
Biochemistry lectures
By
Assistant.Prof Dr. Ban Mahmood Al-joda
Clinical Biochemistry
College of Medicine University of Babylon
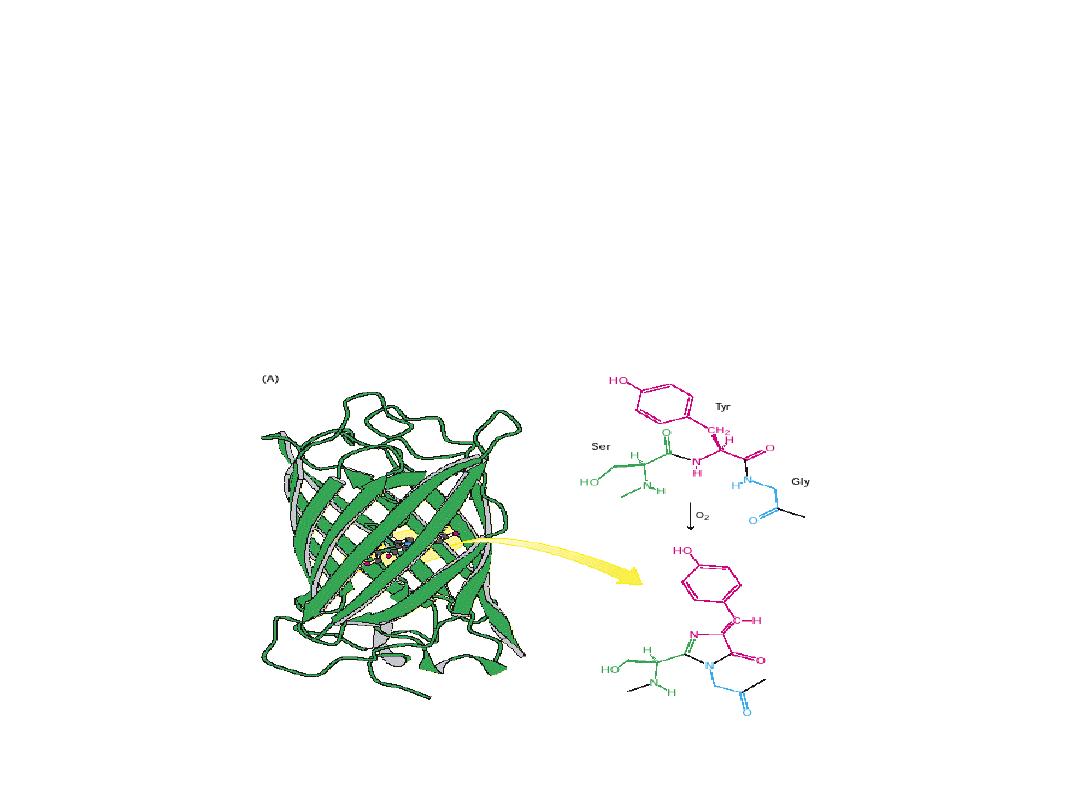
Reaction of amino acids and
properties

objectives
1- Amino acids properties.
2- View the reactions due to carboxyl group,
reactions due to amino group and reactions of SH
group.
3- Define transamination.
4- The clinical value of transamination.
5- The clinical applications of amino acids.

Properties of amino acids
:
A -Physical properties
:
1- Solubility:
Most of the amino acids are
soluble in water and alcohol (polar solvents) ,but
insoluble in nonpolar solvents (benzene).
2- Melting points :
Amino acids generally
melt at higher temperatures ,often above 200ºC.

3-Taste
:
Glycine, alanine, valine , serine, tryptophan, histidine
and proline are sweet in taste; leucine is tasteless;
while isoleucine and arginine are bitter. Sodium
glutamate is a flavoring agent. Aspartame, an artificial
sweetener contains aspartic acid and phenyl alanine.

