1
THI QAR U. MEDICAL COLLEGE HEPATOLOGY LECTURES 2017
DEPARTMENT OF INTERNAL MEDICINE Dr. FAEZ KHALAF, SUBSPACIALITY GIT
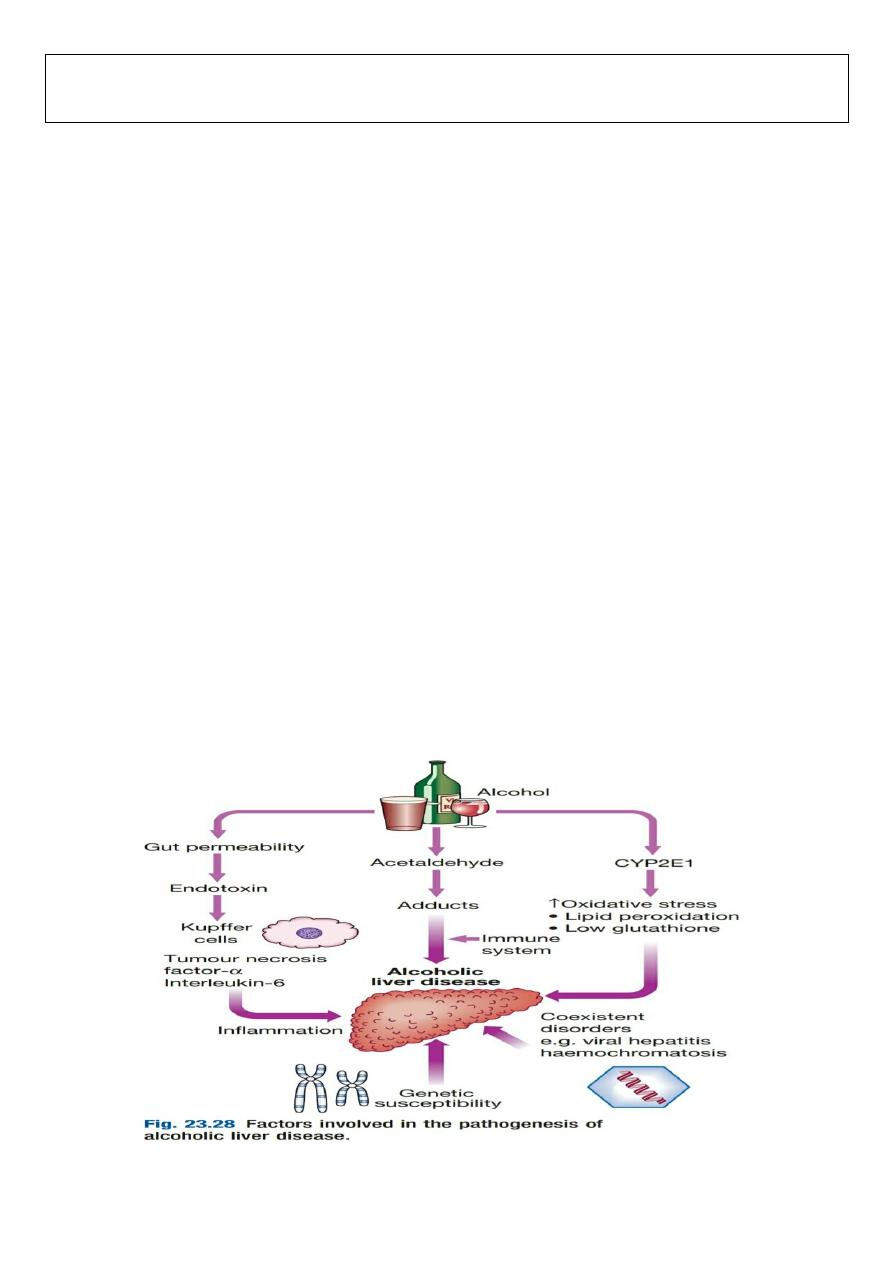
ALCOHOLIC LIVER DISEASE:
Alcohol is one of the most common causes of chronic liver disease worldwide, with consumption continuing to
increase in many countries. Patients with alcoholic liver disease (ALD) may also have risk factors for other liver
diseases (e.g. coexisting NAFLD or chronic viral hepatitis infection), and these may interact to increase disease
severity. In the UK, a unit of alcohol contains 8 g of ethanol. A threshold of
14 units/week in women and 21
units/week in men is generally considered safe
. The risk threshold for developing ALD is variable but begins at 30
g/day of ethanol. However,
there is no clear linear relationship between dose and liver damage
. For many,
consumption
of more than 80 g/day, for more than 5 years, is required to confer significant risk of advanced liver
disease.
