5th stage
Gynecology
د
.
ﺑ
ﺎ
ن
ﻋ
ﺎ
ﻣ
ﺮ
ﻣ
ﻮ
ﺳ
ﻰ
Ectopic Pregnancy
Ectopic pregnancy refers to the implantation of a fertilized egg in a
location outside of the uterine cavity, including the fallopian tubes
(approximately 97.7%), cervix, ovary, cornual region of the uterus, and
abdominal cavity.
Of tubal pregnancies, the ampulla is the most common site of
implantation (80%), followed by the isthmus (12%), fimbria (5%),
cornua (2%), and interstitia (2-3%).
Epidemiology
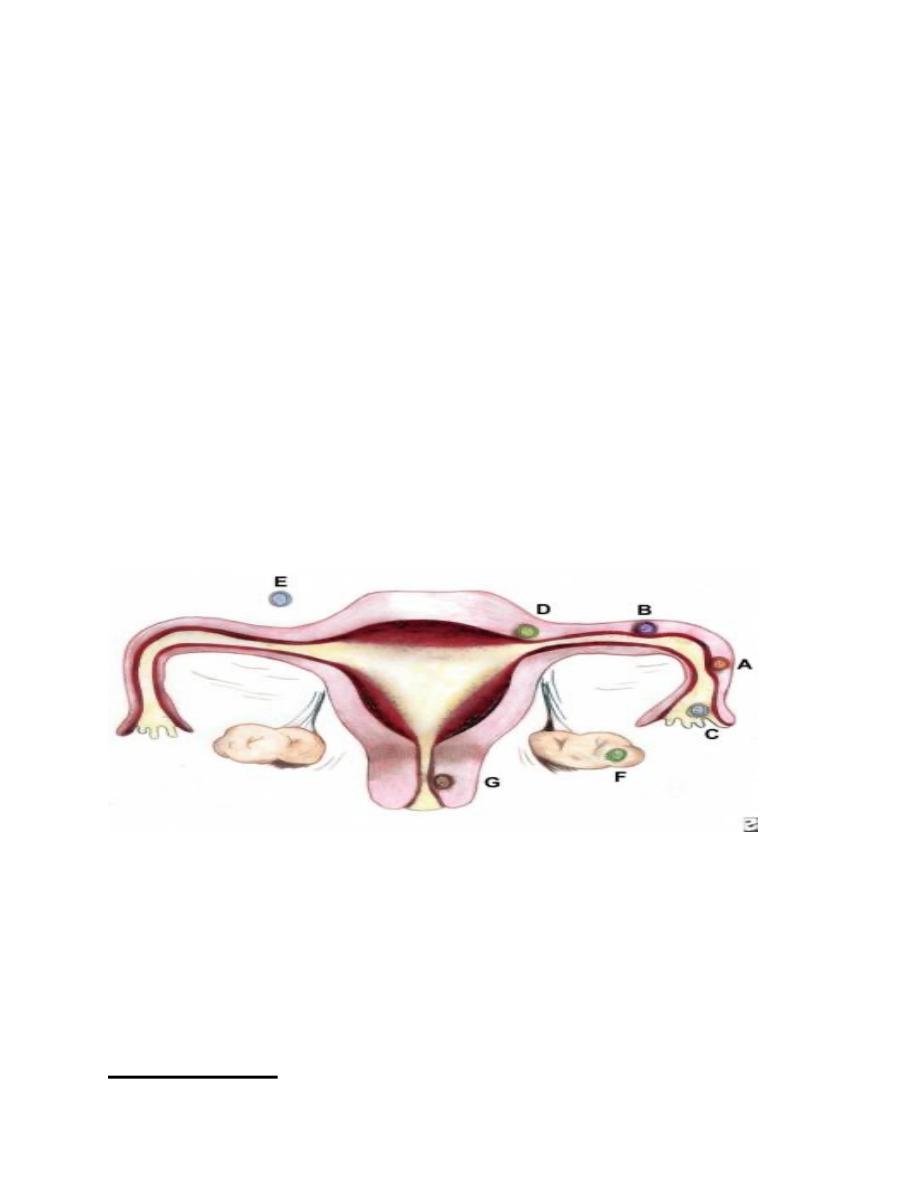
Sites and frequencies of ectopic pregnancy
(A) Ampullary, 80%; (B) Isthmic, 12%; (C) Fimbrial, 5%; (D)
Cornual/Interstitial, 2%; (E) Abdominal, 1.4%; (F) Ovarian,
0.2%; and (G) Cervical, 0.2%. the gestation grows and draws its
blood supply from the site of abnormal implantation. As the
gestation enlarges, it creates the potential for organ rupture,
because only the uterine cavity is designed to expand and
accommodate fetal development.
The incidence of ectopic pregnancy is reported most commonly as
the number of ectopic pregnancies per 1000 conceptions. Since 1970,
when the reported rate in the United States was 4.5 cases per 1000
pregnancies, the frequency of ectopic pregnancy has increased 6-fold,
with ectopic pregnancies approximately 25 cases per 1000 pregnancies.
The increased incidence of ectopic pregnancy has been partially
attributed to improved ability in making an earlier diagnosis. Ectopic
pregnancies that previously would have resulted in tubal abortion or
complete, spontaneous reabsorption and remained clinically
undiagnosed are now detected.
Signs and symptoms
The classic clinical triad of ectopic pregnancy is as follows:
- Abdominal pain
- Amenorrhea
- Vaginal bleeding
Unfortunately, only about 50% of patients present with all 3 symptoms.
Patients may present with other symptoms common to early pregnancy
(eg, nausea, breast fullness).
The presence of the following signs suggests a surgical emergency:
- Abdominal rigidity
- Involuntary guarding
- Severe tenderness
Evidence of hypovolemic shock (eg, hypotension, tachycardia)
Findings on pelvic examination may include the following:
- The uterus may be slightly enlarged and soft
- Uterine or cervical motion tenderness may suggest peritoneal
inflammation
- An adnexal mass may be palpated but is usually difficult to
differentiate from the ipsilateral ovary
- bleeding may be present in the vagina, due to shedding of
endometrial lining stimulated by an ectopic pregnancy
Differential Diagnosis
•
Miscarriage Complications
•
Appendicitis
•
Cervical Cancer
•
Dysmenorrhea
•
Early Pregnancy Loss
Causes of abnormal Implantation sites
The faulty implantation that occurs in ectopic pregnancy occurs
because of a defect in the anatomy or normal function of either the
fallopian tube (as can result from surgical or infectious scarring), the
ovary (as can occur in women undergoing fertility treatments), or the
uterus (as in cases of bicornuate uterus or cesarean delivery scar).
Reflecting this, most ectopic pregnancies are located in the fallopian
tube; the most common site is the ampullary portion of the tube, where
over 80% of ectopic pregnancies occur.
Nontubal ectopic pregnancies are a rare occurrence, with abdominal
pregnancies accounting for 1.4% of ectopic pregnancies and ovarian and

