
GENETICS
Heredity is the transmission of information required to construct
multiple proteins. These proteins have diverse roles and different
subsets, they are utilized by different cell types but all are encoded in
the cells DNA which is organized into discrete structures called
chromosomes.
Chromosomes: every cell nucleus contains a set of chromosomes. Each
chromosome consists of a single molecule of DNA (deoxyribonucleic
acid) together with associated acidic and basic proteins.
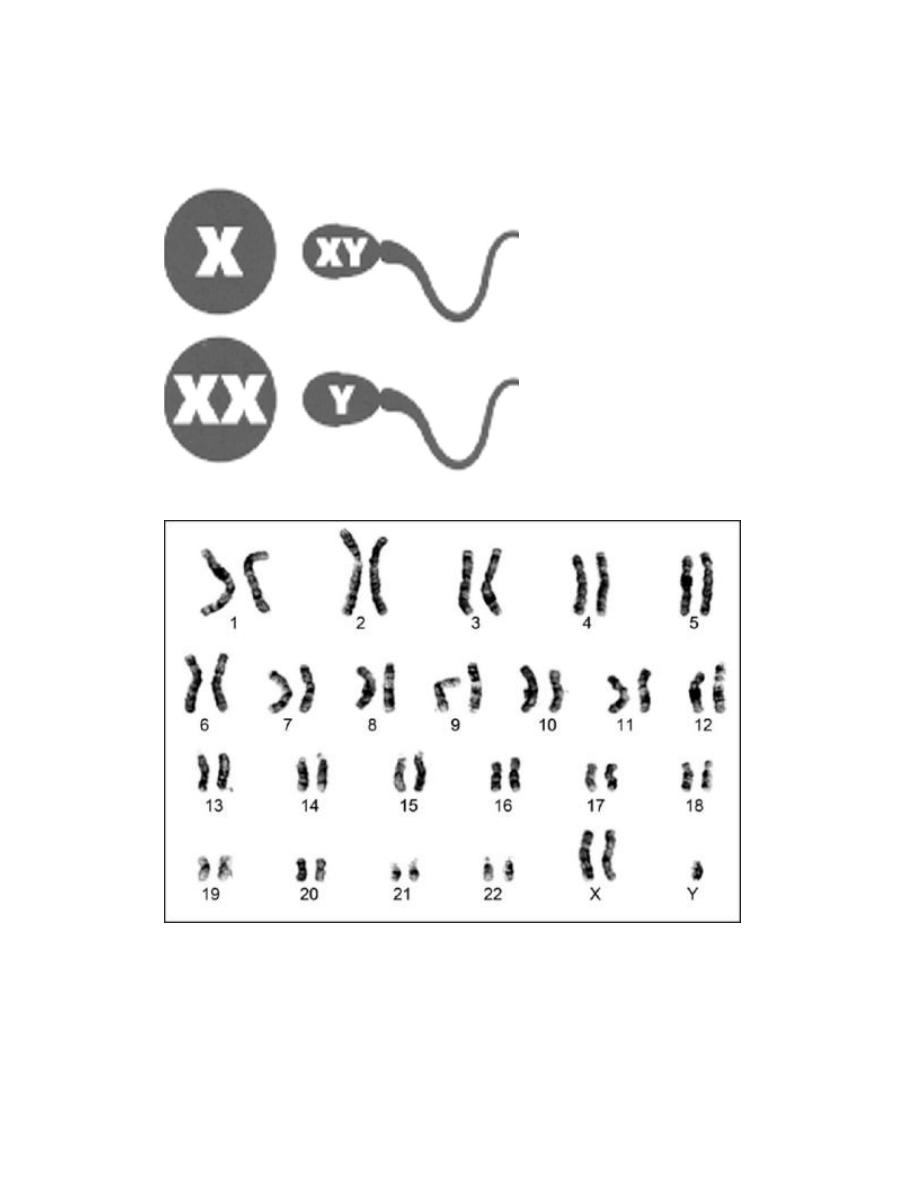
Most human cells contain 46 chromosomes (the diploid number) with
22 pairs of autosomes which are a like in males and females and a pair
of sex chromosomes: XX in female and XY in a male.
Each chromosome has a narrow waist called the centromere which has
a constant position for a given chromosome. The centromere divides
each chromosome into short and long arms.
At mitosis each chromosome replicates to form a pair of sister
chromatids which are held together at the centromere. Although
exchanges of genetic material can occur by crossing over (sister
chromatid exchange) during mitosis, as each sister chromatid is
identical, clinical consequences do not arise. Thus at the end of cell
division each daughter cell has identical set of 46 chromosomes.
In contrast, reduction cell division or meiosis results in cell with a half
set (haploid number) of 23 chromosomes. Meiosis, which is confined to
gonadal cells involved in gametogenesis, consist of two successive
division in which the DNA replicates only once before the first division.

Each mature egg thus normally contains one of each pair of autosomes
and one X and each mature sperm has one of each pair of autosomes
and the X or Y chromosomes. At fertilization the diploid number is
restored and in consequence half of each individual's autosomes are
derived from each parent and a female has an X from each parent.
Whereas a male has a maternal X and a paternal Y sex chromosome.
DNA: each molecule of DNA is composed of two nucleotide chains
which are coiled clockwise around one another to form double helix.
Each nucleotide consists of a nitrogenous base, a molecule of
deoxyribose and a phosphate molecule.
The nitrogenous bases are of two types, purines and pyrimidines. In
DNA there are two purine bases, adenine (A) and guanine (G) and two
pyrimidine bases, thymine (T) and cytosine (C). The nucleotide chains
run in opposite direction and are held together by hydrogen bonds
between A and T or between G and C since A: T and G: C pairing is
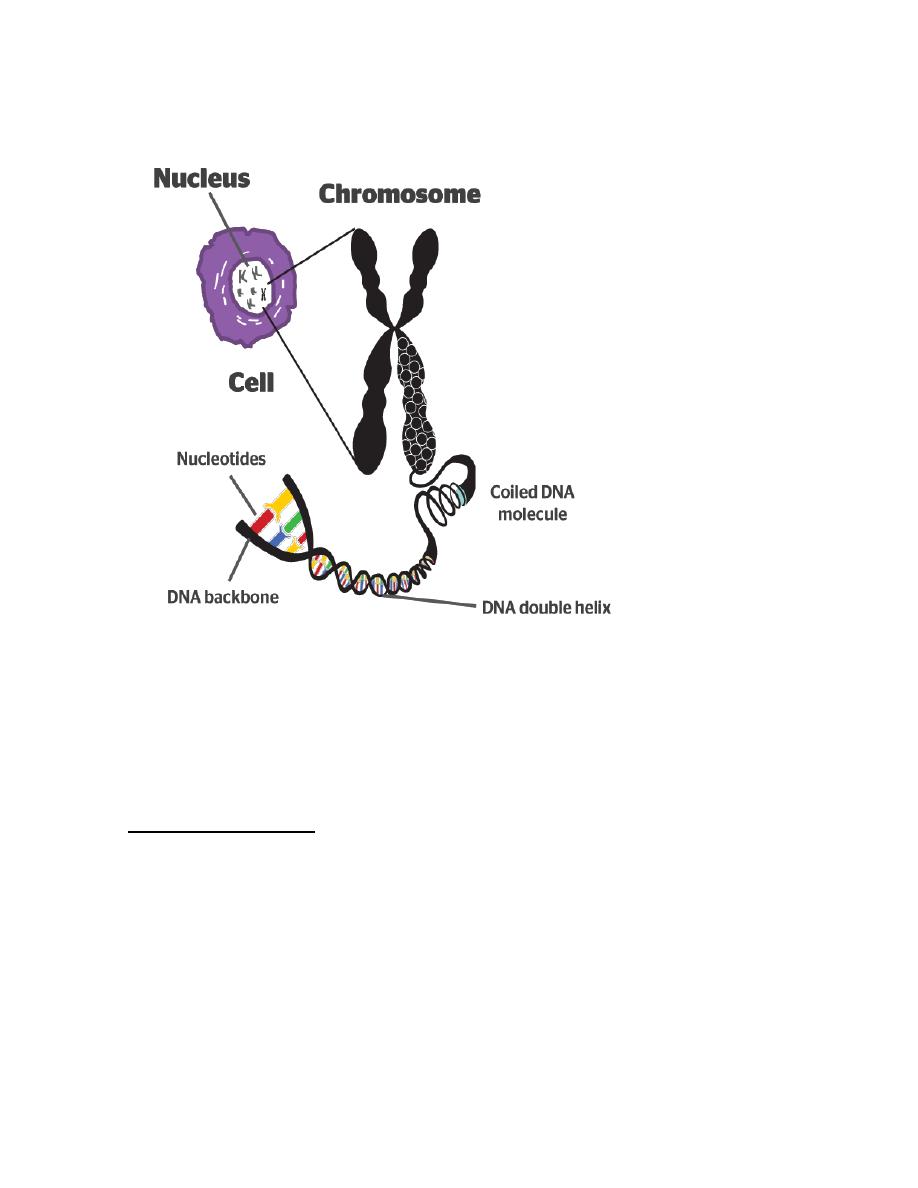
obligatory the parallel strands must be complementary to one another.
Gene: is the unit of the DNA which codes for a protein.
Genetic diseases
:-
Are large group of diseases can be subdivided into:-
A. Single gene defect (dominant & recessive).
B. Chromosomal diseases (numerical & structural)
C. Multifactorial diseases & non traditional disorders.

