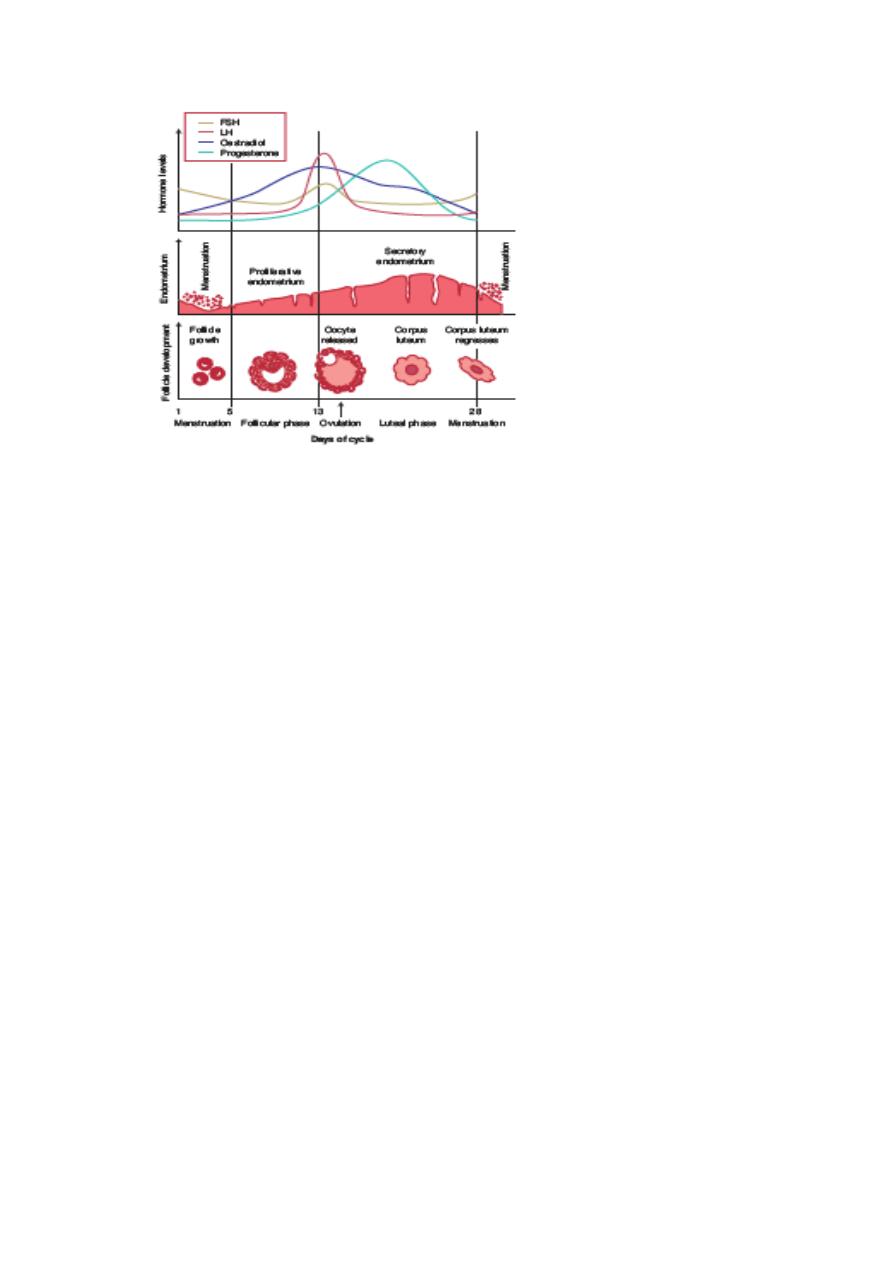
Normal menstrual cycle
Introduction
The external manifestation of a normal menstrual cycle is the presence of regular vaginal bleeding. This occurs as
a result of the shedding of the endometrial lining following failure of fertilization of the oocyte or failure of
implantation. The cycle depends on changes occurring within the ovaries and fluctuation in ovarian hormone
levels, that are themselves controlled by the pituitary and hypothalamus, the hypothalamo–pituitary–ovarian axis
(HPO)
Hypothalamus
The hypothalamus in the forebrain secretes the peptide hormone gonadotrophin-releasing hormone (GnRH),
which in turn controls pituitary hormone secretion. GnRH must be released in a pulsatile fashion to stimulate
pituitary secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH). If GnRH is given in a
constant high dose, it desensitizes the GnRH receptor and reduces LH and FSH release.
