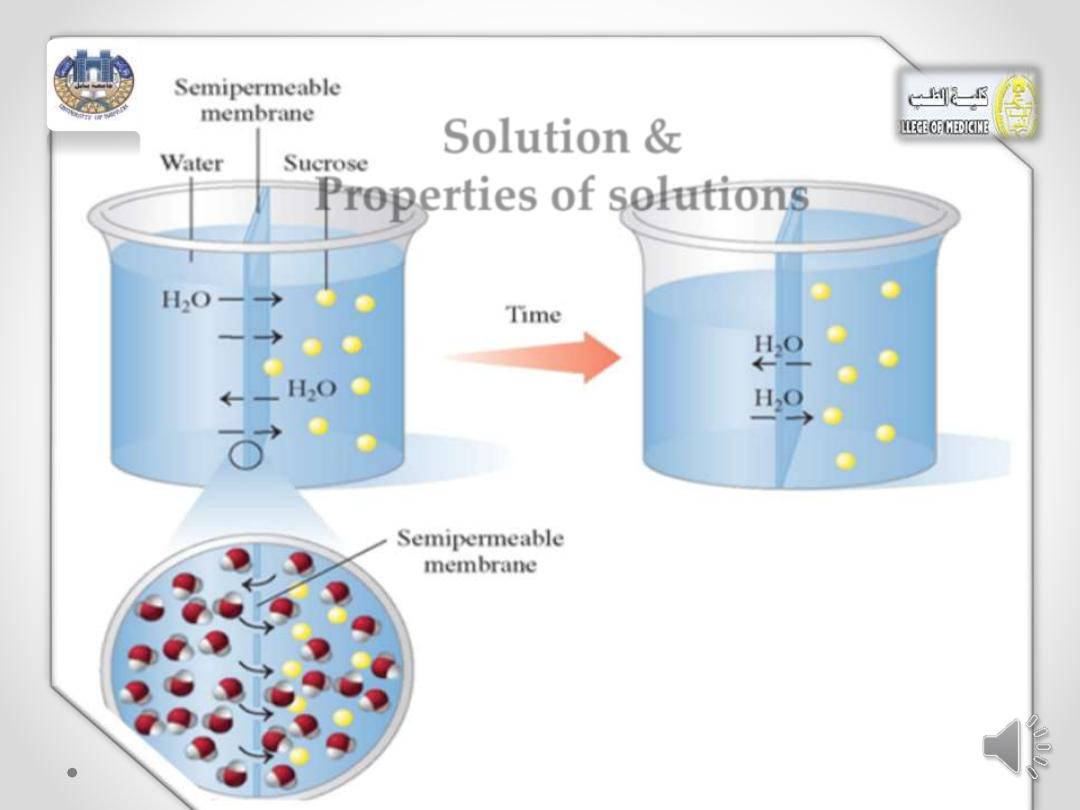
Solution &
Properties of solutions
Dr.Alaa J.Mahrath
Medical Chemistry College of Medicine

LEARNING GOAL
• Identify a mixture as a solution
,
a colloid
, or
a suspension.
• Describe how the number of particles in a solution
affects the
osmotic pressure
of a solution .
i.e. The size and number of solute particles in
different types of mixtures play an important role in
determining the properties of that mixture.
• Electrolytes
• Isotonic , Hypotonic and Hypertonic .
• Dialysis .
AJM Bioorgchem UBCMCD
2

Read this Story about Solution
• When
Michelle
’s kidneys stopped functioning, she was
placed on
dialysis three times a week
. As she enters the
dialysis unit, her dialysis nurse,
Amanda
, asks Michelle
how she is feeling. Michelle indicates that she feels tired
today and has considerable swelling around her ankles.
• The dialysis nurse informs Michelle that her side effects
are due to her body’s inability to regulate the amount of
water in her cells.
• She explains that the amount of water is regulated by the
concentration of electrolytes in her body fluids and the
rate at which waste products are removed from her
body.
AJM Bioorgchem UBCMCD
3
Michelle and Amanda
in Dialysis Unite
AJM Bioorgchem UBCMCD
4

• Amanda explains that although water is essential for the
many chemical reactions that occur in the body, the
amount of water can become too high or too low, due to
various diseases and conditions.
• Because Michelle’s kidneys no longer perform dialysis,
she cannot regulate the amount of electrolytes or waste
in her body fluids.
• As a result, she has an electrolyte imbalance and a
buildup of waste products, so her body is retaining
water. Amanda then explains that the dialysis machine
does the work of her kidneys to reduce the high levels of
electrolytes and waste products.
AJM Bioorgchem UBCMCD
5
Review about Solutions
• solutions are everywhere around us. Most of the gases,
liquids, and solids we see are mixtures of at least one
substance dissolved in another.
• There are different types of solutions.
• The air we breathe is a solution that is primarily oxygen and
nitrogen gases.
• Carbon dioxide gas dissolved in water makes carbonated
drinks.
• When we make solutions of coffee or tea, we use hot water
to dissolve substances from coffee beans or tea leaves.
• In your medicine cabinet, the antiseptic tincture of iodine is a
solution of iodine dissolved in ethanol.
AJM Bioorgchem UBCMCD
6

• Solutions can be described by their concentration, which is the
amount of solute in a specific amount of that solution. These
relationships, which include mass % (m/m), volume % (v/v),
mass/volume % (m/v), and molarity (M), can be used to convert
between the amount of a solute and the quantity of its solution.
• Solutions are also diluted by adding a specific amount of solvent to
a solution.
• You will see also :
• In the processes of osmosis and dialysis, water, essential nutrients,
and waste products enter and leave the cells of the body.
• The kidneys utilize osmosis and dialysis to regulate the amount of
water and electrolytes that are excreted
AJM Bioorgchem UBCMCD
7

• Our body fluids contain water and dissolved
substances such as glucose and urea and
electrolytes such as K
+
, Na
+
, Cl
-
, Mg
2+
, HCO
3
-
, and
HPO
4
-2
.
• Proper
amounts of each of these dissolved
substances and water must be maintained in the
body fluids.
• Small changes in electrolyte levels can seriously
disrupt cellular processes and endanger our
health.
AJM Bioorgchem UBCMCD
8

Defining a Solution and it’s derivatives
• Solutions: are homogeneous mixtures of
substances composed of at least one solute
and one solvent.
• homogeneous mixture: a uniform mixture
of only one phase
• solute: a substance that is dissolved in a
solvent (e.g., salt, NaCl)
• Solvent: the medium in which a solute is
dissolved; often the liquid component of a
solution (e.g., water)
• Please read the type of solute and solvents
in textbook , page (283-284)
AJM Bioorgchem UBCMCD
9
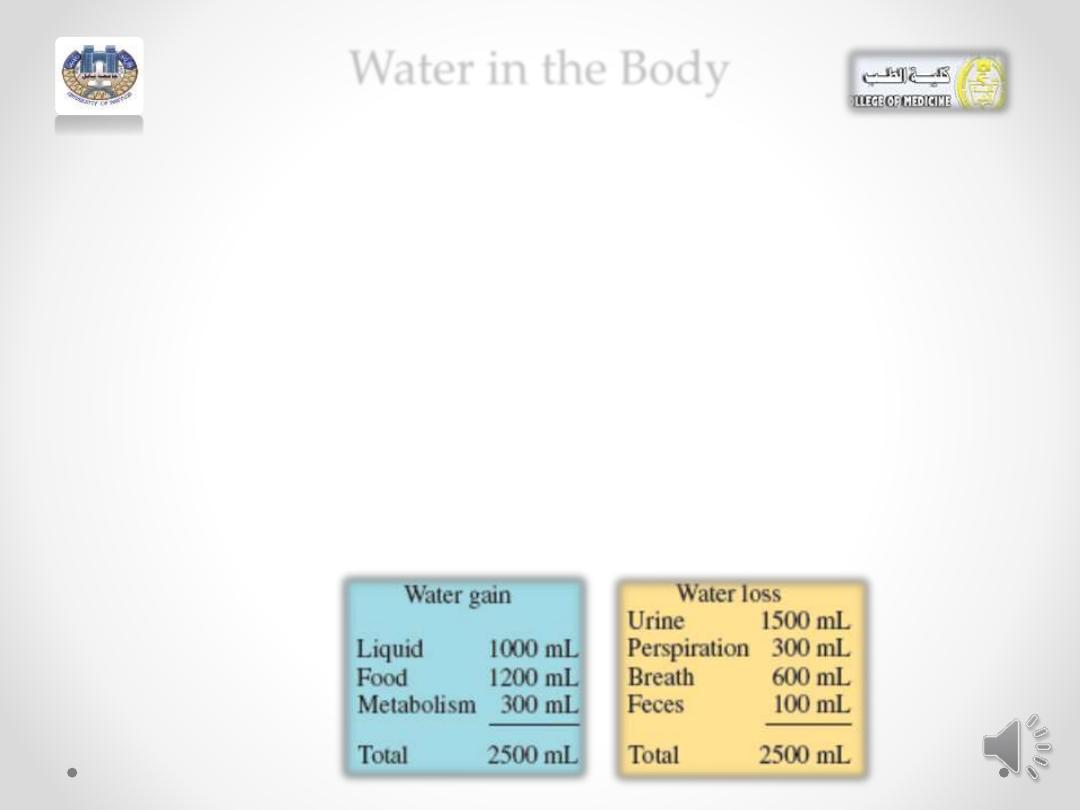
Water in the Body
• Water is one of the most common solvents in nature.
• The average adult is about 60% water by mass, and the
average infant about 75%.
• About 60% of the body’s water is contained within the cells as
intracellular fluids; the other 40% makes up extracellular
fluids, which include the interstitial fluid in tissue and the
plasma in the blood.
• These external fluids carry nutrients and waste materials
between the cells and the circulatory system.
AJM Bioorgchem UBCMCD
10