4- Optical properties :
I-
Amino acids having an asymmetric carbon atom
exhibit optical activity. Asymmetry arises when 4
different groups are attached to the same carbon
atom .
II-
Glycine is the simplest amino acid and has no
asymmetric carbon atom and therefore shows
no optical activity. All others are optically active.
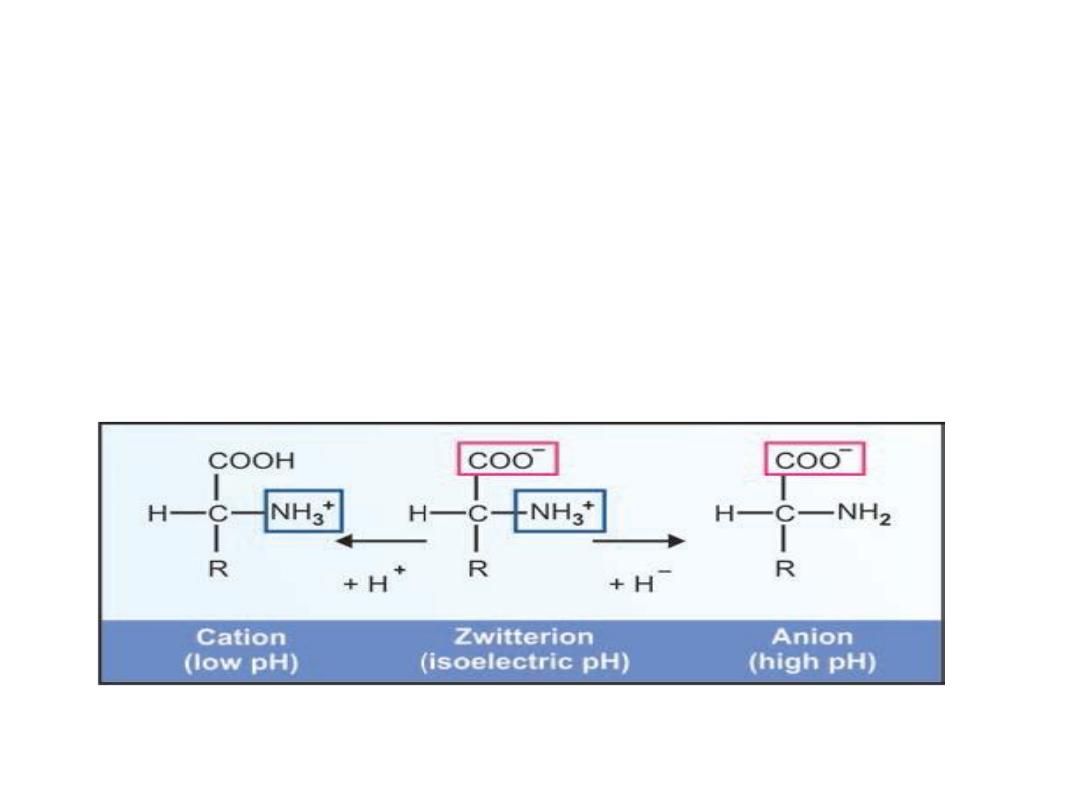
5- Ampholyte and Iso-electric Point
Amino acids can exist as ampholytes or zwitterions
in solution, depending on the pH of the medium. The
pH at which the molecule carries no net charge is
known as iso-electric point or isoelectric pH (pI).
In acidic solution they are cationic in form and in
alkaline solution they behave as anions
Ionic forms of amino acids