Pituitary gland
GnRH stimulation of the basophil cells in the anterior pituitary gland causes synthesis and release of the
gonadotrophic hormones, FSH and LH. This process is modulated by the ovarian sex steroid hormones oestrogen
and progesterone. Low levels of oestrogen have an inhibitory effect on LH production (negative feedback),
whereas high levels of oestrogen will increase LH production (positive feedback). The high levels of circulating
oestrogen in the late follicular phase of the ovary act via the positive feedback mechanism to generate a
periovulatory LH surge from the pituitary.
Unlike oestrogen, low levels of progesterone have a positive feedback effect on pituitary LH and FSH secretion (as
seen immediately prior to ovulation) and contribute to the FSH surge. High levels of progesterone, as seen in the
luteal phase, inhibit pituitary LH and FSH production. Positive feedback effects of progesterone occur via
increasing sensitivity to GnRH in the pituitary. Negative feedback effects are generated through both decreased
GnRH production from the hypothalamus and decreased sensitivity to GnRH in the pituitary. It is known that
progesterone can only have these effects on gonadotropic hormone release after priming by oestrogen.
Inhibin and activin are peptide hormones produced by granulosa cells in the ovaries, with opposing effects on
gonadotrophin production. Inhibin inhibits pituitary FSH secretion, whereas activin stimulates it.

Ovary
Ovaries with developing oocytes are present in the female fetus from an early stage of development. By the end
of the second trimester
in utero, the number of oocytes has reached a maximum and they arrest at the first
prophase step in meiotic division. No new oocytes are formed during the female lifetime.
With the onset of menarche, the primordial follicles containing oocytes will activate and grow in a cyclical
fashion, causing ovulation and subsequent menstruation in the event of non-fertilization. In the course of a
normal menstrual cycle, the ovary will go through three phases:
1
Follicular phase
2
Ovulation
3
Luteal phase.
Follicular phase
The initial stages of follicular development are independent of hormone stimulation.
FSH levels rise in the first days of the menstrual cycle, when oestrogen, progesterone and inhibin levels are low.
This stimulates a cohort of small antral follicles on the ovaries to grow.
Within the follicles, there are two cell types which are involved in the processing of steroids, including oestrogen
and progesterone. These are the theca and the granulosa cells, which respond to LH and FSH stimulation,
respectively. LH stimulates production of androgens from cholesterol within theca cells. These androgens are
converted into oestrogens by the process of aromatization in granulosa cells, under the influence of FSH.
Both FSH and LH are required to generate a normal cycle with adequate amounts of oestrogen.
As the follicles grow and oestrogen secretion increases, there is negative feedback on the pituitary to decrease
FSH secretion. This assists in the selection of one follicle to continue in its development towards ovulation – the
dominant follicle. In the ovary, the follicle which has the most efficient aromatase activity and highest
concentration of FSH-induced LH receptors will be the most likely to survive as FSH levels drop, while smaller
follicles will undergo atresia.
The dominant follicle will go on producing oestrogen and also inhibin, which enhances androgen synthesis under
LH control.
There are other autocrine and paracrine mediators playing a role in the follicular phase of the menstrual cycle.
These include inhibin and activin.

Insulin-like growth factors (IGF-I, IGF-II) act as paracrine regulators. Circulating levels do not change during the
menstrual cycle, but follicular fluid levels increase towards ovulation, with the highest level found in the
dominant follicle.
In the follicular phase, IGF-I is produced by theca cells under the action of LH. Within the theca, IGF-I augments
LH-induced steroidogenesis.