• Every day you lose between 1500 and 3000 mL of water from
the kidneys as urine, from the skin as perspiration, from the
lungs as you exhale, and from the gastrointestinal tract. Serious
dehydration can occur in an adult if there is a 10% net loss in
total body fluid; a 20% loss of fluid can be fatal. An infant
suffers severe dehydration with only a 5 to 10% loss in body
fluid.
• Water loss is continually replaced by the liquids and foods in the
• diet and from metabolic processes that produce water
• in the cells of the body.
• Table 9.2 lists the percentage by mass of water contained
in some foods.
AJM Bioorgchem UBCMCD
11
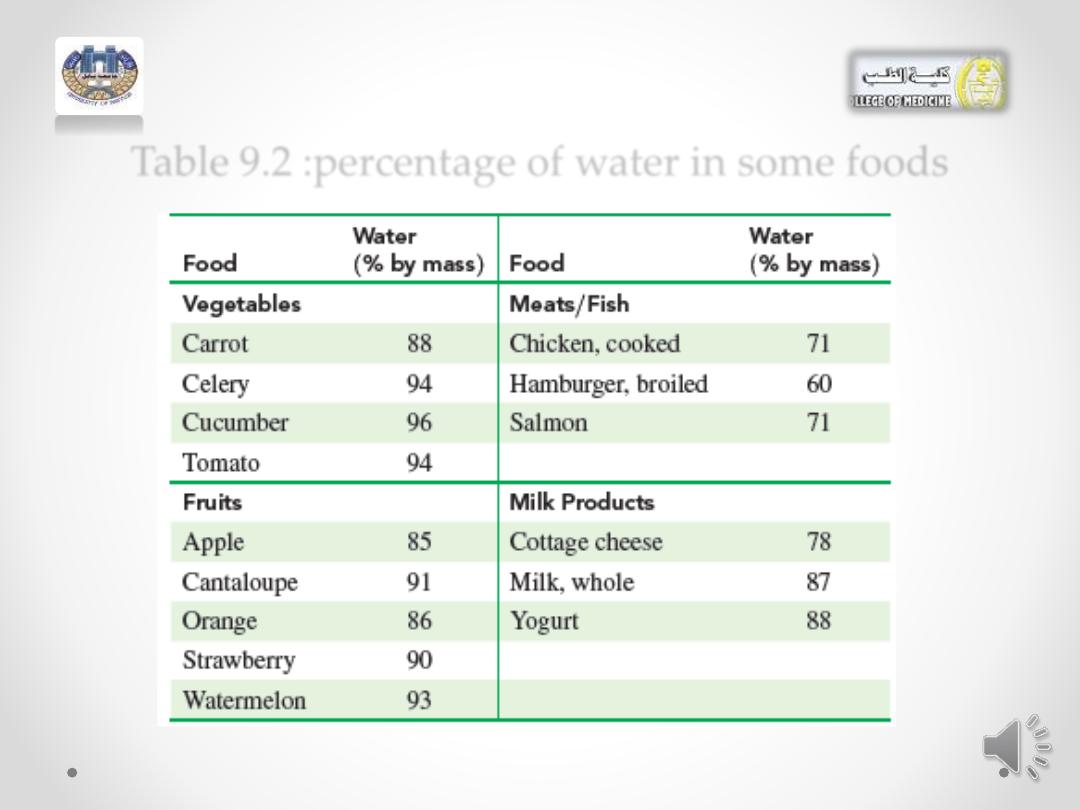
Table 9.2 :percentage of water in some foods
AJM Bioorgchem UBCMCD
12

How to make a solution
• It’s all about ( like dissolve like ) ,it means the polarity of
the solute and the solvent must be similar in order to
form solution. In other word the interaction between
them.
•
Solve Questions and problems page 286
AJM Bioorgchem UBCMCD
13
(a) (b) (c)
Figure 1 :
Like dissolves like. In each test tube, the
lower layer is CH
2
Cl
2
(more dense),
and the upper layer is water (less dense).
(a) CH
2
Cl
2
is nonpolar and water is polar; the two
layers do not mix. (b) The nonpolar solute I
2
(purple) is soluble in the nonpolar solvent CH
2
Cl
2
.
(c) The ionic solute Ni(NO
3
)
2
(green) is soluble in the
polar solvent water

Electrolytes and Nonelectrolytes
• Solutes can be classified by their ability to conduct an
electrical current.
• When electrolytes dissolve in water, the process of
dissociation separates them into ions forming solutions
that conduct electricity.
• When nonelectrolytes dissolve in water, they do not
separate into ions and their solutions do not conduct
electricity.
• To test solutions for the presence of ions, we can use an
apparatus that consists of a battery and a pair of
electrodes connected by wires to a light bulb.
AJM Bioorgchem UBCMCD
14
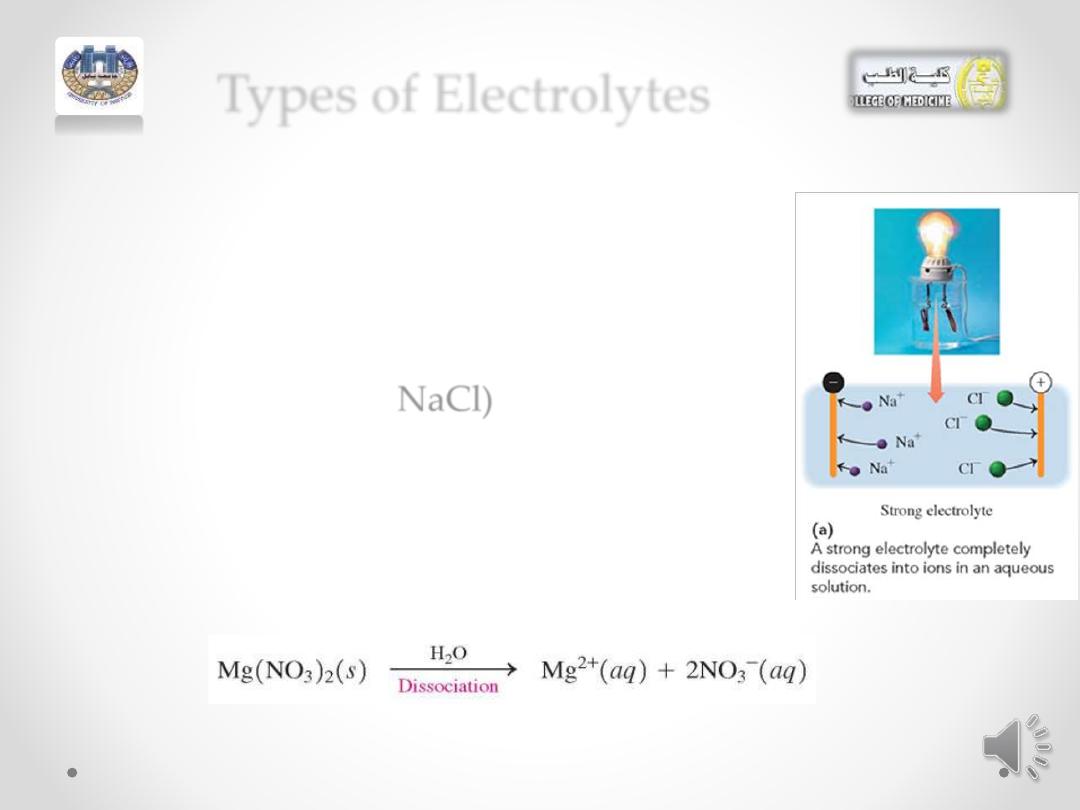
Types of Electrolytes
• Electrolytes can be further classified as
strong
electrolytes
or
weak
electrolytes.
• For a strong electrolyte, such as
sodium chloride (
NaCl)
, there is
100%
dissociation of the solute into ions.
When the electrodes from the
light
bulb
apparatus are placed in the NaCl
solution.
AJM Bioorgchem UBCMCD
15
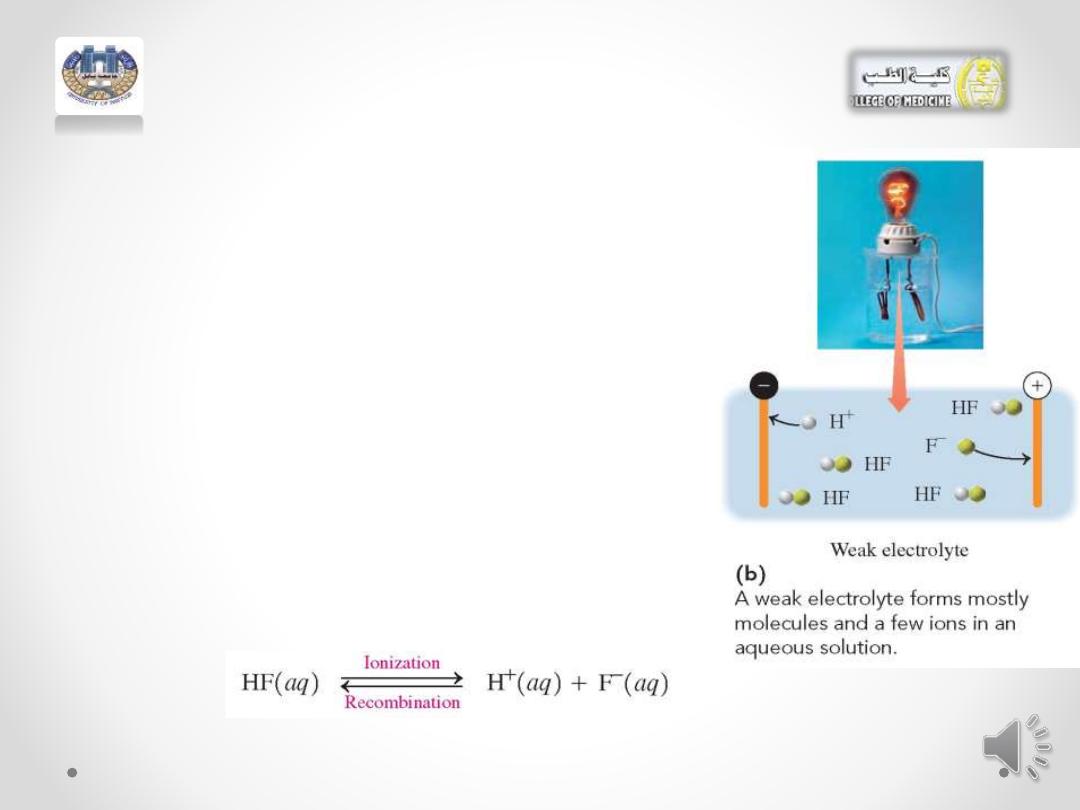
A weak electrolyte :
• is a compound that dissolves in
water mostly as molecules. Only a
few
of
the
dissolved
solute
molecules
undergo
ionization,
producing a small number of ions in
solution .
• In an aqueous solution of the weak
electrolyte HF, a few HF molecules
ionize to produce H+ and F- ions. As
more H+ and F- ions form, some
recombine to give HF molecules.
AJM Bioorgchem UBCMCD
16
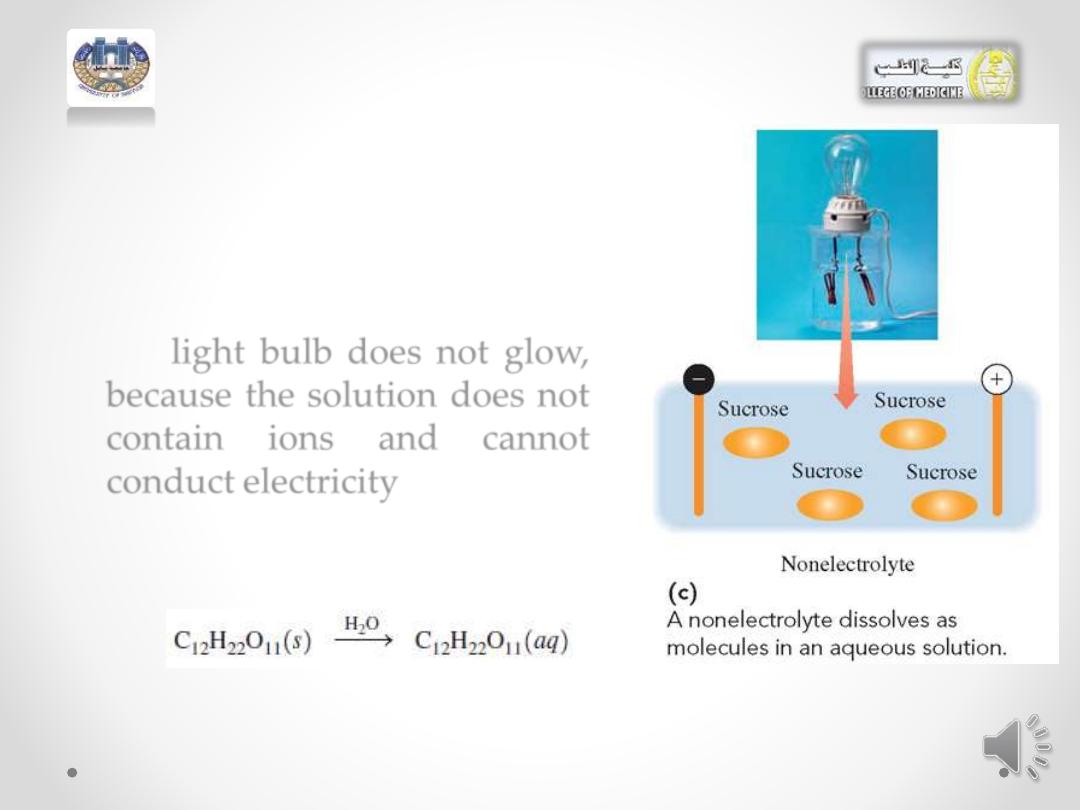
• A nonelectrolyte such as
sucrose (sugar) dissolves in
water
only
as
molecules,
which do not ionize.
• the light bulb does not glow,
because the solution does not
contain
ions
and
cannot
conduct electricity.
AJM Bioorgchem UBCMCD
17
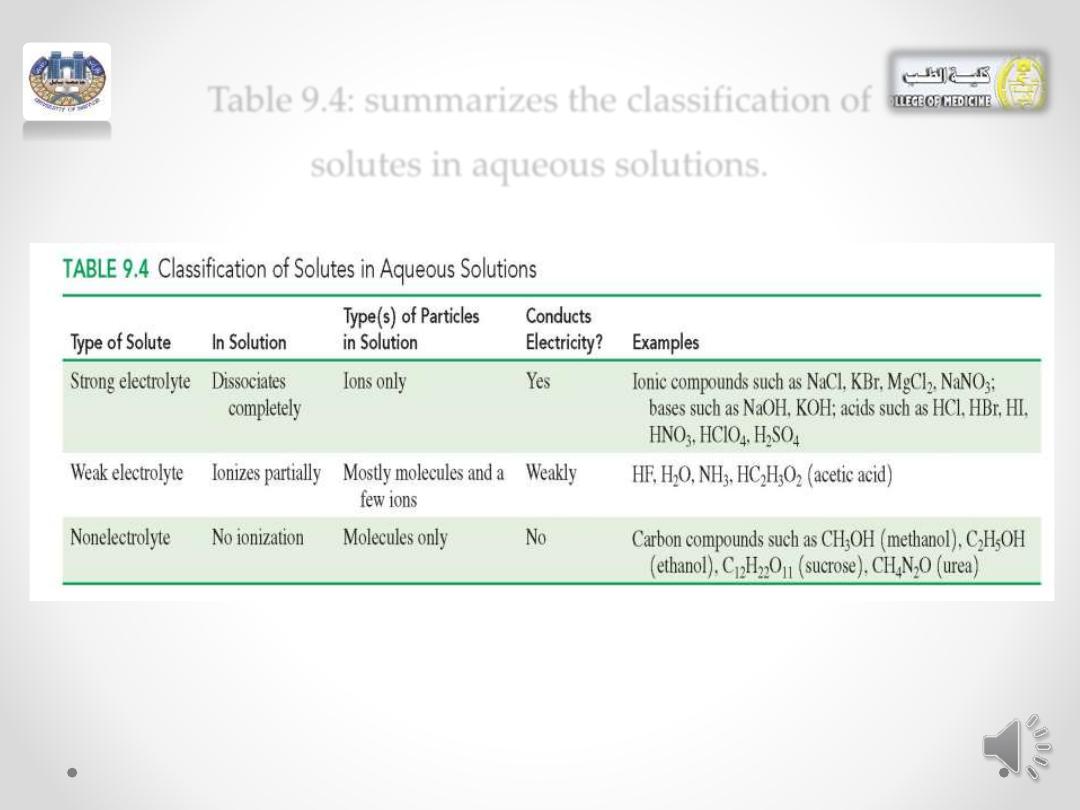
Table 9.4: summarizes the classification of
solutes in aqueous solutions.
AJM Bioorgchem UBCMCD
18