Mutation refers to permanent changes in the DNA. Those that affect germ cells
are transmitted to the progeny and may give rise to inherited diseases. Mutations
in somatic cells are not transmitted to the progeny but are important in the
causation of cancers and some congenital malformations
Thus there is certain changes occur in nitrogenous bases may lead to abnormal
protein synthesis.
Single gene diseases (Unifactorial diseases):-These are caused by a
mutation in a gene. Genes may behave as dominant, i.e. when one of
the alleles becomes mutated it results in a genetic disease; or they may
behave as recessive, i.e. the diseases does not manifest unless both
alleles are affected by the same mutation.
A third category of genes are those which determine an autosomal
character but are situated on the sex chromosome (sex-linked)
The question arises why some genes act in a dominant manner while
others behave in a recessive fashion, i.e. the problem of dominance and
recessiveness. To answer this question, one has to consider and always
remember the following principles:

A Single gene is responsible for formation of a single type of protein,
but since proteins are made of units of polypeptides that could be the
same or different in one molecule of protein, the principle becomes:
A Single gene is responsible for the formation of a single type of
polypeptides, and if we know that our body structures and functions
from the moment of post-fertilization to the full maturity and later on
are determined by proteins one can understand how genes function.
These types of proteins are varied; they could be
1. Structural proteins, like fibrous tissue and elastic tissue proteins
2. Immunoglobulins
3. Signal proteins produced by many oncogenes
4. Receptors
5. Enzymes
6. Hormones
Therefore, the action of the gene being dominant or recessive is
determined by the type of protein it produces and its function.
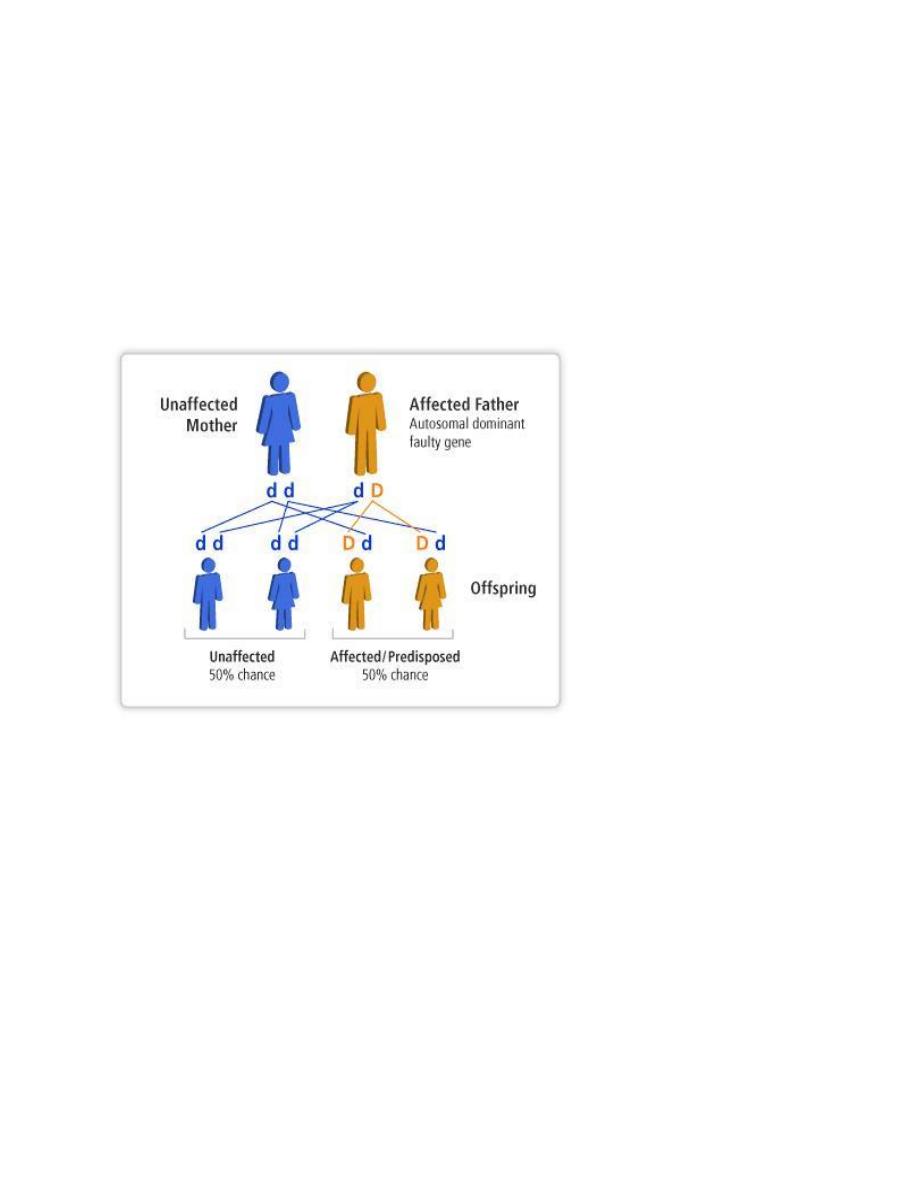
Autosomal dominant disorders:

1. are manifested in a heterozygous state (when one of the alleles
becomes mutated)
2. one of the parents of the affected individual should be affected
and the child appears as diseased individual.
3. both males and females are affected and both can transmitted
the condition. When affected person marries unaffected one,
every child has one chance in two of having the disease.
4. Some times the parents are normal but the child is diseased that
happened because the mutation occur in the cell of that child
alone while then parents are completely normal. The siblings of
this child are neither affected nor at risk of developing the disease
5. Some trait is seen in all individuals that carrying the mutant gene
but it is expressed differently among different individuals:
phenomena called variable expressivity.e.g. polydectaly may be
expressed in toes or in fingers as one or more digitis.
6. Dominant genes usually produce two types of proteins:
1. Major structural proteins, which form or are present in many
parts of the body e.g.is Marfan syndrome in which there is
mutation in fibrillin gene leading to a qualitative & quantitative
defects in fibrillin which result in skeletal abnormality .

2. Enzymes, which are key enzymes in metabolic pathways, under
feedback mechanism, or receptors regulating metabolic
pathways.
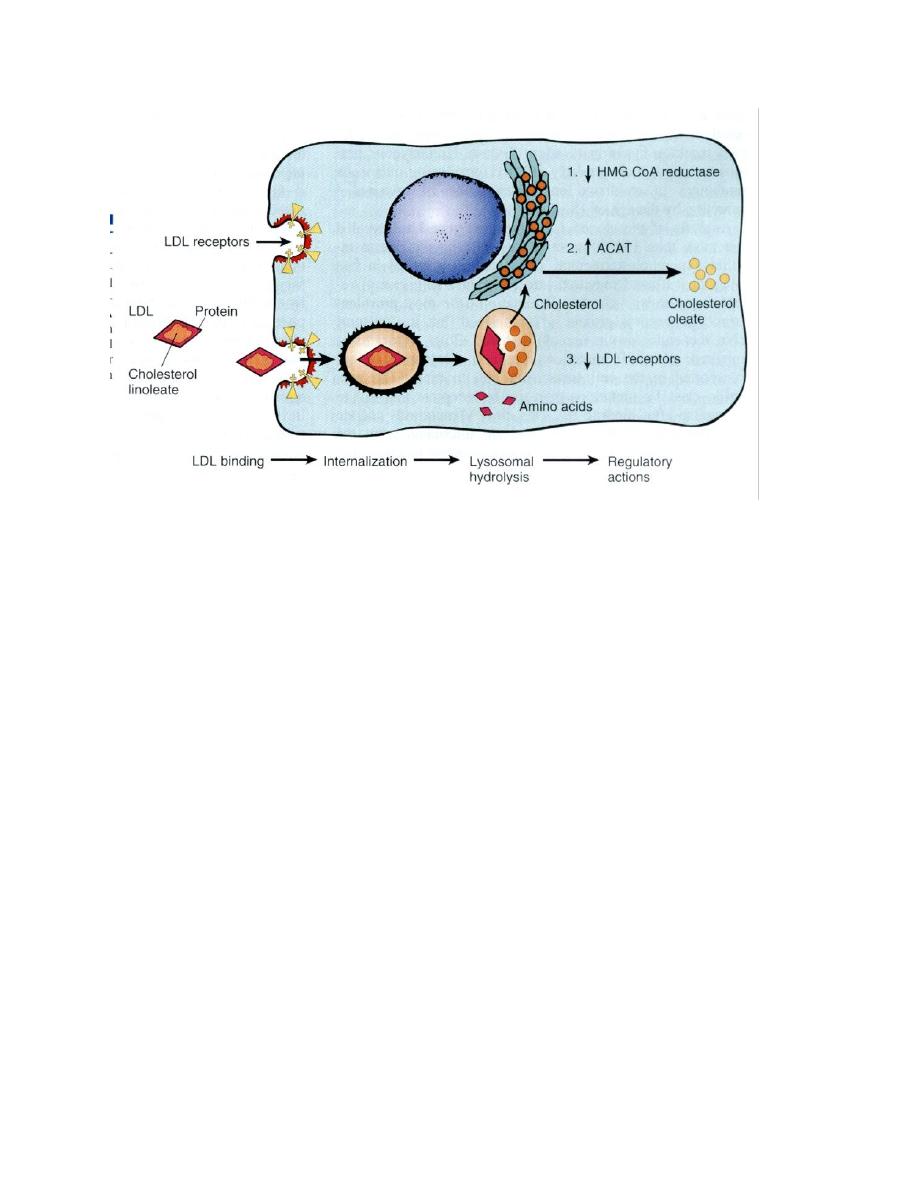
Example of the second is AD
familial hypercholesterolemia
where the receptors for LDL are mutated. They are responsible for
regulation of LDL in the cells and the circulation. To explain the latter
example and how the action of this pathway is executed, let us consider
the pathway of circulating LDL: usually it should enter the cells of the
body for building cellular membranes and nuclear membranes to
replace the old ones that are affected by wear and tear. LDL could not
enter the cells unless it is complexed with receptors on the cell
membrane. Once it is inside the cell, the complex will be degraded into
free cholesterol and amino acid. The latter is the remnant of the
proteinaceous coat of the lipoprotein. The free cholesterol in the cell
constitutes the cholesterol pool of the cell and its level is regulated by
three systems of enzymes, the HMG-CoA reductase, which forms
cholesterol from fatty acids, ACAT, which hydrolyzes cholesterol into
esters and thus rendering it inactive and the number of the receptors
on the surface of the cell. If the pool concentration is low, messages are
sent to activate the HMG-CoA, to inactivate ACAT and increase the
number of receptors on the cell surface. Therefore, when one of the
two alleles responsible for the formation of the receptor protein
becomes mutated, half of the number of receptors are formed only, so
50% of LDL which is used to be internalized inside the cell will remain in
the circulation unable to enter the cells and a state of
hypercholesterolemia results with reading of 400-500 iu/dl of
cholesterol in the blood (normal value 180-220 iu/dl)
Autosomal recessive disorders:
1. are manifested in a homozygous state (they occur when both of
the alleles at a given gene locus are mutants).
2. usually the parents are unaffected clinically because each has only
one mutant gene and so they are a carrier or heterozygote.
3. For two carrier parents the chance for getting an affected child is
1 to 4.
4. As for recessive genes, they are protein enzymes, which usually
share in catabolic pathways and when both alleles are ?defective,
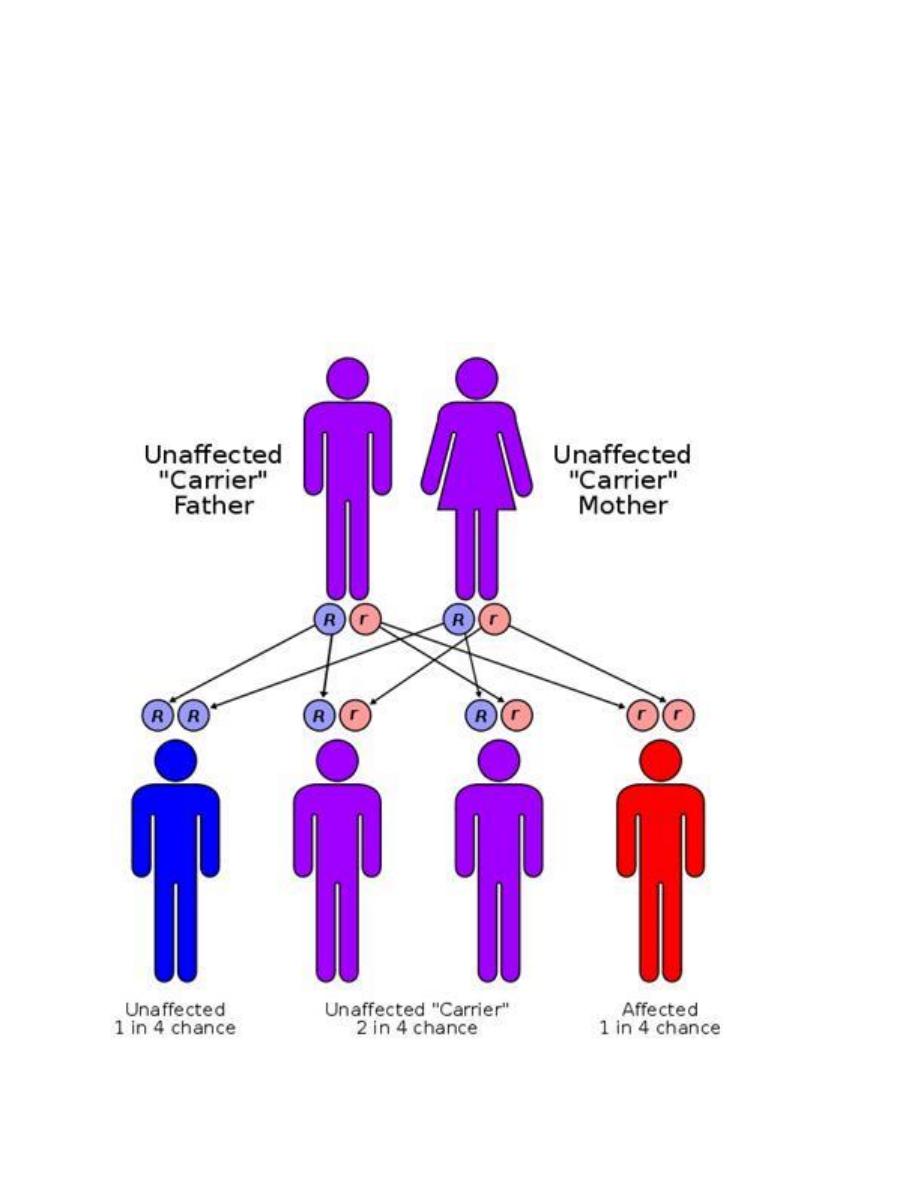
there is no protein, i.e. no enzyme and therefore the catabolic
pathway is obstructed with the accumulation of the biochemical
substrate. Examples of these are mucopolysaccharidosis &
phenylketonuria (PKU) and most of inborn errors of metabolism.