In granulosa cells, IGF-I augments the stimulatory effects of FSH on mitosis, aromatase activity and inhibin
production. In the preovulatory follicle,
IGF-I enhances LH-induced progesterone production from granulosa cells. Following ovulation, IGF-II is produced
from luteinized granulosa cells, and acts in an autocrine manner to augment LH-induced proliferation of
granulosa cells.
Kisspeptins are proteins which have more recently been found to play a role in regulation of the HPO axis, via
the mediation of the metabolic hormone leptin’s effect on the hypothalamus. Leptin is thought to be key in the
relationship between energy production, weight and reproductive health. Mutations in the kisspeptin receptor,
are associated with delayed or absent puberty,
Ovulation
By the end of the follicular phase, which lasts an average of 14 days, the dominant follicle has grown to
approximately 20 mm in diameter. As the follicle matures, FSH induces LH receptors on the granulose cells and
prepare for the signal for ovulation. Production of oestrogen increases until they reach the necessary threshold to
exert a positive feedback effort on the hypothalamus and pituitary to cause the LH surge. This occurs over 24–36
hours, during which time the LH-induced luteinization of granulosa cells in the dominant follicle causes
progesterone to be produced, adding further to the positive feedback for LH secretion and causing a small
periovulatory rise in FSH. Androgens, synthesized in the theca cells, also rise around the time of ovulation .
The LH surge is one of the best predictors of imminent ovulation,
The LH surge has another function in stimulating the resumption of meiosis in the oocyte just prior to its
release.
The physical ovulation of the oocyte occurs after breakdown of the follicular wall occurs under the influence of
LH, FSH and progesterone controlled
proteolytic enzymes, such as plasminogen activators and prostaglandins. There appears to be an inflammatory-
type response within the follicle wall which may assist in extrusion of the oocyte by stimulating smooth muscle
activity. So inhibition of prostaglandin production may result in failure of ovulation. Thus, women wishing to
become pregnant should be advised to avoid taking prostaglandin synthetase inhibitors, such as aspirin and
ibuprofen, which may inhibit oocyte release.
Luteal phase
After the release of the oocyte, the remaining granulose and theca cells on the ovary form the corpus luteum.

The granulosa cells have a vacuolated appearance with accumulated yellow pigment, hence the name corpus
luteum (‘yellow body’). The corpus luteum undergoes extensive vascularization in order to supply granulosa cells
with a rich blood supply for continued steroidogenesis.
Ongoing pituitary LH secretion and granulosa cell activity ensures a supply of progesterone which stabilizes the
endometrium in preparation for pregnancy. Progesterone levels are at their highest in the cycle during the luteal
phase. This also has the effect of suppressing FSH and LH secretion to a level that will not produce further
follicular growth in the ovary during that cycle.
The luteal phase lasts 14 days in most women, without great variation. In the absence of beta human chorionic
gonadotrophin (HCG) being produced from an implanting embryo, the corpus luteum will regress in a process
known as luteolysis. The mature corpus luteum is less sensitive to LH, produces less progesterone, and will
gradually disappear from the ovary. The withdrawal of progesterone has the effect on the uterus of causing
shedding of the endometrium and thus menstruation. Reduction in levels of progesterone, oestrogen and inhibin
feeding back to the pituitary cause increased secretion of gonadotrophic hormones, particularly FSH. New
preantral follicles begin to be stimulated and the cycle begins anew.
Endometrium
The hormone changes effected by the HPO axis during the menstrual cycle will occur whether the uterus is
present or not. However, the specific secondary changes in the uterine endometrium give the most obvious
external sign of regular cycles.
Menstruation
The endometrium is under the influence of sex steroids that circulate in females of reproductive age. Sequential
exposure to oestrogen and progesterone will result in cellular proliferation and differentiation, in preparation for
the implantation of an embryo in the event of pregnancy, followed by regular bleeding in response to
progesterone withdrawal if the corpus luteum regresses. During the ovarian follicular phase, the endometrium
undergoes proliferation (the ‘proliferative phase’); during the ovarian luteal phase, it has its ‘secretory phase’.
