
IRON DEFICIENCY
ANEMIA
IDA
DR MOHAMED GHALIB
INTERNAL MEDICINE
TUCOM

OBJECTIVES
•
Prevalance of IDA
•
Metabolism & absorption of Iron
•
Iron stores
•
Pathogenesis of IDA
•
Causes of IDA
•
Features of IDA
•
Investigations of IDA
•
Treatments of IDA

Around 30% of the total world population is anaemic
and half of these, some 600 million people, have
iron deficiency. The classification of anaemia by the
size of the red cells (MCV) indicates the likely
cause.
Red cells in the bone marrow must acquire a
minimum level of haemoglobin before being
released into the blood stream. While in the marrow
compartment, red cell precursors undergo cell
division, driven by erythropoietin. If red cells cannot
acquire haemoglobin at a normal rate, they will
undergo more divisions than normal and will have a
low MCV when finally released into the blood.

The MCV is low because component parts of
the haemoglobin molecule are not fully
available: that is, iron in iron deficiency,
globin chains in thalassaemia, haem ring in
congenital sideroblastic anaemia and,
occasionally, poor iron utilisation in the
anaemia of chronic disease/anaemia of
inflammation.

Iron deficiency is the leading cause of anemia
worldwide. Although the presentation of
classic iron deficiency anemia is linked with a
microcytic anemia, early iron deficiency is
associated with a normocytic anemia.
Consequently, iron deficiency should be
considered in all patients with anemia, and
iron indices should be a part of the
evaluation of any patient with hypopro-
ductive anemia, regardless of the MCV.

Iron is acquired in the diet from heme sources
(i.e., meat) and from nonheme sources (e.g.,
vegetables such as spinach).
Iron from heme is better absorbed than
nonheme iron.
Iron absorption is increased in iron deficiency,
hypoxia, ineffective erythro-poiesis, and
hereditary hemochromatosis.
Iron is absorbed from the proximal small
intestine; it is transported in the cell bound to
ferroportin and through the plasma bound to
transferrin.

Its uptake into the RBC precursors is mediated
through the transferrin receptor. Iron absorption
from the intestine is further regulated by hepcidin.
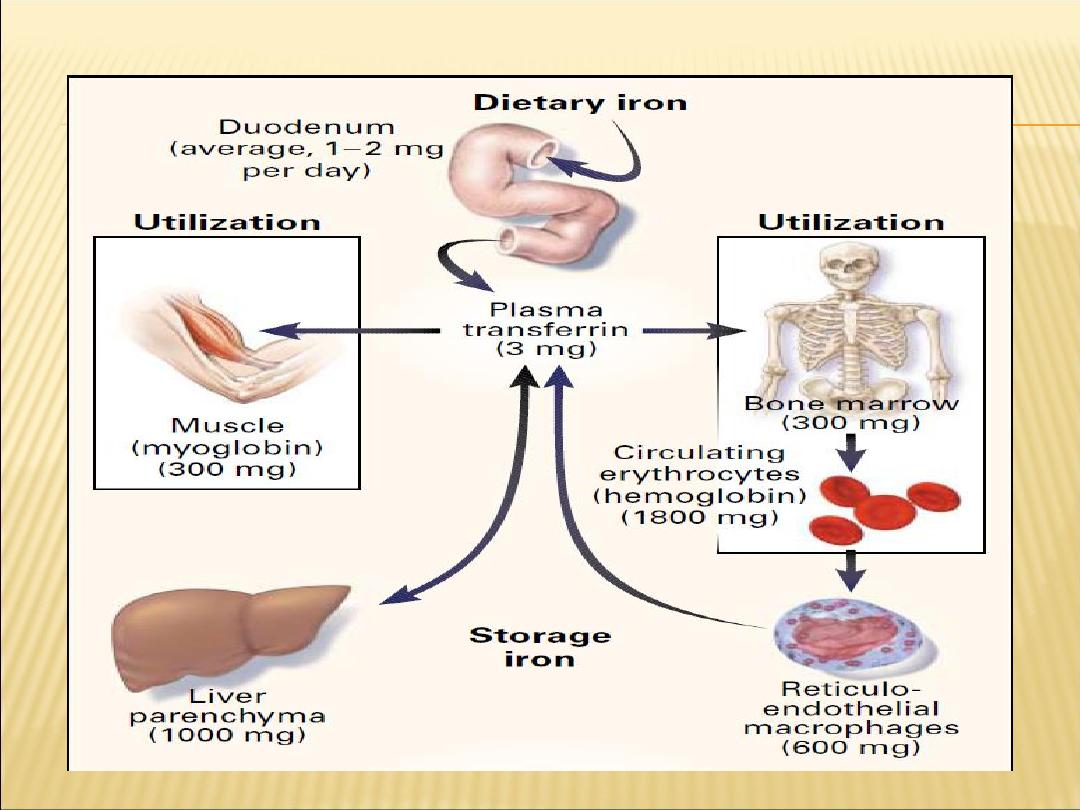
Iron outside hemoglobin-producing cells is stored in
ferritin. Men and women have total-body iron
concentrations of 50 mg/kg and 40 mg/kg,
respectively. Between 60% and 75% of the iron is
found in hemoglobin. A small amount (2 mg/kg) is
found in heme and nonheme enzymes, and 5
mg/kg is found in myoglo-bin. The remainder is
stored in ferritin, which resides primarily in liver,
bone marrow, spleen, and muscle.

