
ANORECTAL ABSCESSES
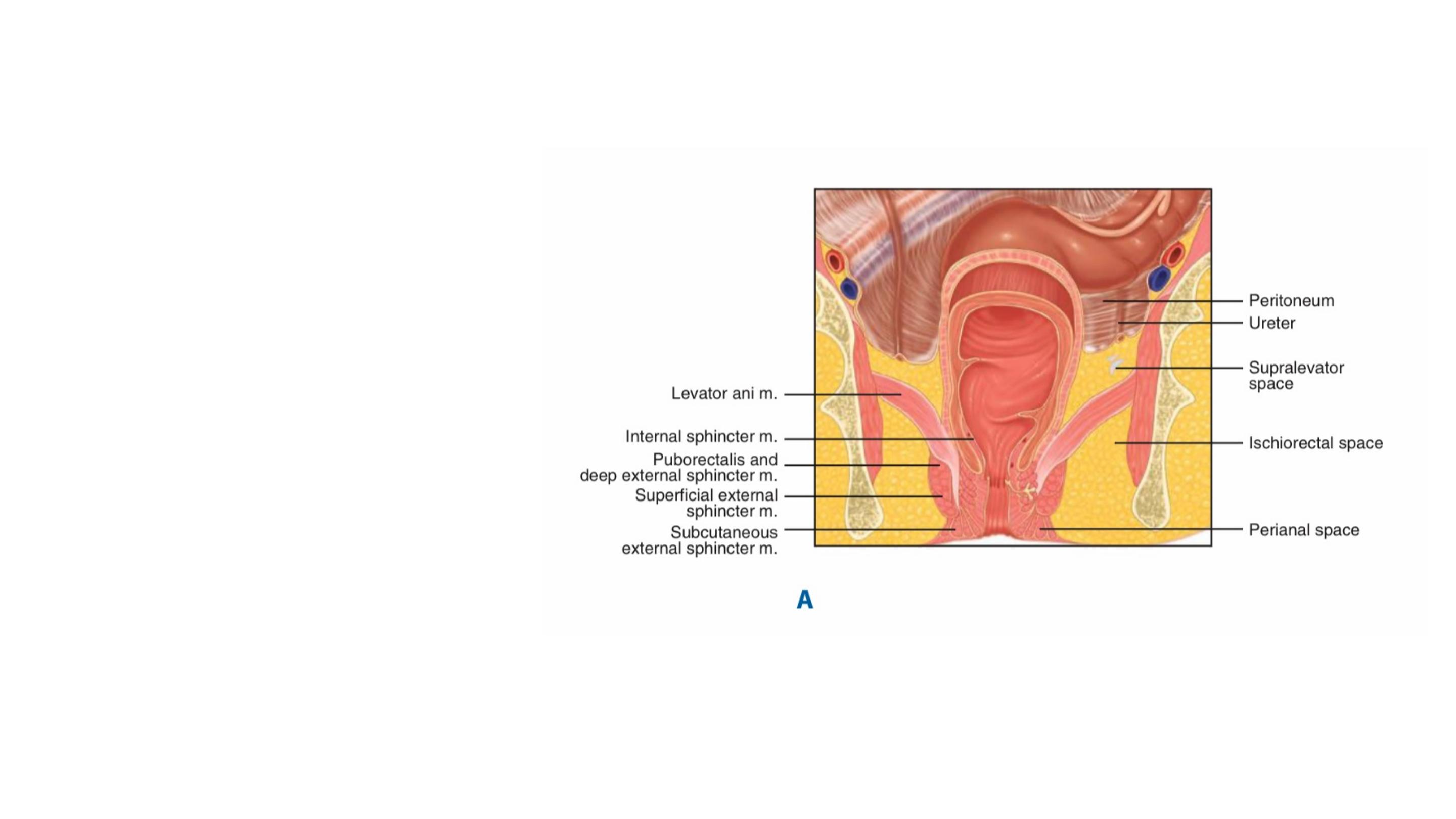
Infection of an anal gland results in the formation of an abscess that
enlarges and spreads along one of several planes in the perianal and
perirectal spaces.
The majority of anorectal suppurative disease results from infections of the
anal glands (cryptoglandular infection) found in the intersphincteric plane.
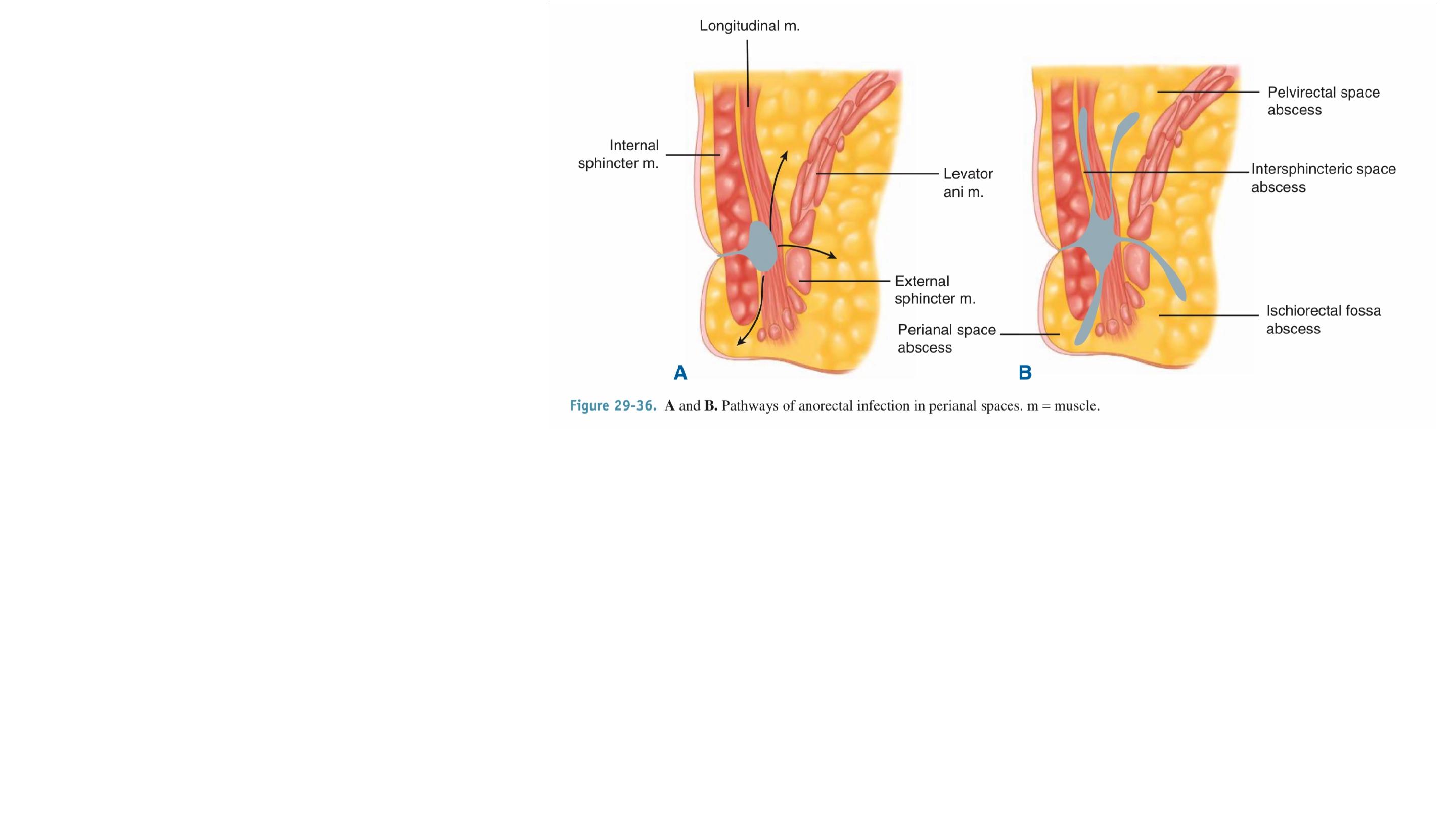
A perianal abscess is the
most common manifestation
and appears as a painful
swelling at the anal verge.
Spread through the external
sphincter below the level of
the puborectalis produces an
ischiorectal abscess.
Pelvic and supralevator abscesses are uncommon and may result from
extension of an intersphincteric or ischiorectal abscess upward or extension of
an intraperitoneal abscess downward
Intersphincteric abscesses occur in the inter- sphincteric space and are
notoriously difficult to diagnose, often requiring an examination under
anesthesia.