The average alcohol consumption of a man with cirrhosis is 160 g/day for over 8 years
. Some of the risk
factors for ALD are:
•
Drinking pattern
. ALD and alcohol dependence are not synonymous; many of those who develop ALD are not
alcohol-dependent and most dependent drinkers have normal liver function. Liver damage is more likely to occur in
continuous rather than intermittent or ‘binge’ drinkers, as this pattern gives the liver a chance to recover. It is
therefore recommended that people should have at least two alcohol-free days each week. The type of beverage
does not affect risk.
• Gender
. The incidence of alcoholic liver disease is increasing in women, who have higher blood ethanol levels than
men after consuming the same amount of alcohol. This may be related to the reduced volume of distribution of
alcohol.
• Genetics
. Alcoholism is more concordant in monozygotic than dizygotic twins. Whilst polymorphisms in the genes
involved in alcohol metabolism, such as aldehyde dehydrogenase, may alter drinking behaviour, they have not been
linked to ALD. Recently, the patatin-like phospholipase domain-containing 3 (
PNPLA3) gene
, also known as
adiponutrin, has been implicated in the pathogenesis
of both ALD and NAFLD.
• Nutrition
. Obesity increases the incidence of liver-related mortality by
over fivefold in heavy drinkers
. Ethanol
itself produces 7 kcal/g (29.3 kJ/g) and many alcoholic drinks also contain sugar, which further increases the calorific
value and may contribute to weight gain. Excess alcohol consumption is frequently associated with nutritional

2
Clinical features
ALD has a wide clinical spectrum ranging from mild abnormalities of LFTs on biochemical testing to advanced
cirrhosis.
The liver is often enlarged in ALD, even in the presence of cirrhosis
. Stigmata of chronic liver disease, such
as palmar erythema, are more common in alcoholic cirrhosis than in cirrhosis of other aetiologies
. Alcohol misuse
may also cause damage of other organs. Three types of ALD are recognized (Box 23.53) but these overlap
considerably, as do the pathological changes seen in the liver.
a)Alcoholic fatty liver disease
Alcoholic fatty liver disease (AFLD) usually presents with elevated transaminases in the absence of hepatomegaly. It
has a good prognosis and steatosis usually disappears after 3 months of abstinence.
b) Alcoholic hepatitis
This presents with jaundice and hepatomegaly; complications of portal hypertension may also be present. It has a
significantly worse prognosis than AFLD. About one third of patients die in the acute episode, particularly those with
hepatic encephalopathy or a prolonged PT. Cirrhosis often coexists; if not present, it is the likely outcome if drinking
continues. Patients with acute alcoholic hepatitis often deteriorate during the first 1–3 weeks in hospital. Even if
they abstain, it may take up to 6 months for jaundice to resolve. In patients presenting with jaundice who
subsequently abstain, the 3- and 5-year survival is 70%. In contrast, those who continue to drink have 3- and 5-year
survival rates of 60% and 34% respectively.
c) Alcoholic cirrhosis
Alcoholic cirrhosis often presents with a serious complication, such as variceal haemorrhage or ascites, and only half
of such patients will survive 5 years from presentation. However, most who survive the initial illness and who
become abstinent will survive beyond 5 years.