The capacity for excreting iron is limited, and
iron overload occurs in patients with
excessive absorption from the
gastrointestinal tract (as a result of ineffective
erythropoiesis or congenital
hemochromatosis) and in those with chronic
transfusions. Iron overload leads to
increased iron deposition in these tissues
and secondary deposition in endocrine
organs, resulting in liver dysfunction,
diabetes, and other endocrine abnormalities.

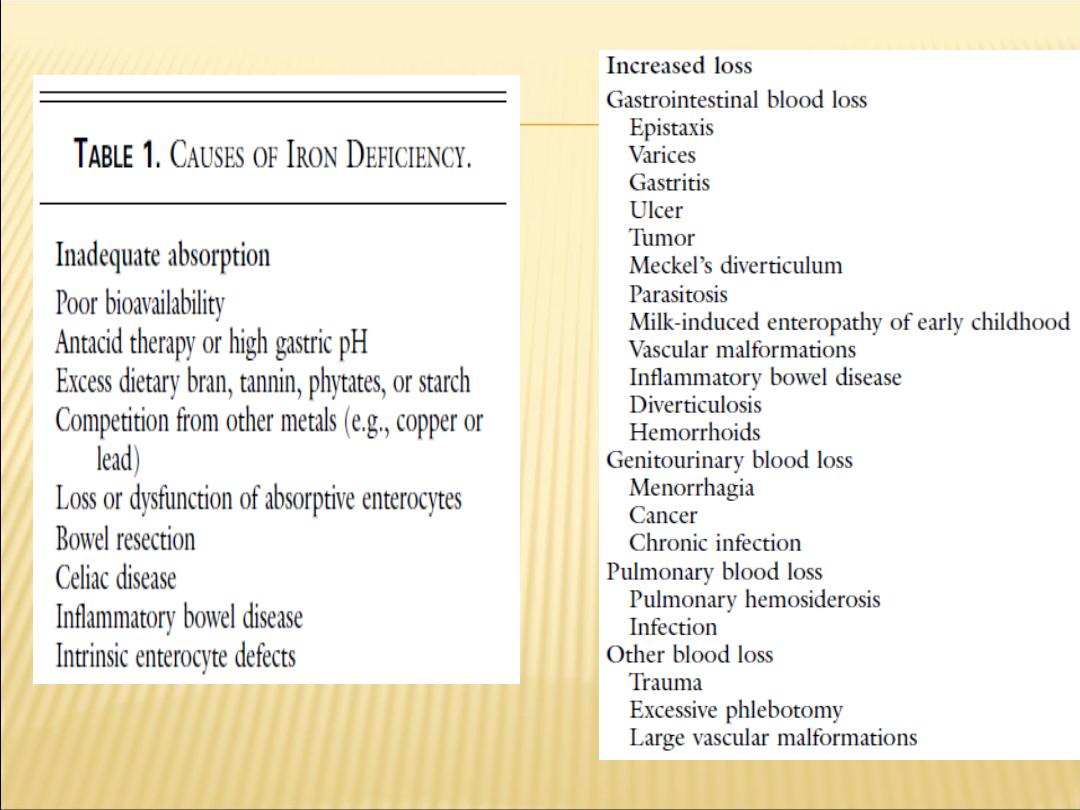
PATHOGENESIS OF IRON DEFICIENCY
1- Blood loss
Occult or overt GI losses, traumatic or surgical losses
2- Failure to meet increased requirements
Rapid growth in infancy and adolescence
Menstruation, pregnancy
3- Inadequate iron absorption
Diet low in heme iron
Gastrointestinal disease or surgery
Excessive cow
’s milk intake in infants