Sepsis unrelated to anal gland infection may occur in :
Submucosal abscess (following haemorrhoidal sclerotherapy, which
usually resolve spontaneously)
Mucocutaneous or marginal abscess (infected haematoma)
Ischiorectal abscess (foreign body, trauma, deep skin-related) and
pelvirectal supralevator sepsis originating in pelvic disease.
Underlying rectal disease, such as neoplasm and particularly
Crohn’s disease, may be the cause.
Patients with generalised disorders, such as diabetes and acquired
immunodeficiency syndrome (AIDS), may present with an anorectal
abscess.

Usually produces a painful, throbbing swelling in the anal region.
The patient often has swinging pyrexia
Subdivided according to anatomical site into perianal,
ischiorectal, submucous and pelvirectal
Underlying conditions include fistula-in-ano (most common),
Crohn’s disease, diabetes, immunosuppression
Always look for a potential underlying problem
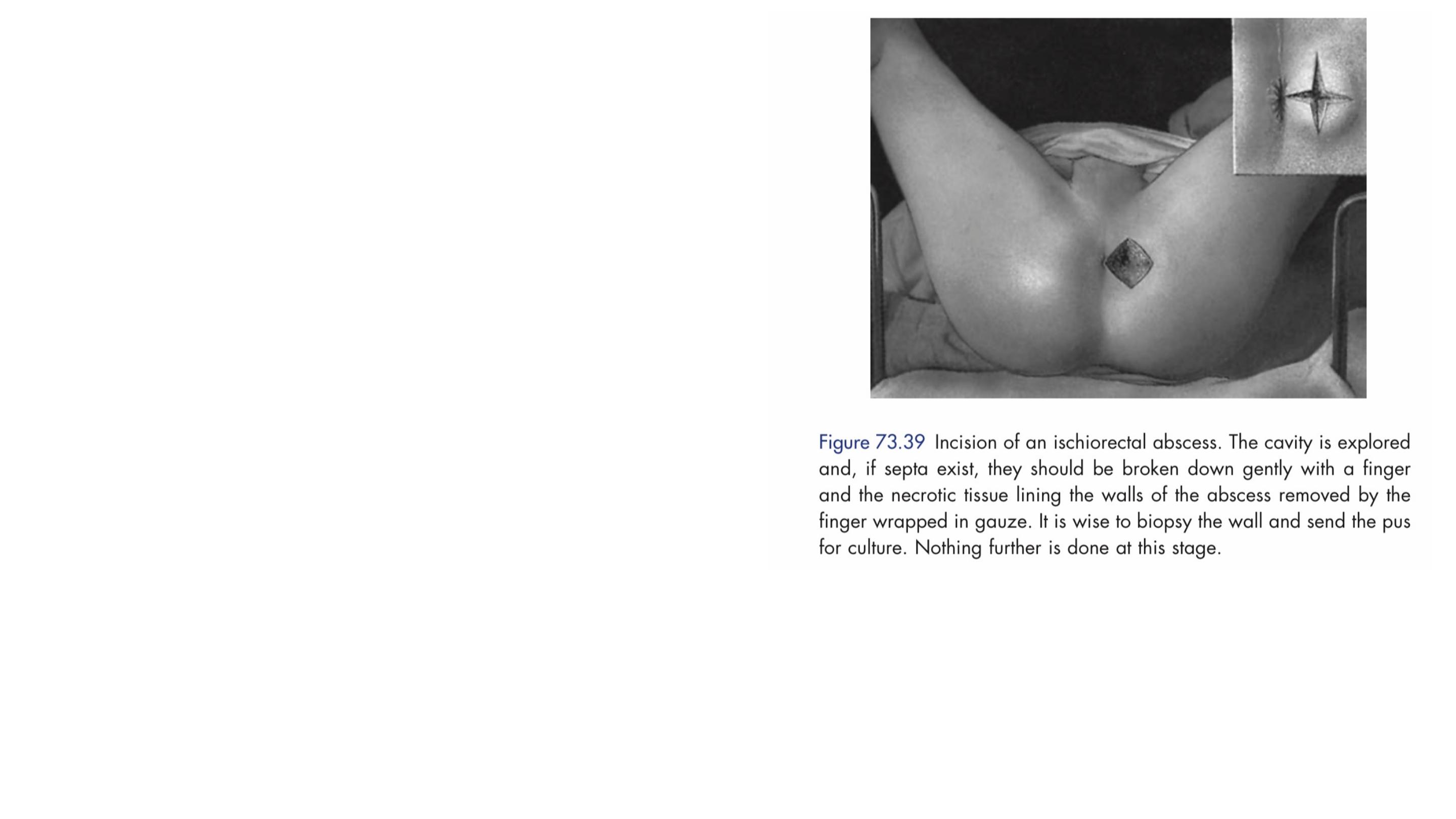
Management of acute anorectal sepsis is
primarily surgical, including careful
examination under anaesthesia,
sigmoidoscopy and proctoscopy, and
adequate drainage of the pus.
Pus is sent for microbiological
culture and tissue from the wall is
sent for histological appraisal to
exclude specific causes.
If the pus subsequently cultures skin-type organisms, there will be no
underlying fistula and the patient can be reassured. If gut flora are cultured, it
is likely, but not inevitable, that there is an underlying fistula.

