CHILDHOOD DIABETES MELLITUS
DEFINITIONThe term diabetes mellitus describes
a metabolic disorder of multiple
etiologies characterized by chronic
hyperglycemia with disturbances of
carbohydrate, fat and protein
metabolism resulting from defects of
insulin secretion, insulin action or
both.
EPIDEMIOLOGY
Diabetes is the most common endocrine problem & is a major health hazard worldwide.Incidence of diabetes is alarmingly increasing all over the globe.
Incidence of childhood diabetes range between 3-50/100,000 worldwide.
CLASSIFICATION OLD(1985)
Type 1, Insulin-dependent (IDDM)
Type 2, Non Insulin-dependent (NIDDM)
obese
non-obese
MODY
IGT
Gestational Diabetes
WHO CLASSIFICATION 2000
Is based on etiology not on type of treatment or age of the patient.Type 1 Diabetes
(idiopathic or autoimmune b-cell destruction)
Type 2 Diabetes
(defects in insulin secretion or action)
Other specific types
WHO CLASSIFICATION/2
Both type 1 & type 2 can be further subdivided into:Not insulin requiring
Insulin requiring for control
Insulin requiring for survival
Gestational diabetes is a separate entity
Impaired Glucose Tolerance (IGT) indicates blood glucose levels between normal & diabetic cut off points during glucose tolerance test.
DIAGNOSTIC CRITERIA
Fasting blood glucose level
Diabetic
Plasma >7.0 mmol
Capillary >6.0 mmol
IGT
Plasma 6.0-6.9 mmol
Capillary 5.6-6.0 mmol
2 hours after glucose load(Plasma or capillary BS)
Diabetic
11.1 (200 mg)IGT
7.8-11.0
Types of Diabetes in Children
Type 1 diabetes mellitus accounts for >90% of cases.Type 2 diabetes is increasingly recognized in children with presentation like in adults.
Permanent neonatal diabetes
Transient neonatal diabetes
Maturity-onset diabetes of the young
Secondary diabetes e.g. in cystic fibrosis or Cushing syndrome.
MODY
Usually affects older children & adolescents
Not rare as previously considered
5 subclasses are identified, one subclass has specific mode of inheritance (AD)
Not associated with immunologic or genetic markers
Insulin resistance is present
TRANSIENT NEONATAL DIABETES
Observed in both term & preterm babies, but more common in pretermCaused by immaturity of islet b-cells
Polyuria & dehydration are prominent, but baby looks well & suck vigorously
Highly sensitive to insulin
Disappears in 4-6 weeks
PERMANENT NEONATAL DIABETES
A familial form of diabetes that appear shortly after birth & continue for lifeThe usual genetic & immunologic markers of Type 1 diabetes are absent
Insulin requiring, but ketosis resistant
Is often associated with other congenital anomalies & syndromes.
TYPE 1 DIABETES: ETIOLOGY
Type 1 diabetes mellitus is an autoimmune disease.It is triggered by environmental factors in genetically susceptible individuals.
Both humoral & cell-mediated immunity are stimulated.
AUTOIMMUNITY
Circulating antibodies against b-cells and insulin.
Immunofluorescent antibodies & lymphocyte infiltration around pancreatic islet cells.
Evidence of immune system activation. Circulating immune complexes with high IgA & low interferon levels.
Association with other autoimmune diseases.
GENETIC FACTORS
Evidence of genetics is shown inEthnic differences
Familial clustering
High concordance rate in twins
Specific genetic markers
Higher incidence with genetic syndromes or chromosomal defects
ENVIRONMENTAL INFLUENCE
Seasonal & geographical variation.Migrants take on risk of new home.
Evidence for rapid temporal changes.
Suspicion of environmental agents causing disease which is confirmed by case-control experimental animal studies.
ENVIRONMENTAL SUSPECTS
VirusesCoxaschie B
Mumps
Rubella
Reoviruses
Nutrition & dietary factors
Cow’s milk protein
Contaminated sea food
OTHER MODIFYING FACTORS
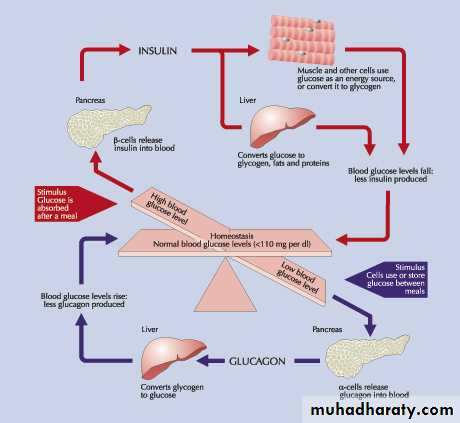
The counter-regulatory hormones:
glucagon
cortisol,
Catecholamines.
thyroxin,
GH & somatostatin.
sex hormones
Emotional stress
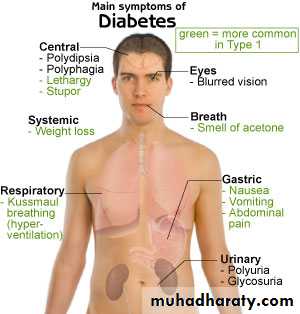
CLINICAL PRESENTATIONS
Classical symptom triad:polyuria, polydipsia and weight loss
DKA
Accidental diagnosis
Anorexia nervosa like illness
DIAGNOSIS
In symptomatic children a random plasma glucose >11 mmol (200 mg) is diagnostic.