FEATURES OF IRON DEFICIENCY ANEMIA
Depends on the degree and the rate of
development of anemia
Symptoms common to all anemias:
pallor, fatigability, weakness, dizziness,
irritability

OTHER FEATURES OF IDA
Pagophagia - craving ice
Pica - craving of nonfood substances
e.g., dirt, clay, laundry starch
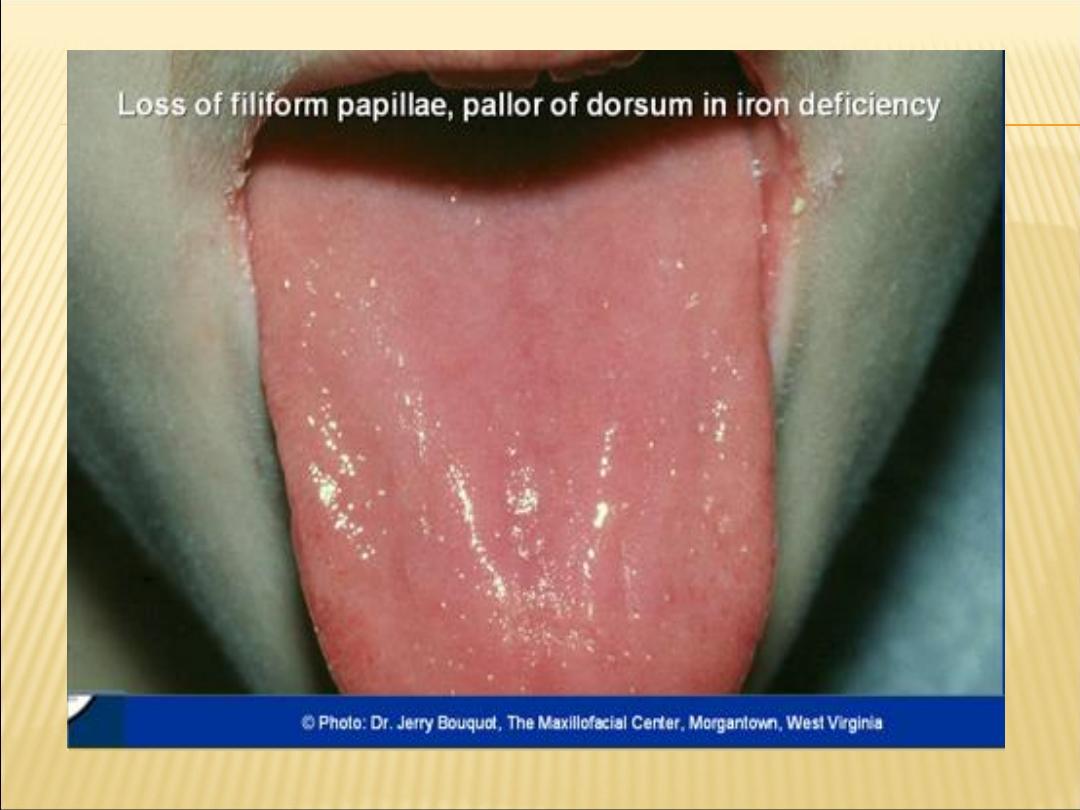
Glossitis - smooth tongue
Restless Legs
Angular stomatitis - cracking of corners of
mouth
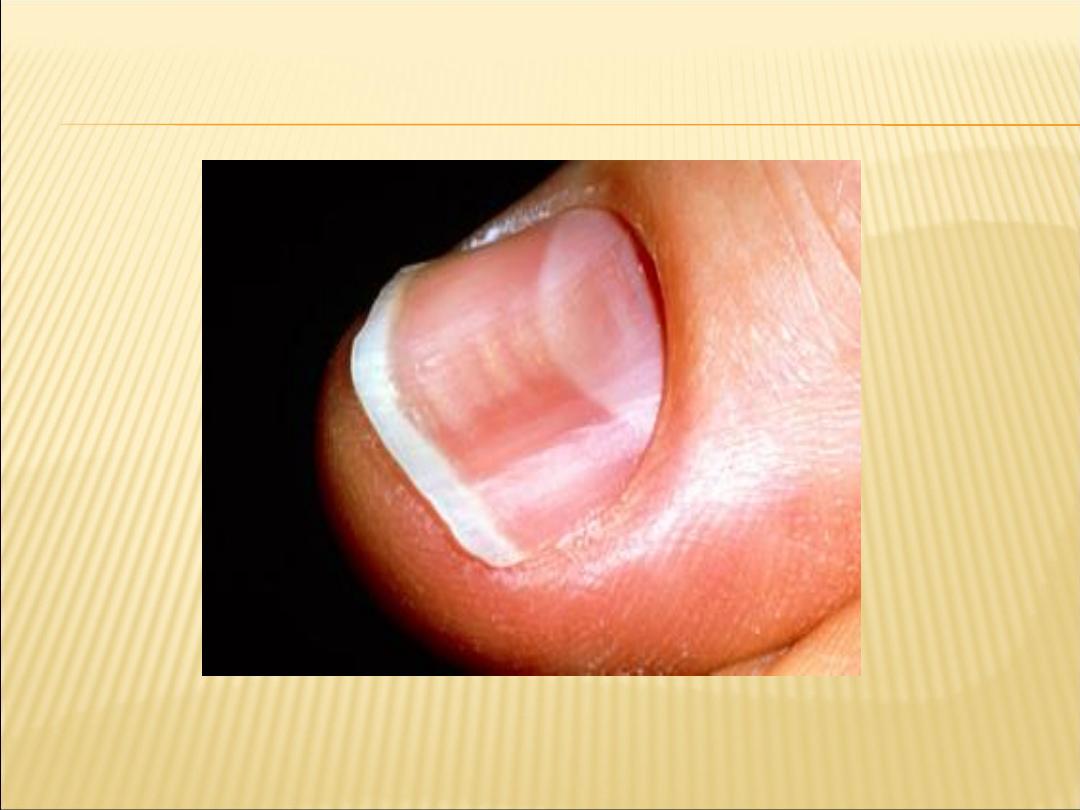