FISTULA-IN-ANO
Is a chronic abnormal communication, usually lined to some degree by
granulation tissue, which runs outwards from the anorectal lumen (the
internal opening) to an external opening on the skin of the perineum or
buttock (or rarely, in women, to the vagina).
May be associated with underlying disease, such as tuberculosis ,
Crohn’s disease or malignancy.
Drainage of an anorectal abscess results in cure for about 50% of
patients. The remaining 50% develop a persistent fistula in ano.

Clinical assessment
A full medical history and proctosigmoidoscopy are necessary to
gain information about sphincter strength and to exclude
associated conditions.
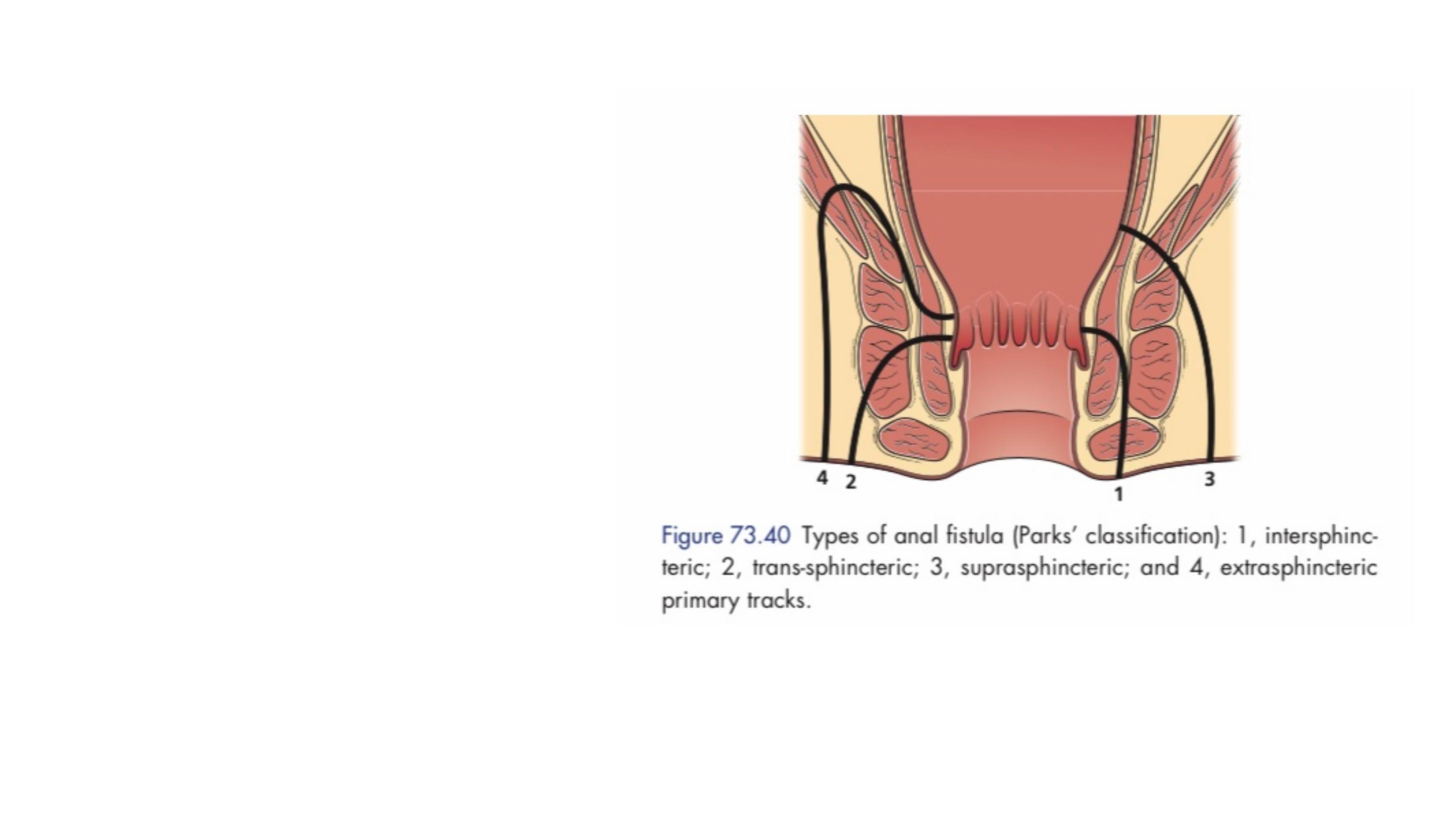
