Lecture 6: Complement system Dr Dhafer A Alghezi
1
Complement system:
The complement system, also known as complement cascade, is a part of the
immune system that enhances (complements) the ability of antibodies and phagocytic
cells to clear microbes and damaged cells from an organism, promote inflammation,
and attack the pathogen's cell membrane. It is a part of the innate immune system, the
complement system can, however, be recruited and brought into action by antibodies
generated by the adaptive immune system.
The complement system consists of approximately 30 serum molecules consisting
nearly 10% of the total serum proteins and forming one of the major defense systems
of the body. Most of the complement glycoproteins are synthesized predominantly by
the liver; but macrophages and many other cell types are also sources of various
complement components, especially at sites of infection and/or inflammation.
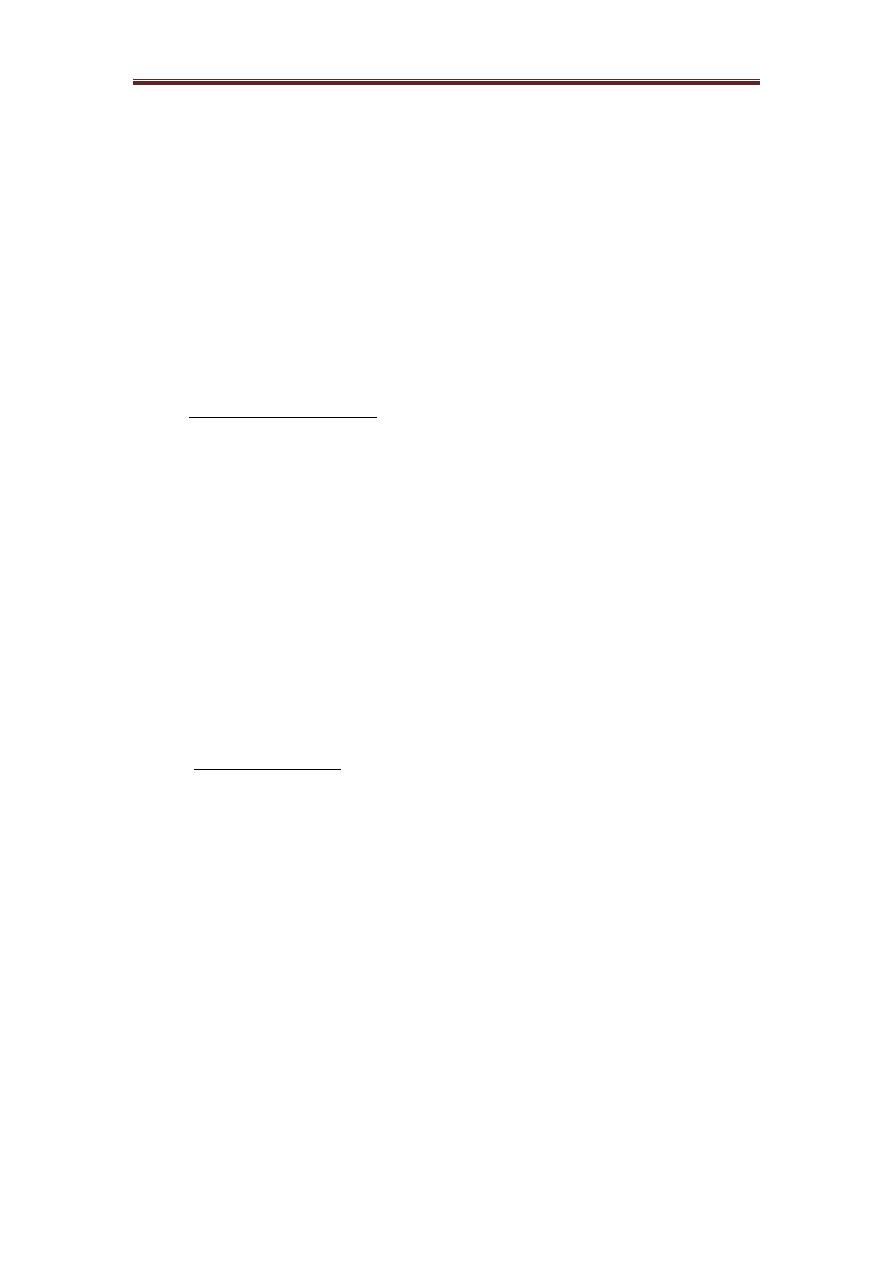
The complement system can be activated by classical, alternative and lectin pathways.
The proteins of the system act in enzyme cascades. Where each step generates
enzyme, which act in the following step of the cascade and all the three pathways
generate enzymes which cleave C3 into two fragments, C3a and C3b as a central step
in the process of complement activation.