Athird category of single gene disorders are Sex-linked diseases in
which genes that determine an autosomal character are situated on the
sex chromosome (sex-linked) and because most of the genes are
carried on the “X” and very few are present on the “Y”, usually sex-
linked is used for “X”-linked both dominant and recessive. Most of the
X-linked disorders are X-linked recessive and are characterized by the
following features:
1. They are transmited by heterozygous female carriers only to sons.
2. An affected male does not transmitted the disorder to sons but all
daughters are carriers.
3. Sons of heterozygous women have one chance in two of receiving
the mutant gene.
Sex linked dominant disease e.g. is the vit D resistant rickets
Y inheritance disease is the hairy ears in males
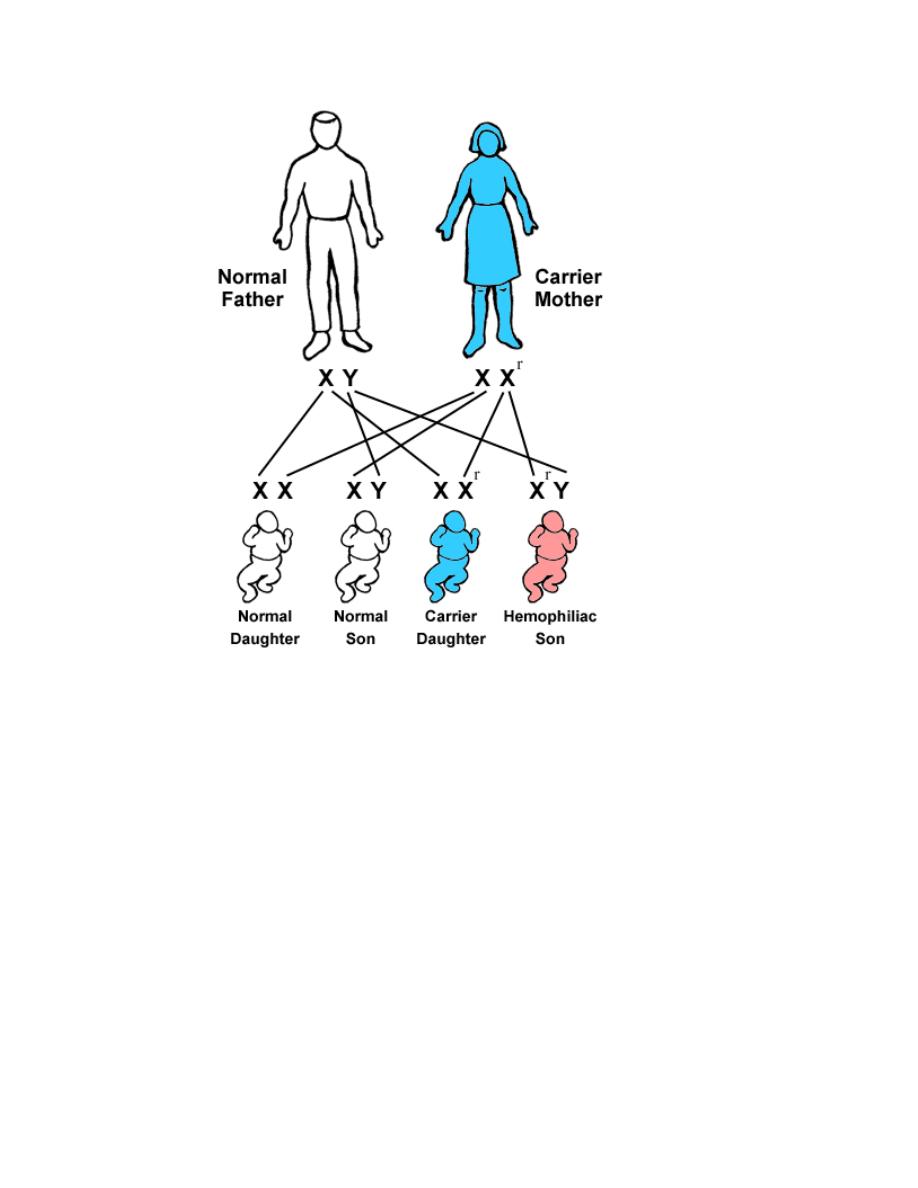
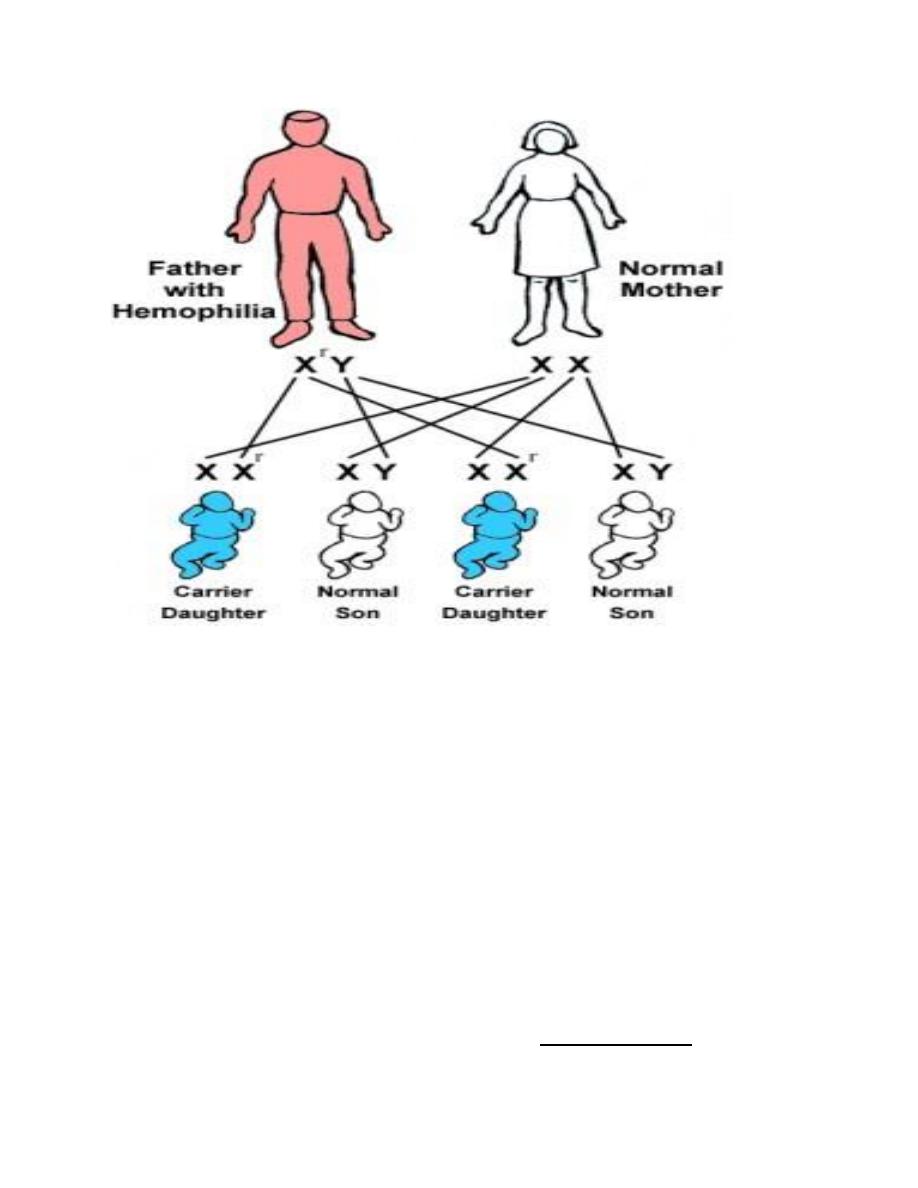
Sex-linked diseases, because most of the genes are carried on the “X” and very
few are present on the “Y”, usually sex-linked is used for “X”-linked both
dominant and recessive. In this type of inheritance, there is a lot of deviation from
the expected and their explanation is forwarded by a hypothesis known as Lyon’s
hypothesis, e.g. in clinical practice, both haemophilia and G6PD-deficiencies are
diseases caused by sex linked genes recessive in nature, i.e. only males who carry
the mutated gene on their “X” are affected clinically while carrier females are
usually silent clinically but transfer the disease to their sons. But it happens that
some cases of both diseases present in female by Lyon’s hypothesis, which states

