
Lecture 5: Ag recognition molecules Dr Dhafer A. Alghezi
1
Antigen recognition molecules:
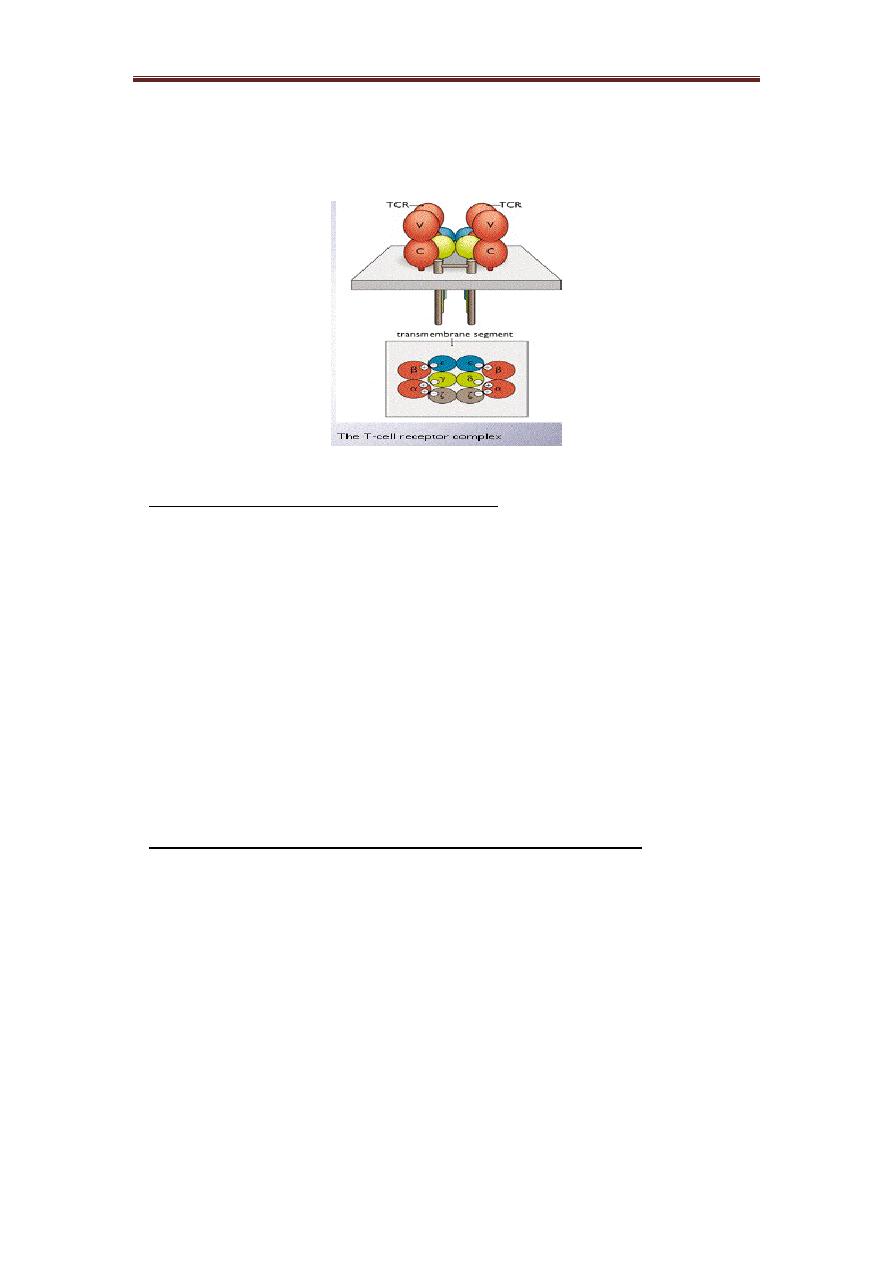
2) T-cells receptor (TCR): It represents another antigen recognition molecule. TCR is
a molecule found on the surface of T cells, or T lymphocytes that are responsible for
recognizing fragments of the antigen as peptides bound to major histocompatibility
complex (MHC) molecules.
