L1. Cardiovascular System DiseasesBy
Assist Prof.Dr.Amin Turki
The Fetal Circulation:
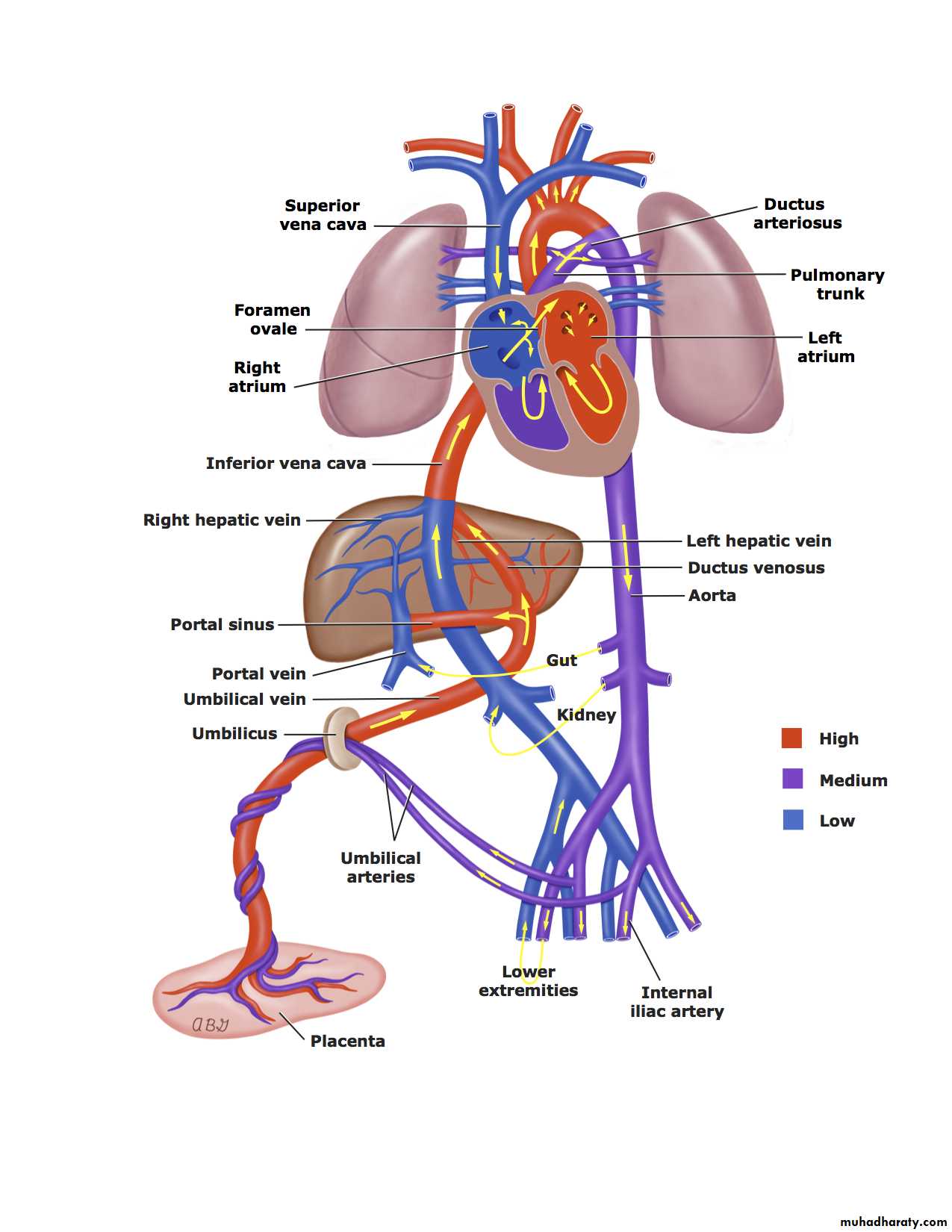
In the fetus, the placenta is responsible for the gas exchange and the lungs are functionless, so the pulmonary vessels are vasoconstricted causing shifting of the blood away from the pulmonary circulation.In the fetal circulation, the Rt. and Lt. ventricles present in parallel circuits rather than series circuits as in newborn or adult. This parallel circulation achieved by THREE cardiovascular structures which are ductus venosus, foramen ovale and ductus arteriosus.
The blood enter to the fetus through the umbilical vein where approximately 50% of it enter to the hepatic circulation and the rest 50% will bypass the liver and joint the inferior vena cava (IVC) through the ductus venosus where it will mixed with poorly oxygenated IVC blood from the lower part of the fetal body.
