THE Cardiovascular system
• By• Dr. Rabei Al Dubooni
• Assist. Prof.
Objectives: The following lectures aim to teach the student about:
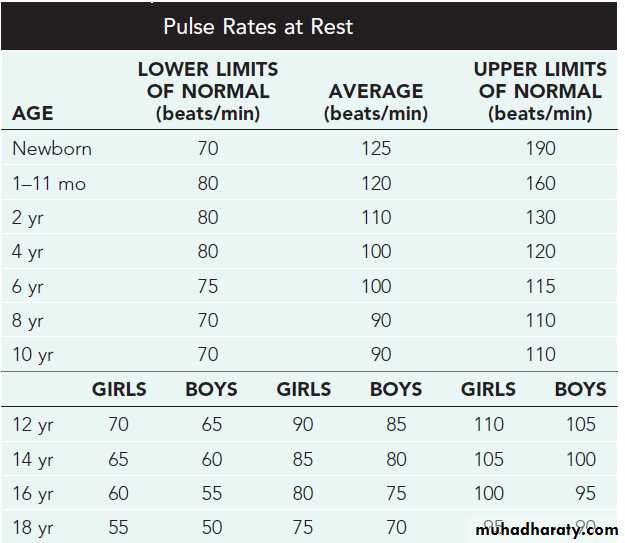
Fetal circulationNormal values of pulse rate in pediatrics
Congenital heart diseases
Cyanotic: TOF, TGA, TA, EA, TAPVR, Approach to neonatal cyanosis
Acyanotic: ASD,VSD, PDA.
Obstructive: Coarctation of Aorta.
Heart failure in infancy and childhood: Etiology, presentation, diagnosis,& treatment.
Rheumatic fever: Etiology, Diagnosis, Treatment, Prevention.
Infective endocarditis : Etiology, Diagnosis, Treatment, Prevention.
Cardiomyopathies : types with special focus on dilated CMP.
Supraventricular tachycardia.
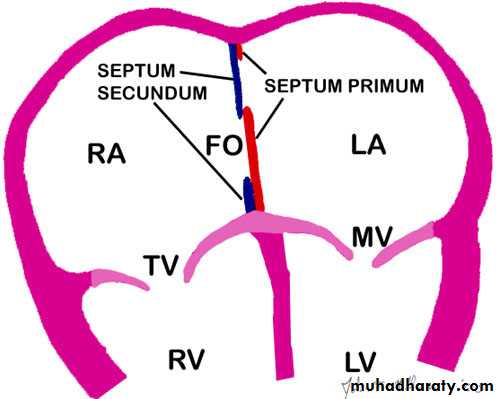
Fetal circulationThree cardiovascular structures unique to the fetus are important for maintaining this parallel circulation: the ductus venosus, foramen ovale, and ductus arteriosus.The highest O2 level provided to the fetus, is only 30-35 mm Hg.
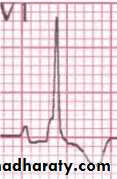
In the human fetus, which has a larger percentage of blood flow going to the brain, RV output is probably closer to 1.3 times LV flow. Thus, during fetal life the right ventricle is not only pumping against systemic blood pressure, but also performing a slightly greater volume of work than the left ventricle. Thus the RV wall is as thick as the LV wall during fetal and immediate neonatal life, explaining the unique features of the neonatal electrocardiogram (showing whatwould be called right ventricular hypertrophy in an adult)
Patency of these fetal pathways may either provide a lifesaving pathway for blood to bypass a congenital defect (patent ductus in pulmonary atresia or coarctation of aorta , foramen ovale in transposition of great vessels ) or present an additional stress to the circulation (patent ductus arteriosus in premature infant, pathway for right to left shunting in infant with pulmonary hypertension). Therapeutic agents may either maintain these fetal pathways (prostaglandin E1 ) or hasten their closure (indomethacin ). This pharmacology explains why indomethacin and similar drugs are contraindicated during the third trimester.