• Example A: Indicate whether solutions of each of the
following contain only ions, only molecules, or mostly
molecules and a few ions. Write the equation for the formation
of a solution for each of the following:
• a. Na
2
SO
4
(s), a strong electrolyte
• b. CH
3
OH(l), a nonelectrolyte .
SOLUTION
•
a. An aqueous solution of Na
2
SO
4
(s) contains only the ions Na
+
and SO
4
2-
•
b. A nonelectrolyte such as CH
3
OH
(
l
)produces only molecules when it dissolves in
water.
AJM Bioorgchem UBCMCD
19
STUDy CHECK 1:
Boric acid, H
3
BO
3
(s), is a weak electrolyte. Would you expect a boric acid solution to
contain only ions, only molecules, or mostly molecules and a few ions?
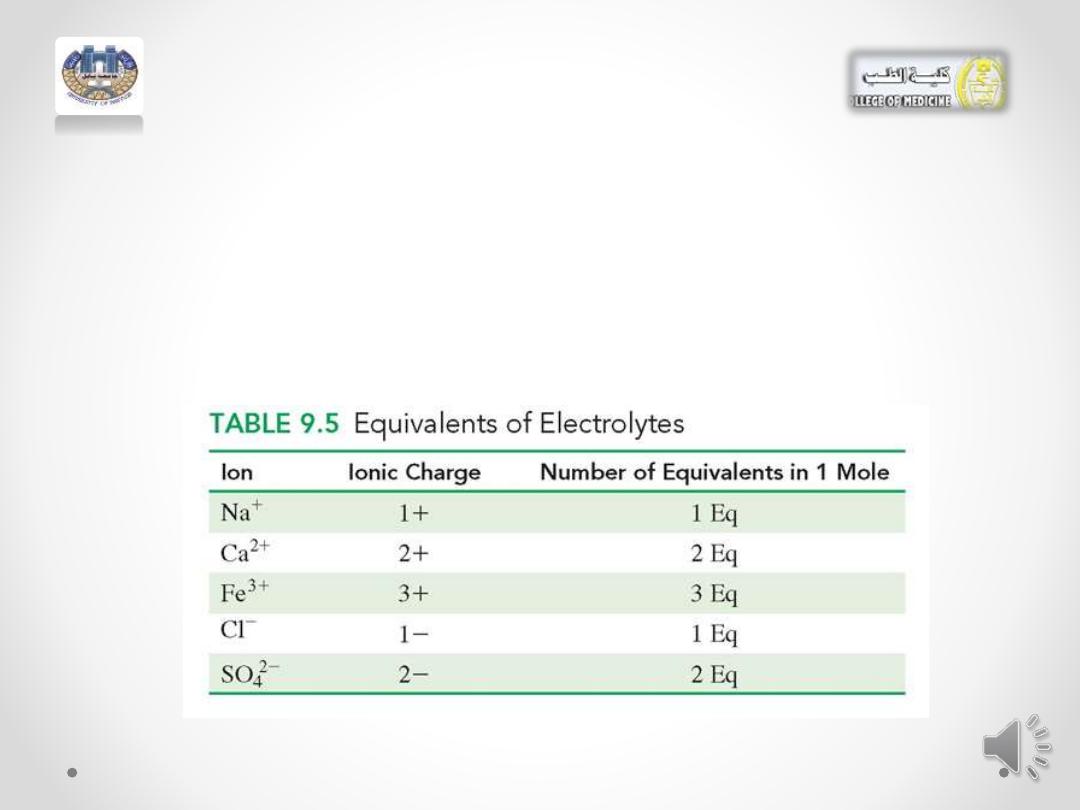
Equivalents
• Body fluids contain a mixture of electrolytes, such as Na
+
, Cl
-
, K
+
,
and Ca
2+
. We measure each individual ion in terms of an equivalent
(Eq), which is the amount of that ion equal to 1 mole of positive or
negative electrical charge , or milliequivalent . 1 Eq= 1000 meq
AJM Bioorgchem UBCMCD
20

• For example: For ions, one equivalent (Eq) is equal to the
number of ions that carry 1 mol of charge.
• 1 mole of Na
+
ions and 1 mole of Cl
-
ions are each 1
equivalent because they each contain 1 mole of charge.
• For an ion with a charge of 2+ or 2-, there are 2
equivalents for each mole.
• In any solution, the charge of the positive ions is always
balanced by the charge of the negative ions. The
concentrations of electrolytes in intravenous fluids are
expressed in milliequivalents per liter (mEq/L); 1 Eq =
1000 mEq.
AJM Bioorgchem UBCMCD
21