that in a female’s autosomal cells, all the “X” chromosomes will be inactivated
except one which remains active during inetrphase.
This process of inactivation takes place early n the post-fertilization period,
19-20 days P.F.
The process of inactivation is random concerning the origin of the “X”
inactivated, i.e. paternal “X”, which comes from the father or maternal; “X”
that comes from the mother.
In a cell, all the daughter cells that descend from it, the same “X” will
remain inactive.
This means that 50% of the “X” chromosomes are inactivated but this does not
necessarily involve all the paternal “X” or all the maternal “X”. in some areas the
paternal X is being inactivated while in other areas, it is the maternal “X” that are
being inactivated, therefore, the body of the female is a mosaic concerning the
function of the active “X”. So, a heterozygote female for type G6PD enzyme A &
B; if we examine different parts of her body for the type of the enzyme, we either
find type A or type B and never both in one part of the body. In contrast males
could either be A or B.
A female who carries the mutated gene for “X” linked and presents clinically the
disease; it happens by chance that in most parts of her body the “X” that carries
the mutated gene remains active, which results in deficiency of the product of the
gene disease. This is because of the randomness of the inactivation. Those
females are known as manifesting carriers in clinical practice.
Etiology:
Mutation refers to permanent changes in the DNA. Those that affect germ cells
are transmitted to the progeny and may give rise to inherited diseases. Mutations
in somatic cells are not transmitted to the progeny but are important in the
causation of cancers and some congenital malformations
Thus there is certain changes occur in nitrogenous bases may lead to abnormal
protein synthesis.
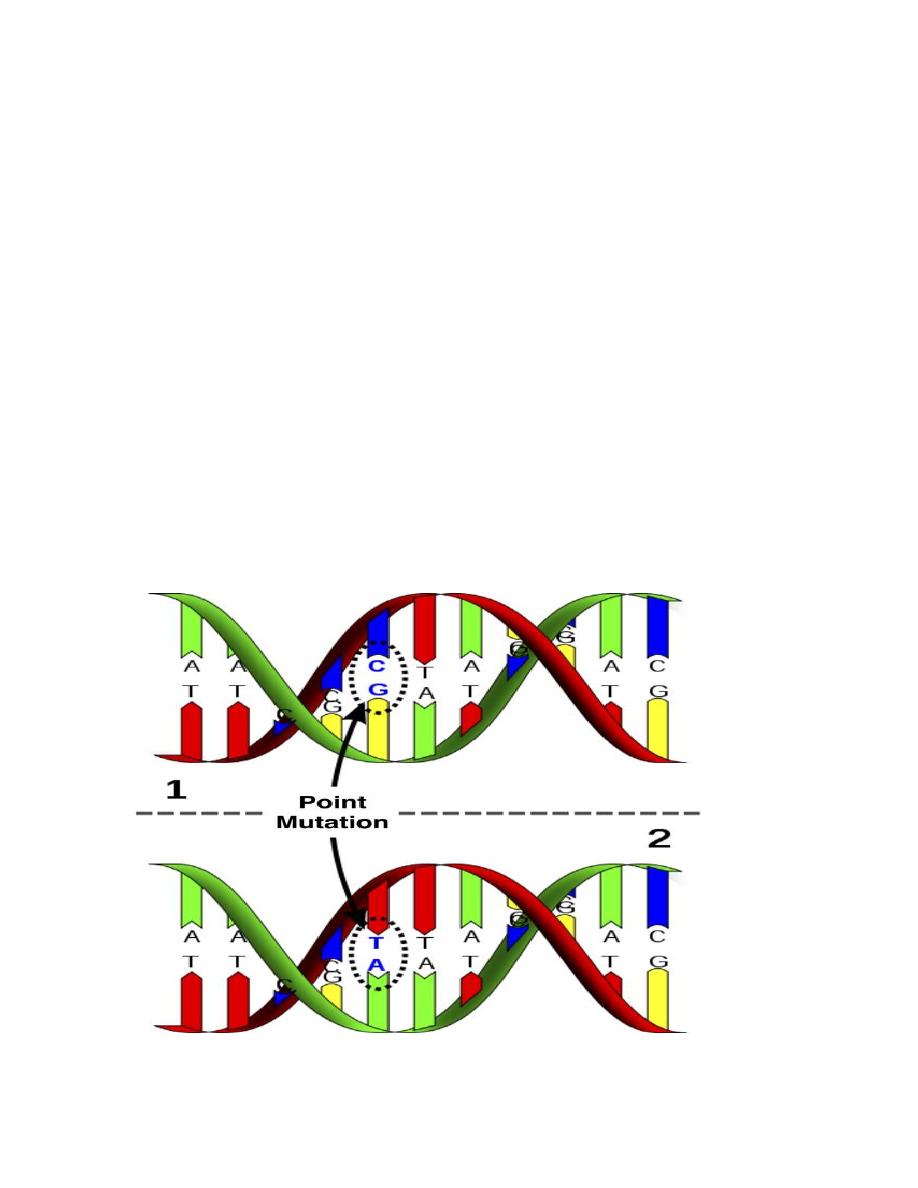
All single gene diseases are due to mutations. They are of different types:
1.
Single point mutation
which is the commonest. They usually result from a
change in one of the nucleotide bases that form the trios (three bases), each
of which codes for a specific amino acid in the protein molecule. Not all of
these changes will result in a mutation that causes a disease.

2-Additional – deletional mutations :
They could be one of three types :
a.
Addition or deletion of a single base.
b.
Addition or deletion of 3 bases or the multiple of 3,
c.
Addition or deletion of a large piece of DNA inside the gene
(intragenic) or in between the genes (intergenic). Again this creates
variability and it is used for genetic testing and diagnosis of genetic
diseases.
Chromosomal Diseases
These are classified into:
a. Numerical abnormalities, which is defined as a gain or loss of a whole
chromosome from the usual number of chromosomes in the karyotype (i.e. 46
chromosomes). Gain or loss in the sex chromosomes especially the X-
chromosome is compatible with life and is relatively common; while loss of an
autosomal chromosome is usually non-viable and a fertilized ovum carrying

