Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
512
CHAPTER 17 Disorders in Immunity
Rh sensitization because the antibodies to these blood group anti-
gens are IgM rather than IgG and are unable to cross the placenta in
large numbers. In fact, the maternal-fetal relationship is a fascinat-
ing instance of foreign tissue not being rejected, despite the exten-
sive potential for contact (Medical Microfile 17.2).
Preventing Hemolytic Disease of the Newborn
Once sensitization of the mother to Rh factor has occurred, all other
Rh
"
fetuses will be at risk for hemolytic disease of the newborn.
Prevention requires a careful family history of an Rh
#
pregnant
woman. It can predict the likelihood that she is already sensitized or
is carrying an Rh
"
fetus. It must take into account other children
she has had, their Rh types, and the Rh status of the father. If the fa-
ther is also Rh
#
, the child will be Rh
#
and free of risk, but if the fa-
ther is Rh
"
, the probability that the child will be Rh
"
is 50% or
100%, depending on the exact genetic makeup of the father. If there
is any possibility that the fetus is Rh
"
, the mother must be pas-
sively immunized with antiserum containing antibodies against the
Rh factor (Rh
o
[D] immune globulin, or RhoGAM*). This anti-
serum, injected at 28 to 32 weeks and again immediately after de-
livery, reacts with any fetal RBCs that have escaped into the mater-
nal circulation, thereby preventing the sensitization of the mother’s
immune system to Rh factor (figure 17.12b). Anti-Rh antibody
must be given with each pregnancy that involves an Rh
"
fetus. It is
ineffective if the mother has already been sensitized by a prior Rh
"
fetus or an incorrect blood transfusion, which can be determined by
a serological test. As in ABO blood types, the Rh factor should be
matched for a transfusion, although it is acceptable to transfuse
Rh
#
blood if the Rh type is not known.
OTHER RBC ANTIGENS
Although the ABO and Rh systems are of greatest medical signifi-
cance, about 20 other red blood cell antigen groups have been
discovered. Examples are the MN, Ss, Kell, and P blood groups.
Because of incompatibilities that these blood groups present, trans-
fused blood is screened to prevent possible cross-reactions. The
study of these blood antigens (as well as ABO and Rh) has given
rise to other useful applications. For example, they can be useful in
forensic medicine (crime detection), studying ethnic ancestry, and
tracing prehistoric migrations in anthropology. Many blood cell
antigens are remarkably hardy and can be detected in dried blood
stains, semen, and saliva. Even the 2,000-year-old mummy of King
Tutankhamen has been typed A
2
MN!
MEDICAL MICROFILE
17.2
Why Doesn’t a Mother Reject Her Fetus?
what ways do fetuses avoid the surveillance of the mother’s immune sys-
tem? The answer appears to lie in the placenta and embryonic tissues.
The fetal components that contribute to these tissues are not strongly
antigenic, and they form a barrier that keeps the fetus isolated in its own
antigen-free environment. The placenta is surrounded by a dense, many-
layered envelope that prevents the passage of maternal cells, and it ac-
tively absorbs, removes, and inactivates circulating antigens.
Think of it: Even though mother and child are genetically related, the fa-
ther’s genetic contribution guarantees that the fetus will contain mole-
cules that are antigenic to the mother. In fact, with the recent practice of
implanting one woman with the fertilized egg of another woman, the sur-
rogate mother is carrying a fetus that has no genetic relationship to her.
Yet, even with this essentially foreign body inside the mother, dangerous
immunologic reactions such as Rh incompatibility are rather rare. In
*
RhoGAM
Immunoglobulin fraction of human anti-Rh serum, prepared from pooled
human sera.
Type II hypersensitivity reactions occur when preformed antibodies
react with foreign cell-bound antigens. The most common type II
reactions occur when transfused blood is mismatched to the recipient’s
ABO type. IgG or IgM antibodies attach to the foreign cells, resulting
in complement fixation. The resultant formation of membrane attack
complexes lyses the donor cells.
Type II hypersensitivities are stimulated by antibodies formed against red
blood cell (RBC) antigens or against other cell-bound antigens
following prior exposure.
Complement, IgG, and IgM antibodies are the primary mediators of
type II hypersensitivities.
The concepts of universal donor (type O) and universal recipient
(type AB) apply only under emergency circumstances. Cross-matching
donor and recipient blood is necessary to determine which
transfusions are safe to perform.
Type II hypersensitivities can also occur when Rh
#
mothers are sensitized
to Rh
"
RBCs of their unborn babies and the mother’s anti-Rh antibodies
cross the placenta, causing hemolysis of the newborn’s RBCs. This is
called hemolytic disease of the newborn, or erythroblastosis fetalis.