Investigations
Investigations aim to establish alcohol misuse,
to exclude alternative or additional coexistent causes of liver disease
and to assess the severity of liver damage
. The clinical history from patient, relatives and friends is important to
establish alcohol misuse duration and severity. Biological markers, particularly
macrocytosis in the absence of
anaemia
, may suggest and support a history of alcohol misuse. A
raised GGT is not specific for alcohol misuse
and
may also be elevated in the presence of other conditions, including NAFLD. The level may not therefore return to
normal with abstinence if chronic liver disease is present, and GGT should not be relied on as an indicator of ongoing
alcohol consumption. The presence of
jaundice may suggest alcoholic hepatitis
. Determining the extent of liver
damage often requires a liver biopsy.
3
In alcoholic hepatitis,
PT and bilirubin are used to calculate a ‘discriminant function’ (DF), also known as the Maddrey
score, which enables the clinician to assess prognosis (PT = prothrombin time; serum bilirubin in μmol/L is divided
by 17 to convert to mg/dL):
DF Increase in PT (= [4.6 X sec)] +Bilirubin (mg/dL)
A
value over 32 implies severe liver disease with a poor prognosis and is used to guide treatment decisions
. A second
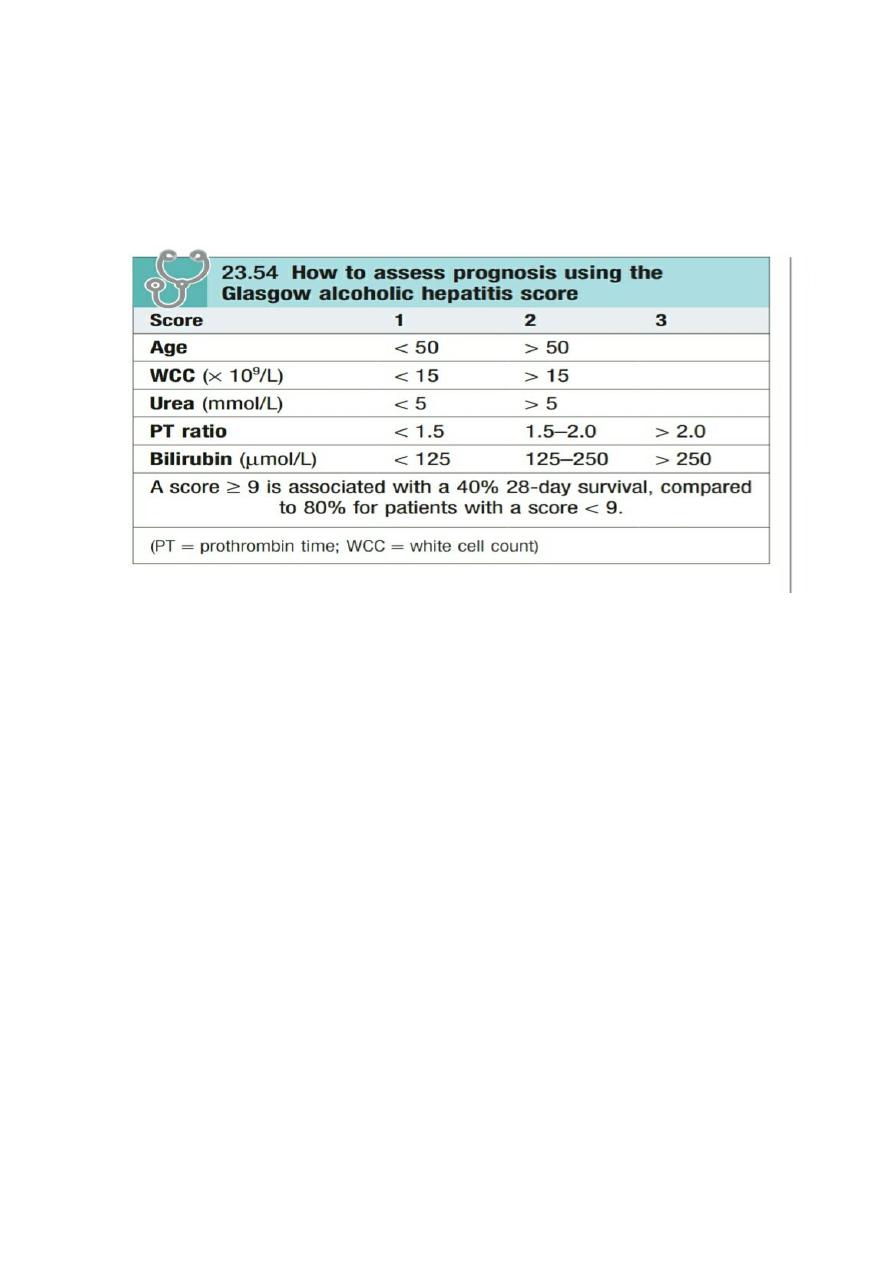
scoring system, the Glasgow score, uses the age, white cell count and renal function, in addition to PT and bilirubin,
to assess prognosis with a cutoff of 9 (Box 23.54).