such karyotype could not sustain pregnancy to full term and usually are lost
very early in pregnancy, i.e. abortion.
Autosomal chromosome trisomy is exemplified by trisomy 21 or Down’s
syndrome, trisomy 18 or Edward’s syndrome, trisomy 13 or Patau’s syndrome.
Sex chromosome trisomy is exemplified by Klienfilter’s syndrome in male and
triple X (XXX) syndrome in female or XYY syndrome in male, while monosomy of
sex chromosome is when a female loses one X resulting in Turner’s syndrome, 45
X known as aneuploidy.
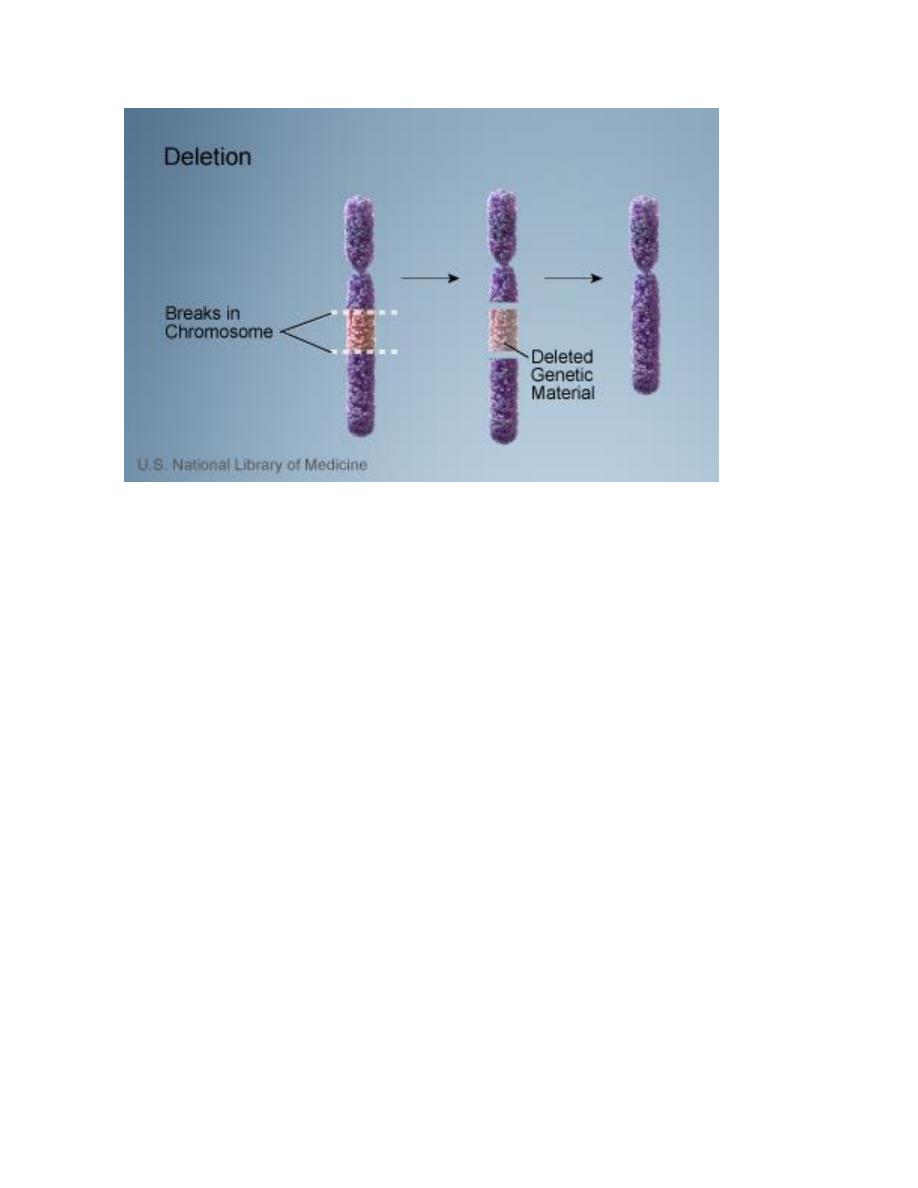
b. Structural abnormalities: it usually results from breakage followed by
rearrangement of material (a cell suffering from structural abnormality has the
normal number of 46 but the chromosomes are morphologically or structurally
abnormal).
These abnormalities are of different types:
A. Deletion: loss of a piece of a chromosome. It is of two types
i. Terminal: single break may delete a terminal segment.
ii. interstitial: where the piece of a chromosome between two breaks is lost
resulting in a syndrome
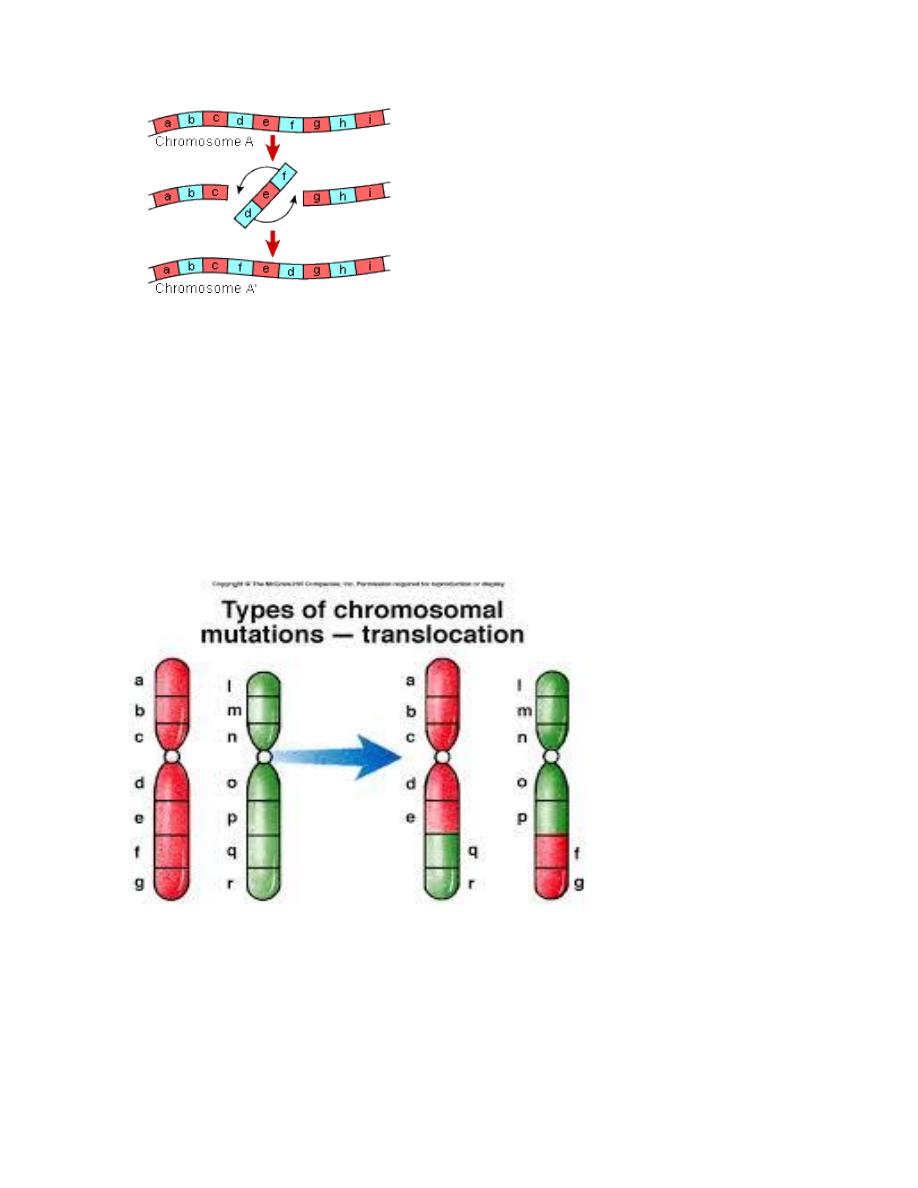
B. Inversion:
This abnormality results from two breaks through out the length of the
chromosome which either involve the centromere area or not and the piece
between the two breaks will rotate 180
o
before it returns to its place. So the
genetic piece which is broken it will rotated so the position of the gene occupied
by this segment is abnormal & the content of the genetic material are changed
according to the piece which is inverted & this will result in abnormal fetus with
signs & symptoms of abnormality.