• Also we can calculate gram equivalent of ion by divided the
molar mass of ion on the charge ion:
• The number of equivalents of a given ion per liter of solution
(Eq/l )can be found by
multiplying the molarity of the ion
(moles per liter) by the charge on the ion.
• For example, a solution containing 25 mEq/L of
Na
+
and 4
mEq /L of
K
+
has a
total positive charge of 29
mEq /L. If Cl
-
is
the only anion, its concentration must be 29 mEq/L.
AJM Bioorgchem UBCMCD
22
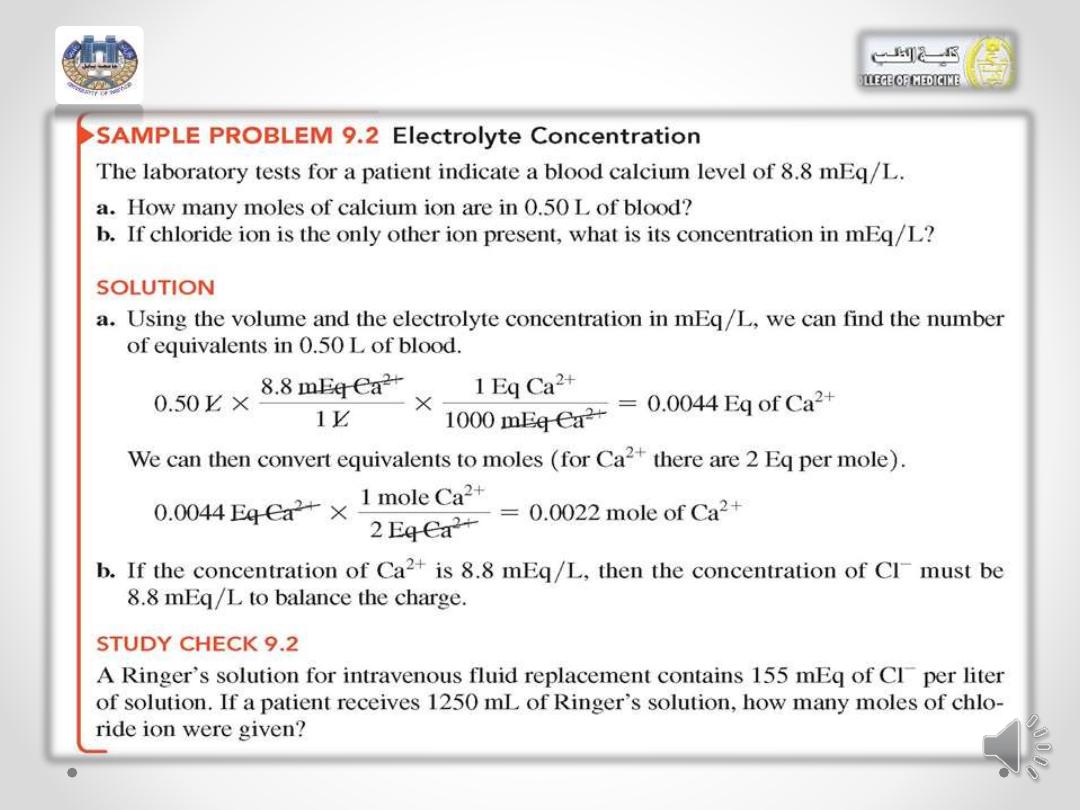
AJM Bioorgchem UBCMCD
23
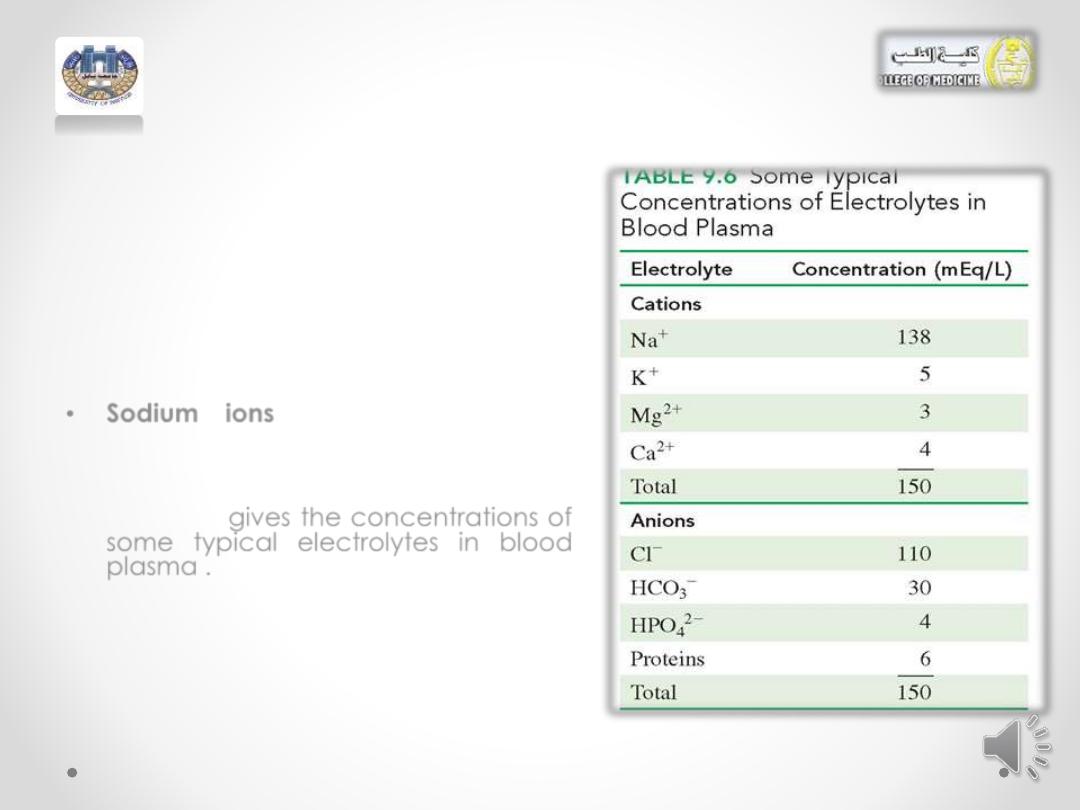
Electrolytes in the Body Fluids
•
Electrolytes in the body play an
important role in maintaining the
proper function of the cells and
organs in the body.
•
Typically, the electrolytes
sodium,
potassium,
chloride,
and
bicarbonate
are measured in a
blood test.
•
Sodium ions regulate the water
content in the body and are
important
in
carrying
electrical
impulses through the nervous system.
•
Table 9.6
gives the concentrations of
some typical electrolytes in blood
plasma .
•
There is a charge balance because
the total number of positive charges
is equal to the total number of
negative charges.
AJM Bioorgchem UBCMCD
24

• Potassium ions
are also involved in the transmission
of electrical impulses and play a role in the
maintenance of a regular heartbeat.
• Chloride ions
balance the charges of the positive
ions and also control the balance of fluids in the
body.
• Bicarbonate
is important in maintaining the proper
pH of the blood.
• Sometimes when vomiting, diarrhea, or sweating is
excessive, the concentrations of certain electrolytes
may decrease. Then fluids such as Pedialyte may
be given to return electrolyte levels to normal.
AJM Bioorgchem UBCMCD
25

• The
concentrations
of
electrolytes present in body
fluids and in intravenous fluids
given to a patient are often
expressed in milliequivalents per
liter (mEq/L) of solution.
•
For
example,
one
liter
of
Pedialyte contains the following
electrolytes: Na
+
45 mEq, Cl
-
35
mEq, and citrate
3-
30 mEq.
• The use of a specific intravenous
solution
depends
on
the
nutritional, electrolyte, and fluid
needs of the individual patient.
AJM Bioorgchem UBCMCD
26
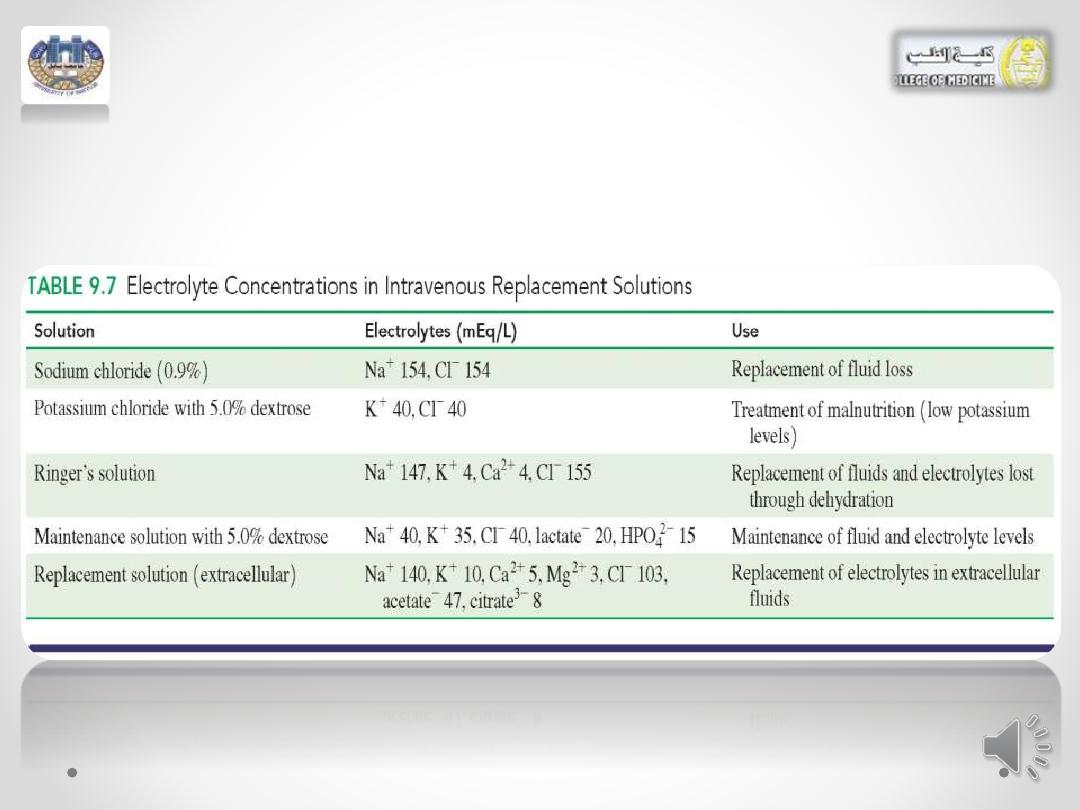
• Examples of various types of solutions are given in
Table 9.7.
AJM Bioorgchem UBCMCD
27

Types of Solution
• We already talk about solutions …
• In a solution, the components cannot be distinguished
one from the other. Syrup is a solution of sugar and
water: The sugar cannot be distinguished from the
water.
• The solution appears transparent, although it may have
a color. The particles are so small that they go through
filters and through
semipermeable membranes.
• A semipermeable membrane allows solvent molecules
such as water and very small solute particles to pass
through, but doesn’t allow the passage of large solute
molecules.
AJM Bioorgchem UBCMCD
28

Colloids Solutions
• The
particles in a colloid
are much larger than
solute particles in a solution.
• Colloidal particles are large molecules, such as
proteins, or groups of molecules or ions.
• Colloids, similar to solutions, are homogeneous
mixtures that do not separate or settle out.
• Colloidal particles are small enough to pass
through filters, but too large to pass through
semipermeable membranes.
AJM Bioorgchem UBCMCD
29

AJM Bioorgchem UBCMCD
30
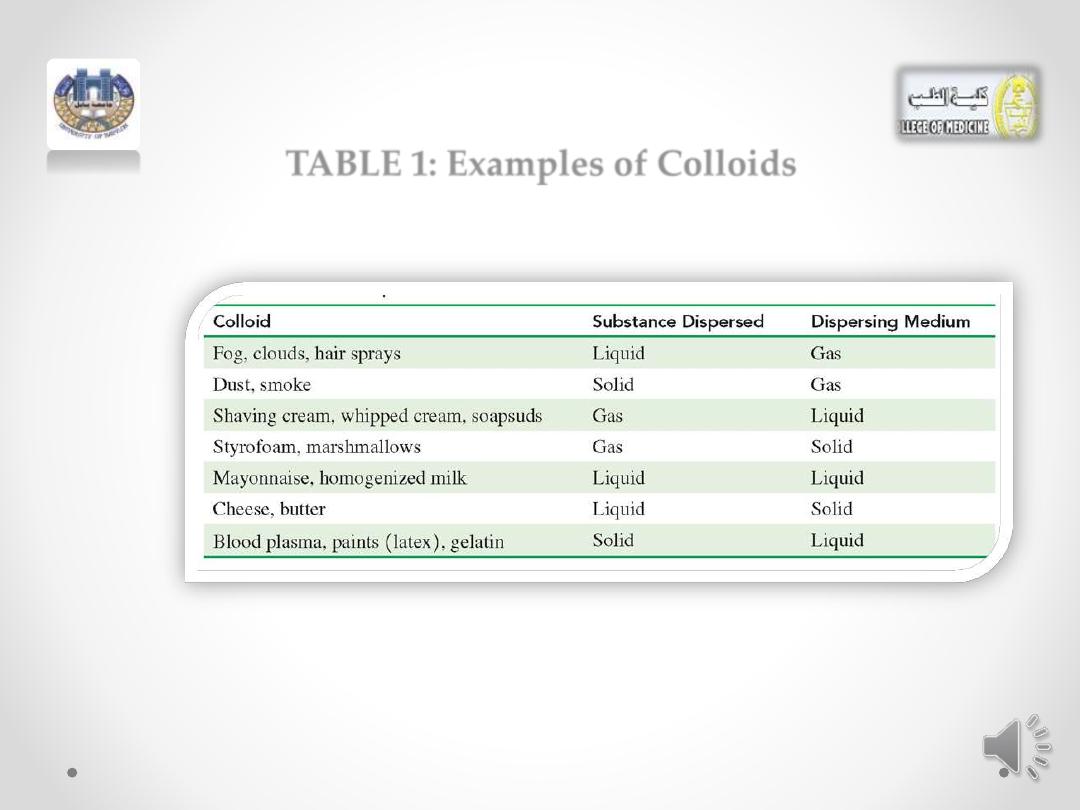
TABLE 1: Examples of Colloids
AJM Bioorgchem UBCMCD
31