Decidualization, the formation of a specialized glandular endometrium, is an irreversible process and apoptosis
occurs if there is no embryo implantation. Menstruation (day 1) is the shedding of the ‘dead’ endometrium and
ceases as the endometrium regenerates (which normally happens by day 5–6 of the cycle).
The endometrium is composed of two layers, the uppermost of which is shed during menstruation. A fall in
circulating levels of oestrogen and progesterone approximately 14 days after ovulation leads to loss of tissue
fluid, vasoconstriction of spiral arterioles and distal ischaemia. This results in tissue breakdown, and loss of the
upper layer along with bleeding from fragments of the remaining arterioles is seen as menstrual bleeding.
Enhanced fibrinolysis reduces clotting.
Vaginal bleeding will cease after 5–10 days as arterioles vasoconstrict and the endometrium begins to
regenerate. Haemostasis in the uterine endometrium is different from haemostasis elsewhere in the body as it
does not involve the processes of clot formation and fibrosis.
Prostaglandin F2, endothelin-1 and plateletmactivating factor (PAF) are vasoconstrictors which are produced
within the endometrium and are thought likely to be involved in vessel constriction, both initiating and
controlling menstruation. They may be balanced by the effect of vasodilator agents, such as prostaglandin E2,

prostacyclin (PGI) and nitric oxide (NO), which are also produced by the endometrium. Progesterone withdrawal
increases endometrial prostaglandin (PG) synthesis and decreases PG metabolism. The COX-2 enzyme and
chemokines are involved in PG synthesis and this is likely to be the target of non-steroidal anti-inflammatory
agents used for the treatment of heavy and painful periods.
Endometrial repair involves both glandular and stromal regeneration and angiogenesis to reconstitute the
endometrial vasculature. VEGF and fibroblast growth factor (FGF) are found within the endometrium and both
are powerful angiogenic agents. Other growth factors, such as transforming growth factors (TGFs), epidermal
growth factor (EGF) and IGFs, and the interleukins may also be important.
The proliferative phase
Menstruation will normally cease after 5–7 days, once endometrial repair is complete. After this time, the
endometrium enters the proliferative phase, when glandular and stromal growth occur. The epithelium lining the
endometrial glands changes from a single layer of columnar cells to a pseudostratified epithelium with frequent
mitoses. The stroma is infiltrated by cells derived from the bone marrow . Endometrial thickness increases rapidly,
from 0.5 mm at menstruation to 3.5–5 mm at the end of the proliferative phase.
The secretory phase
After ovulation (generally around day 14), there is a period of endometrial glandular secretory activity. Following
the progesterone surge, the oestrogen induced cellular proliferation is inhibited and the endometrial thickness
does not increase any further. However, the endometrial glands will become more tortuous, spiral arteries will
grow, and fluid is secreted into glandular cells and into the uterine lumen. Later in the secretory phase,
progesterone induces the formation of a temporary layer, known as the decidua, in the endometrial stroma.
Histologically, this is seen as occurring around blood vessels. Stromal cells show increased mitotic activity,
nuclear enlargement and generation of a basement membrane
Recent research into infertility has identified apical membrane projections of the endometrial epithelial cells
known as pinopodes, which appear after day 21–22 and appear to be a progesterone-dependent stage in
making the endometrium receptive for
embryo implantation .
Immediately prior to menstruation, three distinct layers of endometrium can be seen. The basalis is the lower
25 per cent of the endometrium, which will remain throughout menstruation and shows few changes during the
menstrual cycle. The mid-portion is the stratum spongiosum with oedematous stroma and exhausted glands.
The superficial portion (upper 25 per cent) is the stratum compactum with prominent decidualized stromal
cells. On the withdrawal of both oestrogen and progesterone, the decidua will collapse, with vasoconstriction
and relaxation of spiral arteries and shedding of the outer layers of the endometrium