CHAPTER CHECKPOINTS
Type III Hypersensitivities: Immune
Complex Reactions
Type III hypersensitivity involves the reaction of soluble antigen
with antibody and the deposition of the resulting complexes in base-
ment membranes of epithelial tissue. It is similar to type II, because
it involves the production of IgG and IgM antibodies after repeated
exposure to antigens and the activation of complement. Type III
differs from type II because its antigens are not attached to the sur-
face of a cell. The interaction of these antigens with antibodies
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
Type III Hypersensitivities: Immune Complex Reactions
513
produces free-floating complexes that can be deposited in the tis-
sues, causing an
immune complex reaction or disease. This cate-
gory includes therapy-related disorders (serum sickness and the
Arthus reaction) and a number of autoimmune diseases (such as
glomerulonephritis and lupus erythematosus).
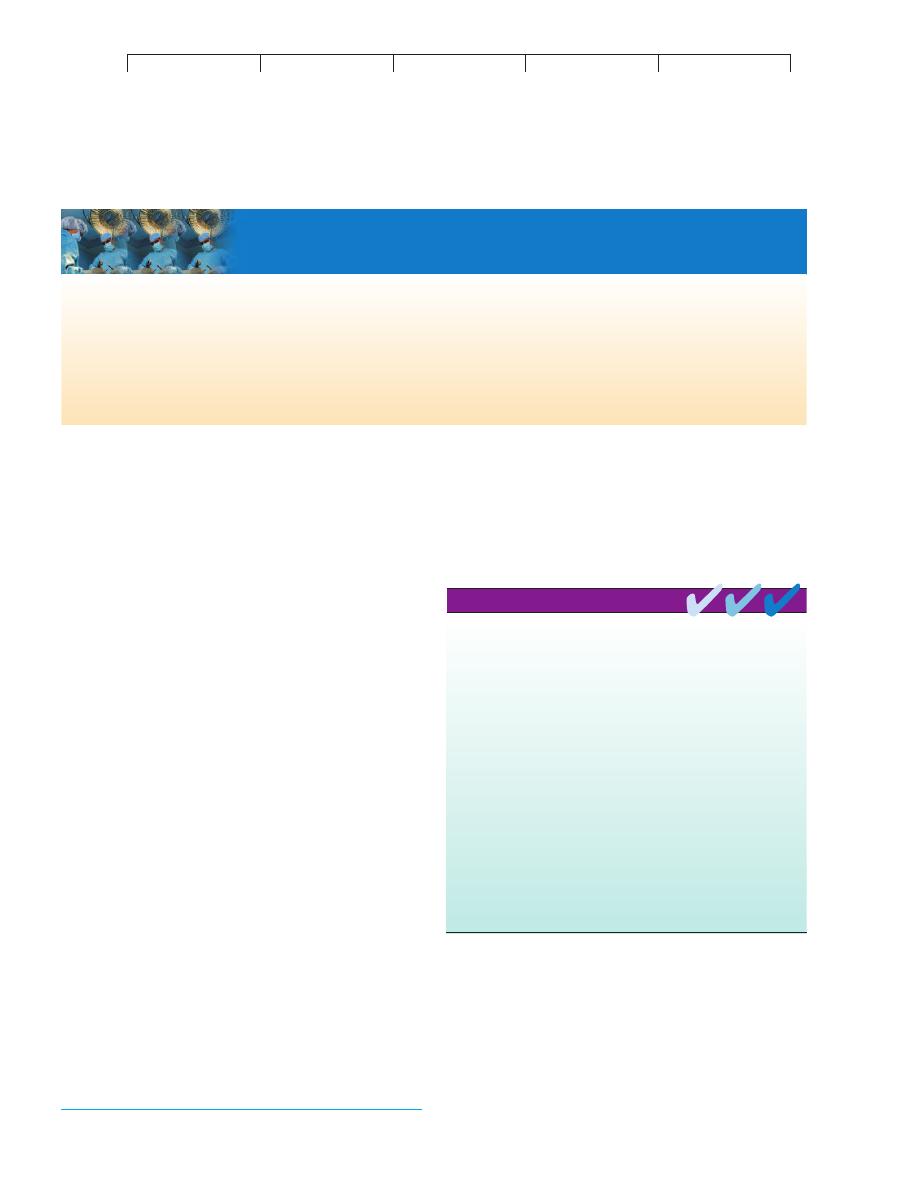
MECHANISMS OF IMMUNE COMPLEX DISEASE
After initial exposure to a profuse amount of antigen, the immune
system produces large quantities of antibodies that circulate in the
fluid compartments. When this antigen enters the system a second
time, it reacts with the antibodies to form antigen-antibody com-
plexes (figure 17.13). These complexes summon various inflamma-
tory components such as complement and neutrophils, which
would ordinarily eliminate Ag-Ab complexes as part of the normal
immune response. In an immune complex disease, however, these
complexes are so abundant that they deposit in the basement mem-
branes* of epithelial tissues and become inaccessible. In response
to these events, neutrophils release lysosomal granules that digest
tissues and cause a destructive inflammatory condition. The symp-
toms of type III hypersensitivities are due in great measure to this
pathologic state.
TYPES OF IMMUNE COMPLEX DISEASE
During the early tests of immunotherapy using animals, hyper-
sensitivity reactions to serum and vaccines were common. In ad-
dition to anaphylaxis, two syndromes, the
Arthus reaction
3
and
serum sickness, were identified. These syndromes are associated
with certain types of passive immunization (especially with ani-
mal serum).
