Professor Dr. Ali Abid Saadoon
Epidemiology and Control of Viral Hepatitis A infectionHepatitis A Infection (HAV) (Infectious Hepatitis)
The Causative Agent:Hepatitis A Virus:
27 nm picornavirus single stranded RNA virus
Nonenveloped, acid and heat stable
Reservoir of infection:
Humans are the only reservoirs of infection (Acute cases because there is No chronic carrier state)Hepatitis A Transmission
Close personal contactHousehold or sexual contact
Daycare centers
Fecal-oral contamination of food or water
Food handlers
Raw shellfish
Blood-borne (rare)
Injecting drug users
might develop mayalgia, arthralgia & alteration of smell & taste.
Later (after several days to one week) : Jaundice with dark urine & clay stool.
Asymptomatic (anicteric) infection is common especially in children.
Symptomatic (Icteric) infection occurs in:
<10% of Children under 6 years of age,
50-60% of Children from 6-14 years old,
70%-80% of adults and children over 14,
Incubation Period:15-50 days or (2-6 weeks)
Clinical Features:
Initially (pre-ecteric stage): Sudden onset of fever, anorexia, malaise, nausea, vomiting, & abdominal discomfort. Some patients
Consequences of hepatitis A infection
Complete recovery without sequelae or recurrence in the majority of casesImmunity after infection lasts for life
No chronic carrier state.
Case fatality rate increases with increasing age
Age-specific Mortality Due to Hepatitis A
Age group Case-Fatality(years) (per 1000)
<5 3.0
5-14 1.6
15-29 1.6
30-49 3.8
>49 17.5
Total 4.1
Period of Communicability:
HAV is found in stool 2 weeks after exposure to infection reaching a peak levels the week or two before onset of symptoms.
Infectivity is higher towards the end of the incubation period.
The number of viruses falls after the patient becomes jaundiced. Most cases are non-infectious after the first week of jaundice.
When the patient becomes jaundiced &comes under the care of the doctor he is probably no longer excreting the virus & no longer infectious
Diagnosis
Clinical features (The clinical course of acute hepatitis A is indistinguishable from that of other types of acute viral hepatitis )
Urine bilirubin in the pre-ecteric stage
Liver function test
Diagnosis is established by the detection of :
IgM Anti HAV : appears 4 wks after exposure and may remain detectable for 4-6 months. It indicates acute infection
IgG Anti-HAV : peaks during convalescence and persists for life. Indicates immunity acquired from past infection or immunization
Control measures
General Preventive measures:Good sanitation & personal hygiene with special emphasis on hand washing particularly among food handlers.
Provision of safe water supply & proper sewage disposal.
Specific Preventive measures:
Measures for patients:Isolation is not necessary
Strict personal hygiene is required in homes where a case has occurred
Treatment of patients
- No specific treatment
- Bed rest is essential
Measures for contacts
:
Investigation of contacts searching for other cases
Post-exposure prophylaxis:
Immune globulin 0.02 ml/kg IM within 2 weeks of exposure.
Post-exposure prophylaxis (PEP) is any prophylactic measure started immediately after exposure to a disease (such as a disease caused by virus), in order to prevent the disease in healthy individuals
Indications:
Sexual contactsClose household contacts.
Staff and children at day care center exposed to a patient with Hepatitis A.
Food handlers exposed to a patient with Hepatitis A.