cervical sites accounting for 0.2% each. Some ectopic pregnancies
implant in the cervix (< 1%), in previous cesarean delivery scars, or in a
rudimentary uterine horn; although these may be technically in the
uterus, they are not considered normal intrauterine pregnancies.
In the absence of modern prenatal care, abdominal pregnancies can
present at an advanced stage (>28 wk) and have the potential for
catastrophic rupture and bleeding.
Risk factors
Multiple factors contribute to the relative risk of ectopic pregnancy. In
theory, anything that hampers or delays the migration of the fertilized
ovum (blastocyst) to the endometrial cavity can predispose a woman to
ectopic gestation. The following risk factors have been linked to ectopic
pregnancy:
1- Pelvic inflammatory disease
A history of major tubal infection decreases fertility and increases
abnormal implantation. The most common cause of PID is an antecedent
infection caused by Chlamydia trachomatis. Patients with chlamydial
infection have a range of clinical presentations, from
asymptomatic cervicitis to salpingitis and florid PID. More than 50% of
women who have been infected are unaware of the exposure.
Other organisms that cause PID, such as Neisseria gonorrhea, also
increase the risk of ectopic pregnancy, and a history of salpingitis
increases the risk of ectopic pregnancy 4-fold. The incidence of tubal
damage increases after successive episodes of PID (ie, 13% after 1
episode, 35% after 2 episodes, 75% after 3 episodes).
2- History of previous ectopic pregnancy