1. The classical pathway:
Immunoglobulins and native complement components are normally found in the
serum and in the lymph, but these molecules do not interact with each other until the
antibodies interact with their corresponding antigens and undergo the necessary
secondary and tertiary conformational changes. The classical pathway is activated by
immune complexes and the activation is initiated by the binding of C1 to domains in
IgG (CH2-domain) or IgM (CH3-domain) which are complexes with Ag. Detailed
chemical studies have revealed that C1 is actually a complex of three different types
of molecules [C1q, dimerized C1r (C1r2), and dimerized C1s (C1s2)] held loosely
together through noncovalent bonds and requiring a physiological Ca2 concentration
for their proper association.
The recognition unit of the complement pathway is the C1q which is a molecule with
a six globular head group and when a specific Ab (IgG or IgM) interacts with its
corresponding Ag, binding sites for the globular head group of the C1q are exposed
on the Fc-region of the Ab molecule. At least two molecules of IgG or CRP or one
molecule of IgM are required for binding C1q. after binding to Fc-region of Ab
molecule, a conformational change occurs in C1q and this C1q change causes the pro-
enzyme C1r to become the enzymatically active C1r. the substrate for the enzyme C1r
is C1s, which is then cleaved to become the serine esterase (C1s).
C1q contains several distinct portions; there is a collagen-like stalk/stem region that
branches into a six-branched umbrella-shape. Within the umbrella portion of the stem,
an association with the C1r2 and C1s2 proenzymes occurs. Each of the six collagen-
like branches of C1q terminates in a globular head region.
The activation unit, the active enzyme of C1s cleaves two proteins, C4 (into C4a &
C4b) and C2 (into C2a & C2b), in a magnesium-dependent reaction. C4b and C2a
Lecture 6: Complement system Dr Dhafer A Alghezi
2
combine to form an active enzyme, C4b2a, which is the classical pathway, C3-
convertase that cleaves many molecules of C3 (into C3a & C3b).
The C3b is either form a covalent bond with an Ag or with bystander surfaces
(e.g. erythrocytes) in immune adherence or can bind to C4b2a to form C4b2a3b, a C5-
convertase, an enzyme cleaved C5 (into C5a & C5b). C5b binds to a molecule of C6
to form a stable bimolecular complex which bind to C7 to form a trimolecular
complex (C5b67), this trimolecular complex binds hydrophobically to a membrane
since the complex is amphiphilic, this allow it to insert into cell membranes. C8 now
joins the complex and unwinds into the cell membrane. Thus, forming a functional
trans-membrane channel and itself cause disruption and lysis of membranes, an effect
which is greatly enhanced by the incorporation of C9 and if more than six molecules
of C9 enter the complex, typically doughnut-shaped pores are formed. C9 is not
essential for the lytic event, but it does accelerate lysis.
2. The alternative pathway
The alternative pathway is activated near 'protected' surfaces such as bacterial or
fungal cell walls, bacterial lipopolysacharide, some virus-infected cells and rabbit
RBCs. It has been shown that the (activating surfaces) is actually a protective surface,
protecting spontaneously hydrolyzed C3 (non-enzymatically cleaved into C3a and
C3b) from being inactivated by the control proteins.
In presence of factor D and magnesium, C3b-like molecule can cleave factor
B (into Ba and Bb), Bb binds to the C3b to form an alternative pathway C3-
convertase C3bBb. The C3bBb is a very unstable and quickly inactivated by control
proteins, unless it's bound to activating surface and stabilized by P (properdin), the
C3bBbP enzymatic complex can cleave additional molecules of C3 and if a second
C3b is inserted into the C3-convertase, it becomes C3bBb3bP, this becomes a C5-
convertase that can cleave C5 into C5a and C5b. the membrane attack unite for the
alternative pathway begins with C5b and progresses through C6,7,8 and C9 in exactly
the same sequence as it does for the classical pathway.
3. The lectin pathway:
The newest discovered pathway for activating the second, fourth, and third
complement components is the lectin complement pathway, which involves a serum
MBL (mannan-binding lectin) and other serum lectins called ficolins. The activation
of the lectin pathway does not involve antigen-antibody interactions.
Lectin pathway is activated by bacterial carbohydrates, the molecule which
initiates the pathway mannan-binding lectin (MBL), the MBL is associated with two
proenzymes MASP-1 and MASP-2 MBL-associated serine proteases). When MBL
binds to terminal mannose group on bacterial carbohydrates it activates MASP-1&2
which go on to activate the classical pathway in an Ab-independent fashion. But it has
been shown that activated MASP-2 cleaves C4 and C2 while activated MASP-1
cleave C3 and C2.
Note: C1q itself is also able to bind directly to some microorganisms,
including mycoplasma and retroviruses, in an Ab-independent fashion).