C.Translocation: This is defined as exchange of segments of chromosomes
between two non-homologous chromosomes so there is a translocation of some
oncogenes from their normal habitat to a new situation where they are induced
to function in uncontrolled manner leading to malignancies. This is usually seen in
leukemias and lymphomas, e.g. Philadelphia chromosome.
D. Isochromosome: This abnormality results from aberrant division of the
centromere which is the last part of the chromosome that divides in the mitosis
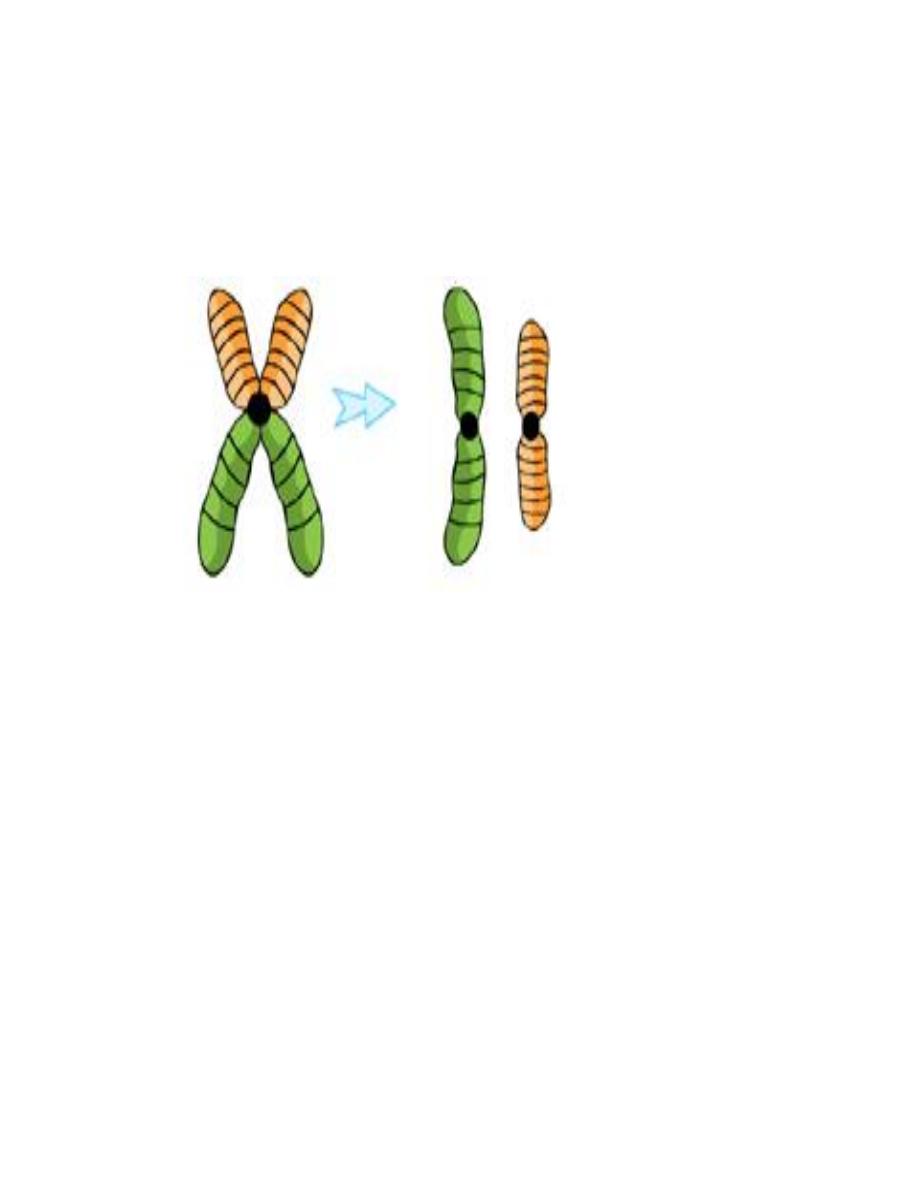
to separate the two sister chromatids into individual chromosomes. This aberrant
division takes place in a horizontal way rather than the perpendicular natural way.
So the resulting two chromosomes are imbalanced, one formed of two short arms
and the other of two long arms. Each of them is an isochromsome.
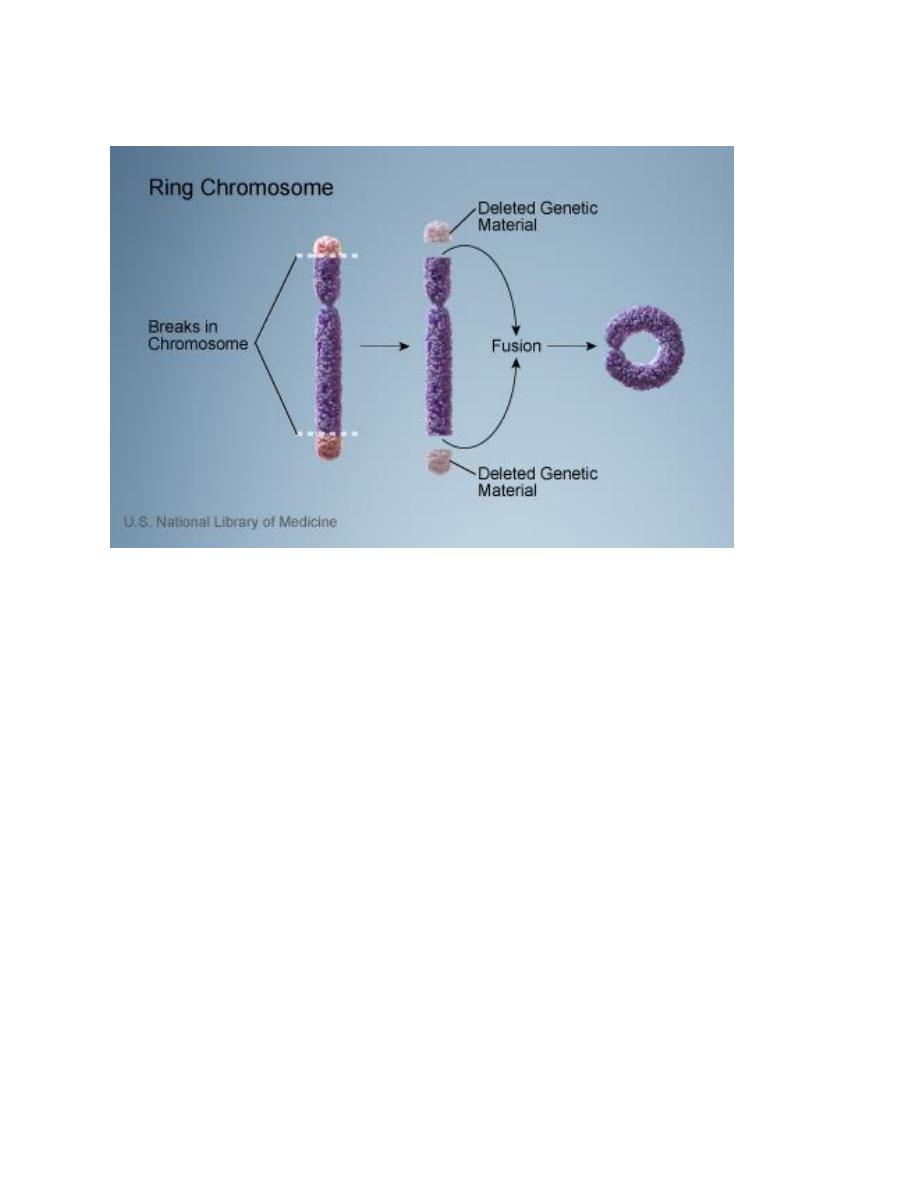
E. Ring-chromosome: It results from deletion of both ends of a
chromosome and then the ends, because of the adhesive nature of the exposed
DNA, will stick together forming a ring.
Cytogenetic disorders involving autosomes:
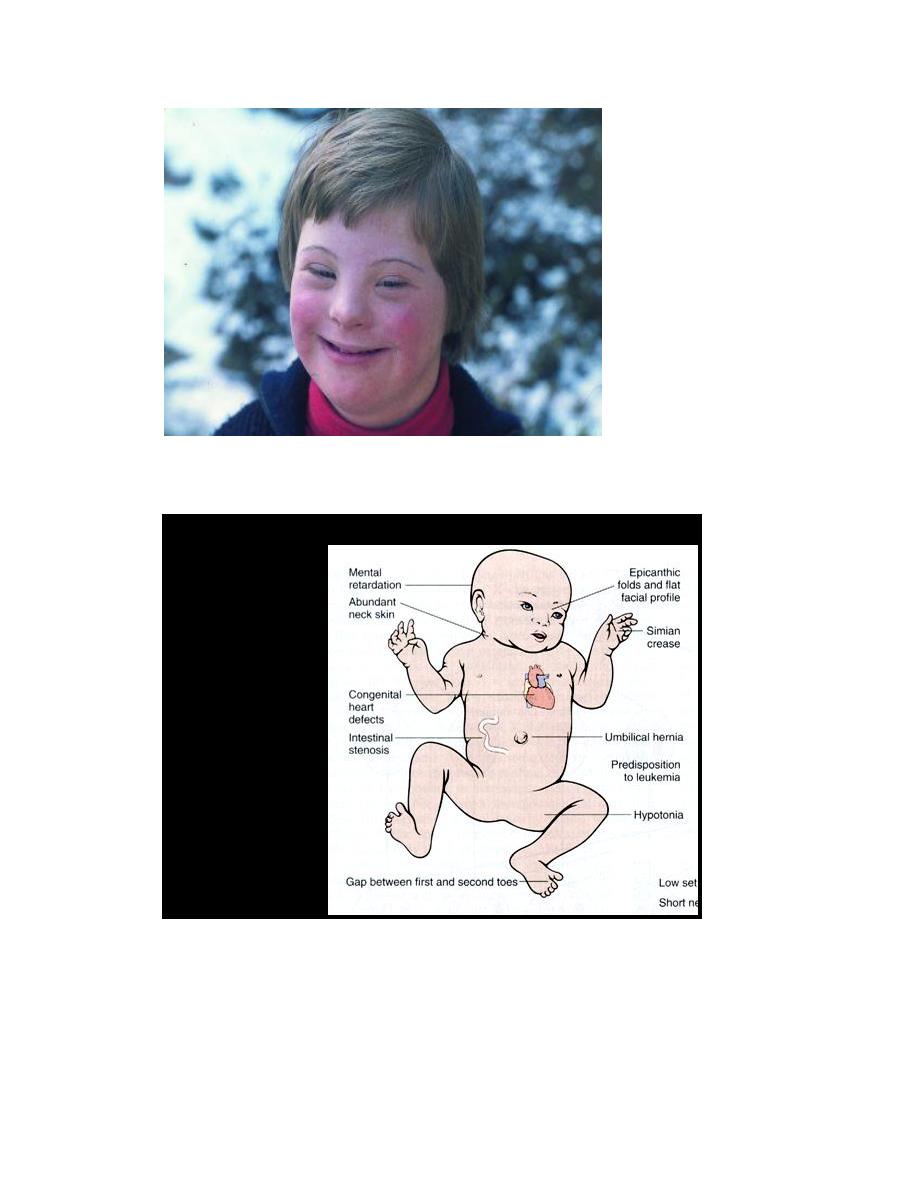
Down syndrome (Trisomy 21), karytype (47, xx or xy, +21):
is the most common of the chromosomal disorders. About 95% of affected
persons have trisomy 21 resulting from meiotic nondisjunction. The parents of
such children have normal karyotype and are normal in all respects. Increasing of
maternal age has a strong influence on the incidence of Down syndrome. The
correlation with maternal age suggests that in most cases the meiotic
nondisjunction of chromosome 21 occurs in the ovum.