This combined blood enter the Rt. Atrium and is mainly directed by Eustachian valve (flap of tissue at the Rt atrial-IVC junction) across the foramen ovale into the Lt atrium then to Lt ventricle, then ejected into the ascending aorta to supplies mainly the fetal upper body and brain.
Fetal superior vena cava (SVC) blood enter the RT atrium and then mainly into the RT ventricle through the tricuspid valve then ejected into the pulmonary artery. Because the pulmonary circulation is vasoconstricted, so only 5% of the pulmonary artery blood enter the lungs and the remaining blood shifting through the ductus arteriosus to the descending aorta (Rt-to-Lt shunt) to supply the lower part of the fetal body and placenta via 2 umbilical arteries.
So, the upper part of the fetal body (including coronary arteries, cerebral arteries and upper extremities) is supplied exclusively by the Lt ventricle, while the lower part of the fetal body is supplied mainly by the Rt ventricle.
At Birth: the following changes will occurs:
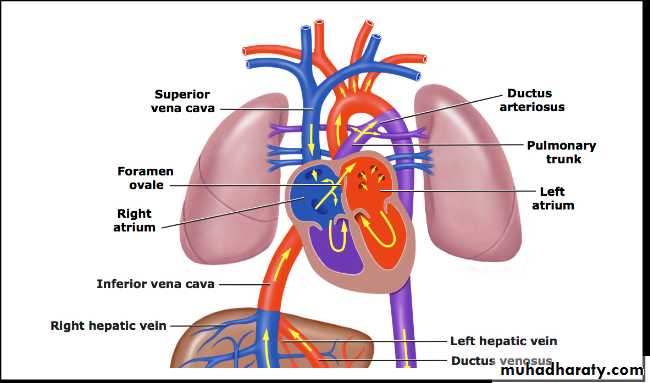
Mechanical expansion of the lungs and increases in the arterial Po2 lead to rapid decrease in the pulmonary vascular resistance (PVR) to become much lower than systemic vascular resistance (SVR).Removal of the placenta lead to increase SVR more than PVR and the closure of ductus venosus.
** These changes will lead to:
The blood will shift from Lt-to-Rt through the ductus arteriosus due to increased SVR and rapid decrease in the PVR and the ductus will closed over several days to form ligamentum arteriosum and now the entire Rt ventricle output will flows into the pulmonary circulation.Increase the volume of pulmonary blood flow returning to the Lt atrium from the lungs will increase the Lt atrium volume and pressure causing functioning closure of foramen ovale although it may remain anatomically patent for several years.
Classification of the Congenital Herat Diseases (CHD):
Acyanotic CHD:Acyanotic CHD causing increased volume load:
Atrial Septal Defect (ASD).
Ventricular Septal Defect (VSD).
Atrio-Ventricular Septal Defect (AV canal).
Patent Ductus Arteriosus (PDA).
Pathophysiology: Presence of communication between the pulmonary and systemic circulations causing shifting of oxygenated blood back to the lungs (Lt-to-Rt shunt) with increased pulmonary blood flow.
Acyanotic CHD causing increased pressure load:
Valvular pulmonary stenosis.
Valvular aortic stenosis.
Coarctation of the aorta.
Tricuspid stenosis.
Mitral stenosis.
Pulmonary veins obstruction.
Pathophysiology: Presence of obstruction to the normal blood flow, which is either obstruction to ventricular out flow( more common, as in the first 3 causes) or obstruction to ventricular inflow (less common, as in the last 3 causes).
Cyanotic CHD:
Cyanotic CHD with decreased pulmonary blood flow:
Tetralogy of Fallot (TOF).
Pulmonary atresia with intact septum.
Tricuspid atresia.
Total anomalous pulmonary venous return with obstruction.
Pathophysiology: In this group, there is must be presence of obstruction to the pulmonary blood flow and a communication between the pulmonary and systemic circulation through it the systemic deoxygenated venous blood shunt from right to left enter the systemic circulation (via PDA, foramen ovale, ASD and VSD).
Cyanotic CHD with increased pulmonary blood flow:
Transposition of Great Arteries (TGA).Single ventricle.
Truncus arteriosus.
Total anomalous pulmonary venous return without obstruction.
Pathophysiology: This group is not associated with obstruction of pulmonary blood flow. Cyanosis caused by either abnormal ventricular-arterial connection, or from total mixing of systemic venous and pulmonary venous blood within the heart.
