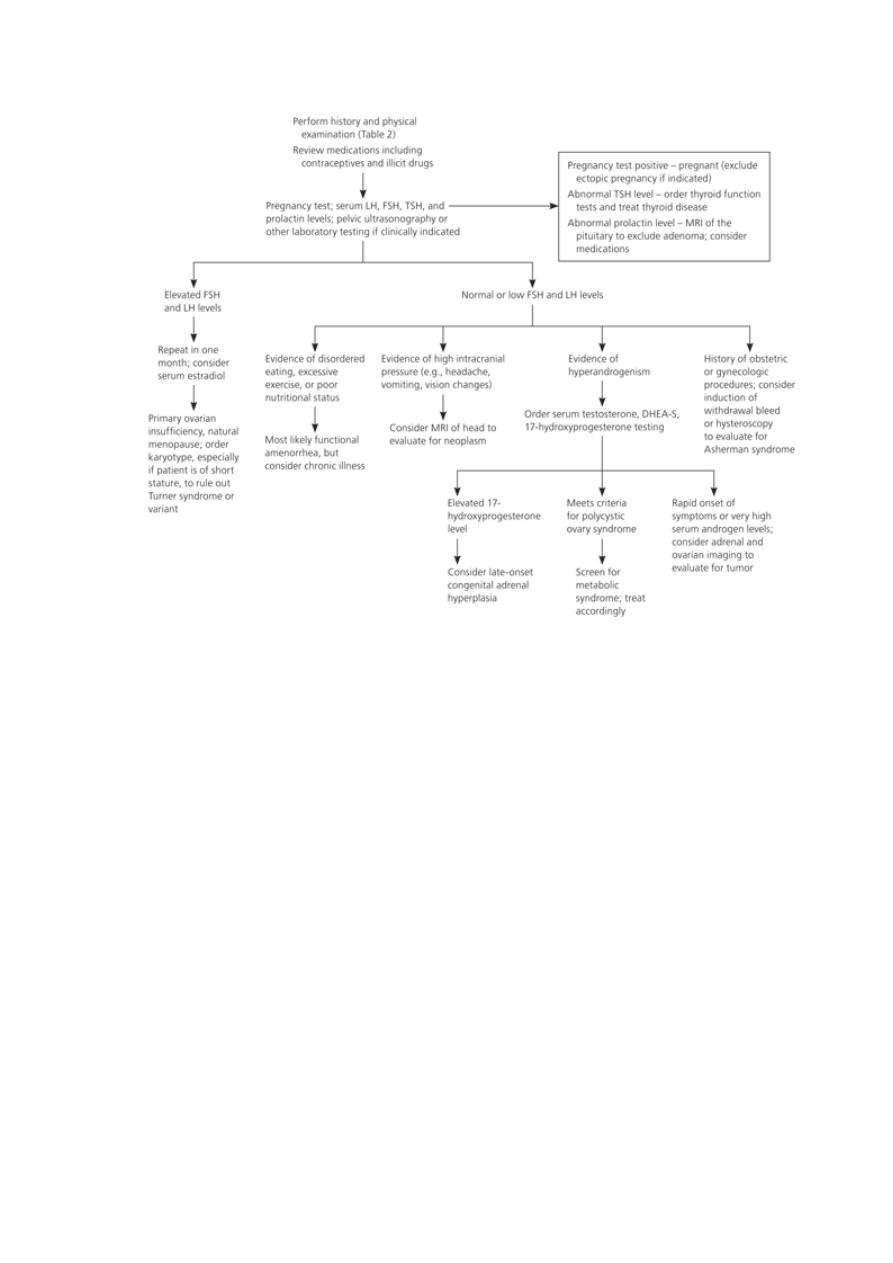
A diagnostic approach to secondary amenorrhea. (DHEA-S = dehydroepiandrosterone sulfate; FSH =
follicle-stimulating hormone; LH = luteinizing hormone; MRI = magnetic resonance imaging; TSH =
thyroid-stimulating hormone.