B- Chemical properties:
•
The general reactions of amino acids are mostly due
to the presence of two functional groups namely
carboxylic (-COOH) and amino (-NH2) group.
General reactions of amino acids
I- Reactions due to –COOH group
1- Decarboxylation:
The amino acids will
undergo alpha decarboxylation to form the
corresponding amine. Thus some important
amines are produced from amino acids. For
example,
Histidine
Histamine + CO
2
Tyrosine Tyramine + CO
2
Tryptophan Tryptamine + CO
2
Lysine Cadaverine + CO
2
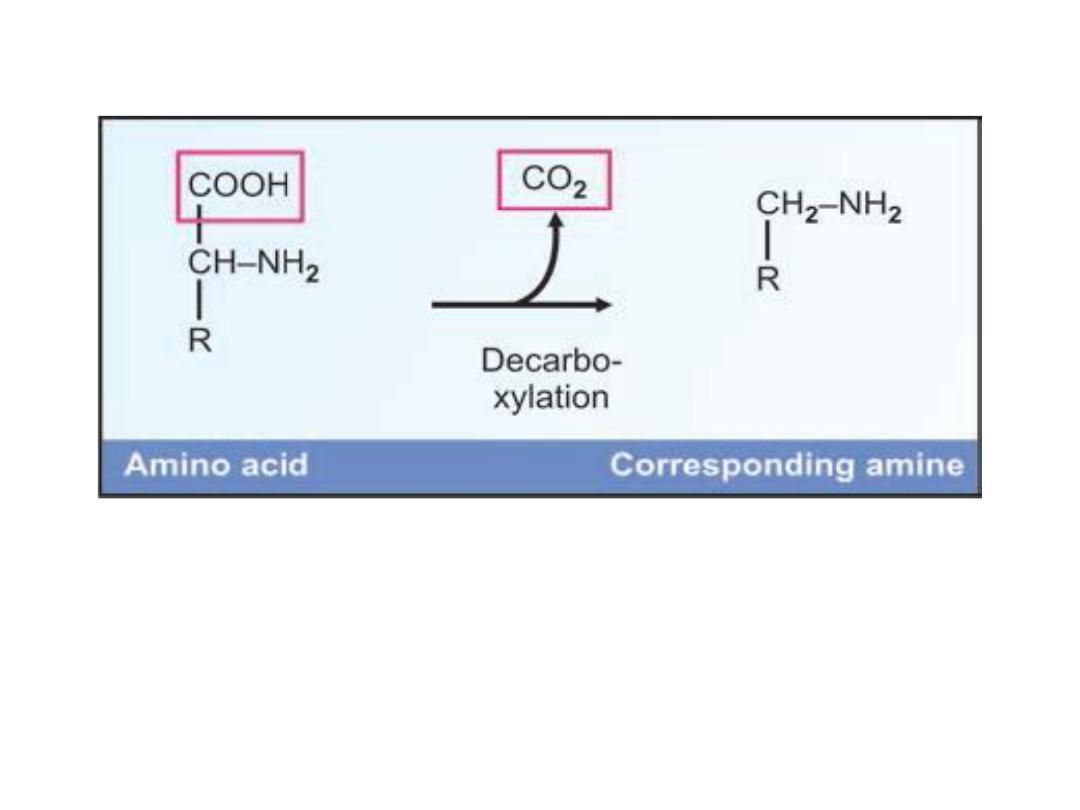
Decarboxylation of amino acid

2- Amide Formation:
The -COOH group of dicarboxylic amino acids
(other than alphacarboxyl) can combine with
ammonia to form the corresponding amide. For
example ,
Aspartic acid + NH
3
Asparagine
Glutamic acid + NH
3
Glutamine
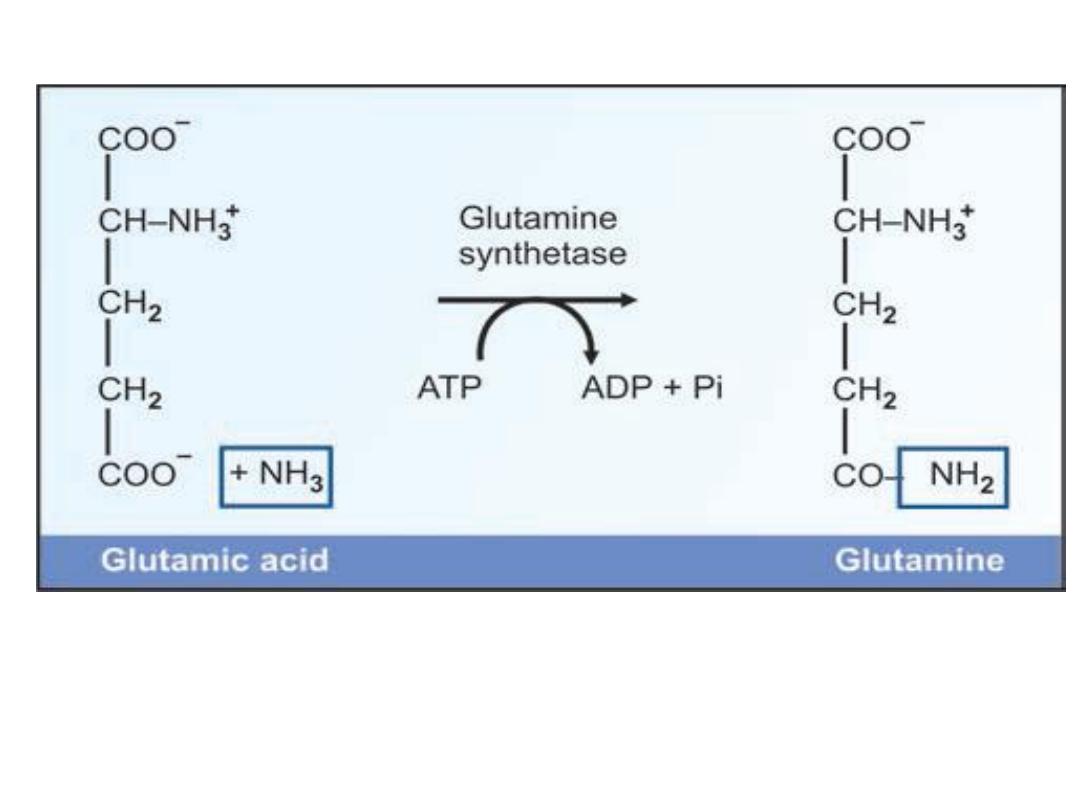
Formation of glutamine