A modified OGTT (fasting & 2h) may be needed in asymptomatic children with hyperglycemia if the cause is not obvious.
Remember: acute infections in young non-diabetic children can cause hyperglycemia without ketoacidosis.
NATURAL HISTORY
Diagnosis & initiation of insulinPeriod of metabolic recovery
Honeymoon phase
State of total insulin dependency
METABOLIC RECOVERY
During metabolic recovery the patient may develop one or more of the following:Hepatomegaly
Peripheral edema
Loss of hair
Problem with visual acuity
These are caused by deposition of glycogen & metabolic re-balance.
HONEYMOON PERIOD
Observed in 50-60% of newly diagnosed patients & it can last up to one year but it always ends.Due to b-cell reserve optimal function & initiation of insulin therapy.
Leads to normal blood glucose level without exogenous insulin.
Can confuse patients & parents if not educated about it early.
COMPLICATIONS OF DIABETES
Acute:
DKA
Hypoglycemia
Late-onset:
Retinopathy
Neuropathy
Nephropathy
Ischemic heart disease & stroke
TREATMENT GOALS
Prevent death & alleviate symptomsAchieve biochemical control
Maintain growth & development
Prevent acute complications
Prevent or delay late-onset complications
TREATMENT ELEMENTS
EducationInsulin therapy
Diet and meal planning
Monitoring
HbA1c every 2-months
Home regular BG monitoring
Home urine ketones tests when indicated
EDUCATION
Educate child & care givers about:
Diabetes
Insulin
Life-saving skills
Recognition of Hypo & DKA
Meal plan
Sick-day management
INSULIN
A polypeptide made of 2 b-chains.Discovered by Bants & Best in 1921.
Animal types (porcine & bovine) were used before the introduction of human-like insulin (DNA-recombinant types).
Recently more potent insulin analogs are produced by changing aminoacid sequence.
FUNCTION OF INSULIN
• Insulin being an anabolic hormone stimulates protein & fatty acids synthesis.• Insulin decreases blood sugar
• By inhibiting hepatic glycogenolysis and gluconeogenesis.
• By stimulating glucose uptake, utilization & storage by the liver, muscles & adipose tissue.
TYPES OF INSULIN
Short acting (neutral, soluble, regular)
Peak 2-3 hours & duration up to 8 hours
Intermediate acting
Isophane (peak 6-8 h & duration 16-24 h)
Biphasic (peak 4-6 h & duration 12-20 h)
Semilente (peak 5-7 h & duration 12-18 h)
Long acting (lente, ultralente & PZI)
Peak 8-14 h & duration 20-36 h
INSULIN REGIMENS
Twice daily: either NPH alone or NPH+SI.Thrice daily: SI before each meal and NPH only before dinner.
Intensive 4 times/day: SI before meals + NPH or Glargine at bed time.
Continuous s/c infusion using pumps loaded with SI.
ADVERSE EFFECTS OF INSULIN
HypoglycemiaLipoatrophy
Lipohypertrophy
Obesity
Insulin allergy
Insulin antibodies
Insulin induced edema
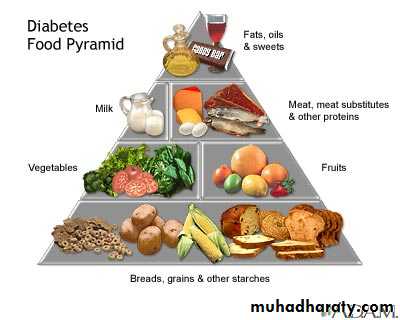
DIET REGULATION
Regular meal plans with calorie exchange options are encouraged.
50-60% of required energy to be obtained from complex carbohydrates.
Distribute carbohydrate load evenly during the day preferably 3 meals & 2 snacks with avoidance of simple sugars.
Encouraged low salt, low saturated fats and high fiber diet.
EXERCISE
Decreases insulin requirement in diabetic subjects by increasing both sensitivity of muscle cells to insulin & glucose utilization.It can precipitate hypoglycemia in the unprepared diabetic patient.
It may worsen pre-existing diabetic retinopathy.
MONITORING
Compliance (check records)HBG tests
HbA1 every 2 months
Insulin & meal plan
Growth & development
Well being & life style
School & hobbies
PREVENTION OF DIABETES
Primary prevention
Identification of diabetes gene
Tampering with the immune system
Elimination of environmental factor
Secondary prevention
Immunosuppressive therapy
Tertiary prevention
Tight metabolic control & good monitoring