Serum sickness and the Arthus reaction are like anaphylaxis
in requiring sensitization and preformed antibodies. Characteristics
that set them apart are: (1) They depend upon IgG, IgM, or IgA
(precipitating antibodies) rather than IgE; (2) they require large
doses of antigen (not a miniscule dose as in anaphylaxis); and
(3) they have delayed symptoms (a few hours to days). The Arthus
reaction and serum sickness differ from each other in some impor-
tant ways. The Arthus reaction is a localized dermal injury due to
inflamed blood vessels in the vicinity of any injected antigen.
Serum sickness is a systemic injury initiated by antigen-antibody
complexes that circulate in the blood and settle into membranes at
various sites.
The Arthus Reaction
The Arthus reaction is usually an acute response to a second injec-
tion of vaccines (boosters) or drugs at the same site as the first in-
jection. In a few hours, the area becomes red, hot to the touch,
swollen, and very painful. These symptoms are mainly due to the
destruction of tissues in and around the blood vessels and the re-
lease of histamine from mast cells and basophils. Although the re-
action is usually self-limiting and rapidly cleared, intravascular
blood clotting can occasionally cause necrosis and loss of tissue.
Serum Sickness
Serum sickness was named for a condition that appeared in soldiers
after repeated injections of horse serum to treat tetanus. It can also
be caused by injections of animal hormones and drugs. The im-
mune complexes enter the circulation, are carried throughout the
body, and are eventually deposited in blood vessels of the kidney,
heart, skin, and joints (figure 17.13). The condition can become
chronic, causing symptoms such as enlarged lymph nodes, rashes,
painful joints, swelling, fever, and renal dysfunction.
AN INAPPROPRIATE RESPONSE
AGAINST SELF, OR AUTOIMMUNITY
The immune diseases we have covered so far are all caused by
foreign antigens. In the case of
autoimmunity, an individual actu-
ally develops hypersensitivity to himself. This pathologic process
accounts for
autoimmune diseases, in which autoantibodies and,
in certain cases, T cells mount an abnormal attack against self anti-
gens. The scope of autoimmune diseases is extremely varied. In
Ab
Ag
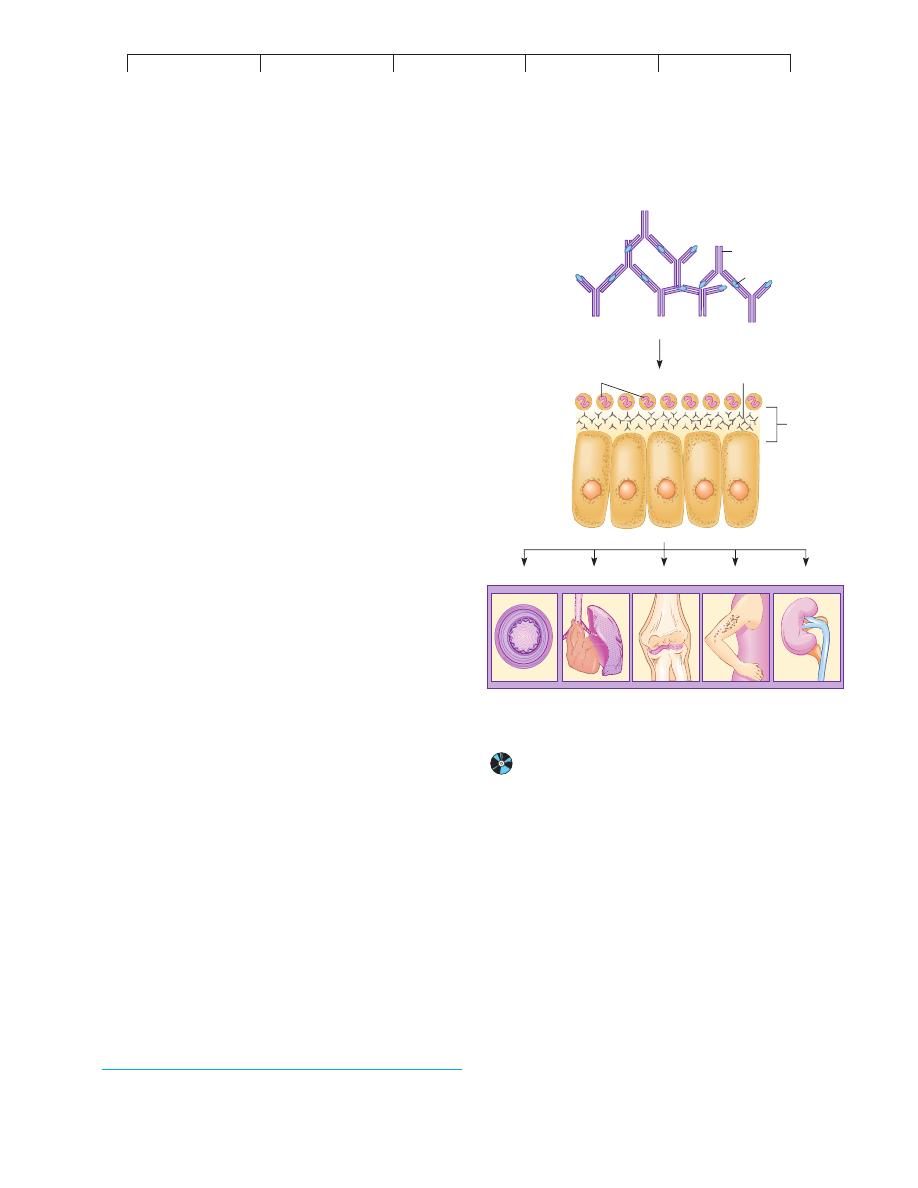
Immune complexes
Lodging of complexes
in basement membrane
Ag/Ab complexes
Neutrophils
Basement
membrane
Epithelial
tissue
Blood vessels Heart/Lungs
Joints
Skin
Kidney
Major organs that can be targets
of immune complex deposition
FIGURE 17.13
The background of immune complex disease.