Management
Cessation of alcohol consumption is the single most important treatment and prognostic factor
. Life-long abstinence
is the best advice. General health and life expectancy are improved when this occurs, irrespective of the stage of
liver disease.
Abstinence is even effective at preventing progression, hepatic decompensation and death once
cirrhosis is present
. In the acute presentation of ALD it is important to identify and
anticipate alcohol withdrawal and
Wernicke’s encephalopathy
, which need treating in parallel with the liver disease and any complications of cirrhosis.
Nutrition
Good nutrition is very important, and enteral feeding via a fine-bore nasogastric tube may be needed in severely ill
patients.
Corticosteroids
These are of value in patients with severe alcoholic hepatitis (Maddrey’s discriminative score > 32) and increase
survival. A similar improvement in 28-day survival from 52% to 78% is seen when steroids are given to those with a
Glasgow score of more than 9.
Sepsis is the main side-effect of steroids, and existing sepsis and variceal
haemorrhage are the main contraindications to their use.
If the bilirubin has not fallen 7 days after starting steroids,
the drugs are unlikely to reduce mortality and should be stopped.
Pentoxifylline
Pentoxifylline, which has a weak anti-TNF action, may be beneficial in severe alcoholic hepatitis. It reduces the
incidence of hepatorenal failure and its use is not complicated by sepsis. It is not known whether corticosteroids,
pentoxifylline or a combination is superior in the treatment of alcoholic hepatitis.
4
Liver transplantation
The role of liver transplantation in the management of ALD remains controversial.
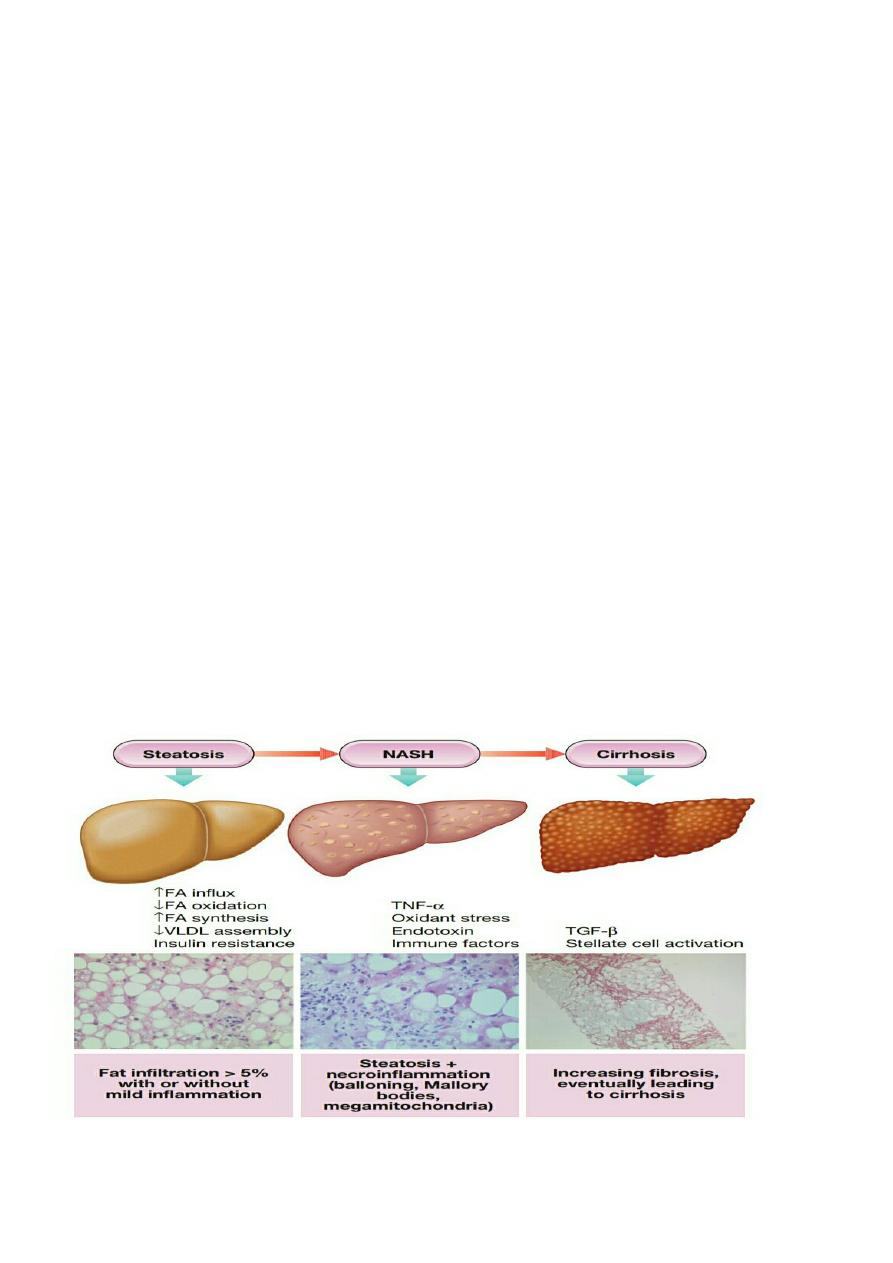
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD):
(NAFLD) represents a spectrum of liver disease encompassing simple
fatty infiltration (steatosis
), fat and
inflammation (nonalcoholic steatohepatitis, NASH
) and
cirrhosis
, in the absence of excessive alcohol consumption