Suspensions Solutions
• Suspensions are
heterogeneous
that are very
different from solutions or colloids.
• The particles of a suspension are so large that they
can often be seen with the naked eye.
• They are trapped by filters and semipermeable
membranes.
• You can find suspensions among the medications in
a hospital or in your medicine cabinet.
• These
include
Kaopectate,
calamine
lotion,
antacid mixtures, and liquid penicillin.
AJM Bioorgchem UBCMCD
32

• shake well before using so that the particles form a
suspension.
• Water-treatment plants make use of the properties
of suspensions to purify water.
• Aluminum sulfate or iron(III) sulfate are added to
untreated water, they react with impurities to form
large suspended particles called floc.
• (Table 2 )compares the different types of mixtures
AJM Bioorgchem UBCMCD
33
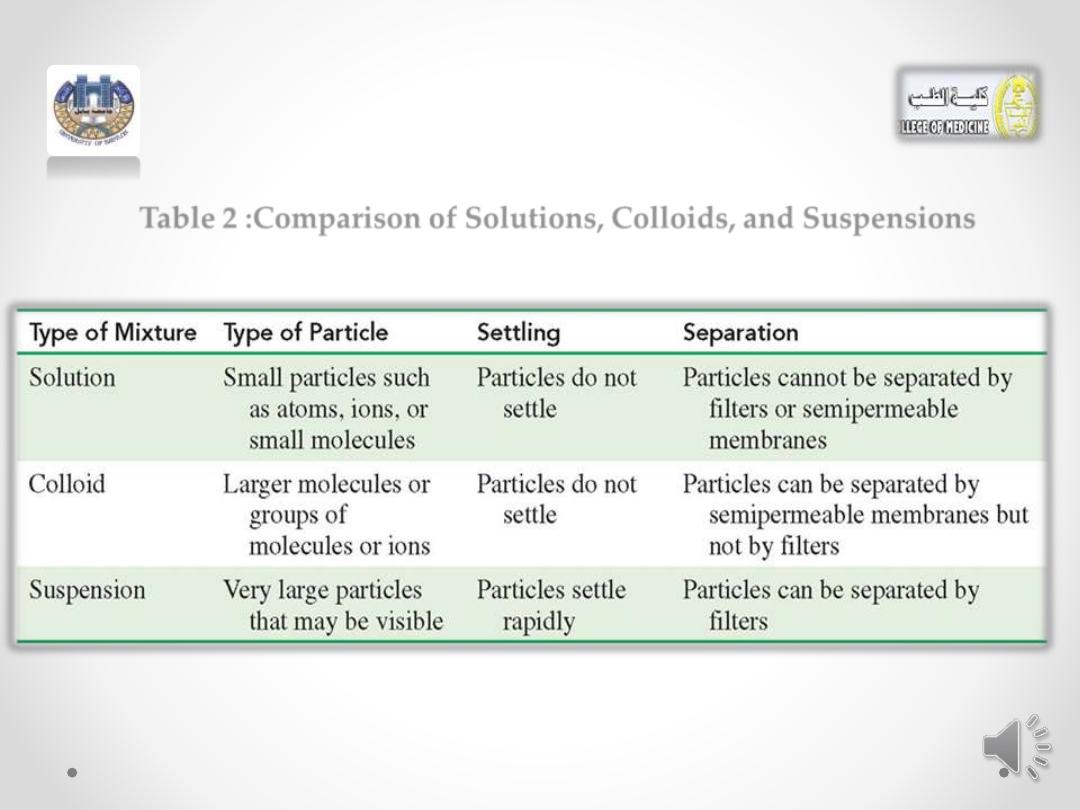
Table 2 :Comparison of Solutions, Colloids, and Suspensions
AJM Bioorgchem UBCMCD
34

AJM Bioorgchem UBCMCD
35

If you have mixture
AJM Bioorgchem UBCMCD
36
Figure 1 : Properties of different types of mixtures: (a)suspensions settle out; (b) suspensions are
separated by a filter; (c) solution particles go through a semipermeable membrane, but colloids and
suspensions do not
Colloids and Solution in the body
• In the body, colloids are retained by semipermeable
membranes.
• For example, the
intestinal lining
allows solution
particles to pass into the blood and lymph circulatory
systems.
• However,
the colloids from foods are too large to
pass through the membrane
,
and they remain in the
intestinal tract.
• Digestion breaks down large colloidal particles, such
as starch and protein
, into smaller particles, such as
glucose and amino acids
that can pass through the
intestinal membrane and enter the circulatory system.
AJM Bioorgchem UBCMCD
37

• Certain foods, such as bran, a fiber, cannot be
broken down by human digestive processes, and
they move through the intestine intact.
• Because large proteins, such as enzymes, are
colloids, they remain inside cells.
• However, many of the substances that must be
obtained by cells, such as
oxygen, amino acids,
electrolytes, glucose, and minerals
, can pass
through cellular membranes.
• Waste products, such as urea and carbon dioxide,
pass out of the cell to be excreted.
AJM Bioorgchem UBCMCD
38

Osmotic Pressure
• The movement of water into and out of the cells of
plants as well as the cells of our bodies is an
important biological process that also depends on
the solute concentration.
• In a process called osmosis, water molecules move
through a semipermeable membrane from the
solution with the lower concentration of solute into a
solution with the higher solute concentration
AJM Bioorgchem UBCMCD
39
AJM Bioorgchem UBCMCD
40
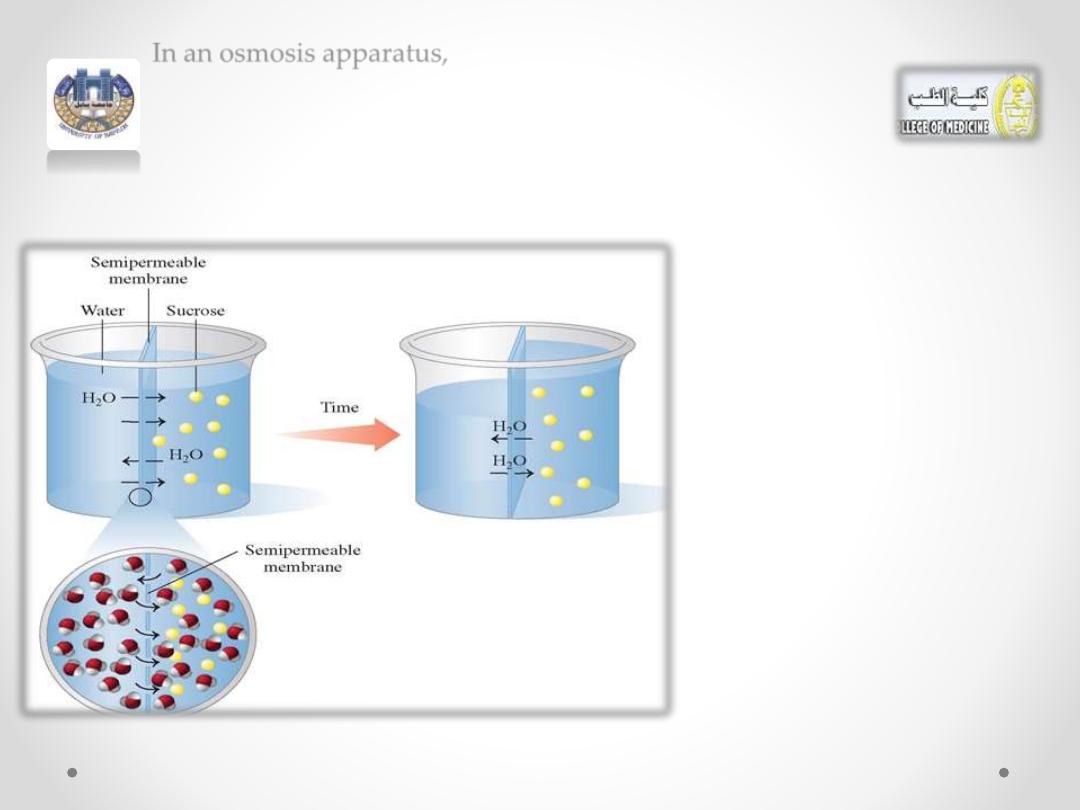
In an osmosis apparatus,
water is placed on one side of a semipermeable membrane
and a sucrose (sugar) solution on the other side.
The
semipermeable
membrane allows water
molecules to flow back and
forth but blocks the sucrose
molecules
because
they
cannot pass through the
membrane.

AJM Bioorgchem UBCMCD
41
AJM Bioorgchem UBCMCD
42
• Because the sucrose solution has a higher solute
concentration, more water molecules flow into the
sucrose solution than out of the sucrose solution.
• The volume level of the sucrose solution rises as the
volume level on the water side falls.
• The increase of water dilutes the sucrose solution to
equalize (or attempt to equalize) the concentrations
on both sides of the membrane.
• Eventually the height of the sucrose solution creates
sufficient pressure to equalize the flow of water
between the two compartments. This pressure, called
osmotic pressure.
• The osmotic pressure depends on the
concentration of
solute particles in the solution
.
• The greater the number of particles dissolved, the higher
its osmotic pressure.
• the sucrose solution has a higher osmotic pressure than
pure water, which has an osmotic pressure of zero.
• Water flows into the solution with a higher solute
concentration until the flow of water becomes equal in
both directions.
• In a process called
reverse osmosis
, a pressure greater
than the osmotic pressure is applied to a solution so that
it is forced through a purification membrane
AJM Bioorgchem UBCMCD
43

Isotonic Solutions
• Because the cell membranes in
biological systems are semipermeable, osmosis is an
ongoing process. The solutes in body solutions such as
blood, tissue fluids, lymph, and plasma all exert
osmotic pressure.
• Most intravenous (IV) solutions used in a hospital are
isotonic solutions, which exert the same osmotic
pressure as body fluids such as blood.
• The most typical isotonic solutions are 0.9% (m/v)
NaCl solution, and 5% (m/v) glucose
AJM Bioorgchem UBCMCD
44