Classification
Parks classification based on
the centrality of intersphincteric
anal gland sepsis (the internal
opening is usually at the
dentate line), which results in a
primary track whose relation to
the external sphincter defines
the type of fistula and which
influences management.
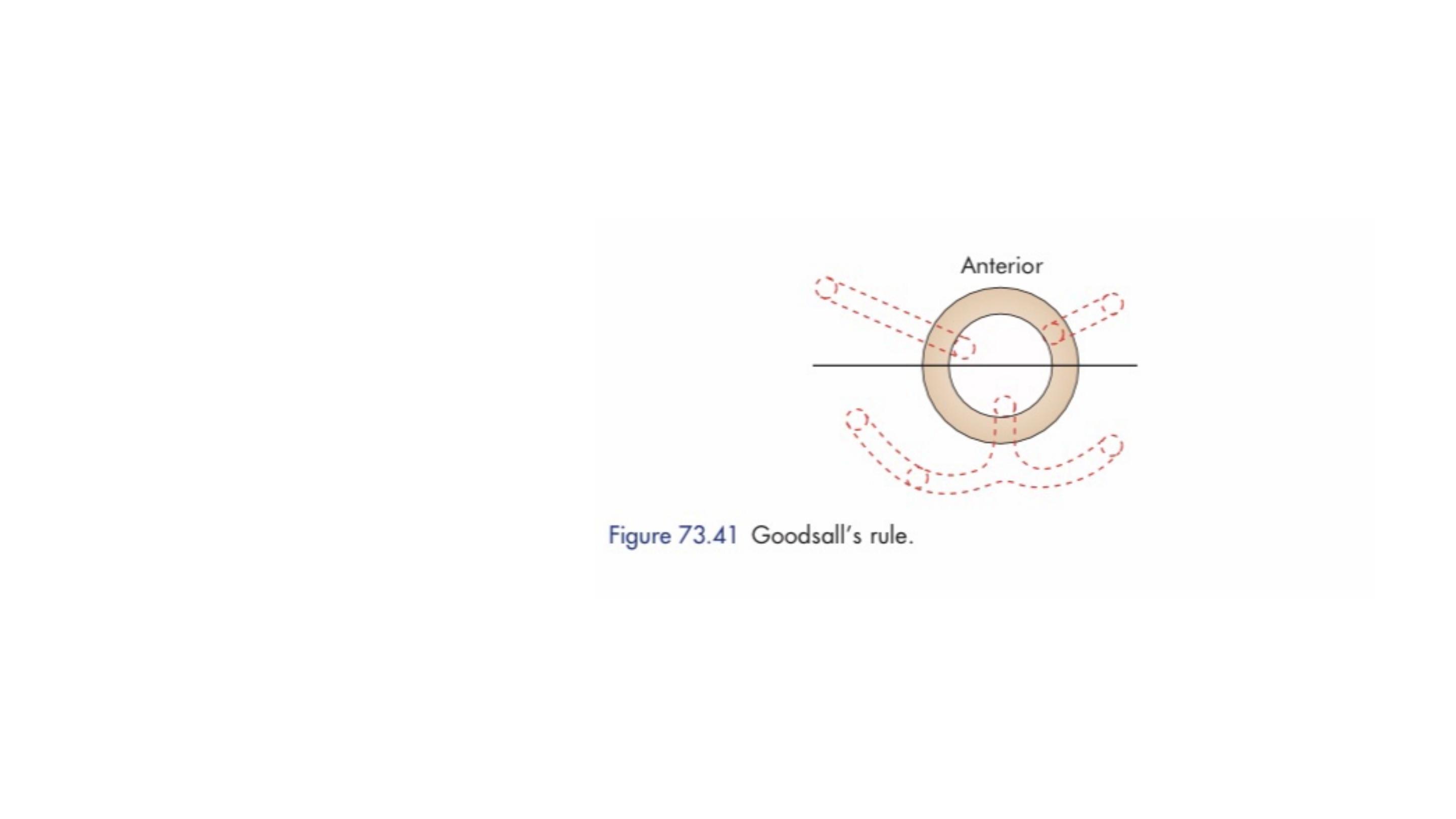
Goodsall’s rule
used to indicate the likely position of
the internal opening according to the position of the
external opening(s), is helpful but not infallible.
In general, fistulas with an external opening anteriorly connect to the internal
opening by a short, radial tract. Fistulas with an external opening posteriorly
track in a curvilinear fashion to the posterior midline.