In general,
circulating immune complexes become lodged in the basement
membrane of the epithelia and cause vascular damage and organ
malfunction.
3. Named after Maurice Arthus, the physiologist who first identified this localized
inflammatory response.
*
Basement membranes
are basal partitions of epithelia that normally filter out
circulating antigen-antibody complexes.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
514
CHAPTER 17 Disorders in Immunity
general, they can be differentiated as systemic, involving several
major organs, or organ-specific, involving only one organ or tissue.
They usually fall into the categories of type II or type III hypersen-
sitivity, depending upon how the autoantibodies bring about injury.
Some major autoimmune diseases, their targets, and basic pathol-
ogy are presented in table 17.4.
Genetic and Gender Correlation
in Autoimmune Disease
In most cases, the precipitating cause of autoimmune disease re-
mains obscure, but we do know that susceptibility is determined by
genetics and influenced by gender. Cases cluster in families, and
even unaffected members tend to develop the autoantibodies for
that disease. More direct evidence comes from studies of the major
histocompatibility gene complex. Particular genes in the class I and
II major histocompatibility complex (see figure 15.3) coincide with
certain autoimmune diseases. For example, autoimmune joint dis-
eases such as rheumatoid arthritis and ankylosing spondylitis are
more common in persons with the B-27 HLA type; systemic lupus
erythematosus, Graves disease, and myasthenia gravis are associ-
ated with the B-8 HLA antigen. Why autoimmune diseases (except
ankylosing spondylitis) afflict more females than males also re-
mains a mystery. Females are more susceptible during childbearing
years than before puberty or after menopause, suggesting a possible
hormonal relationship.
The Origins of Autoimmune Disease
Very low titers of autoantibodies in otherwise healthy individuals
suggest some normal function for them. A moderate, regulated
amount of autoimmunity is probably required to dispose of old
cells and cellular debris. Disease apparently arises when this regu-
latory or recognition apparatus goes awry. Attempts to explain the
origin of autoimmunity include the following theories.
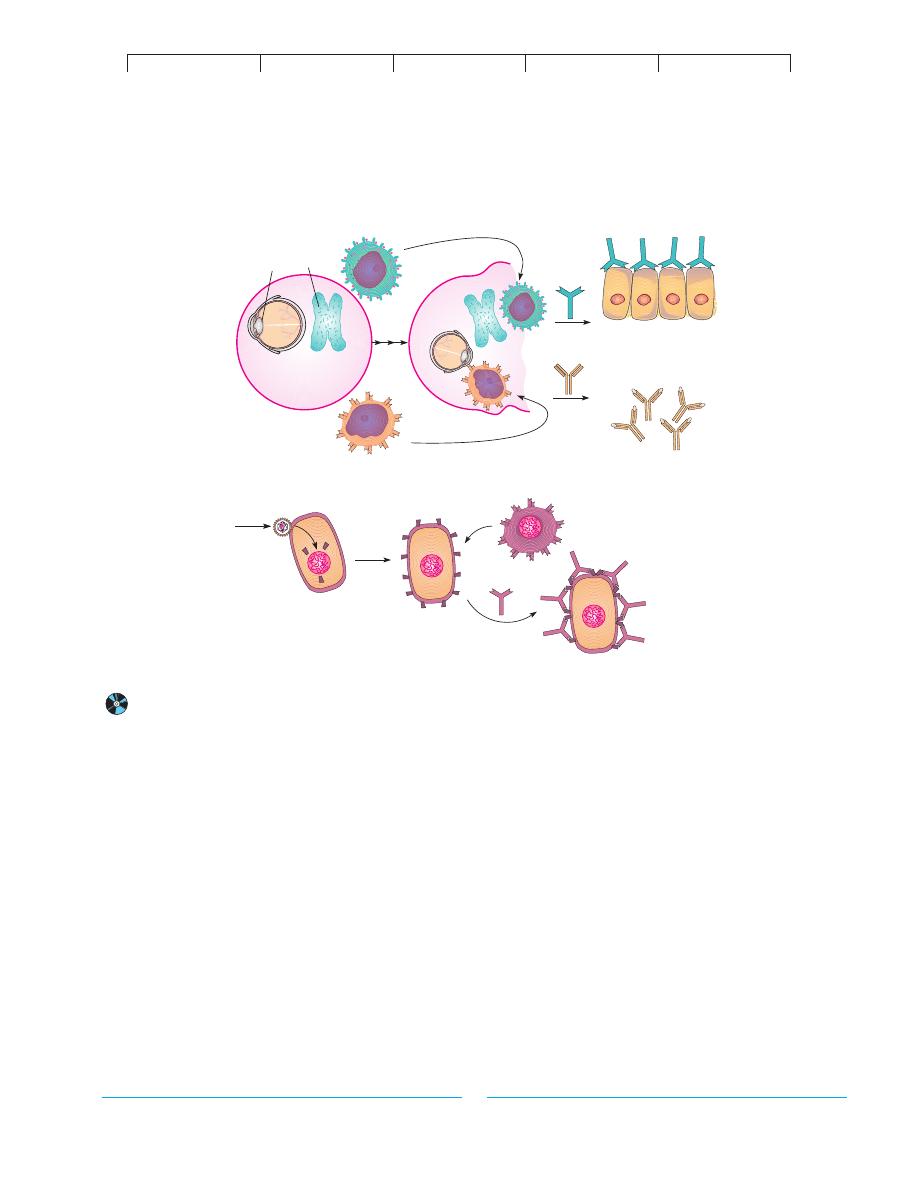
The sequestered antigen theory explains that during embry-
onic growth, some tissues are immunologically privileged; that is,
they are sequestered behind anatomical barriers and cannot be
scanned by the immune system (figure 17.14a). Examples of
these sites are regions of the central nervous system, which are
shielded by the meninges and blood-brain barrier; the lens of the
eye, which is enclosed by a thick sheath; and antigens in the thy-
roid and testes, which are sequestered behind an epithelial barrier.