(typically a threshold of < 20 g/day for women and < 30 g/day for men is adopted). While simple steatosis has not
been associated with liver-related morbidity, NASH is linked with progressive liver fibrosis, cirrhosis and liver cancer,
as well as increased cardiovascular risk
. The true extent of associated morbidity is not well defined however, in one
study NASH was associated with a greater than tenfold increased risk of liver-related death (2.8% vs. 0.2%) and a
doubling of cardiovascular risk over a mean follow-up of 13.7 years.
NAFLD is strongly associated with obesity,
dyslipidaemia, insulin resistance and type 2 (non-insulin dependent) diabetes mellitus
, and so may be considered to
be the hepatic manifestation of the
‘metabolic syndrome’
. Increasingly sedentary lifestyles and changing dietary
patterns mean that the prevalence of obesity and insulin resistance has increased, making NAFLD the leading cause
of liver dysfunction in the non-alcoholic, viral hepatitis-negative population in Europe and North America. Estimates
vary between populations; however, one large European study found
NAFLD to be present in 94% of obese patients
(BMI) > 30 kg/m2), 67% of overweight patients (BMI > 25 kg/m2) and
25% of normal-weight patients
. The overall
prevalence of NAFLD in patients with type 2 diabetes ranges from 40% to 70%. Histological NASH was found in 3–
16% of apparently healthy potential living liver-donors in Europe and 6–15% in USA.
Pathophysiology:
Cellular damage triggers a mixture of immune-mediated hepatocellular injury and cell death, which leads to stellate
cell activation and hepatic fibrosis a combination of several different ‘hits', including:
1. oxidative stress due to free radicals produced during fatty acid oxidation
2. direct lipotoxicity
3. gut-derived endotoxin
4. Cytokine release (TNF-α etc.)
5. Endoplasmic reticulum stress.

5
Clinical features: NAFLD is
frequently asymptomatic
, although it may be associated with f
atigue and mild right upper
quadrant discomfort
. It is
commonly identified as an incidental biochemical abnormality during routine blood tests
.