After 1 ectopic pregnancy, a patient incurs a 7- to 13-fold increase in
the likelihood of another ectopic pregnancy.
3- History of tubal surgery and conception after tubal
ligation
Previous tubal surgery has been demonstrated to increase the risk of
developing ectopic pregnancy such as fimbrioplasty, tubal
reanastomosis, and lysis of peritubal or periovarian adhesions.
4- Smoking
Cigarette smoking has been shown to be a risk factor for ectopic
pregnancy development. Studies have demonstrated an elevated risk
ranging from 1.6 to 3.5 times that of nonsmokers. A dose-response
effect has also been suggested.
5- Use of oral contraceptives or an intrauterine device
All contraceptive methods lead to an overall lower risk of pregnancy and
therefore to an overall lower risk of ectopic pregnancy. However, among
cases of contraceptive failure, women at increased risk of ectopic
pregnancy compared with pregnant controls included those using
progestin-only oral contraceptives, progestin-only implants, or IUDs and
those with a history of tubal ligation .
Nevertheless, if a woman ultimately conceives with an IUD in place, it
is more likely to be an ectopic pregnancy.
6- Use of fertility drugs or assisted reproductive
technology

Ovulation induction with clomiphene citrate or injectable gonadotropin
therapy has been linked to a 4-fold increase in the risk of ectopic
pregnancy in a case-control study. This finding suggests that multiple
eggs and high hormone levels may be significant factors. In addition, the
risk of ectopic pregnancy and heterotopic pregnancy (ie, pregnancies
occurring simultaneously in different body sites) dramatically increases
when a patient has used assisted reproductive techniques—such as such
as in vitro fertilization (IVF) .
7- Increasing age
The highest rate of ectopic pregnancy occurs in women aged 35-44
years. A 3- to 4-fold increase in the risk of developing an ectopic
pregnancy exists compared with women aged 15-24 years. One
proposed explanation suggests that aging may result in a progressive
loss of myoelectrical activity in the fallopian tube; myoelectrical activity
is responsible for tubal motility.
8- Salpingitis isthmica nodosum
Salpingitis isthmica nodosum is defined as the microscopic presence of
tubal epithelium in the myosalpinx or beneath the tubal serosa. These
pockets of epithelium protrude through the tube, similar to small
diverticula. The etiology of salpingitis isthmica nodosum is unclear, but
proposed mechanisms include postinflammatory and congenital changes,
as well as acquired tubal changes, such as those observed with
endometriosis
9- Other

Other risk factors associated with increased incidence of ectopic
pregnancy include anatomic abnormalities of the uterus such as a T-
shaped or bicornuate uterus, fibroids or other uterine tumors, previous
abdominal surgery, failure with progestin-only contraception, and
ruptured appendix.
Diagnosis
1----Serum β-HCG levels
In a normal pregnancy, the β-HCG level doubles every 48 hours until
it reaches 10,000-20,000mIU/mL. In ectopic pregnancies, β-HCG levels
usually increase less. Mean serum β-HCG levels are lower in ectopic
pregnancies than in healthy pregnancies.
No single serum β-HCG level is diagnostic of an ectopic pregnancy.
Serial serum β-HCG levels are necessary to differentiate between normal
and abnormal pregnancies and to monitor resolution of ectopic
pregnancy once therapy has been initiated.
The discriminatory zone of β-HCG
:-
the level above which an
imaging scan should reliably visualize a gestational sac within the uterus
in a normal intrauterine pregnancy) is as follows: 1500-1800 mIU/mL
with transvaginal ultrasonography, but up to 2300 mIU/mL with
multiple gestates , 6000-6500 mIU/mL with abdominal ultrasonography.
Absence of an intrauterine pregnancy on a scan when the β-HCG level
is above the discriminatory zone represents an ectopic pregnancy or a
recent abortion.
2----Ultrasonography