Preexposure
Immune GlobulinVACCINATION
Inactivated HAV Vaccine
Parenteral
Safe & well tolerated
Protective efficacy rate 95-100%
Protective antibodies are seen within 15 days of vaccination
Protection for >10 years
Dose & schedule
Age: ≥ 2 years
2 doses at 6-12 months interval
Indications for Hepatitis-A Vaccine
Persons with chronic liver disease (e.g. hepatitis B or C)
Homosexuals
Travelers to highly endemic areas
Drug users( drug addicts), particularly injection drug users
Persons at occupational risk for infection
2nd lec
Hepatitis B infectionCausative Agent
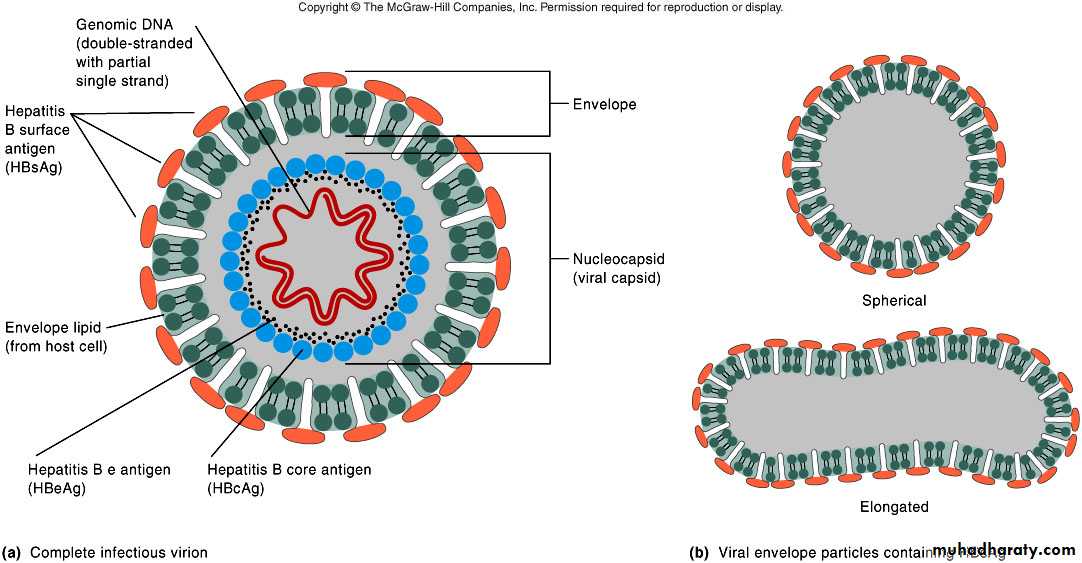
Hepatitis B virus (HBV)Double stranded DNA genome
Enveloped
Virus contains 3 important HBV antigens
HBsAg: Surface antigen
HBcAg:Core antigen
HBeAg:e antigen
Global Distribution of Chronic Hepatitis B Carriers
More than 2 billion people worldwide have been infected with HBV, and an estimated 350-400 million of those people have become chronic carriers of the virus.The prevalence varies widely from region to region of the world. It is highly endemic in China, Southeast Asia, and Africa, but occurs less frequently in North America, Western Europe, and Australia.
>1 million people die annually of HBV-related chronic liver disease
Reservoir :
Man is the only reservoir of infectionSource of infection:
Acute symptomatic & A symptomatic cases
Patients with chronic infection
Modes of Transmission:
Hepatitis B is transmitted through exposure to body fluids containing the virus:Perinatal
Sexual transmission
Parentral/percutaneous,
blood / blood products, needle stick, injection drug use, tattooing and piercing with contaminated instruments, acupuncture.
Non-sexual person-to-person contact, e.g., household contact, Shared toothbrushes, razors, and towels
Concentration of Hepatitis B Virus in Various Body Fluids
Low or not detectable
Moderate
high
Sweat
Semen
Blood
tears
Vaginal discharge
Serum
Breast milk
Saliva
Wound exudate
Incubation period:
1-6 months ) Average 2-6 months)Clinical Features :
• Similar to hepatitis A• Symptomatic infection increases with the increase in age
• In children < 5 yrs: <10% develop clinical jaundice
5 yrs and above : 30%-50% develop clinical jaundice
Outcomes of HBV Infection
Three outcomes:
• Recovery from symptomatic & asymptomatic infection
• Death from fulminant acute hepatitis
• Chronic carrier, usually for life
Age is the major risk factor in determining the carrier state, the younger the age , the higher the risk of becoming a carrier:
• neonates and infants ; 80-90%
• 1-4 year olds ; 30-50%
• adults ; 2-5%
Diagnosis
• Abnormal liver function test• Serological tests: many serological tests are used for the diagnosis of acute and chronic hepatitis B infection.