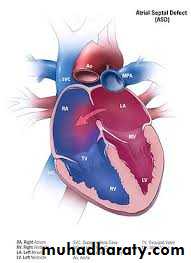
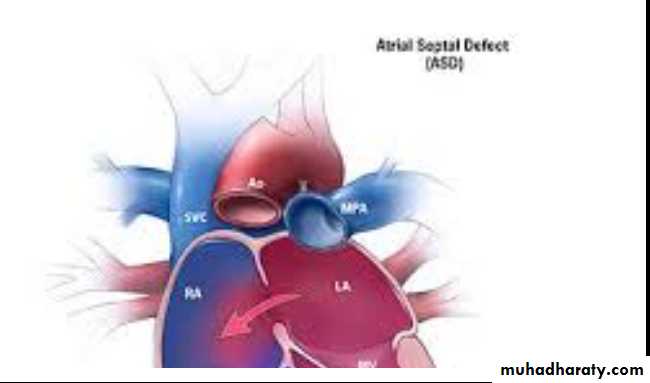
Atrial Septal Defect (ASD)
ASDs represent approximately 10% of all congenital heart defects.
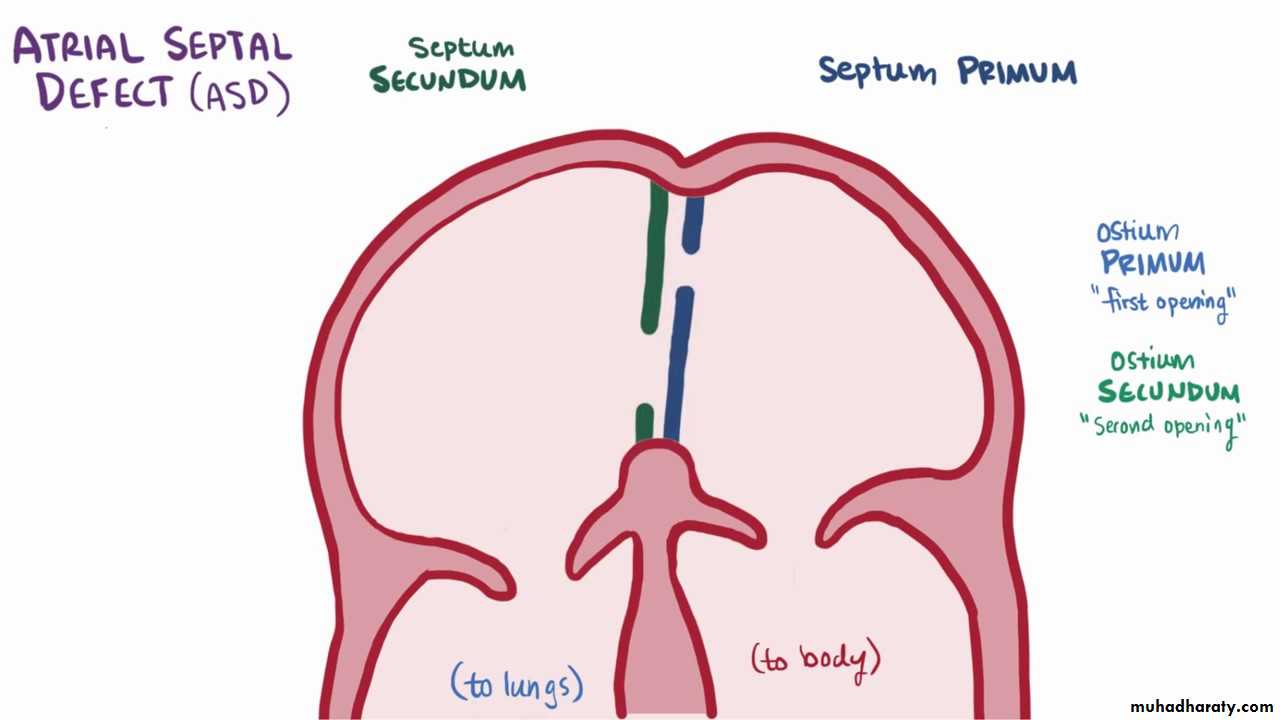
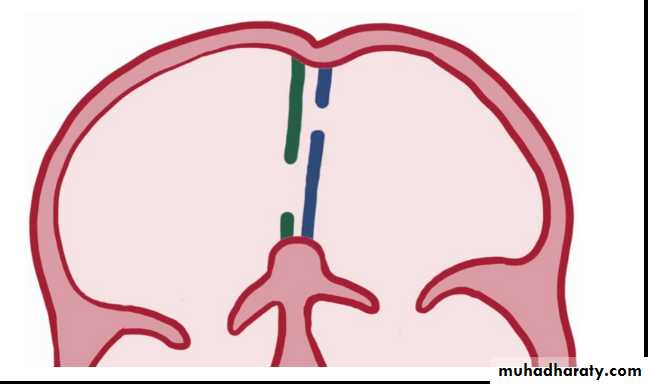
Types:
Ostium secondum ASD: is the most common type, in which the hole in the region of the foramen ovale.Ostium primum ASD: in which the hole located near the endocardial cushions, and it may be part of a complete atrioventricular canal defect
Sinus venosus ASD: is the least common type, which may be associated with anomalous pulmonary venous return.