Eventually the antigen becomes exposed by means of infection,
trauma, or deterioration, and is perceived by the immune system
as a foreign substance.
According to the clonal selection theory, the immune system
of a fetus develops tolerance by eradicating all self-reacting lym-
phocyte clones, called forbidden clones, while retaining only those
clones that react to foreign antigens. Some of these clones may sur-
vive, and since they have not been subjected to this tolerance
process, they can attack tissues with self antigens.
The theory of immune deficiency proposes that mutations in
the receptor genes of some lymphocytes render them reactive to
self or that a general breakdown in the normal T-suppressor func-
tion sets the scene for inappropriate immune responses.
Some autoimmune diseases appear to be caused by molecu-
lar mimicry, in which microbial antigens bear molecular determi-
nants similar to normal human cells. An infection could cause for-
mation of antibodies that can cross-react with tissues. This is one
purported explanation for the pathology of rheumatic fever. Au-
toimmune disorders such as type I diabetes and multiple sclerosis
TABLE 17.4
Selected Autoimmune Diseases
Type of
Disease
Target
Hypersensitivity
Characteristics
Systemic lupus erythematosus
Systemic
III
Inflammation of many organs; antibodies against red and
(SLE)
white blood cells, platelets, clotting factors, nucleus
Rheumatoid arthritis and
Systemic
III
Vasculitis; frequent target is joint lining; antibodies against
ankylosing spondylitis
other antibodies (rheumatoid factor)
Scleroderma
Systemic
II
Excess collagen deposition in organs; antibodies formed
against many intracellular organelles
Hashimoto’s thyroiditis
Thyroid
II
Destruction of the thyroid follicles
Graves disease
Thyroid
II
Antibodies against thyroid-stimulating hormone receptors
Pernicious anemia
Stomach lining
II
Antibodies against receptors prevent transport of vitamin B
12
Myasthenia gravis
Muscle
II
Antibodies against the acetylcholine receptors on the
nerve-muscle junction alter function
Type I diabetes
Pancreas
II
Antibodies stimulate destruction of insulin-secreting cells
Type II diabetes
Insulin receptor
II
Antibodies block attachment of insulin
Multiple sclerosis
Myelin
II
T cells and antibodies sensitized to myelin sheath destroy
neurons
Goodpasture syndrome
Kidney
II
Antibodies to basement membrane of the glomerulus damage
(glomerulonephritis)
kidneys
Rheumatic fever
Heart
II
Antibodies to group A Streptococcus cross-react with heart
tissue
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
Type III Hypersensitivities: Immune Complex Reactions
515
are likely triggered by viral infection. Viruses can noticeably alter
cell receptors, thereby causing immune cells to attack the tissues
bearing viral receptors (figure 17.14b).