In about 4% of all patients with trisomy 21, the extra chromosomal material
is due to translocation of the long arm chromosome 21 to chromosome 22 or 14
and the remaining 1% of trisomy 21 patients are mosaics.
Trisomy 21 is the leading cause of mental retardation. The patient has a
combination of epicanthic folds and flat facial profile which is quite characteristic.
Congenital malformations are common. Approximately 40% of patients have
cardiac malformations, which are responsible for most of the death in early
childhood. They found that approximately 80% of those without congenital heart
disease can expect to survive 30 years but most of them develop Alzheimer
disease and frank dementia.
Serious infections and increased risk of developing acute leukemias are
another cause of morbidity and mortality in patients with trisomy 21.
Autosomal trisomies
Trisomy 21
Down
syndrome
Cytogenetic disorders involving sex chromosome:

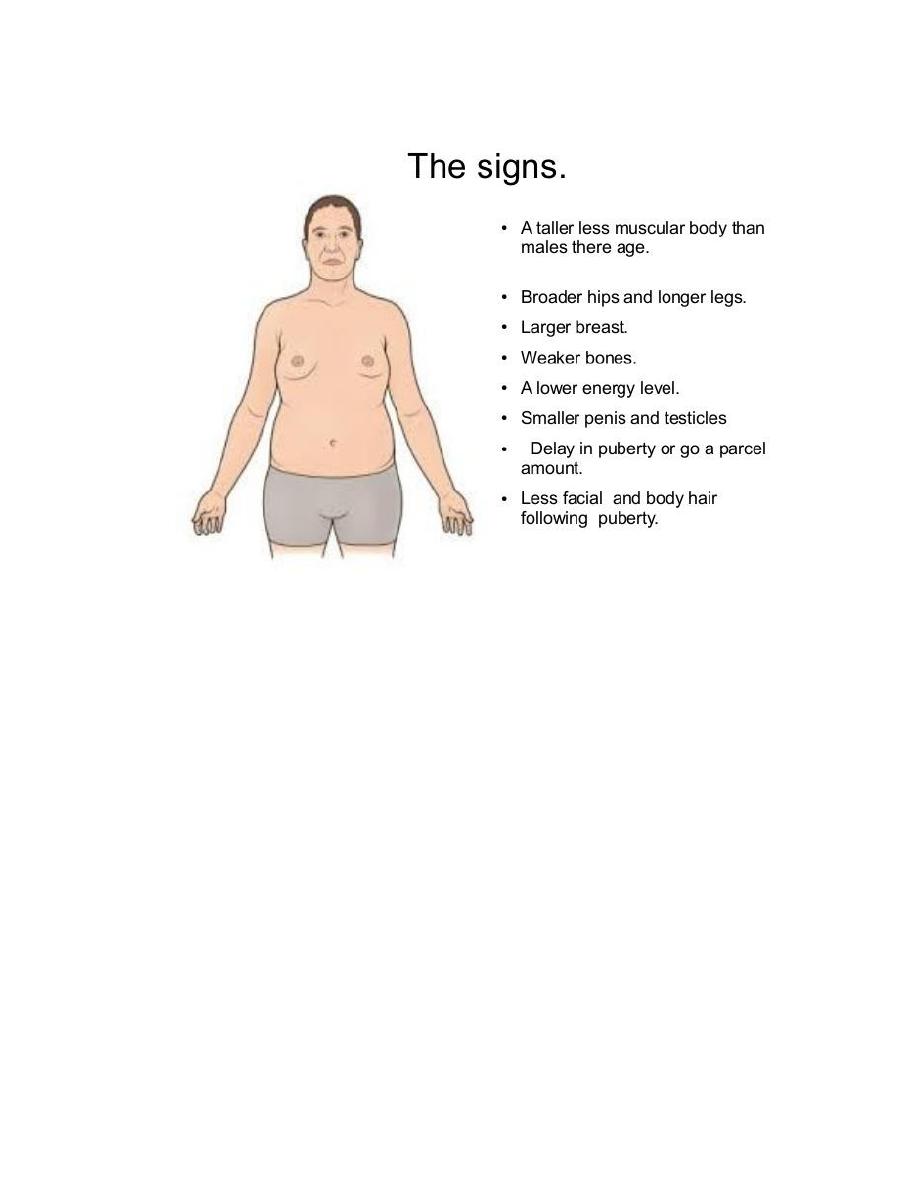
Klinefelter syndrome: this syndrome is best defined as male hypogonadism
that develop when there are at least two X chromosomes and one or more Y
chromosomes. Most patients are 47, XXY. This karyotype results from
nondisjunction of sex chromosomes during meiosis. The extra X chromosome may
be of maternal or paternal origin. Advanced maternal age and history of
irradiation of either parent may contribute to the meiotic error resulting in this
condition. Approximately 15% of patients show mosaic patterns (i.e. 46,XY/47,XXY
or 47,XXY/48,XXXY) and the presence of a 46,XY line in mosaics is usually
associated with a milder clinical condition.
Although the following description applies to most patients, it should be
noted that klinefelter syndrome is associated with a wide rang of clinical
manifestations. In some it may be expressed only as hypogonadism, but most
patients have an increased length between the soles and the pubic bone, which
creates the appearance of an elongated body. Reduced facial body and pubic hair
with gyneocomastia are also frequently noted. Testicular atrophy, the serum
testosterone levels are lower than normal while urinary gonadotropin levels are
elevated. So the principle clinical effect of this syndrome is sterility. Only rarely
the patients are fertile and these are presumably mosaics with a large proportion
of 46, XY cells. This syndrome may be associated with mental retardation but the
degree of intellectual impairment is typically mild and in some cases is
undetectable. The reduction in intelligence is correlated with the number of extra
X chromosomes. Thus, in patients with the most common variant (XXY),
intelligence is nearly normal, but in those with rare variant forms involving
additional X chromosomes, significantly subnormal levels of intelligence, as well
as more sever physical abnormalities are found.
Turner syndrome:
It is characterized by primary hypogonadism in phenotypic females, results
from partial or complete monosomy of the short arm of the X chromosome. In
approximately 57% of patients, the entire X chromosome is missing, resulting in a
45,X karyotype. These patients are the most severely affected. Typical clinical
features associated with 45X Turner syndrome include significant growth
retardation, leading to abnormal short stature; swelling of the nap of the neck
due to distended lymphatic channels (in infancy) that is seen as webbing of the
neck in older children; low posterior hair line; shieldlike chest with widely spaced
nipples; high arched palate; lymphedema of the hands and feet; and a variety of

congenital malformations such as horseshoe kidney, bicuspid aortic valve and
coarctation of the aorta. Affected females develop normal secondary sex
characteristics; the genitalia remains infantile, breast development is minimal and
little pubic hair appears. Most have primary amenorrhea, and morphologic
examination reveals transformation of the ovaries into white streaks of fibrous
stroma devoid of follicles. The mental status of these patients is usually normal.
Curiously, hypothyroidism caused by autoantibodies is noted in 25% to 30%. In
adult patients a combination of short stature and primary amenorrhea should
prompt strong suspicion of Turner syndrome. The diagnosis is established by
karyotyping.
Approximately 43% of patients with turner syndrome either are mosaics
(one of the cell lines being 45,X) or have structural abnormalities of the X
chromosome.
Turner syndrome
Congenital malformations:
This topic is discussed within the context of genetic diseases because it has some
bearings to those diseases. Not all congenital malformations, as it may come to
the mind of the student, are caused by genetic aetiology; but only a small percent
of them are so. The majorities of congenital malformations are caused by
environmental aetiology, and from this angle comes their relationship to genetic
diseases.
If congenital malformations are represented by a big circle (that encompasses
genetic disease in addition to others) Figure (1), the largest group i.e. 2/3 of the
surface area is of unknown aetiology, while only a small sector represents
diseases of genetic basis.
Definition:
It is a deformation of structure or function of an organ present at birth.
genetic
diseases
unknown
chemical
drugs
physical
prenatal
infection
maternal
disease

It may be on the surface of the body, e.g. cleft lip or inside the body, e.g.
horseshoe kidney; it may be macrocellular, i.e. recognized by the naked eye e.g.
club foot or microcellular i.e. recognized only by microscopical examination, e.g.
sponge kidney where the defect is abnormal connection between the collecting
tubules and the urineferous tubules leading to thin microscopical dilatation that
gives the kidney a spongy feeling; it could be diagnosed by mere naked eye, e.g.
microcephaly
or microphthalmia
or it may need special procedure for
diagnosis, e.g. congenital heart defects; it may present at birth showing signs and
symptoms, e.g. tracheo-esophageal fistula or the signs and symptoms may
present later in life (but the defect is actually present at birth), e.g. adult
polycystic kidney; it may be familial or non-familial, i.e. either there are multiple
cases in one family or it is the only case in the family (sporadic); and lastly it could
be genetic, i.e. direct descent from parents through genetic defect or could be
non-genetic, i.e. caused by environmental causes, e.g. microcephaly; some cases
are genetic while others are due to uterine infection during pregnancy (prenatal
infection).
Causes
The etiological factor that results in congenital malformation is known as a
teratogen.
Some teratogens may act as mutagen or carcinogen.
The effect of a teratogen is most effective on growing tissue with rapid division
rate but not all teratogens affecting different foeti cause the same severity of
action, i.e. the action of one teratogen is variable in different foeti. This is due to
the following factors
a. Dose of the teratogen: larger doses of course causes severer effects.