Koilonychia - thin, brittle, spoon-shaped
fingernails
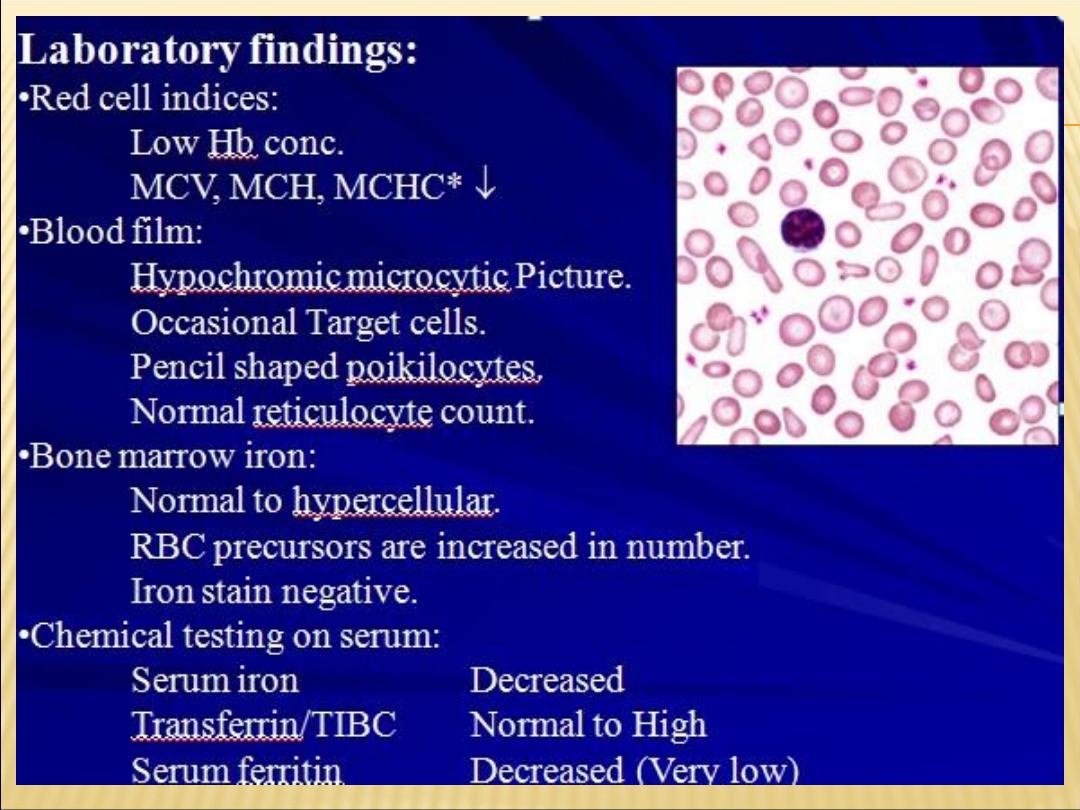
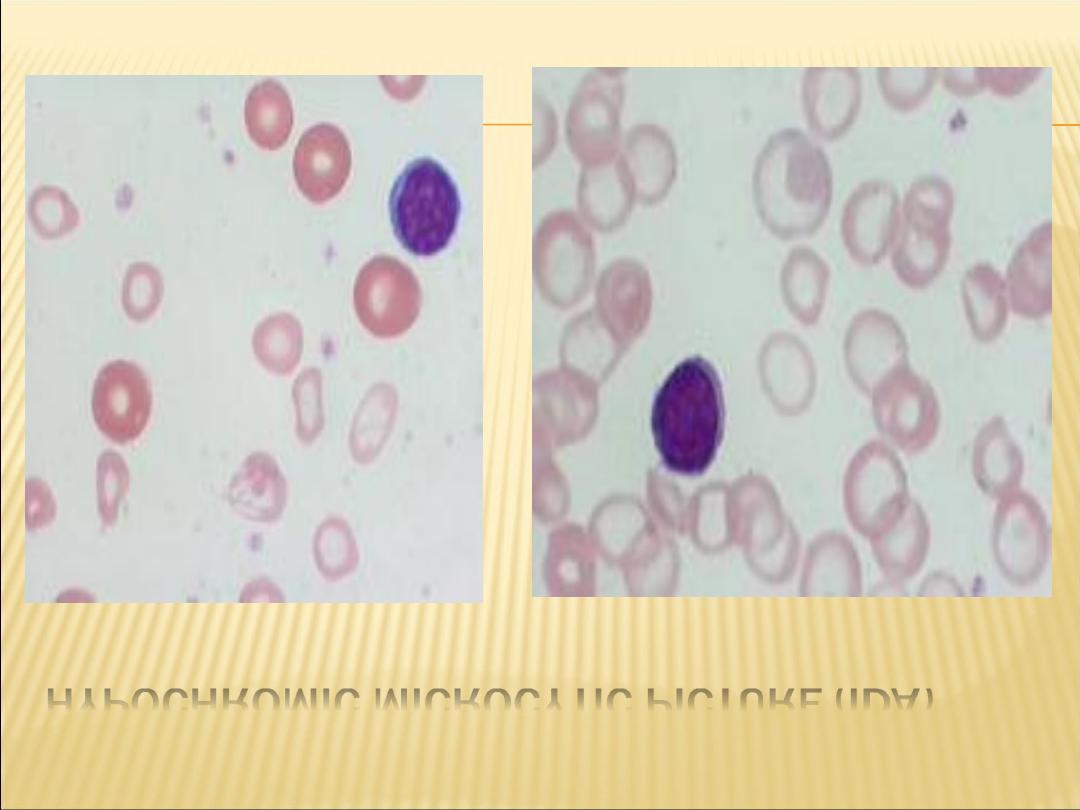
HYPOCHROMIC MICROCYTIC PICTURE (IDA)
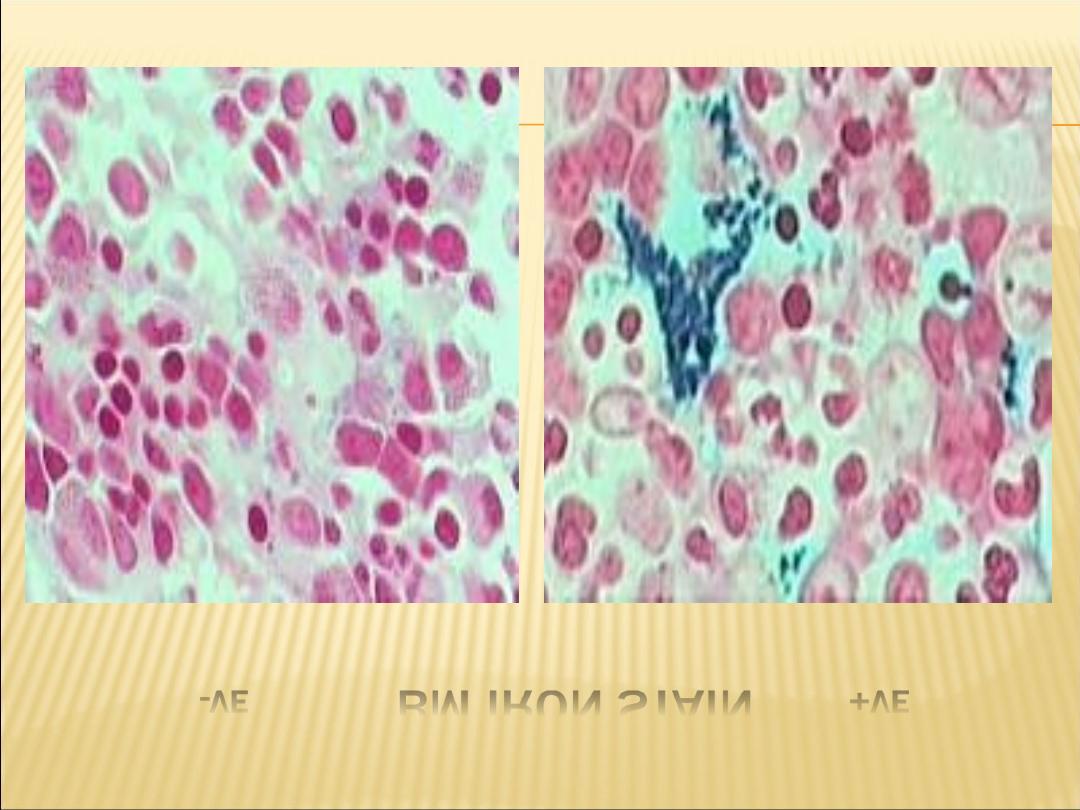
-VE
BM IRON STAIN
+VE