Examples of Autoimmune Disease
Systemic Autoimmunities
One of the severest chronic autoim-
mune diseases is
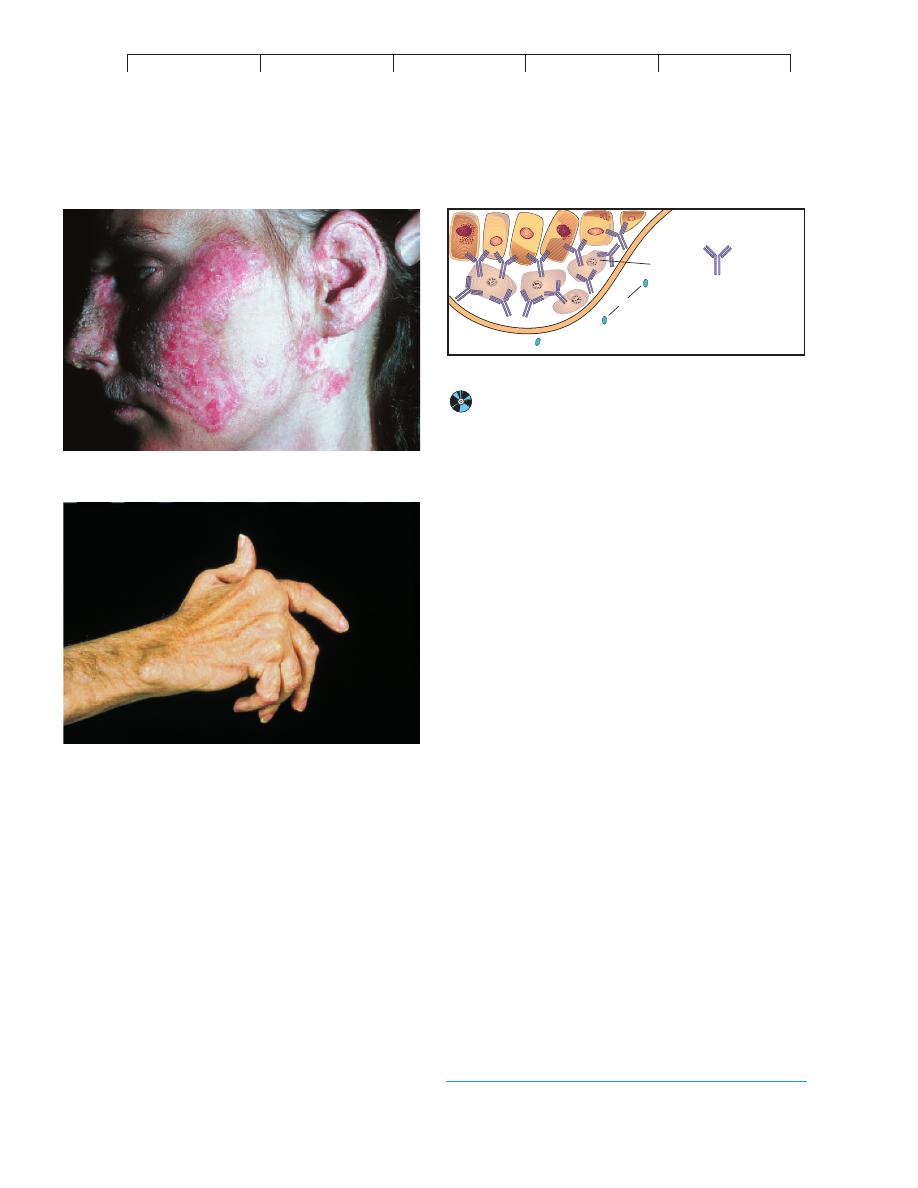
systemic lupus erythematosus* (SLE, or lupus).
This name originated from the characteristic rash that spreads
across the nose and cheeks in a pattern suggesting the appearance
of a wolf (figure 17.15a). Although the manifestations of the dis-
ease vary considerably, all patients produce autoantibodies against
a great variety of organs and tissues. The organs most involved are
the kidneys, bone marrow, skin, nervous system, joints, muscles,
heart, and GI tract. Antibodies to intracellular materials such as the
nucleoprotein of the nucleus and mitochondria are also common.
In SLE, autoantibody-autoantigen complexes appear to be
deposited in the basement membranes of various organs. Kidney
failure, blood abnormalities, lung inflammation, myocarditis, and
skin lesions are the predominant symptoms. One form of chronic
lupus (called discoid) is influenced by exposure to the sun and pri-
marily afflicts the skin. The etiology of lupus is still a puzzle. It is
not known how such a generalized loss of self-tolerance arises,
though viral infection or loss of T-cell suppressor function are sus-
pected. The fact that women of childbearing years account for 90%
of cases indicates that hormones may be involved. The diagnosis of
SLE can usually be made with blood tests. Antibodies against the
nucleus (ANA) and various tissues (detected by indirect fluorescent
antibody or radioimmune assay techniques) are common, and a
positive test for the lupus factor (an antinuclear factor) is also very
indicative of the disease.
Rheumatoid arthritis,* another systemic autoimmune dis-
ease, incurs progressive, debilitating damage to the joints. In some
patients, the lung, eye, skin, and nervous system are also involved.