II- Reactions Due to Amino Group
1- Transamination:
The alpha amino group of amino acid can
be transferred to alpha keto acid to form the corresponding new
amino acid and alpha keto acid . This is an important reaction in
the body for the inter-conversion of amino acids and for
synthesis of non-essential amino acids.
amino acid 1 + keto acid 2 amino acid 2 + keto acid 1
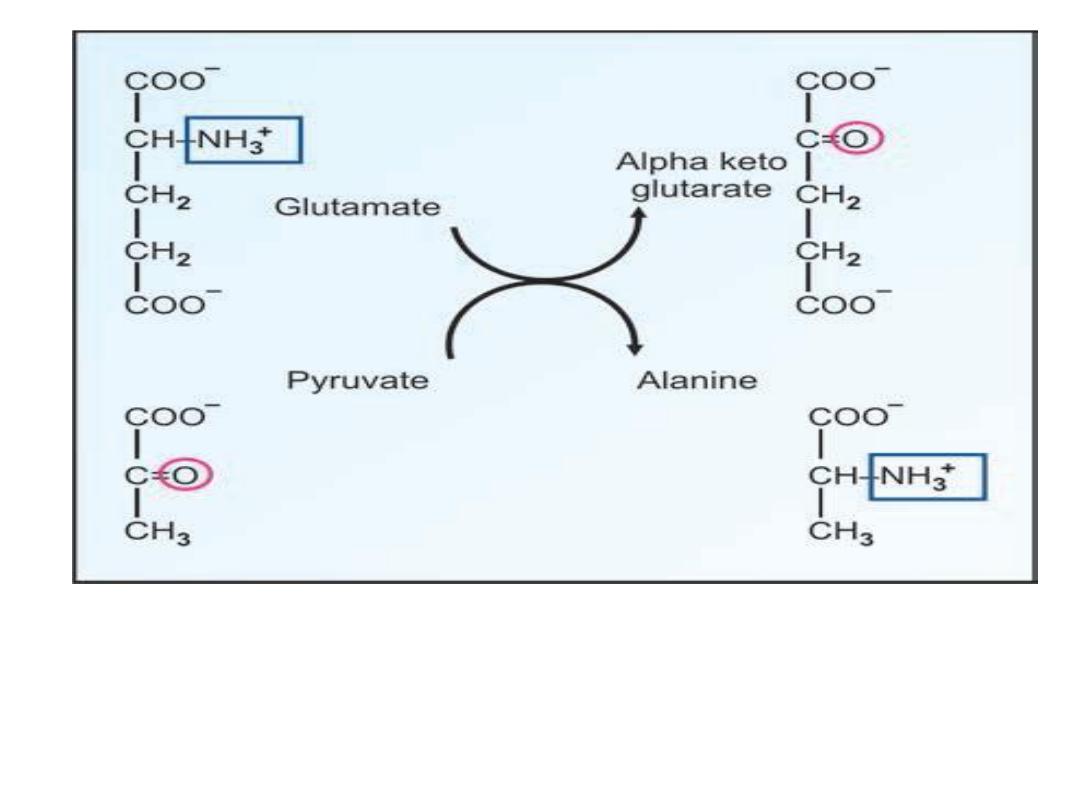
Transamination reaction