• Although they do not contain
the same kinds of particles, a
0.9% (m/v) NaCl solution as well
as a 5% (m/v) glucose solution
are both 0.3 M (Na
+
and Cl
-
ions
or glucose molecules).
AJM Bioorgchem UBCMCD
45
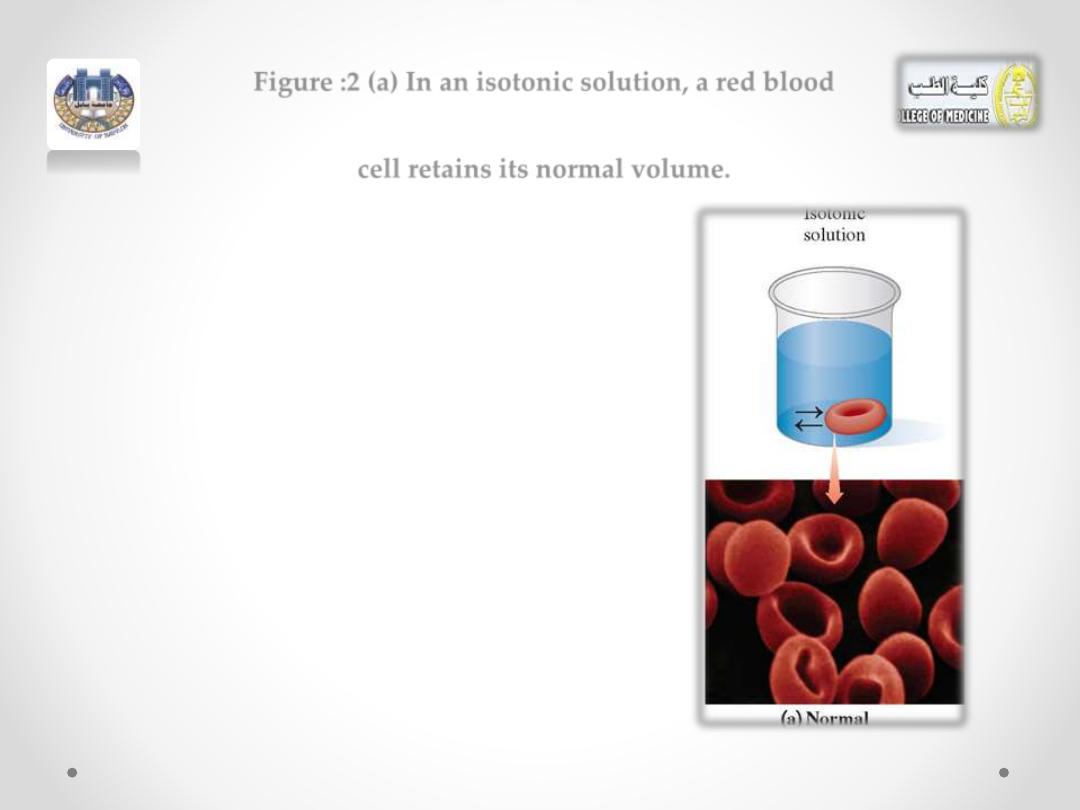
Figure :2 (a) In an isotonic solution, a red blood
cell retains its normal volume.
AJM Bioorgchem UBCMCD
46
A red blood cell placed in an isotonic
solution retains its volume because there is
an equal flow of water into and out of the
cell (see Figure 2a).
(Figure 2a)

AJM Bioorgchem UBCMCD
47

Hypotonic Solutions
• If a red blood cell is placed in a
solution that is not isotonic, the
differences in osmotic pressure
inside and outside the cell can
drastically alter the volume of the
cell.
• When a red blood cell is placed
in a hypotonic solution, which
has a lower solute concentration
water flows into the cell by
osmosis.
•
The increase in fluid causes the
cell to swell, and possibly burst—
a process called hemolysis (see
Figure 2b).
AJM Bioorgchem UBCMCD
48
(Figure 2b).
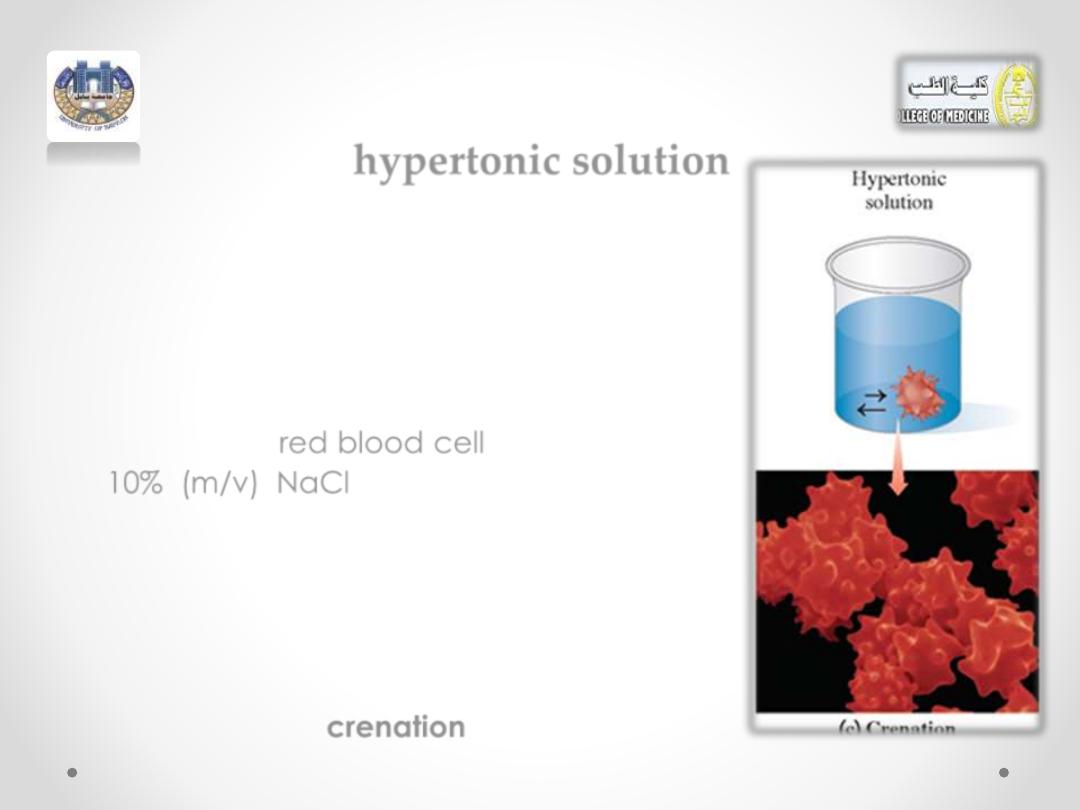
hypertonic solution
• If a red blood cell is placed in a
hypertonic solution, which has a higher
solute concentration, water flows out of
the cell into the hypertonic solution by
osmosis.
•
Suppose a
red blood cell
is placed in a
10% (m/v) NaCl solution. Because the
osmotic pressure in the red blood cell is
the same as a 0.9% (m/v) NaCl solution,
the 10% (m/v) NaCl solution has a much
greater osmotic pressure.
• As water leaves the cell, it shrinks, a
process called
crenation
(see Figure.2c)
AJM Bioorgchem UBCMCD
49
(Figure.2c)
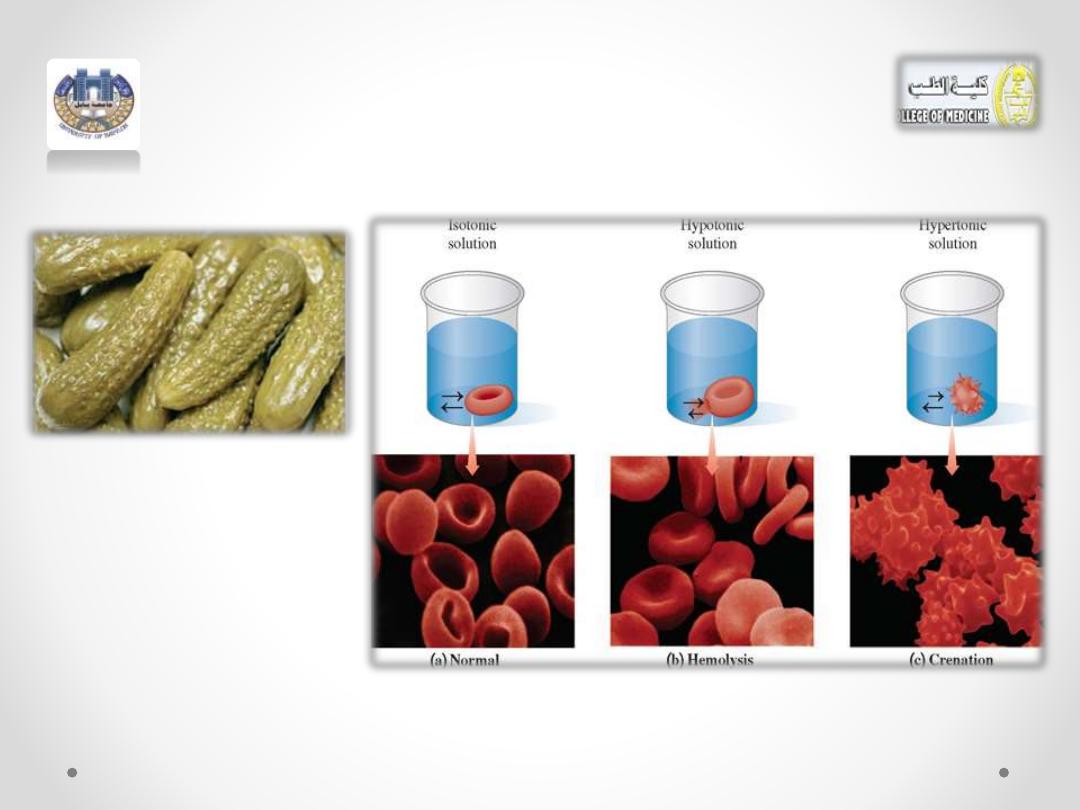
• A similar process occurs when making pickles,
which uses a hypertonic salt solution that
causes the cucumbers to shrivel as they lose
water.
AJM Bioorgchem UBCMCD
50

SAMPLE PROBLEM
• Describe each of the following solutions as isotonic,
hypotonic, or hypertonic. Indicate whether
a red
blood cell
placed in each solution will undergo
hemolysis, crenation, or no change.
• a. 5% (m/v) glucose solution
• b. 0.2% (m/v) NaCl solution
• SOLUTION:
• Study Case 2: What will happen to a red blood cell
placed in a 10% (m/v) glucose solution?
AJM Bioorgchem UBCMCD
51