Lecture 6: Complement system Dr Dhafer A Alghezi
3
Primary Function of the Complement System
The primary function of the complement system is to bind and neutralize any foreign
substance that activates it, and then to effectively cause those neutralized
complement-coated substances to tightly adhere to phagocytes, thereby enhancing
phagocytosis. In this regard, the third complement component (C3) is a major factor
due to its position in the classical, alternative, and lectin pathways and because of its
relatively high concentration in serum.
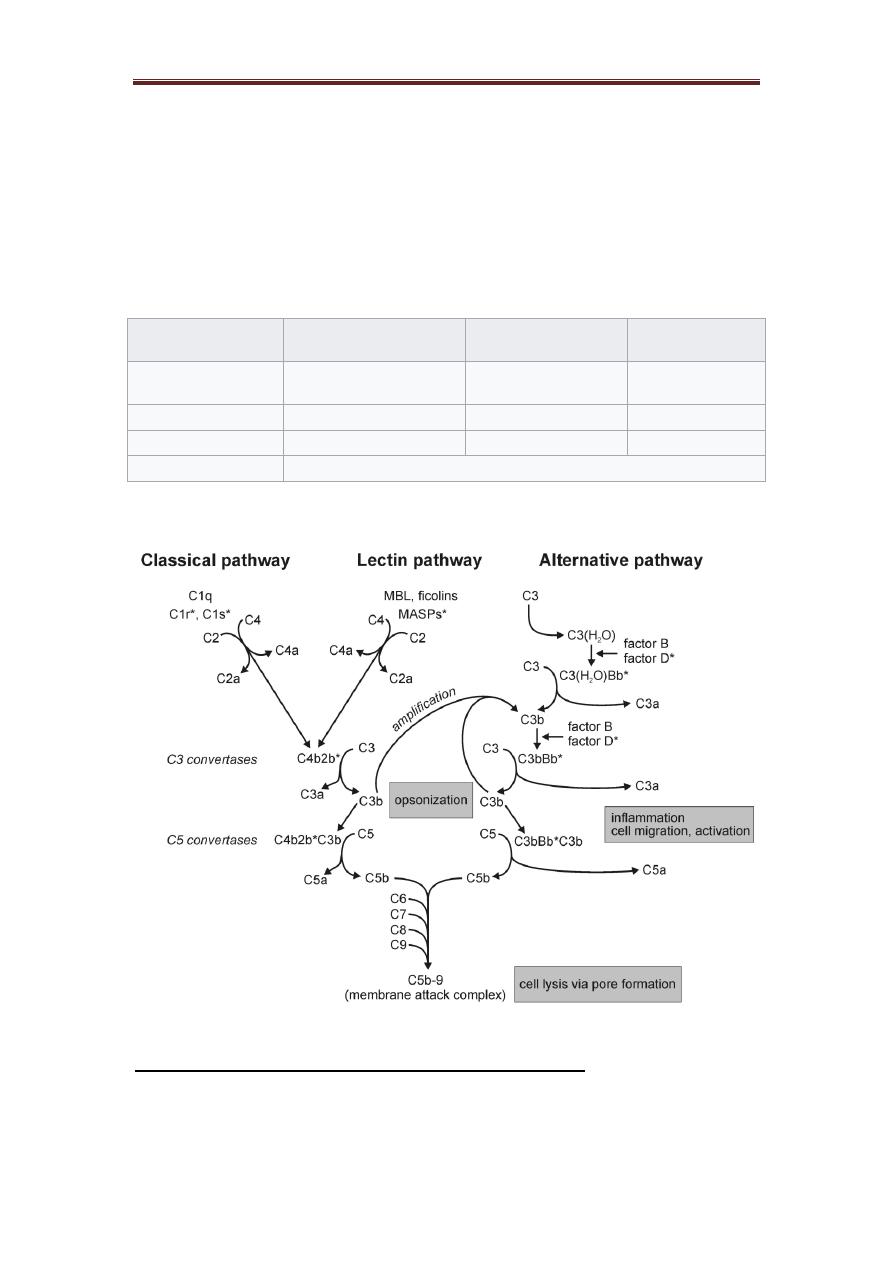
The biological consequences of complement activation are:
1. opsonization: the complement component coating the surface of a target such
as bacteria. Phagocytic cells carrying receptors for these complement components are
Activation
pathway
Classic
Alternative
Lectin
Activator
Ag–Ab Complex
spontaneous hydrolysis
of C3
MBL-Mannose
Complex
C3-convertase
C4b2a
C3bBb
C4b2a
C5-convertase
C4b2a3b
C3bC3bBb
C4b2a3b
MAC development
C5b+C6+C7+C8+C9

Lecture 6: Complement system Dr Dhafer A Alghezi
4
there able to bind to the bacteria, and once it become opsonized, more effective
phagocytosis occur.