In the joint form of the disease, autoantibodies form immune com-
plexes that bind to the synovial membrane of the joints and activate
phagocytes and stimulate release of cytokines. Chronic inflamma-
tion leads to scar tissue and joint destruction. The joints in the
hands and feet are affected first, followed by the knee and hip joints
(figure 17.15b). The precipitating cause in rheumatoid arthritis is
B cell
Sequestered tissues
with hidden
antigen
B cell
Autoantibodies
Thyroid cells
Lens protein
Viral infection
of cell
Antibody
binds cells
bearing viral
receptor; cells
destroyed
B-cell clone
that recognizes
foreign receptor
Altered self antigens
Viral Infection Theory
A
ntib
ody formed
L
o
ss
o
f a
na
to
m
ica
l barr
ier
(b)
Sequestered Antigen Theory
(a)
Lens
Thyroid
gland
FIGURE 17.14
Possible explanations for autoimmunity.
(a) Self antigens are sequestered and later incorrectly identified as a foreign antigen by B lymphocytes.
(b) Self antigens are altered by viral infection, which causes an immune response against the perceived foreign antigens.
*
systemic lupus erythematosus
(sis-tem
-ik loo-pis air -uh-theem-uh-toh-sis)
L. lupus, wolf, and erythema, redness.
*
rheumatoid arthritis
(roo
-muh-toyd ar-thry-tis) Gr. rheuma, a moist discharge, and
arthron, joint.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
516
CHAPTER 17 Disorders in Immunity
not known, though infectious agents such as Epstein-Barr virus
have been suspected. The most common feature of the disease is the
presence of an IgM antibody, called rheumatoid factor (RF), di-
rected against other antibodies. This does not cause the disease but
is used mainly in diagnosis. Some relief can be achieved with anti-
inflammatory agents, immunosuppressive drugs, and gold salt in-
jections in some individuals.
Autoimmunities of the Endocrine Glands
On occasion, the
thyroid gland is the target of autoimmunity. The underlying cause
of
Graves disease is the attachment of autoantibodies to receptors
on the follicle cells that secrete the hormone thyroxin. The abnor-
mal stimulation of these cells causes the overproduction of this hor-
mone and the symptoms of hyperthyroidism. In
Hashimoto
thyroiditis, both autoantibodies and T cells are reactive to the thy-
roid gland, but in this instance, they reduce the levels of thyroxin by
destroying follicle cells and by inactivating the hormone. As a re-
sult of these reactions, the patient suffers from hypothyroidism.
The pancreas and its hormone, insulin, are other autoim-
mune targets. Insulin, secreted by the beta cells in the pancreas,
regulates and is essential to the utilization of glucose by cells.
Diabetes mellitus is caused by a dysfunction in insulin production
or utilization (figure 17.16). Type I diabetes (also termed insulin-
dependent diabetes) is associated with autoantibodies and sensi-
tized T cells that damage the beta cells. A complex inflammatory
reaction leading to lysis of these cells greatly reduces the amount
of insulin secreted.
Neuromuscular Autoimmunities
Myasthenia gravis* is named
for the pronounced muscle weakness that is its principal symptom.
Although the disease afflicts all skeletal muscle, the first effects are
usually felt in the muscles of the eyes and throat. Eventually, it can
progress to complete loss of muscle function and death. The classic
syndrome is caused by autoantibodies binding to the receptors for
acetylcholine, a chemical required to transmit a nerve impulse
across the synaptic junction to a muscle (figure 17.17). The immune
attack so severely damages the muscle cell membrane that trans-
mission is blocked and paralysis ensues. Current treatment usually
includes immunosuppressive drugs and therapy to remove the au-
toantibodies from the circulation. Experimental therapy using im-
munotoxins to destroy lymphocytes that produce autoantibodies
shows some promise.
Multiple sclerosis* (MS) is a paralyzing neuromuscular dis-
ease associated with lesions in the insulating myelin sheath that
surrounds neurons in the white matter of the central nervous sys-
tem. The underlying pathology involves damage to the sheath by
(a)
(b)
FIGURE 17.15
Common autoimmune diseases.
(a) Systemic lupus erythematosus.
One symptom is a prominent rash across the bridge of the nose and on
the cheeks. These papules and blotches can also occur on the chest
and limbs. (b) Rheumatoid arthritis commonly targets the synovial
membrane of joints. Over time, chronic inflammation causes thickening
of this membrane, erosion of the articular cartilage, and fusion of the
joint. These effects severely limit motion and can eventually swell and
distort the joints.
Type I Diabetes
Damaged
islet cell
Autoantibody
specific for
islets
Insulin
FIGURE 17.16
The autoimmune component in diabetes mellitus, type I.
Autoantibodies produced against the beta cells of the islets of
Langerhans destroy the cells and greatly reduce insulin synthesis.
*
myasthenia gravis
(my
-us-thee-nee-uh grah-vis) Gr. myo, muscle, astheneia,
weakness, and gravida, heavy.