Special investigations
Clinical examination will give some indication of functional anal sphincter
length, resting tone and voluntary squeeze
Anal manometry objectively gives useful information about sphincter integrity
and can also be used to delineate fistulae.
Magnetic resonance imaging (MRI) is acknowledged to be the ‘gold standard’ for
fistula imaging, but it is limited by availability and cost and is usually reserved for
difficult recurrent cases. The great advantage of MRI is its ability to demonstrate
secondary extensions, which may be missed at surgery and which are the cause
of persistence.
Fistulography and computed tomography (CT) both have limitation but are
useful techniques if an extrasphincteric fistula is suspected.

Full examination under anaesthesia should be repeated before surgical
intervention.
Patients with minimal symptoms, especially if they have compromised
sphincters, may be managed expectantly.
Eradication of sepsis requires surgery, the aim of which must be
balanced with the preservation of continence.
Most fistulae are relatively straightforward to deal with; however, a
minority are extremely problematic.
Management

Fistulotomy
, or laying open, is the surest way of getting rid of a fistula, but, by
definition, it involves division of all those structures lying between the external
and internal openings.
Fistulectomy, This technique involves coring out of the fistula.

Loose setons
are tied such that there is no tension upon the
encircled tissue; there is no intent to cut the tissue.
Tight or cutting setons
are placed with the intention of cutting
through the enclosed muscle.

Simple intersphincteric fistulas can often be treated by fistulotomy.
Fistulas that include less than 30% of the sphincter muscles can
often be treated by sphincterotomy without significant risk of
major incontinence
High transsphincteric fistulas and suprasphincteric which
encircle a greater amount of muscle, are more safely treated by
initial placement of a seton

Fibrin glue and a variety of collagen-based plugs also have been used to
treat persistent fistulas with variable results.
Higher fistulas may be treated by an endorectal advancement flap.
A more recent technique, ligation of the intersphincteric fistula tract (LIFT),
also shows promise.
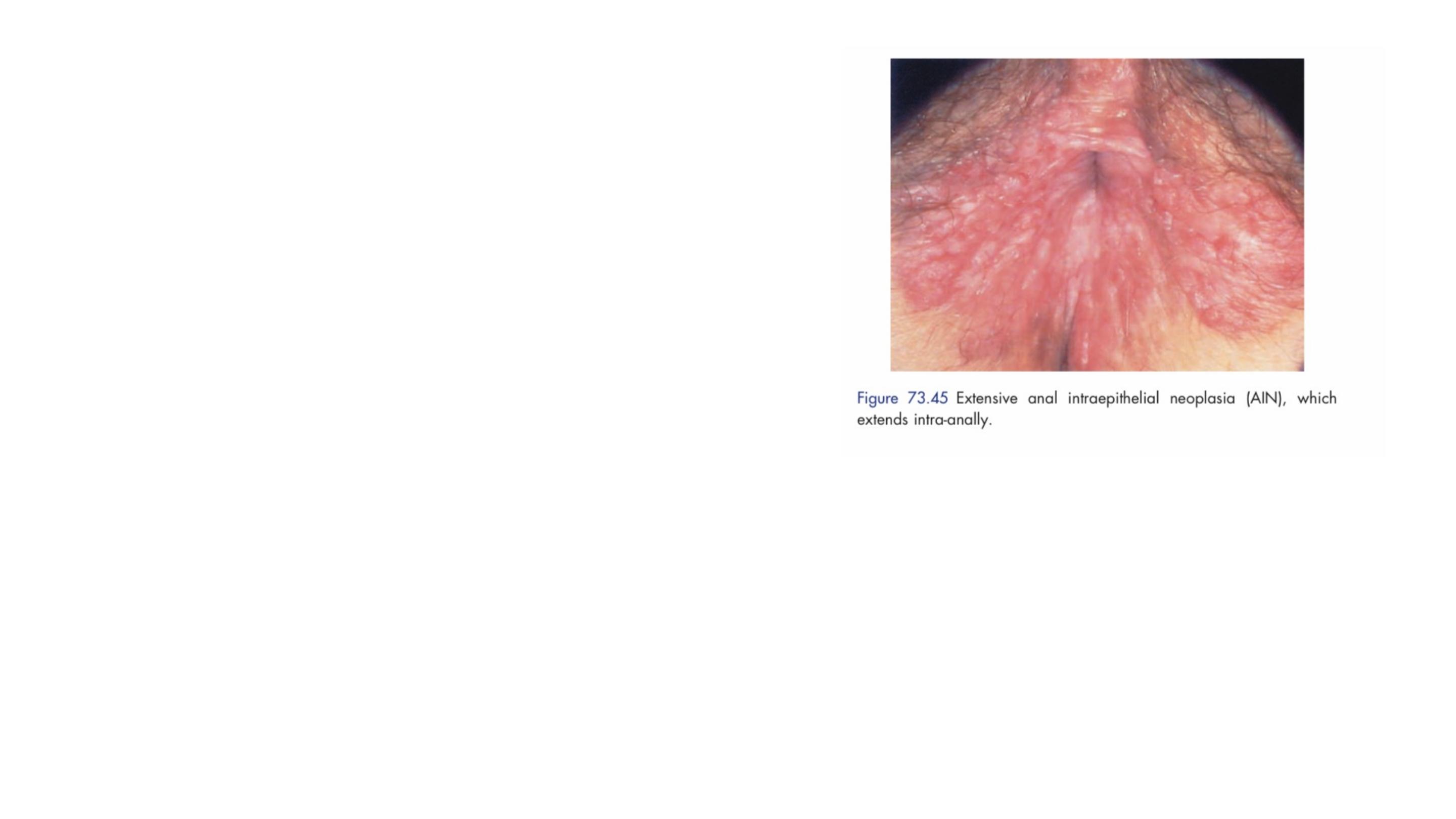
ANAL INTRAEPITHELIAL NEOPLASIA
Anal intraepithelial neoplasia is a multifocal
virally induced dysplasia of the perianal or
intra-anal epidermis which is associated with
the human papilloma virus (most frequently
subtypes 6, 11, 16 and 18).
At-risk groups include patients with HIV, as well as immunocompromised
patients.
It is classified according to the degree of dysplasia on biopsy into AIN I, AIN II and
AIN III, according to the lack of keratocyte maturation and extension of the
proliferative zone from the lower third (AIN I) to the full thickness of the epithelium
(AIN III),