Alternatively, patients with progressive
NASH may present late in the natural history of the disease with
complications of cirrhosis and portal hypertension, such as variceal haemorrhage, or HCC
. The average age of NASH
patients is 40–50 years (50–60 years for NASH–cirrhosis); however, the emerging epidemic of childhood obesity
means that NASH is present in increasing numbers of younger patients. Most patients with NAFLD have insulin
resistance and exhibit features of the metabolic syndrome. Recognized independent risk factors for disease
progression are age over 45 years, presence of diabetes (or severity of insulin resistance), obesity (BMI > 30 kg/m2)
and hypertension. These factors help with identification of ‘high-risk’ patient groups.
NAFLD is also associated with
polycystic ovary syndrome, obstructive sleep apnea and small-bowel bacterial overgrowth.
Investigations
First towards exclusion of excess alcohol consumption and other liver diseases (including viral, autoimmune and
other metabolic causes), and then at confirming the presence of NAFLD, discriminating simple steatosis from NASH
and determining the extent of any hepatic fibrosis that is present.
Biochemical tests
There
is no single diagnostic blood test for NAFLD
. Elevations of serum
ALT and AST are modest
, and usually less
than twice the upper limit of normal.
ALT levels fall as hepatic fibrosis increases and the characteristic AST: ALT ratio
of less than 1 seen in
NASH reverses (AST: ALT > 1) as disease progresses towards cirrhosis
, meaning that
steatohepatitis with advanced disease may be present even in those with normal-range ALT levels
. Other laboratory
abnormalities that may be present include non-specific
elevations of GGT
,
low-titre ANA in 20–30%
of patients and
elevated ferritin levels
.
Imaging
Ultrasound is most often used and provides a qualitative assessment of hepatic fat content, as the liver appears
‘bright’ due to increased echogenicity;
however, sensitivity is limited when fewer than 33% of hepatocytes
are
steatotic. Alternatives include
CT, MRI or MR spectroscopy
, which offer greater sensitivity for detecting lesser
degrees of steatosis, but these are resource intensive and not widely used in routine practice. Currently, no routine
imaging modality can distinguish simple steatosis from steatohepatitis or accurately quantify hepatic fibrosis short of
cirrhosis.
Liver biopsy
Liver
biopsy remains the ‘gold standard’ investigation for diagnosis and assessment of degree of inflammation
and
extent of liver fibrosis. The histological definition of NASH is based on a combination of three lesions (steatosis,
hepatocellular injury and inflammation. with a mainly centrilobular, acinar zone 3, distribution. Specific features
include
hepatocyte ballooning degeneration
with or without acidophil bodies or spotty necrosis and a mild, mixed
inflammatory infiltrate. These may be accompanied by
Mallory–Denk bodies
.
Perisinusoidal fibrosis is a
characteristic feature of NASH.
Histological scoring systems are widely used to assess disease severity semi-
quantitatively. It is important to note that hepatic fat content tends to diminish as cirrhosis develops and so NASH is
likely to be under-diagnosed in the setting of advanced liver disease, where it is thought to be the underlying cause
of 30–75% of cases in which no specific aetiology is readily identified (so-called ‘cryptogenic cirrhosis’
).
Management
Identification of NAFLD should
prompt screening for and treatment of cardiovascular risk factors in all patients.
Non-pharmacological treatment
Current treatment comprises lifestyle interventions to promote weight loss and improve insulin sensitivity through
dietary changes and physical exercise.
Sustained weight reduction of 7–10% is associated with significant
improvement in histological and biochemical NASH severity.

6
Pharmacological treatment
No pharmacological agents are currently licensed specifically for NASH therapy
. Treatment directed at coexisting
metabolic disorders,
such as dyslipidaemia and hypertension
, should be given. Although use of HMG-CoA reductase
inhibitors (statins) does not ameliorate NAFLD, there does not appear to be any increased risk of hepatotoxicity or
other side-effects from these agents, and so they may be used to treat dyslipidaemia. Specific insulin-sensitising
agents, in particular
glitazones,
may help selected patients, while positive results
with high-dose vitamin E (800
U/day) have been tempered by evidence that high doses may be associated with an increased risk of prostate cancer
and all-cause mortality, which has limited its use.