2- Oxidative Deamination:
The alpha amino group is removed from the amino acid
to form the corresponding keto acid and ammonia
.
In the body, Glutamic acid is the most common amino
acid to undergo oxidative deamination.
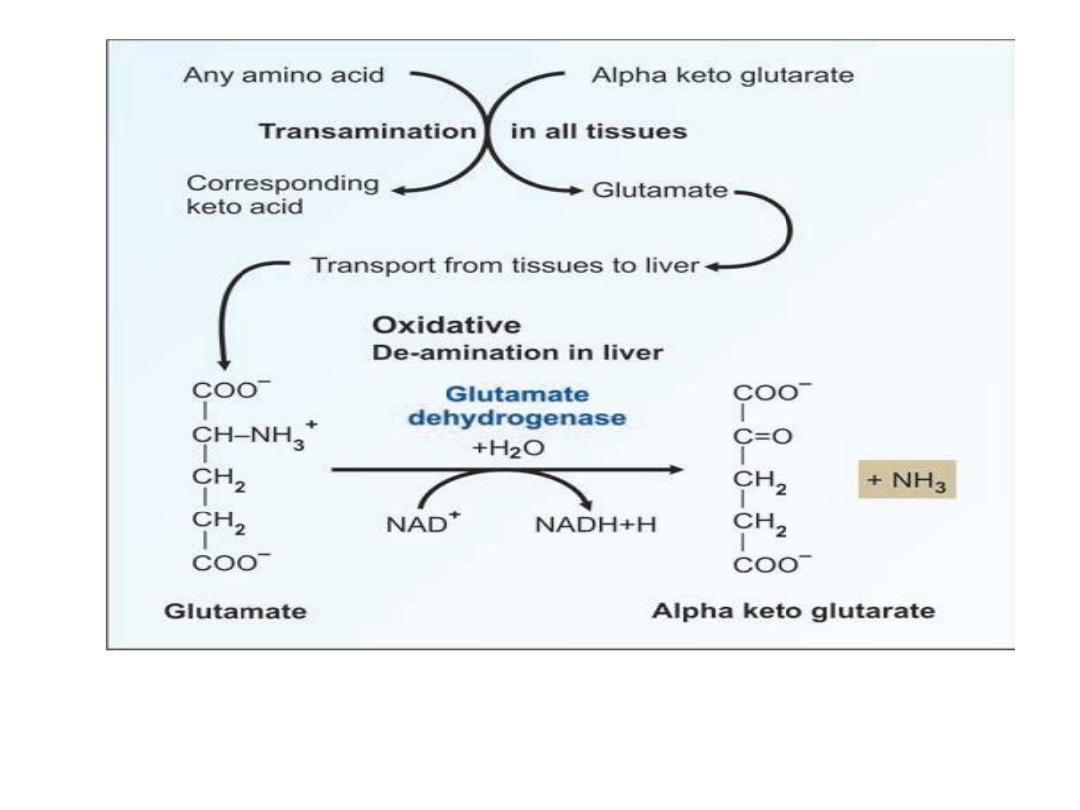
Transamination + deamination = transdeamination
Clinical Significance of Transamination
Aspartate amino transferase (AST) and Alanine amino
transferase (ALT) are induced by glucocorticoids which favor
gluconeogenesis. AST and ALT are markers of liver injury .
Diagnostic value:
Transaminases are normally intracellular enzymes.
They are elevated in the blood
when damage to the cells
producing these enzymes occurs.
**
Increase level of both ALT & AST
indicates
possible damage to the liver cells.
**
Increase level of AST alone
suggest damage to heart
muscle ,skeletal muscle or kidney
.