b. Time of exposure to the teratogen,
e.g. the action of the Mullerian
inhibiting factor (MIF) which is an enzyme produced by the foetal
testis at a certain time (7
th
week post-fertilization) to effect the
regression of the female primitive reproductive organ in a male
foetus. If the testis fails to produce this enzyme at that time, then it
will have no effect and the female reproductive organs will remain
inside the abdomen of the male infant resulting in ambiguous
genitalia.
c. Host susceptibility: some foeti are more susceptible than others for
the same dose and therefore they are more severely affected.
d. Interaction with other factors, environmental or genetic constitution
of the foetus.
Types of teratogens
I. Genetic,
these could be:
1. Single gene that is either:
a. autosomal dominant (AD).
b. autosomal recessive (AR).
c. sex-linked dominant (XD).
d. sex-linked recessive (XR).
2. Chromosomal abnormality that could be:
a. numerical.
b. structural.
II. Environmental teratogens
; these causes:
1. In-utero infection :
Prenatal infection of the fetus with bacteria, viruses, or parasites may occur
during pregnancy due to maternal infection that is transferred to the fetus
through the placenta causing fetal infection. The most common is the viral
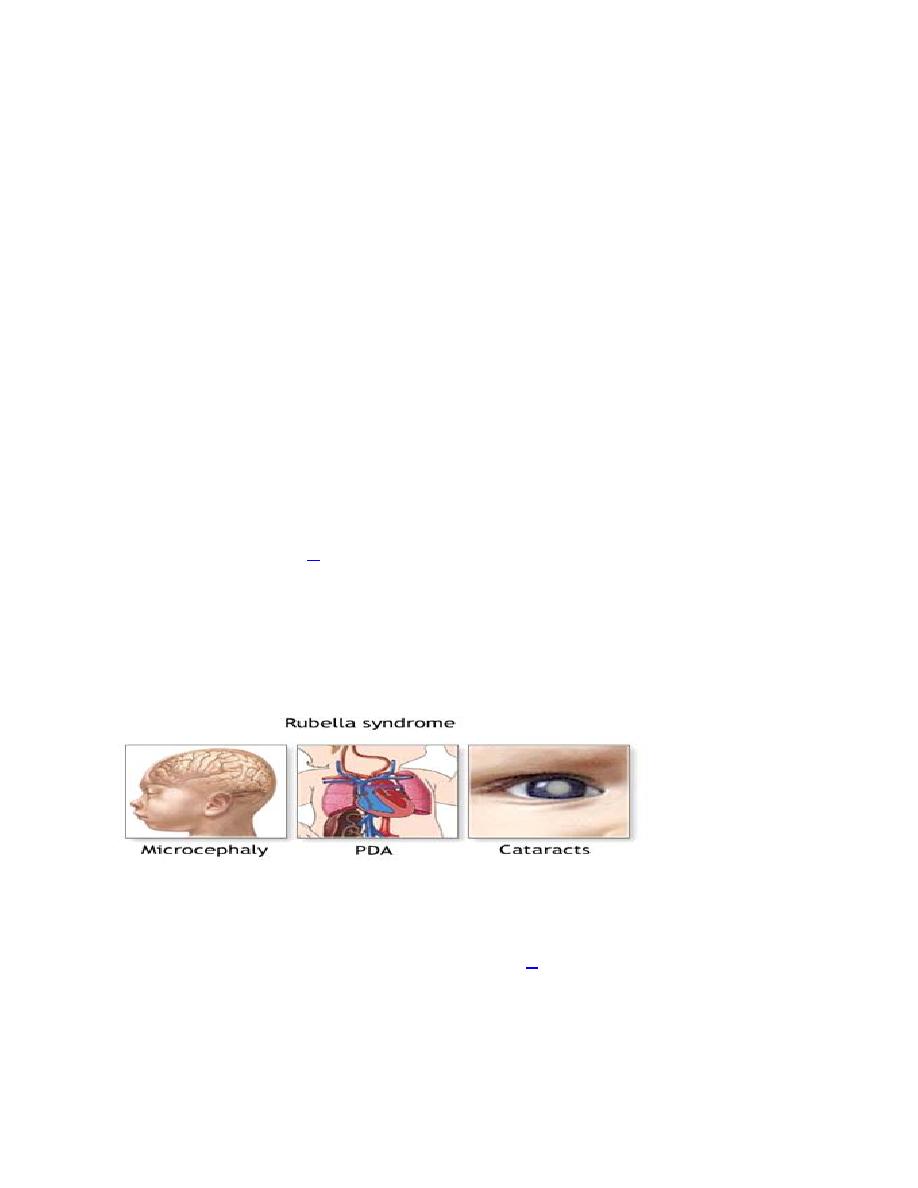
infection with rubella virus (German measles), and influenza virus.
Rubella usually causes severe malformation the earlier it infects the fetus The
modern immunization against rubella of girls at reproductive age is one of the
very successful methods to prevent this type of malformation.
Usually, rubella causes congenital heart defect, mainly septal defects,
microcephaly, cataract
, or infection of the chambers of the eyes leading to
blindness, deafness and mental retardation.
Influenza virus usually causes cleft lip and palate
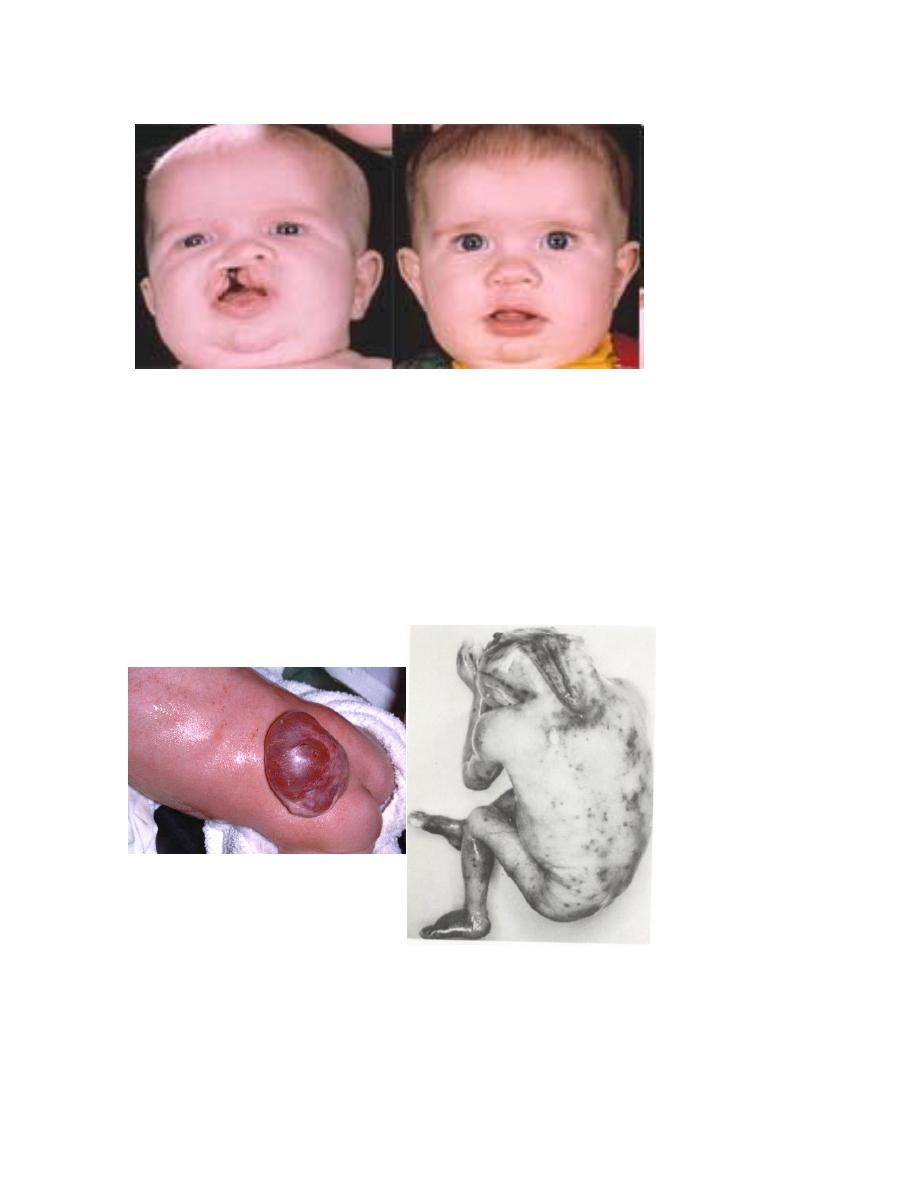
Unilateral Cleft Lip And Palate
Pre and Post-repair
Neural Tube Defects
Meningomyelocele
Anencephaly

Toxoplasmosis is a parasite infestation contracted from animals, usually sheep or
cats; it may cause microcephaly, jaundice, and mental retardation.
Microcephaly
Physical teratogens :
These could be in the form of:
a.
Heat,
whether sauna bathing, or from weather or fever (if it does not
cause abortion) usually causes neurological abnormalities and neural
tube defects.
b.
Physical pressure
that may be caused from inside the uterus or
outside it, pressing the growing foetus and thus preventing its proper
growth.as uterine fibroid.
c.
Ionizing radiation
, whether diagnostic or therapeutic. Radiation or
accidents usually causes malformation. Radiation causes
microcephaly, anophthalmia and spina bifida. The most sensitive

period for the foetus is the 1
st
two weeks post-fertilization to the 4
th
-
5
th
weeks of conception.
2. Chemical teratogens, these could be :
a. Non-medicinal: like insecticide, and household chemical used in
cleaning and industrial chemical. Some of those usually contain
organic phosphorous, which is very toxic. These usually are ingested
accidentally to contaminate food and water, also alcohol and
cigarette smoking in this category, both cause intrauterine growth
retardation and also delayed mental development in later life after
birth with small stature.
b. Medicinal chemicals : one should consider that any drug is not safe
during the early weeks post-fertilization and medication should be
taken cautiously of the most properly documented drug in causing
congenital malformation is :
i. Thalidomide: a drug used for sedation of hyperemesis
gravidarum, usually caused amelia and phocomelia
Phocomelia

Anti-convulsant, Anticoagulant, Cytotoxic drugs and hormones: like progesterone,
estrogen and cortisone.
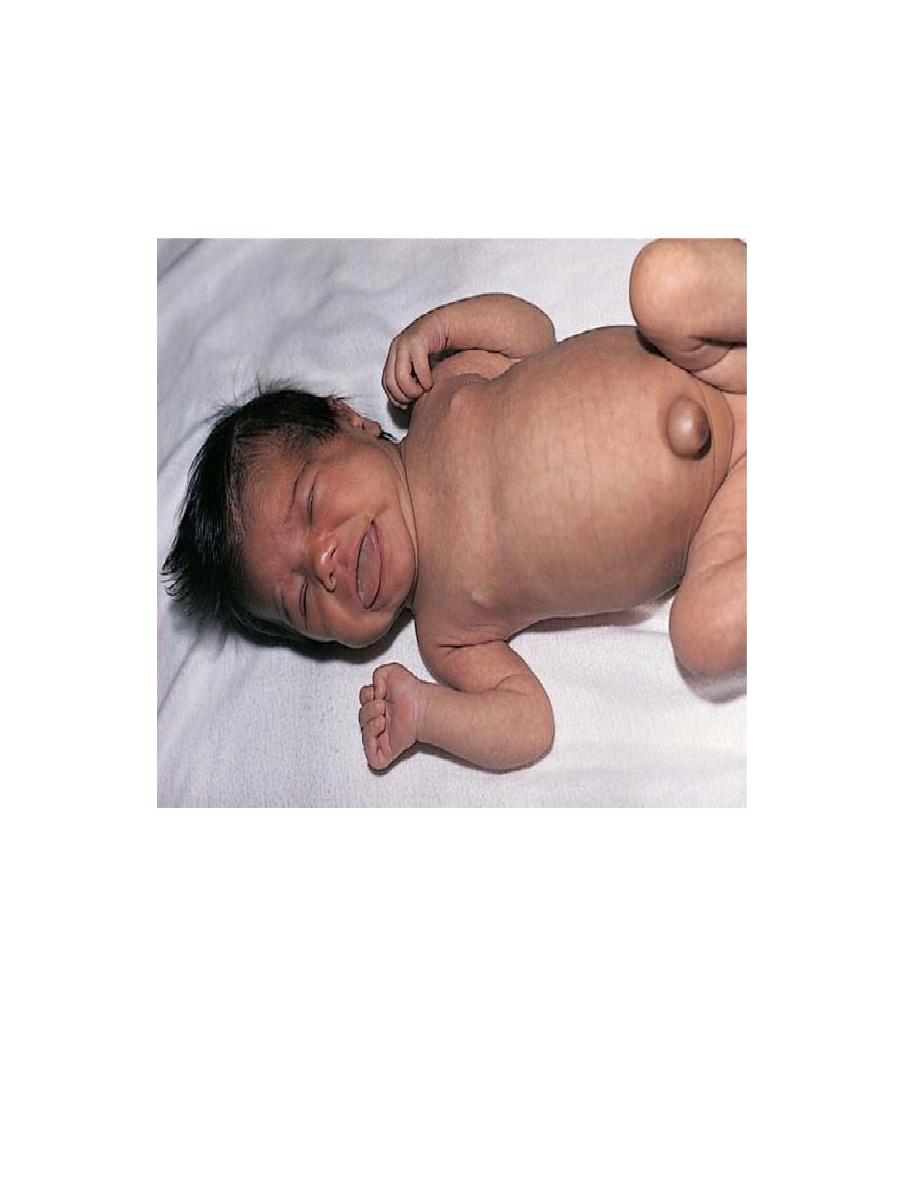
3. Maternal disorders
a. Diabetes, whether treated or not. Cause macrosomia of fetus
Macrosomic baby
(infant of diabetic
mother) with caudal
regression. Notice
femoral hypoplasia
b. Maternal phenylketonuria (PKU), which is a genetic disease that
could be treated. Females may reach reproduction with normal state
but their high phenylalanine that results from their deficiency of
phenylalanine hydroxylase enzyme will destroy the developing brain
to cause mental retardation in the fetus.
c. Thyroid: maternal hypothyroidism will cause a high degree of thyroid
stimulating hormone that will suppress the fetal thyroid gland
resulting in a state of hypothyroidism after a short period of
hyperstimulation (cretinism).