Ostium
PrimumASD
Ostium
secondum
ASD
Pathophysiology:
The degree of left-to-right shunting is dependent on:The size of the defect, the relative compliance of the right and left ventricles, and the
relative vascular resistance in the pulmonary and systemic circulations.
In large defects, a considerable shunt of oxygenated blood flows from the left to the right atrium. This blood is added to the usual venous return to the right atrium and is pumped by the right ventricle to the lungs. With large defects, the ratio of pulmonary to systemic blood flow (Qp : Qs) is usually between 2 : 1 and 4 : 1.
Clinical features:
ASD is often asymptomatic and discovered during physical examination.Even extremely large ASD, it rarely produce clinical heart failure during childhood, but subtle failure to thrive may be present.
Various degrees of exercise intolerance might present in older children.
On Examination:
There is might be mild left precordial bulge due to cardiomegaly.
Rt ventricular impulse can palpated on Lt. lower sternal border.
On auscultation:
Fixed splitting of second heart sound (S2).
Soft (grade I or II) systolic ejection murmur best heard in the middle and upper sternal border due to increase blood flow across the Rt ventricle into the pulmonary artery and not due to low pressure flow across the ASD.
Mid-diastolic murmur at Lt lower sternal border due to increase flow across the tricuspid valve.
Diagnosis:
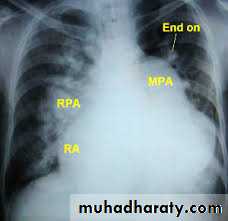
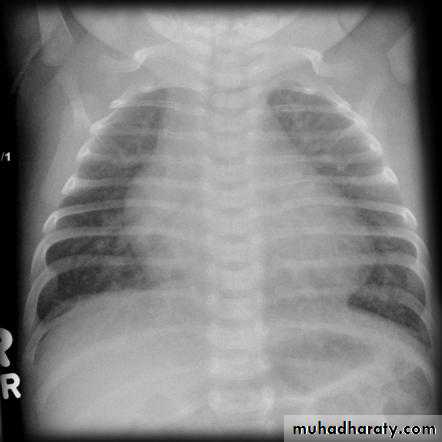
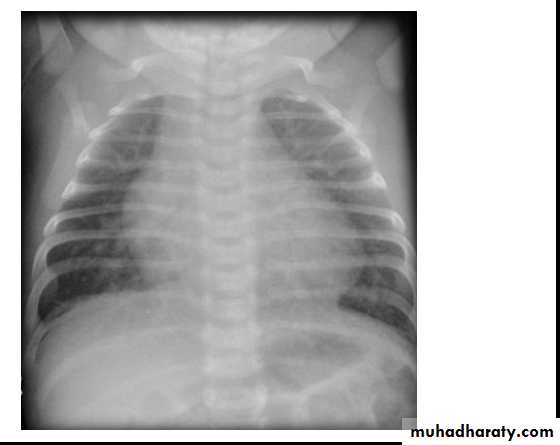
CXR: shows cardiomegaly, Rt atrial enlargement, prominent pulmonary artery and increased pulmonary
ECG shows Rt axis deviation and Rt ventricular hypertrophy.
Echocardiography demonstrate the definitive size and location of the defect.
Treatment:
Asymptomatic child required no treatment.Transcatheter or surgical closure indicated in:
All symptomatic children.
Asymptomatic patient with Qp:Qs ratio of 2:1 and more.
Severe Rt ventricular enlargement.
Prognosis:
Small-moderate ASD in term infants may close spontaneously.Secondum ASD is well tolerated during childhood and complications don’t appear until the third decade or later including pulmonary HT, atrial dysrhythmias, tricuspid or mitral insufficiency and heart failure.