Structure of TCR:
TCR is a heterodimeric molecule comprising an alpha (α) chain and a beta (β)
chain or gamma and delta chains linked by a disulphide bond. In humans, the great
majority of cells generate an alpha (α) chain and a beta (β) chain TCR, whereas in 5%
of T cells the TCR consists of gamma and delta (γ/δ) chains. Each polypeptide chain
consists of two extracellular Ig-like domains anchored in the plasma membrane by a
transmembrane peptide which has a short cytoplasmic tail.
The overall feature of the structure shows that the α and β chains fold into a
structure that resembles the Fab-region of Ab. The N-terminal domains of the α and β
polypeptides are known as CDRs or hypervariable region. These are clustered together
to form an MHC-Ag-binding site which is analogous to the Ag-binding site on Ab.
TCR is associated physically with a series of polypeptides, called CD3. The
CD3 component shows no diversity. CD3 contains four invariant polypeptides, called
gamma, delta, epsilon, and sigma. The structure of the CD3-TCR complex is (alpha,
beta) 2, gamma, delta, (epsilon) 2, (sigma) 2. The negatively charged CD3-chains are
essential for the assembly of the TCR complex with positively charged chains. The
CD3 sigma gene is on a different chromosome from the CD3 alpha, delta, and epsilon-
gene complex.
The intracytoplasmic section of sigma chain is called immunoreceptor tyrosine
activation motifs (ITAM a) target for phosphorylation by specific protein kinases and
it's essential for T-cell activation. ITAM is also present in Ig-alpha and beta (CD79)
which are involved in B-cell activation and with CD16 which involved in the activation
of macrophage and NK-cells.
The α, βTCR is present on more than 95% of the peripheral T-cells and on the
majority of TCR-expressing thymocytes. By contrast, T-cells expressing the gamma
delta-TCR often have defined anatomical locations, they are abundant in various
epithelia, such as the epidermis (in mice) intestinal epithelium, uterus and tongue. They
Lecture 5: Ag recognition molecules Dr Dhafer A. Alghezi
2
produce a lower level of some cytokines than alpha-beta T-cells; they may be involved
in the control and modulation of responses mediated by alpha-beta T-cells.
B & T cell receptors have several similarities:
❖ Even though the TCR is a heterodimer, the TCR chains has constant and
variable regions
❖ TCR germ line DNA for each of the chains is organized into multigene families
with multiple V region segments (with rearrangement of V, J – for α& γ chains
and V, D, J for β & δ chains) with one or more C region gene segments.
❖ The mechanisms for T cell V region gene segment rearrangement are similar to
B cells rearranging Ig germline DNA
❖ The mechanisms generating TCR diversity are similar to those seen in
generating Ab diversity.
❖ It's structurally related since both are folded into domains.
T cell receptor differs from the B cell receptor in different ways:
❖ The T cell does not secrete its receptor like the B cell does so any assessment
of receptor structure and specificity has to rely on complex cellular assays.
❖ TCR has one antigen binding site, whereas, Igs have at least two.
❖ T cell can recognize and bind antigens just when are presented by MHC,
whereas, Ig can recognize free antigens.
❖ TCR can be activated by protein antigens (not CHO antigens). In contrast, Ig
can recognize intact molecules proteins, carbohydrates, and lipids.
Lecture 5: Ag recognition molecules Dr Dhafer A. Alghezi
3
Genes of TCR:
The alpha and gamma loci have sets of V and J-genes (like an Ig - light chain) and beta
and delta loci have set of V, D, and J-genes (like Ig-heavy chain). Diversification of the
TCR gene occurs by recombination between V, D, and J segments. Extensive diversity
is generated in the joining process, as not only are VDJ-arrangement possible, but also
V-J and VDDJ-joins.
Major histocompatibility complex molecules (MHC) or Human Leukocyte
Antigens (HAL)
The human MHC, which is known as the human leukocyte Antigen (HLA) -system
is a cluster of genes that are located on a short arm of the chromosome 6. The molecules
presented Ag to T-cells are mostly encoded within the MHC. This gene complex
containing more than 100 separate gene loci. MHC I&II are highly polymorphic cell-
surface structures. The remaining genes in the complex are very diverse. They including
some that encode complement system molecules (C4, C2, and factor-B), some
cytokines (TNF), enzyme, heat-shock proteins and some molecules involved in Ag
processing, they have collectively been called MHC III gene products.
A) MHC I
The HLA or H2 (MHC of mice) molecules are heterodimers formed by two
nonidentical polypeptide chains: an α chain and β chain. In MHC I, there are HLA-A,
HLA-B and HLA-c loci.