TREATMENT
Oral iron supplementation, with administration
of ferrous sulfate or ferrous gluconate two or
three times daily, is the treatment for iron
deficiency. Patients should be educated
about the potential gastrointestinal side
effects, including diarrhea or constipation,
and some may benefit from a gradual
increase in the dose based on tolerance. Iron
should be administered for several months
after resolution of anemia to allow for the
reconstitution of iron stores.

In patients with malabsorption, a complete
inability to tolerate oral iron, or iron demands
that outstrip replacement with oral
supplements, parenteral iron may be
administered. The paren-teral administration
of iron, especially iron dextran, has been
associated with anaphylaxis. However,
newer preparations such as sodium ferric
gluconate, iron sucrose, ferumoxytol, and
ferric carboxymaltose are significantly safer.
All male patients and postmenopausal women
with iron deficiency require evaluation for a
source of gastrointestinal bleeding.

The haemoglobin should rise by around 10 g/L every
7
–10 days and a reticulocyte response will be
evident within a week. A failure to respond
adequately
may
be
due
to
non-adherence,
continued blood loss, malabsorption or an incorrect
diagnosis. Patients with malabsorption, chronic gut
disease or inability to tolerate any oral preparation
may need parenteral iron therapy. Previously, iron
dextran or iron sucrose was used, but new
preparations
of
iron
isomaltose
and
iron
carboxymaltose have fewer allergic effects and are
preferred. Doses required can be calculated based
on the patient
’s starting haemoglobin and body
weight. Observation for anaphylaxis following an
initial test dose is recommended.