2. Chemotaxis: C5a is a potent chemotactic factor and it induces the directed
migration of neutrophils and monocytes into the area of inflammation.
3. Anaphylaxis: C3a, C5a a biologically active peptides and these anaphylatoxin
mediate inflammation by inducing the release of mediators from basophiles and mast
cells, causing smooth muscle contraction and increase vascular permeability.
4. Immune adherence: it’s the covalent bonding between C3b and nearby
soluble immune complexes or particulate surfaces. Since C3b has receptors on human
erythrocyte, B-lymphocytes, monocytes, glomerular epithelial cells and mast cells.
One biologic purpose for immune adherence is to facilitate removal of soluble
immune complexes and immune adherence provides a mechanism for the soluble
complexes to bind to erythrocytes, facilitating removal of these complexes by the
reticuloendothelial system.
5. Kinin activation: The fragment of C2b interacts with plasmin to produce
kinin-like activity. The biologic activity of C2b results in smooth muscle contraction,
mucous gland secretion, increased vascular permeability, and pain.
6. Lysis of target cells: the final step in complement activation causes the
assembly of a membrane attack complex (MAC), which can insert itself into lipid
bilayers, such as the outer membrane of gram-negative bacteria or a viral envelope,
and then osmotic disruption of the target cell causing lysis.
Complement components are synthesized in the liver, with the exception of C1,
which is synthesized in the epithelial cells of the intestine. Limited quantities of
complement components, including C1q can be synthesized by activated macrophage-
monocyte.
Control of complement activation
The first mean of control is if activated complement (enzyme) does not combine
with its substance within milliseconds, the activity is lost.
Several proteins serve as inhibitors or inactivators of specific reactions or products
involved in the complement cascade like:
❖ C1-inhibitor (C1INH): form irreversible complex with both C1r and C1s
and block their enzyme activities and dissociate them from C1q.
❖ Factor H & I: serve to control tightly the enzyme that cleave C3 and C5
(factor I: Inactivates C3b and C4b. Whereas, factor H accelerates the decay
of alternative C3-convertase C3bBb3b by dissociating Bb from the
enzyme.).
❖ C4-binding protein (C4BP): bind to and inactivate C4.
❖ Anaphylatoxin inactivators (carboxypeptidase) effect C4a, C3a, C5a by
removal of a single amino acid (carboxyterminal arginine).
Lecture 6: Complement system Dr Dhafer A Alghezi
5
❖ MAC inhibitor: a serum protein can bind to fluid phase C5b67, prevent its
attachment to membrane protein or C8.
❖ Inhibition of assembly: like the (protectin, CD59) and (delay accelerating
factors, DAF).
Clinical diseases associated with complement activity:
1. C1INH-deficiency or angioneurotic edema:
This is a rare genetic disorder due to a genetically inherited C1-INH deficiency, of
which two main variants are known. Deficiency of C1INH leads to uncontrolled
activation of the classical PW. In the most common, the genetic inheritance of a silent
gene results in a significantly lower level of C1-INH. The second variant is
characterized by normal levels of C1-INH protein, but 75% of the molecules are
dysfunctional, that is, will not inhibit activated C1r2 or C1s2 because of an aberrant
amino acid substitution.
Thus, C1s continues to cleavage C4 and C2 resulting in C2a release leading to
kinin like activity and C4a release leading to anaphylactic reaction Activity. Both
producing swelling (extra-ordinary amount of fluid in tissue spaces as a result of
increase
vascular
Permeability
and
smooth
muscle
contraction).
No
immunodeficiency, and decreased C2 & C4, with Normal C3 (downstream C4BP
works). The hereditary form called hereditary angioedema (HAE), its autosomal
dominant trait. Whereas, acquired from associated with lympho-proliferative diseases.
2. Paroxysmal nocturnal hemoglobinuria (PNH)
It is a clonal disorder of hemopoietic cells, in which there is increased
susceptibility to damage by complement due to deficiency of glycosyl phosphatidyl
inositol (GPI) which binds delay accelerating factors (DAF or CD 55) and protectin
(CD59) to membrane. The clinical feature of this illness is the episodic
hemoglobinuria mainly at morning, hemolytic anemia, increased susceptibility to
infection due to decreased number or function of WBC.
3. Nephritic factors (NF)
❖ It is a pathological enhancing protein.
❖ It is IgG with specific to alternative PW C3-convertase.
❖ It prevents inactivation of C3 - convertase by H and I control proteins.
❖ Presence of this factor leads to C3 deficiency and recurrent infection, in
addition to lipodystrophy and emaciation.