The class I heavy chain consists of three extracellular domains, designated alpha1,
2&3, a transmembrane region and a cytoplasmic tail. The alpha 2&3 domains have
intrachain disulphide bonds. The alpha 3 domain is structurally homologous to Ig-
constant domains, and contains a site which interacts with CD8 on cytotoxic T-cells.
β2-microglobulin is post synthetically and noncovalently associated with the major
polypeptide chain.
The most polymorphic areas of the molecule are located within and on the edges of a
groove formed at the junction of the helical α1 and β2 domains. This groove is usually
occupied by a short peptide (10–11 residues), usually of endogenous origin. The α3
domain shows much less genetic polymorphism and, together with β2-microglobulin,
is like a frame supporting the deployment in space of the more polymorphic α1 and α2
Lecture 5: Ag recognition molecules Dr Dhafer A. Alghezi
4
domains. In addition, the α3 domain has a binding site for the CD8 molecule
characteristic of cytotoxic T cells.
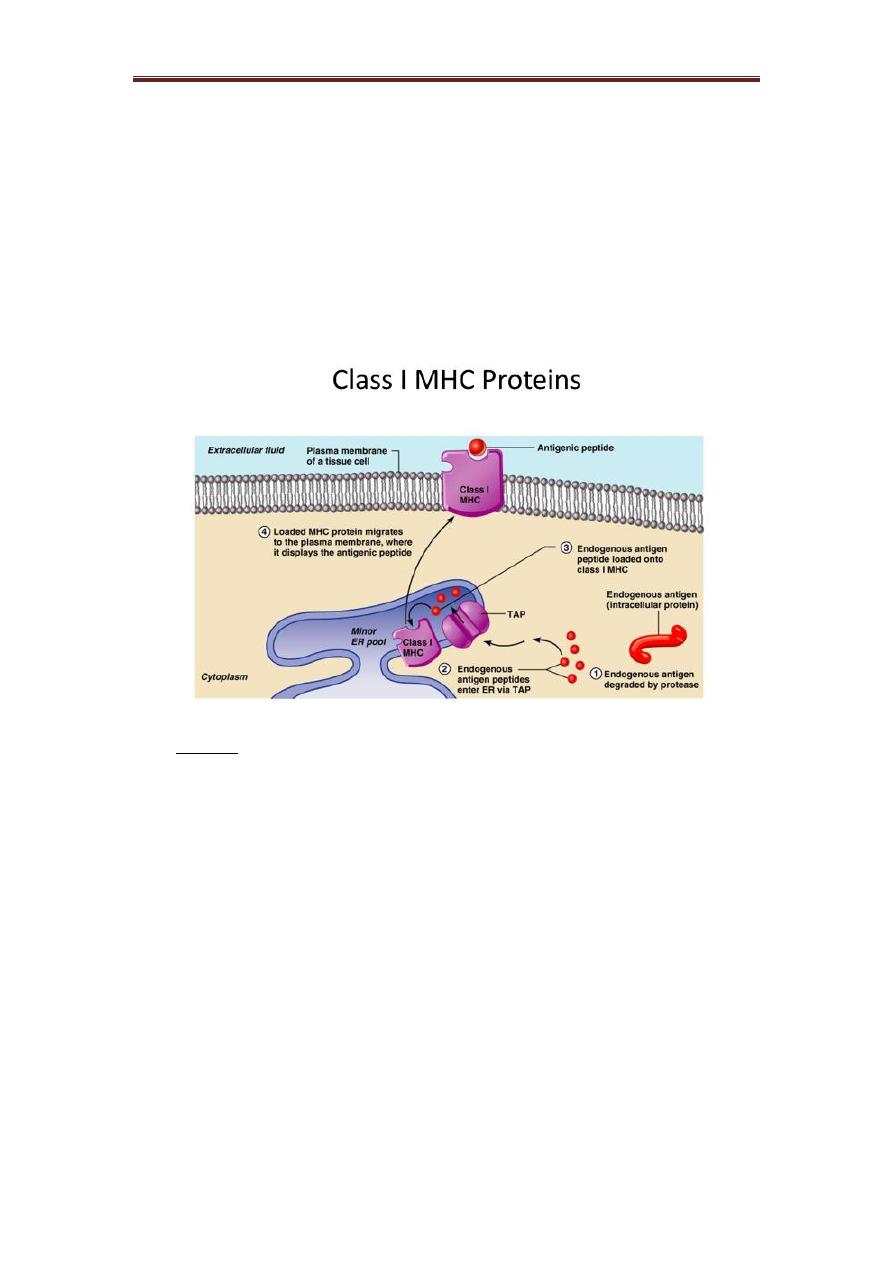
Class I protein:
It is a glycoprotein found on the surface of all nucleated cells. The peptides which
bind to MHC I molecules come from proteins synthesized within the cell, which are
broken down and transported via TAP (transporter associated with antigen processing)
to the endoplasmic reticulum, thus peptide of an appropriate size to occupy the MHC
I-binding groove.