AUTOIMMUNE LIVER AND BILIARY DISEASE:
Autoimmune hepatitis and (primary biliary cirrhosis and primary sclerosing cholangitis).
Autoimmune hepatitis
:
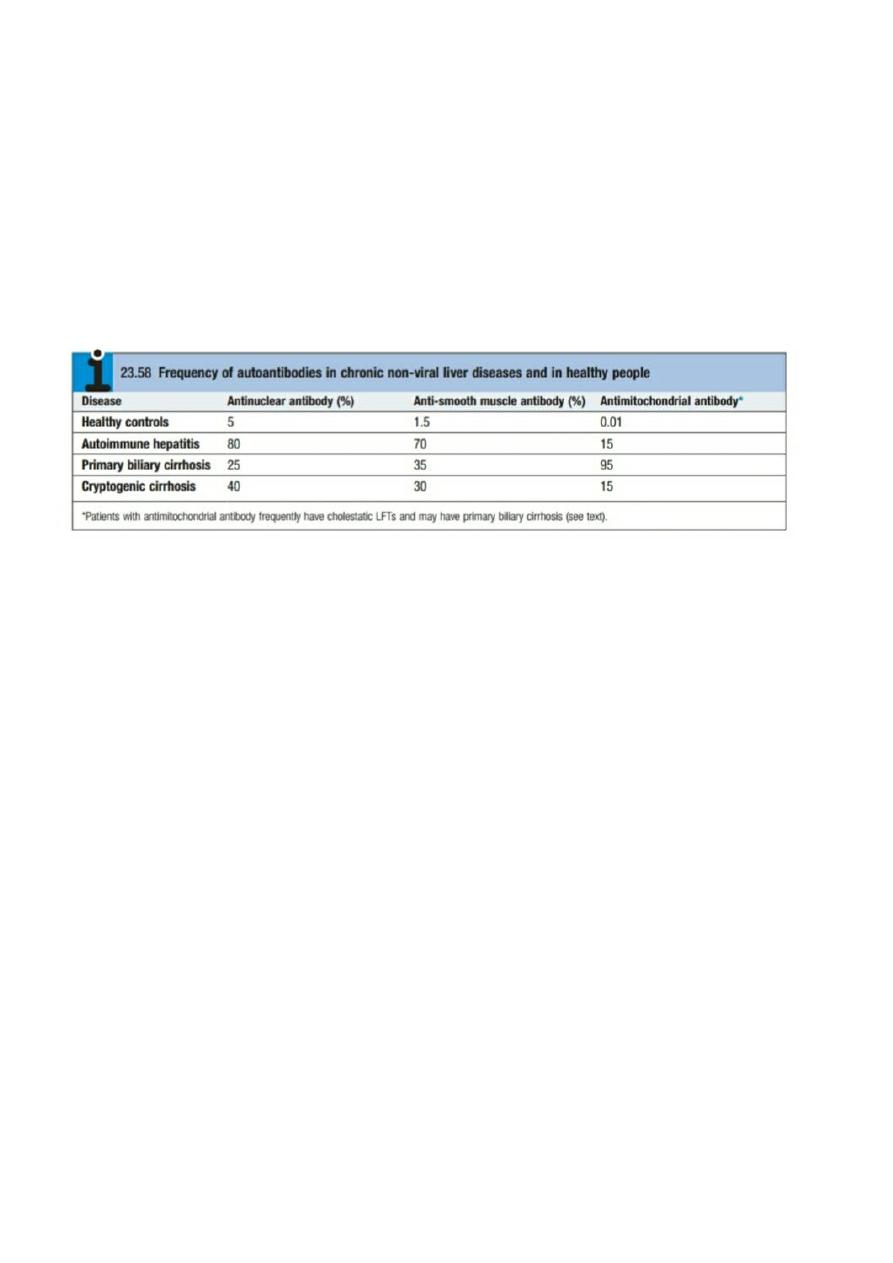
Is a disease of immune-mediated liver injury characterised by the presence of serum antibodies and peripheral
blood T lymphocytes reactive with self-proteins, a strong association with other autoimmune diseases (Box 23.57),
and high levels of serum immunoglobulins – in particular,
elevation of IgG
.