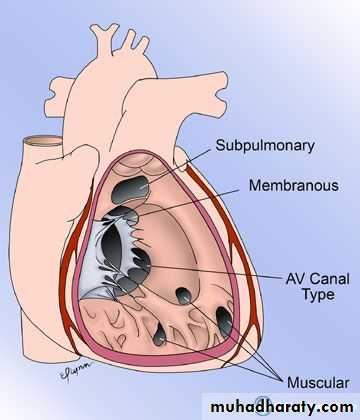
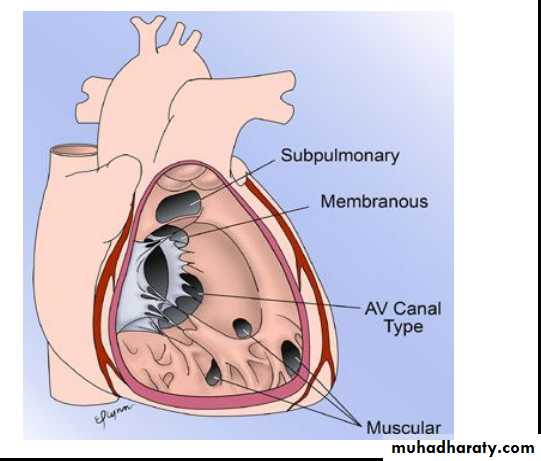
Ventricular Septal Defects (VSD):
Is the most common cardiac malformation, accounts for 25% of CHD.Types:
Membranous type: Is the most common type located in the membranous portion of the septum in the posteroinferior position.
Supracristal type: Is less common, located superior to crista supraventricularis, just beneath the pulmonary valve.
Muscular type: is located in the mid portion or apical region of the ventricular septum and it may be single or multiple (Swiss cheese septum)
Pathophysiology: The onset and severity of clinical manifestations depends on the magnitude of Lt-to-Rt shunt which determined by:
Size of the VSD: Small defects (usually <5 mm) is called pressure restrictive VSD (i.e. the Rt ventricular pressure is normal and higher pressure in Lt ventricle which derive the shunt Lt-to-Rt which is limited by the VSD size).
Large VSD (usually >10mm) is called nonrestrictive VSD in which the Rt and Lt ventricular pressures are equalized and the direction and magnitude of the shunt is determined by the pulmonary to systemic vascular resistance ratio (Qp:Qs).
Pulmonary to systemic vascular resistance ratio (Qp:QS): when the pulmonary vascular resistance (PVR) is high it will limit the Lt-to-Rt shunt. After birth, the PVR is high due to pulmonary vasoconstriction, so there is limitation of the Lt-to-Rt shunt through the VSD. When the PVR begin to fall during the first 6-8 wk after birth, the shunt will be increase and the clinical manifestations will appears.
When the PVR=SVR (Qp:Qs is 1:1), the shunt will become bidirectional and patient become cyanosed (Eisenmenger physiology).
When the PVR>SVR (Qp:Qs is 2:1 or more), the shunt will reverse to become Rt-to-Lt and the cyanosis will increase more.
Clinical Features:
Small VSD with minimal Lt-to-Rt shunt and normal pulmonary pressure:The patient is asymptomatic and discovered during routine examination.
Characteristic loud, harsh, halosystolic murmur, best heard over the lower Lt sternal border and frequently associated with thrill
Large VSD with excessive pulmonary blood flow and pulmonary HT:
Symptoms of heart failure in early infancy (Dyspnea, feeding difficulties, poor growth and profuse sweating) and recurrent chest infections.
Cyanosis is usually absent, but duskiness may note during infection or crying.
Lt precordium bulge and palpable parasternal lift is common.
The halosystolic murmur is less harsh than that of small VSD.
Mid-diastolic, low-pitched murmur at the apex due to increased blood flow across the mitral valve.
Diagnosis:
CXR: may be normal in small VSD, but in large VSD there is may be cardiomegaly and increased pulmonary vascularity.
ECG: It may be normal in small VSD, but in large VSD there is biventricular hypertrophy with notched or peaked P wave.
Echocardiography: for size and location of the defect, direction and magnitude of the shunt, and measurement of pulmonary pressure.
Natural coarse(natural history) of VSD: It depend on the size of VSD.
30-50% of small VSDs closed spontaneously, mostly during the first 2yr of life.Small muscular VSDs are more likely to close (up to 80%) than membranous VSDs (up to 35%)
Closed VSD often have ventricular septal aneurysm which limit the shunt magnitude.
Most children with small restrictive VSD remain asymptomatic.
Non operated VSD might increase incidence of arrhythmia, subaortic stenosis and exercise intolerance.
Treatment:
Prophylactic antibiotic to prevent subacute bacterial endocarditis (SBE).
Treatment of heart failure in large VSD by diuretics and digoxin.
Surgical closure indicated in continued poor growth or pulmonary HT despite medical treatment.