B) MHC II
The Class II genes in human are DP, DQ & DR. MHC II is a heterodimer of heavy α
and light (β) glycoprotein chains. The α and β chains have the same overall structures.
An extracellular portion comprising two domains (α1&2 or β1&2) is connected by a
short sequence to a transmembrane region and the cytoplasmic domain.
There are many differences between the primary structure of both MHC I& II. First,
class II gene products are not associated with β2-microglobulin. Each polypeptide chain
has two extracellular domains (α1 and α2; β1 and β2). The NH2 terminal ends of the
α1 and β1 domains contain hypervariable regions. Both chains are encoded by genes in
the MHC region on chromosome 6. The class II groove is more open than that of class
I, so that longer peptides can be accommodated. As the class II groove is not closed at
the ends, peptides bound to MHC II molecule extend out of the ends of the groove. The
B2-domain does contain a binding site for CD4. Thus CD4 and CD8 are important

Lecture 5: Ag recognition molecules Dr Dhafer A. Alghezi
5
eliminates in Ag presentation, because they are involved in the recruitment of kinases,
which signal T-cell activation.
Class II protein:
It’s a glycoprotein found in certain cells such as macrophage, B cells, dendritic cells
of spleen and Langerhans cells of skin. It’s an exogenous protein that comes from
outside the cells. Peptides bind to MHC II come from proteins which have been
internalized by the cell and then degraded. These peptides are less uniform in size and
may be trimmed once they have bound to the MHCII-molecule.
Invariant Chain (Ii, CD74)
The invariant chain (Ii) is a polypeptide involved in the formation and transport of
MHC class II protein. The cell surface form of the invariant chain is known as CD74.
In the endoplasmic reticulum (ER), a region of Ii interacts with the binding groove to
prevent binding of endogenous peptides. This region acts as “chaperone” to allow the
MHC class II molecule + Ii to leave the ER. The Ii is cleaved by cathepsin S (cathepsin
L in cortical thymic epithelial cells), leaving only a small fragment called CLIP
remaining bound to the groove of MHC class II molecules. The rest of the Ii is degraded.
CLIP blocks peptide binding until HLA-DM interacts with MHC II, releasing CLIP and
allowing other peptides to bind. The stable MHC class-II with antigen complex is then
presented on the cell surface. Without CLIP, MHC class II aggregates, disassemble,
and/or denature in the endosomes, and proper antigen presentation is impaired.

Lecture 5: Ag recognition molecules Dr Dhafer A. Alghezi
6
Interaction of the TCR with MHC and antigen:
The first and second complementarity determining regions (CDRs) of the TCR
α and β chains are positioned over residues near the N and C-terminal of the presented
polypeptide, and the third CDR, each chain lying at the centre of the TCR-binding site,
are positioned over the central residues of the peptide which protrude from the groove.
Residues from each of the CDR are positioned to interact with residues from the MHC
molecule.
Each T-cell may express 100000 receptors and each APCs has a similar number of
MHC molecules. CD4 or CD8 can help stabilize the interaction of the TCR and MHC-
peptide.
The affinity of Ag-MHC for the TCR is very important because it determines the
degree to which that the T-cell becomes activated. Each lymphocyte binds to just one
Ag using its TCR.
The biological importance of MHC
❖ Antigen recognition by T cells
CD8 T cells…………… MHC I molecules
CD4 T cells……………...MHC II molecules
❖ It’s also important in autoimmune diseases which occur in people who carry
MHC genes such as HLA- B27 in Ankylosing spondylitis.
❖ Success of organ transplantation is determined by the compatibility of MHC
genes of donor and recipient.