AJM Bioorgchem UBCMCD
52
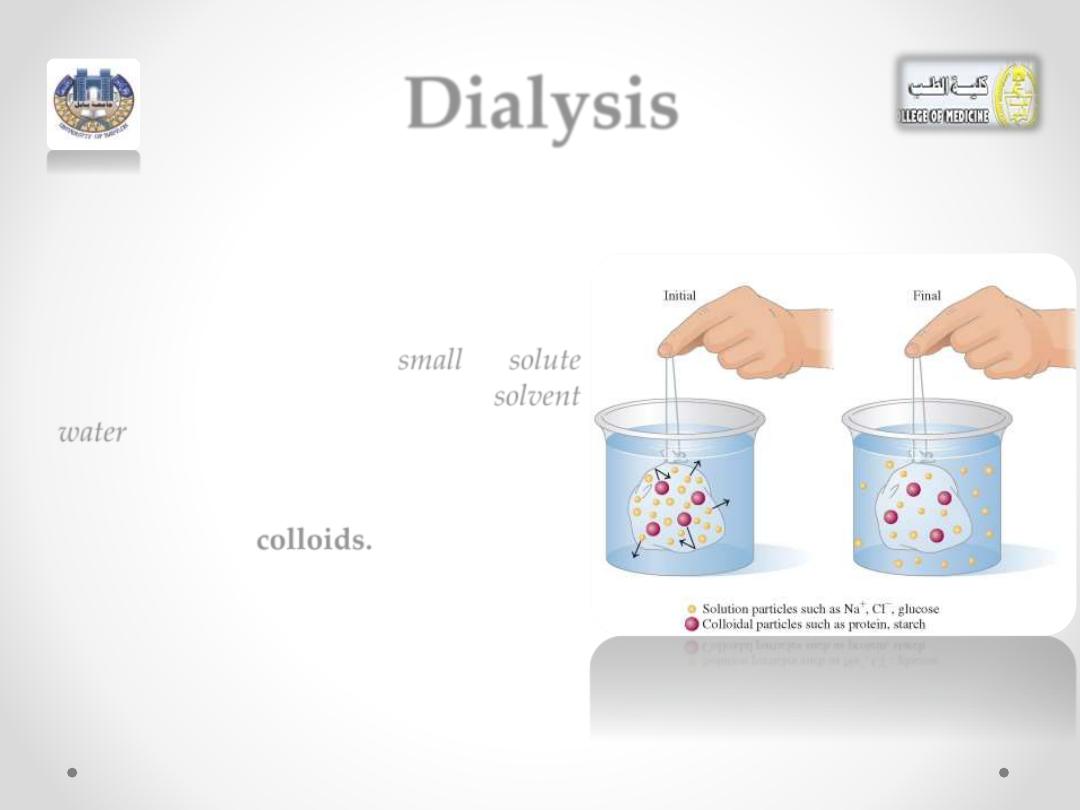
Dialysis
AJM Bioorgchem UBCMCD
53
o Dialysis is a process that is similar to
osmosis. In dialysis, a semipermeable
membrane,
called
a
dialyzing
membrane,
permits
small
solute
molecules and
ions
as well as
solvent
water
molecules to pass through, but it
retains
large particles
, such as
colloids.
o Dialysis is a way to separate
solution
particles from
colloids.
o Suppose we fill a cellophane bag with
a solution containing NaCl, glucose,
starch, and protein and place it in pure
water.
AJM Bioorgchem UBCMCD
54
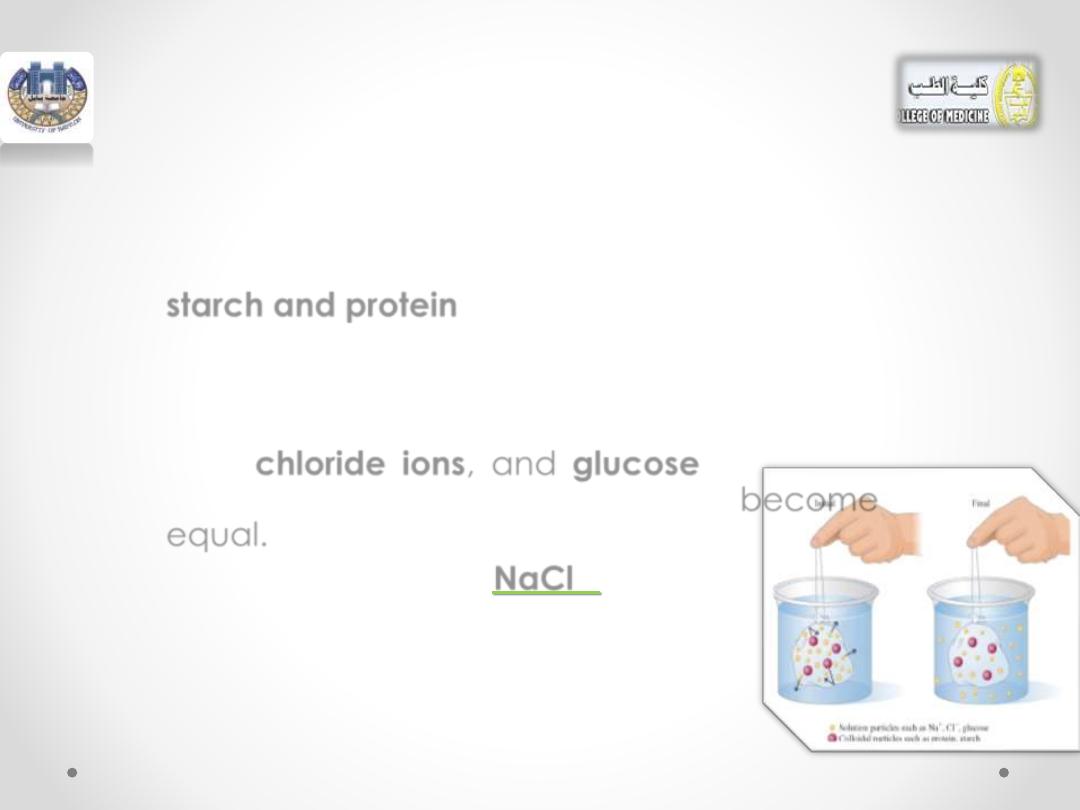
• Cellophane is a dialyzing membrane, and
the
sodium ions
,
chloride ions
, and
glucose
molecules will pass through it into the
surrounding water.
• However, large colloidal particles, like
starch and protein
, remain inside.
• Water
molecules
will
flow
into
the
cellophane bag.
•
Eventually the concentrations of
sodium
ions,
chloride ions
, and
glucose
molecules
inside and outside the dialysis bag become
equal.
• To remove more
NaCl
or
glucose,
the
cellophane bag must be placed in a fresh
sample of pure water.
Dialysis by Kidneys and Artificial Kidney
AJM Bioorgchem UBCMCD
55
The fluids of the body undergo dialysis by
the membranes of the kidneys, which
remove
waste materials
,
excess salts
,
and
water.
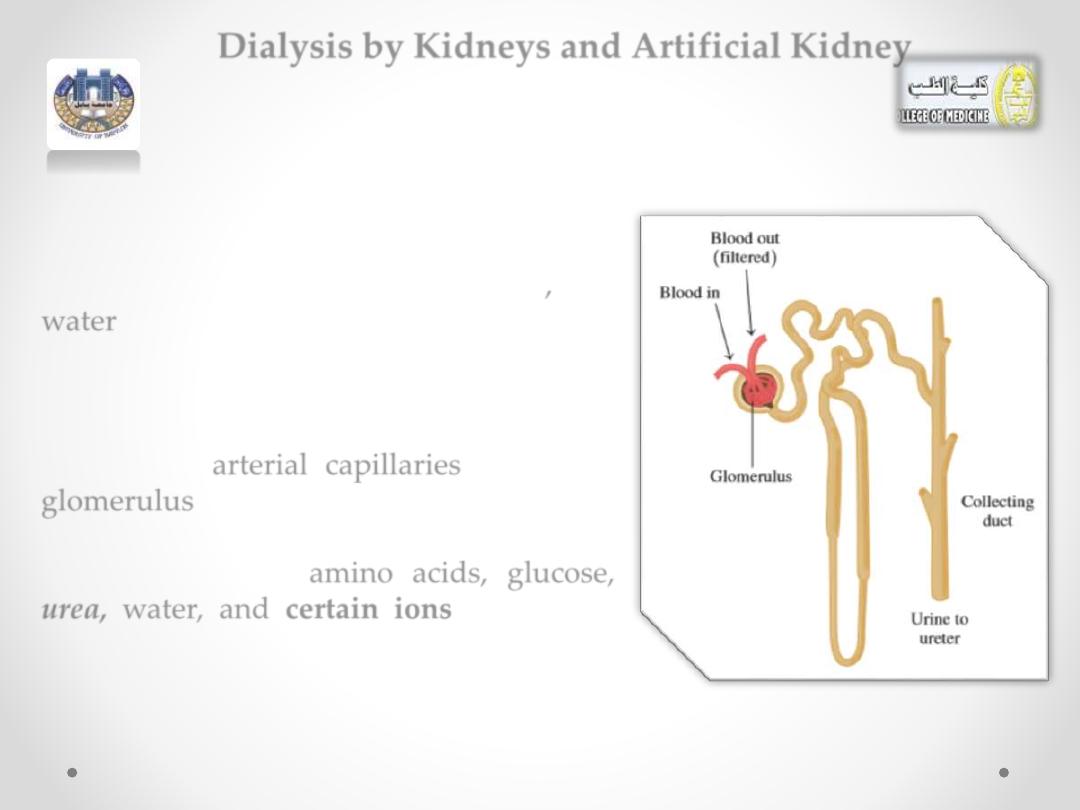
In an adult, each kidney contains about
2
million nephrons
.
At the top of each nephron, there is a
network of arterial capillaries called the
glomerulus.
As blood flows into the glomerulus, small
particles, such as
amino acids,
glucose,
urea,
wate
r, and
certain ions,
will move
through the capillary membranes into the
nephron.
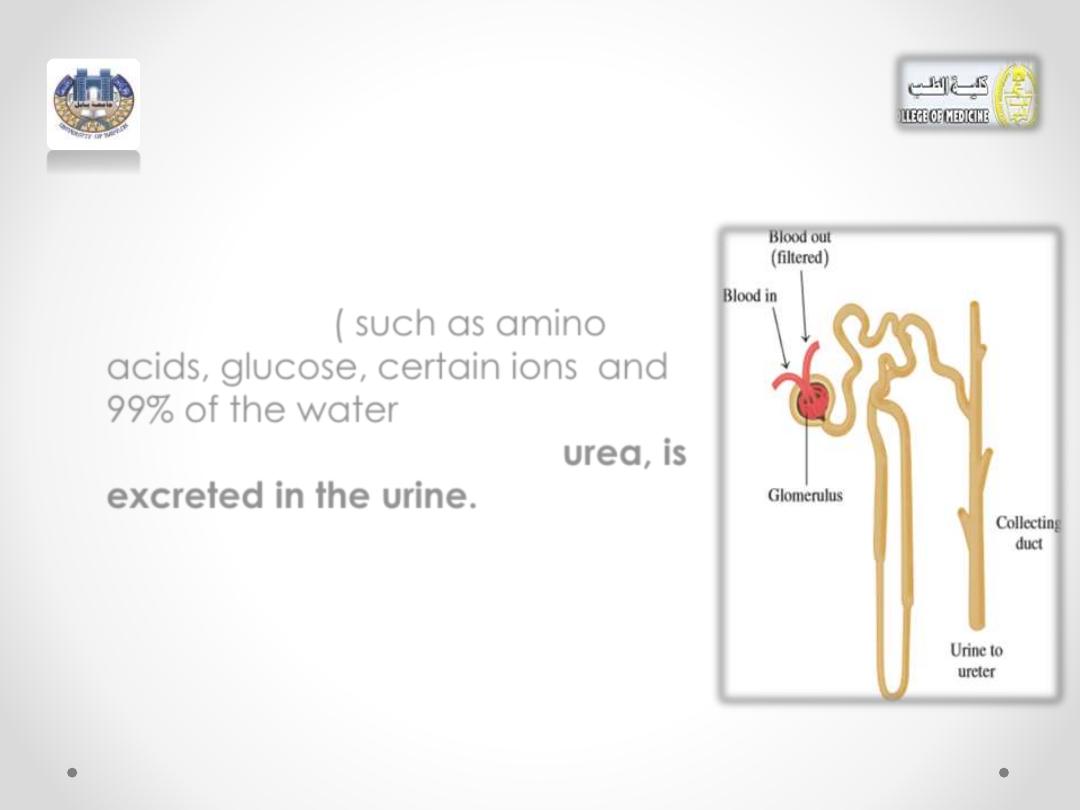
• As this solution moves through the
nephron, substances still of value
to the body
(
such as
amino
acids,
glucose
,
certain ions
,
and
99% of the water
) are reabsorbed.
The major waste product,
urea,
is
excreted in the
urine.
AJM Bioorgchem UBCMCD
56