Ultrasonography is probably the most important tool for diagnosing an
extrauterine pregnancy. Transvaginal ultrasonography, or endovaginal
ultrasonography, can be used to visualize an intrauterine pregnancy by
24 days postovulation or 38 days after the last menstrual period (about 1
week earlier than transabdominal ultrasonography). An empty uterus on
endovaginal ultrasonographic images in patients with a serum β-HCG
level greater than the discriminatory cut-off value is an ectopic
pregnancy until proved otherwise. Color-flow Doppler ultrasonography
improves the diagnostic sensitivity and specificity of transvaginal
ultrasonography, especially in cases in which a gestational sac is
questionable or absent.
3----Laparoscopy
Laparoscopy remains the criterion standard for diagnosis; however, its
routine use on all patients suspected of ectopic pregnancy may lead to
unnecessary risks, morbidity, and costs. Moreover, laparoscopy can miss
up to 4% of early ectopic pregnancies. Laparoscopy is indicated for
patients who are in pain or hemodynamically stable.
4---Culdocentesis
Aspiration of peritoneal fluid from posterior vaginal pouch
(haemoperitonial fluid )
Management :-
Therapeutic options in ectopic pregnancy are as
follows:
1- Expectant management
2- Medical management
3- Surgery

Expectant management::-Candidates for successful expectant
management should be
-asymptomatic and have no evidence of rupture or hemodynamic
instability.
-Low serum HCG < 1500 IU/L
-Candidates should demonstrate objective evidence of resolution (eg,
declining β-HCG levels).Close follow-up and patient compliance are of
paramount importance, as tubal rupture may occur despite low and
declining serum levels of β-HCG.
Medical management::--Local injection of PG , potassium chloride ,
hyperosmolar glucose or methotrexate .
Methotrexate is the standard medical treatment for unruptured ectopic
pregnancy. A single-dose IM injection is the more popular regimen. The
ideal candidate should have the following:
1- Hemodynamic stability
2-No severe or persisting abdominal pain
3-The ability to follow up multiple times
4-Normal baseline liver and renal function test results
5-Mass size < 5 cm .
6-Serum HCG < 3000 IU/L
7-NO evidence of cardiac activity .
Absolute contraindications to methotrexate therapy include
1- Existence of an intrauterine pregnancy
2- Immunodeficiency

3- Moderate to severe anemia, leukopenia, or thrombocytopenia
4- Sensitivity to methotrexate
5- Active pulmonary or peptic ulcer disease
6- Clinically important hepatic or renal dysfunction
7- Breastfeeding
8- Evidence of tubal rupture
Surgical treatment::--
Laparoscopy
has become the recommended
surgical approach in most cases.
Laparotomy
is usually reserved for patients who are
1--hemodynamically unstable
2--patients with cornual ectopic pregnancies;
3--preferred method for surgeons inexperienced in laparoscopy
4--patients in whom a laparoscopic approach is difficult such as morbid
obesity and adhesion .
Salpengectomy (best) or salpengestomy ( if contralateral tube is
unhealthy )
Fertility following ectopic pregnancy
Previous history of infertility has been found to be the most
significant factor affecting postsurgical fertility when the contralateral
fallopian tube is normal, the subsequent fertility rate is independent of
the type of surgery. Intrauterine pregnancy rate following ectopic
pregnancy between 50-70% .
Recurrent ectopic pregnancy occur in 6-16 % of women with
previous history of ectopic .