Approximately 10 per cent of AIN III lesions will progress to anal
carcinoma at five years.
Focal disease may be excised and local excision is effective for
lesions <30 per cent of the circumference of the anus.
More widespread disease can be dealt with surgically by wide
local excision and closure of the resultant defect by flap or skin
graft, with or without covering colostomy.

Anal cancer
■
Uncommon tumour, which is usually a squamous cell carcinoma
■
Anal SCC is associated with HPV (especially subtypes 16, 18, 31 or 33) in
70–90 per cent of cases
■
More prevalent in patients with HIV infection
■
Pain and bleeding are the most common symptoms and mass, pruritus or
discharge is less common.
■ May affect the anal verge or anal canal
■
On examination, anal margin tumours look like malignant ulcers.
■
Anal canal tumours are palpable as irregular indurated tender ulceration.

Lymphatic spread is to the inguinal lymph nodes.
Despite good results with chemoradiotherapy 20–25 per cent of
patients will have local disease relapse. After thorough
assessment, these patients may require radical abdominoperineal
resection,
Treatment is by chemoradiotherapy in the first instance.

Adenocarcinomata
within the anal canal are usually extensions
of distal rectal cancers.
Rarely, adenocarcinoma may arise from anal glandular epithelium
or develop within a longstanding (usually complex) anal fistula
Treatment is as for low rectal cancers (i.e. abdominoperineal excision of
the rectum (APER) with or without previous radiotherapy or
chemoradiotherapy), but prognosis is less good.

Pilonidal Disease
It consists of a hair-containing sinus or abscess occurring in the
intergluteal cleft.
The cleft creates a suction that draws hair into the midline pits when a
patients sits. These ingrown hairs may then become infected and
present acutely as an abscess in the sacrococcygeal region.
An acute abscess should be incised and drained as soon as the
diagnosis is made.

Chronic pilonidal sinus
The simplest method involves unroofing the tract, curetting the base,
and marsupializing the wound.
Alternatively, a small lateral incision can be created and the pit excised.
Complex and/or recurrent sinus tracts may require more extensive
resection and closure with a Z-plasty, advancement flap, or rotational
flap.