AJM Bioorgchem UBCMCD
57

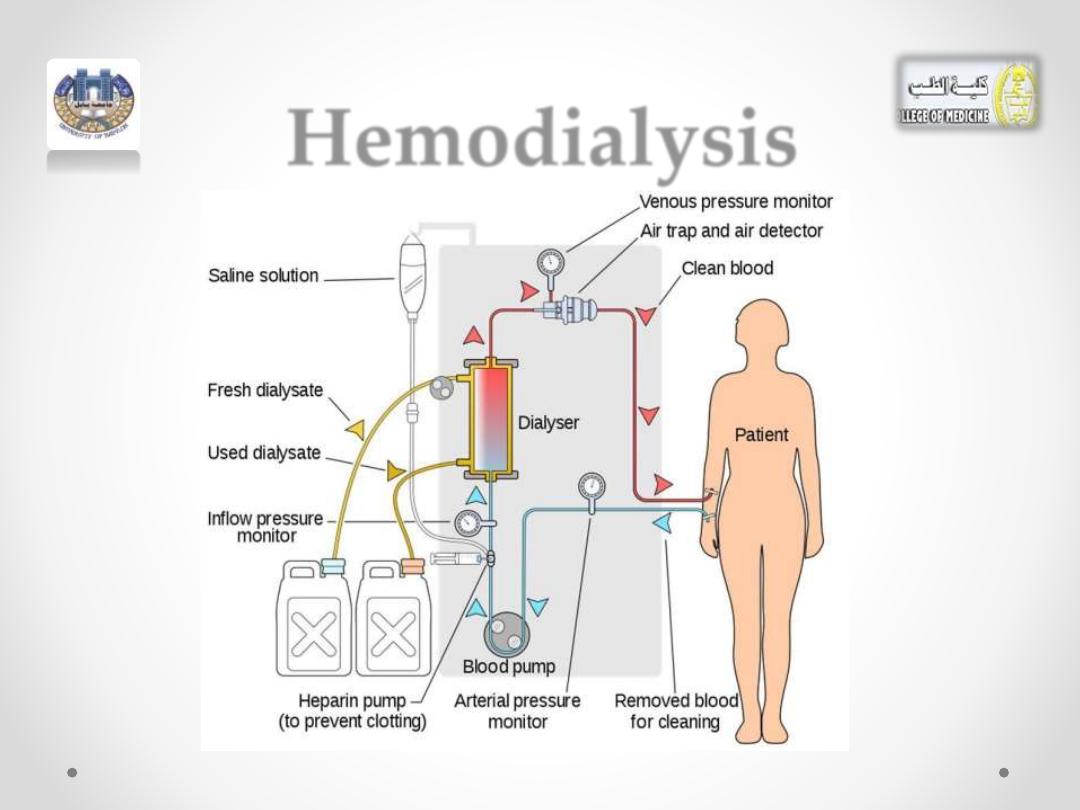
Hemodialysis
•
If the kidneys fail
to dialyze waste products, increased
levels of
urea
can become life-threatening in a
relatively short time.
• A person with kidney failure must use an artificial
kidney, which cleanses the blood by hemodialysis.
• A typical artificial kidney machine contains a large
tank filled with water containing selected electrolytes.
• In the center of this dialyzing bath (
dialysate)
, there is
a dialyzing coil or membrane made of cellulose tubing.
AJM Bioorgchem UBCMCD
58
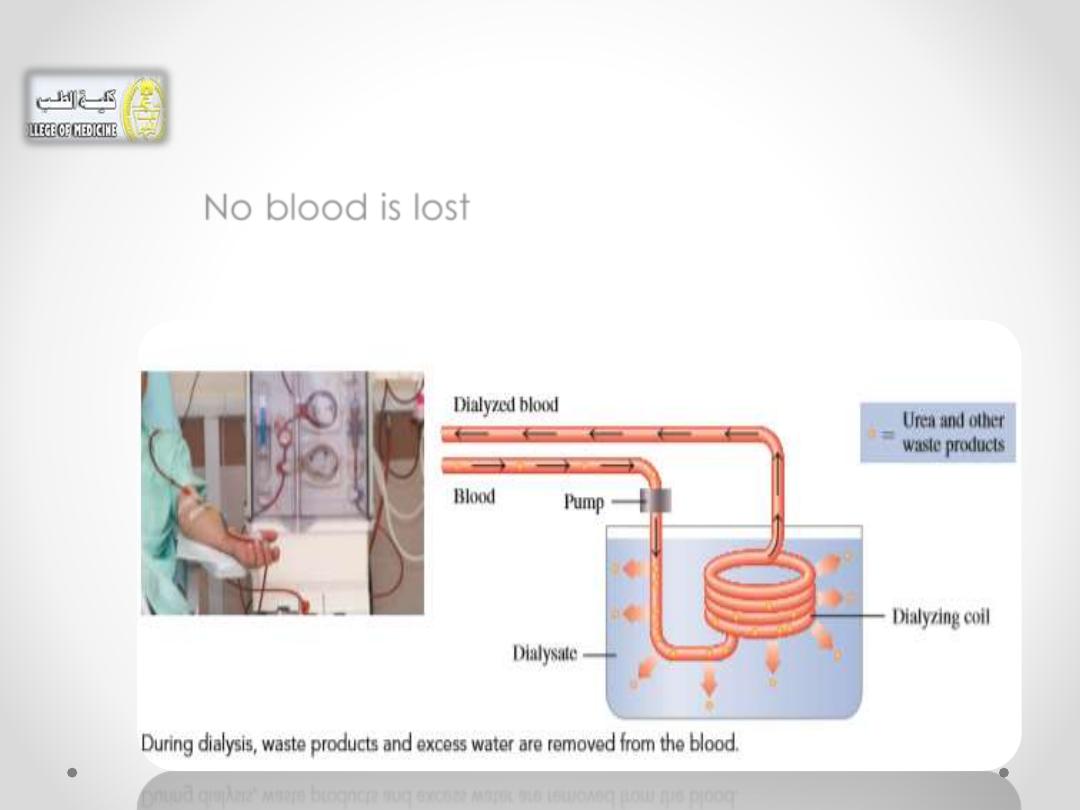
• As the patient’s blood flows through the
dialyzing coil, the highly concentrated waste
products dialyze out of the blood.
• No blood is lost because the membrane is not
permeable
to large particles
such as
red blood
cells.
AJM Bioorgchem UBCMCD
59

Dialysis patients do not produce much urine. As a result,
they retain large amounts of water between dialysis
treatments, which produces a strain on the heart.
The intake of fluids for a dialysis patient may be restricted
to as little as a few teaspoons of water a day.
In the dialysis procedure, the pressure of the blood is
increased as it circulates through the dialyzing coil so
water can be squeezed out of the blood. For some
dialysis patients, 2 to 10 L of water may be removed
during one treatment.
Dialysis patients have from two to three treatments a
week, each treatment requiring
about 5 to 7 h
.
Some of the newer treatments require less time.
For many patients, dialysis is done at home with a home
dialysis unit.
AJM Bioorgchem UBCMCD
60
Hemodialysis
AJM Bioorgchem UBCMCD
61
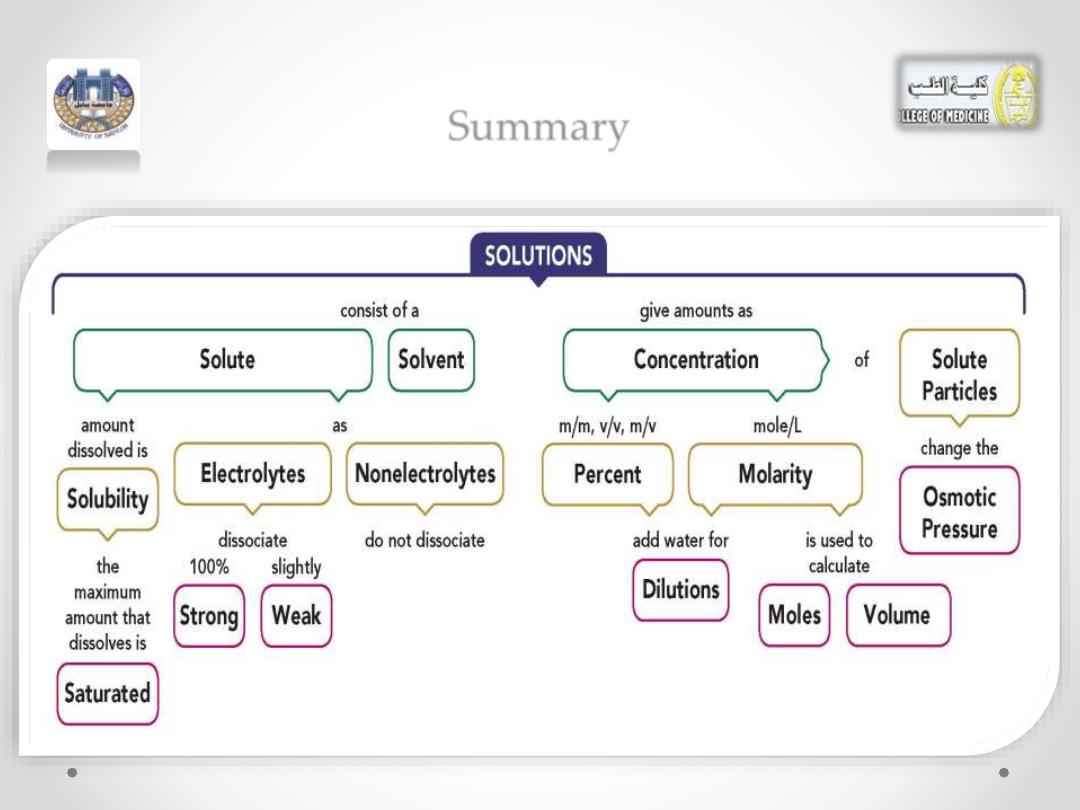
Summary
AJM Bioorgchem UBCMCD
62

QUESTIONS AND PROBLEM (H.W)
• 1. Identify the following as characteristic of a solution, a colloid,
or a suspension:
a. a mixture that cannot be separated by a semipermeable
membrane
b. a mixture that settles out upon standing.
• 2. Identify the following as characteristic of a solution, a colloid, or
a suspension:
a. Particles of this mixture remain inside a semipermeable
membrane but pass through filters.
b. The particles of solute in this solution are very large and visible .
• 3. A 10% (m/v) starch solution is separated from a 1% (m/v)
starch solution by a semipermeable membrane. (Starch is a
colloid.)
a. Which compartment has the higher osmotic pressure?
b. In which direction will water flow initially?
c. In which compartment will the volume level rise?
AJM Bioorgchem UBCMCD
63

Thank You For your
Attention
AJM Bioorgchem UBCMCD
64