3- Formation of carbamino compound:
Carbon dioxide adds to the alpha amino group of amino
acids to form carbamino compounds. The reaction occurs at
alkaline pH and serves as a mechanism for the transport of
carbon dioxide from tissues to the lungs by hemoglobin .
Hb—NH
2
+ CO
2
Hb—NH—COOH (Carbamino-Hb)

III- Reactions Due to Side Chains
1-Transmethylation
2- Ester formation by the OH group
3- Reaction of the amide group
4- Reactions of SH group

Reactions of SH group:
Cysteine has a sulfhydryl (SH)
group and it can form a disulphide (S-S) bond with
another cysteine residue. The two cysteine residues can
connect two polypeptide chains by the formation of
interchain disulphide bonds
or links . The dimer
formed by two cysteine residues is sometimes called
Cystine
or
Dicysteine
.
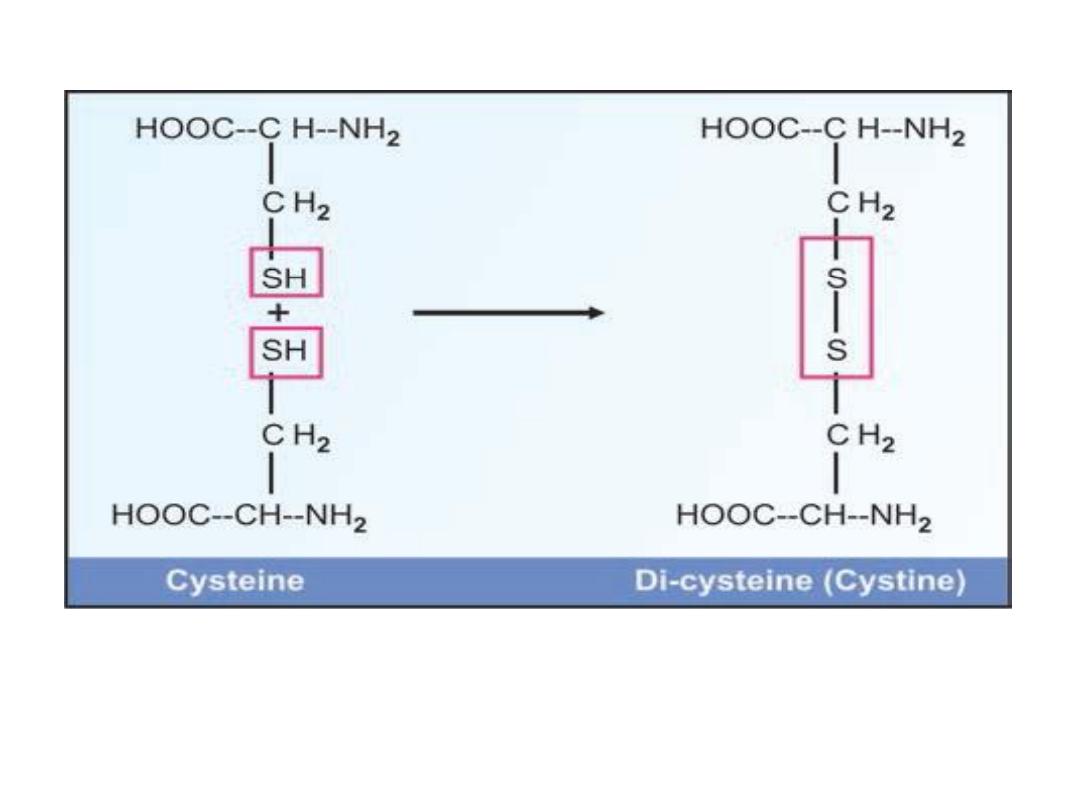
Formation of disulphide bridges

Clinical applications for some amino acids
1- Glycine is used for heme, purine and creatin
synthesis.
Creatine synthesis from = (Glycine, Argenine ,Methionine) .
2-
Glutathione can be produced using enzymatic
methods in the presence of ATP and its three precursor
amino acids (
L-glutamic acid, L-cysteine, glycine
).
Function
in detoxification, antioxidant defense .

3- Catecholamine (CA) is a monoamine compound, that
has a catechol (benzene with two -OH side groups) and
a side-chain amine.
CA
are derivatives of Tyrosine
including
dopamine
,
norepinephrine
, and
epinephrine
.
CA
are require for the body to adapt to a great variety of
acute and chronic stress.
4- Tryptophan serves as the precursor for the synthesis
of
serotonin
and
melatoninby.
5- Histamine is derived from the decarboxylation of the
amino acid
histidine
.

REFERENCES
1- DM Vasudevan, Sreekumari S, Kannan Vaidyanathan ,
Textbook of Biochemistry
for Medical Students , 6
th
ed. 2011 .
2- Lippincott’s Reviews of Biochemistry, 3
rd
ed. , 2018.