• HBsAg - used as a general marker of infection (acute and chronic infection).
• Anti-HBc IgM - marker of acute infection.
• Anti-HBcIgG – a marker of past or chronic infection.
• HBeAg - indicates active replication of virus and therefore infectiveness.
• Anti-Hbe – indicates that the virus no longer replicating. However, the patient can still be positive for HBsAg .
• Anti-HBs- used to indicate recovery and/or immunity to HBV infection.
• HBV-DNA - indicates active replication of virus, more accurate than HBeAg . Used mainly for monitoring response to therapy.
Period of communicability
• Body fluids &blood of affected individuals are infective several weeks before onset of symptoms & remain infective throughout the acute clinical stage & during the chronic carrier state.
• The infectivity of chronic carriers varies from highly infectious ( HBeAg +ve ) to relatively lack infectivity ( Anti-HBe +ve
Treatment
• No specific treatment for typical acute viral hepatitis.• Symptomatic treatment is recommended.
• Patient isolation or hospitalization is rarely necessary.
Preventive and control measures
• Vaccination• Screening of high risk populations
• ٍScreening of blood products
Health education to:
Avoid sharing of items that might get contaminated with blood like toothbrush, razor, & nail clippers).
Avoid getting tattoos because transmission may occur through unsterilized tattoo or piercing instruments.
Avoid sharing needles or syringes
Vaccination (pre-exposure prophylaxis)
Highly effective recombinant vaccine is available:• Protective efficacy rate 85-95%
• Immunogenic in healthy subjects( >95%)
• Safe and well tolerated
Vaccination is recommended for:
• high risk groups only (In areas of low-endemicity).
Cont.
All Neonates as universal vaccination in many countries (In hyper-endemic &moderately endemic areas ).
Recommended dosage & schedule
3 IM doses 0,1,6 months
Dose 0.5ml for children from birth - 10 years
1 ml for older children and adults.
In Iraq
3 IM doses to infants at birth, 2 months, and 6 months.
Hepatitis B Risk groups
• People exposed to blood as a result of their occupation or of medical treatment; health care workers; recipients of blood products; haemodialysis patients & staff.• Household contacts & sexual partners of infected persons.
• Homosexuals & sexually promiscuous
• Injecting drug users (IDUs); drug addicts
• Babies born to HBsAg-positive mothers.
Post-exposure prophylaxis
Include :Hepatitis B Immunoglobulin (HBIG)
Hepatitis B Vaccine
• Hepatitis B Immunoglobulin (HBIG) may be used to protect persons who are exposed to hepatitis B. It is particularly effective within 48 hours of the incident.
• Protective efficacy exceeds 95%
• Dose
Post-exposure prophylaxis
- 0.06 ml/kg as early as possible after exposure
- Infants 0.5 ml
Indications
Neonates of HBsAg-positive mothers (should be given within <12 hours
Accidental needle brick (within 24 hours)
Susceptible sexual contacts of cases (within 14 days)
Household contacts
Post exposure prophylaxis for accidental needle brick
The measures depend on the vaccination status of the exposed person :Unvaccinated exposed person:
Hepatitis B immunoglobulin (HBIG) 0.06 ml/kg IM immediately . Initiate hepatitis B vaccination series within 7 days
Previously vaccinated exposed person:
HBIG 0.06 ml/kg IM immediately plus HB Vaccine booster
OR HBIG 0.06 ml/kg IM immediately and repeat it after 1 month.Post exposure prophylaxis for houshold contacts:
For Infants (<1 year) : HBIG & vaccination (3 doses)
Others: Vaccination only (3doses)