*
sclerosis
(skleh-roh
-sis) Gr. sklerosis, hardness.

Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
Type IV Hypersensitivities: Cell-Mediated (Delayed) Reactions
517
both T cells and autoantibodies that severely compromises the ca-
pacity of neurons to send impulses. The principal motor and sen-
sory symptoms are muscular weakness and tremors, difficulties in
speech and vision, and some degree of paralysis. Most MS patients
first experience symptoms as young adults, and they tend to experi-
ence remissions (periods of relief) alternating with recurrences of
disease throughout their lives. Convincing evidence from studies of
the brain tissue of MS patients points to a strong connection
between the disease and infection with human herpesvirus 6 (see
chapter 24). The disease can be treated passively with monoclonal
antibodies that target T cells, and a vaccine containing the myelin
protein has shown beneficial effects. Immunosuppressants such as
cortisone and interferon B may also alleviate symptoms.
Type IV Hypersensitivities: Cell-Mediated
(Delayed) Reactions
The adverse immune responses we have covered so far are ex-
plained primarily by B-cell involvement and antibodies. But type
IV hypersensitivity involves primarily the T-cell branch of the im-
mune system. Type IV immune dysfunction has traditionally been
known as delayed hypersensitivity because the symptoms arise one
to several days following the second contact with an antigen. In
general, type IV diseases result when T cells respond to self tissues
or transplanted foreign cells. Examples of type IV hypersensitivity
include delayed allergic reactions to infectious agents, contact der-
matitis, and graft rejection.
DELAYED-TYPE HYPERSENSITIVITY
Infectious Allergy
A classic example of a delayed-type hypersensitivity occurs when a
person sensitized by tuberculosis infection is injected with an
extract (tuberculin) of the bacterium Mycobacterium tuberculosis.
The so-called
tuberculin reaction is an acute skin inflammation at
the injection site appearing within 24 to 48 hours. So useful and di-
agnostic is this technique for detecting present or prior tuberculosis
that it is the chosen screening device (figure 17.18a). Other infec-
tions that use similar skin testing are leprosy, syphilis, histoplasmo-
sis, toxoplasmosis, and candidiasis. This form of hypersensitivity
arises from time-consuming cellular events involving the
T
D
class
of cells. After these cells receive processed microbial antigens from
macrophages, they release broad-spectrum cytokines that attract
inflammatory cells to the site—particularly mononuclear cells,
fibroblasts, and other lymphocytes. In a chronic infection (tertiary
syphilis, for example), extensive damage to organs can occur
through granuloma formation.
Contact Dermatitis
The most common delayed allergic reaction,
contact dermatitis, is
caused by exposure to resins in poison ivy or poison oak (Spotlight
on Microbiology 17.3), to simple haptens in household and per-
sonal articles ( jewelry, cosmetics, elasticized undergarments), and
to certain drugs. Like immediate atopic dermatitis, the reaction to
these allergens requires a sensitizing and a provocative dose. The
hypersensitivity reactions. Autoimmune T-cell responses are type IV
hypersensitivity reactions.
Susceptibility to autoimmune disease appears to be influenced by gender
and by genes in the MHC complex.
Autoimmune disease may be an excessive response of a normal immune
function, the appearance of sequestered antigens, “forbidden’’ clones
of lymphocytes that react to self antigens, or the result of alterations in
the immune response caused by infectious agents, particularly viruses.
Examples of autoimmune diseases include systemic lupus erythematosus,
rheumatoid arthritis, diabetes mellitus, myasthenia gravis, and multiple
sclerosis.
Type III hypersensitivities are induced when a profuse amount of antigen
enters the system and results in large quantities of antibody formation.
Type III hypersensitivity reactions occur when large quantities of antigen
react with host antibody to form small, soluble immune complexes that
settle in tissue cell membranes, causing chronic destructive
inflammation. The reactions appear hours or days after the antigen
challenge.
The mediators of type III hypersensitivity reactions include soluble IgA,
IgG, or IgM, and agents of the inflammatory response.
Two kinds of type III hypersensitivities are localized (Arthus) reactions
and systemic (serum sickness). Arthus reactions occur at the site of
injected drugs or booster immunizations. Systemic reactions occur
when repeated antigen challenges cause systemic distribution of the
immune complexes and subsequent inflammation of joints, lymph
nodes, and kidney tubules.
Autoimmune hypersensitivity reactions occur when autoantibodies or host
T cells mount an abnormal attack against self antigens. Autoimmune
antibody responses can be either local or systemic type II or type III
CHAPTER CHECKPOINTS
Normal
Myasthenia gravis
Myoneural junction
Acetylcholine
Receptors
Autoantibody
specific for
receptor
Paralysis
Muscle
Contraction
Postsynaptic
membrane
Neuron
X
X
X
Signal
FIGURE 17.17
Proposed mechanisms for involvement of autoantibodies in
myasthenia gravis.
Antibodies developed against receptors on
the postsynaptic membrane block them so that acetylcholine cannot bind
and muscle contraction is inhibited.