Although most commonly seen in women,
particularly in the second and third decades of life
, it
can develop in either sex at any age
. The reasons for the
breakdown in immune tolerance in autoimmune hepatitis
remain unclear
, although cross-reactivity with viruses such
as HAV and EBV in immunogenetically susceptible individuals (typically those with (
HLA)-DR3 and DR4, particularly
HLA-DRB3*0101 and HLA-DRB1*0401) has been suggested as a mechanism.
Pathophysiology:
Several subtypes of this disorder have been proposed that have differing immunological markers. The formal
classification into disease types has fallen out of favour in recent years.
The most frequently seen autoantibody
pattern is high titre of antinuclear and anti-smooth muscle antibodies, typically associated with IgG
hyperglobulinaemia (type I autoimmune hepatitis in the old classification), frequently seen in young adult females.
Disease characterised by the presence of anti-LKM (liver–kidney microsomal) antibodies
,
recognising cytochrome
P450- IID6 expressed on the hepatocyte membrane, is typically seen in paediatric populations and can be more
resistant to treatment than ANA-positive disease
.
Adult onset of anti-LKM can be seen in chronic HCV infection
. This
was classified as type II disease in the old system. More recently, a pattern of antibody reactivity with antisoluble
liver antigen has been described in typically adult patients, often with aggressive disease and usually lacking
autoantibodies of other specificities.
Clinical features
The onset is
usually insidious
, with
fatigue, anorexia and jaundice
. In about
one-quarter of patients, the onset is
acute, resembling viral hepatitis
, but resolution does not occur. This
acute presentation can lead to extensive liver
necrosis and liver failure
. Other features include
fever, arthralgia, vitiligo and epistaxis
.
Amenorrhea can occur
.
Jaundice is mild to moderate or occasionally absent, but
signs of chronic liver disease, especially spider naevi and
7
hepatosplenomegaly,
can be present. Associated autoimmune disease, such as Hashimoto’s thyroiditis or
rheumatoid arthritis, is often present and can modulate the clinical presentation.
Investigations
Serological tests for autoantibodies are often positive (Box 23.58), but low titres of these antibodies occur in some
healthy people and in patients with other inflammatory liver diseases.
ANA
also occur in connective tissue diseases
and other autoimmune diseases (with an identical pattern of homogenous nuclear staining) while anti-smooth
muscle antibody has been reported in infectious mononucleosis and a variety of malignant diseases. Anti-
microsomal antibodies
(anti-LKM)
occur particularly in children and adolescents.
Elevated serum IgG levels
are an
important diagnostic and treatment response feature if present, but the diagnosis is still possible in the presence of
normal IgG levels. If the diagnosis of
autoimmune hepatitis is suspected, liver biopsy should be performed. It
typically shows interface hepatitis, with or without cirrhosis
.
Management
Treatment with corticosteroids is life-saving in autoimmune hepatitis, particularly during exacerbations of active and
symptomatic disease
. Initially,
prednisolone 40 mg/day is given orally
; the dose is then gradually reduced as the
patient and LFTs improve. Maintenance therapy should only be instituted once LFTs are normal (as well as IgG if
elevated). Approaches to maintenance include
reduced-dose prednisolone (ideally below 5–10 mg/day
), usually in
the context of azathioprine 1.0–1.5 mg/kg/day
. Azathioprine can also be used as the sole maintenance
immunosuppressive agent in patients with low-activity disease. Newer agents such as mycophenolate mofetil
(MMF)
are increasingly being used but formal evidence to inform practice in this area is lacking. Patients should be
monitored for acute exacerbations (LFT and IgG screening with patients alerted to the possible symptoms) and such
exacerbations should be treated with corticosteroids
. Although treatment can significantly reduce the rate of
progression to cirrhosis, end-stage disease can be seen in patients despite treatment.