Cardiovascular System Assessment
HistoryMaternal history :Maternal complications such as gestational diabetes, teratogenic medications, systemic lupus erythematosus, or substance abuse canbe associated with cardiac problems.Cyanosis : Central or Peripheral ClubbingSyncopeChest painHistory
Children do not present with the typical features of congestive heart failure as seen in adults.Age is very important when assessing child.
Infants:
Feeding difficulties
Easily fatigued
Sweating while feeding
Rapid respirations
Older children:
Shortness of breath
Dyspnea on exertion
Physical examination:
Cardinal features of heart failure in children:
• Tachycardia• Rapid respiration
• Tender hepatomegaly
• Pulmonary rales
REMEMBER THAT During physical examination:
− Need to refer to normal heart and respiratory rates for ages to determine tachycardia and tachypnea.− Height and weight should be assessed to determine proper growth.
− Always get upper and lower extremity blood pressures and pulses.
− Hepatomegaly suggests heart failure. Splenomegaly in infective
endocarditis
− Always palpate for femoral pulses and compare with radials.
− Examine for cyanosis and clubbing.
Diagnostic tests
Pulse OximetryChest radiograph for:
° Heart size : on a good inspiratory film, the ct ratio should be less than 55% in infants under 1 year of age and less than 50% in older children and adolescents
° heart shape: ex: BOOT-shaped, Egg on side.
° Lung fields
° Ribs for notching
° Position of great vessels
Electrocardiogram
Echocardiography
Others : MRI, MRA ,cardiac catheterization, angiography, exercise testing
Congenital Heart Diseases
Classification of congenital heart diseases• Group I : Left to right shunts (acyanotic): ASD, VSD, PDA
• Group II: Right to lefts shunts (cyanotic): TOF, TGA,TA, EA
• Group III: Obstructive lesions: AS, PS, COA
Incidence : 8/1000 births
Etiology:
Multifactorial inheritance ( genetic predisposition + environmental factors).
Common environmental factors include: Maternal illness (eg, diabetes, rubella, SLE)Maternal intake of teratogenic agents (eg, lithium, retinoic acid, alcohol, anticonvulsants).Paternal age may also be a risk factor
Genetic Factors
Numerical chromosomal abnormalities, such as Down syndrome (trisomy 21), trisomy 18, trisomy 13, and monosomy X (Turner syndrome), are strongly associated with congenital heart disease. However, these abnormalities account for only about 5% of patients with CHD. Microscopic deletions on chromosomes or single-gene mutations. Often, the microscopic deletions and mutations cause congenital syndromes affecting multiple organs in addition to the heart. Examples include Digeorge syndrome (microdeletion in 22q11.2) and Williams syndrome (microdeletion in 7p11.23). Single-gene defects that cause syndromes associated with CHD like (Marfan syndrome).Acyanotic Congenital Heart Disease
• Left-to-Right Shunt Lesions• Atrial Septal Defect (ASD)
• Ventricular Septal Defect (VSD)
• Atrioventricular Septal Defect (AV Canal)
• Patent Ductus Arteriosus (PDA)
Atrial Septal Defect
• ASD is an opening in the atrial septum permitting free communication of blood between the atria. Seen in 10% of all CHD.There are 3 major types:
• Secundum ASD – at the Fossa Ovalis, most common.• • Primum ASD – is lower in position.
• • Sinus Venosus ASD – high in the atrial septum, associated with anomalous venous return & is the least common.
CLINICAL PRESENTATION
• Females outnumber males 3 : 1 in incidence• • Most are asymptomatic but may have easy fatigability or mild growth failure.
• • Cyanosis does not occur unless pulmonary hypertension is present.
• Examination:
• • Hyperactive precordium, RV heave, fixed widely split S2.
• • II-III/VI systolic ejection murmur at Left SB (2nd intercostal) produced by the increased flow across the RV outflow tract into the pulmonary artery .
• • A mid-diastolic murmur heard over LLSB indicates a large defect.
Diagnosis
• Chest x-ray—varying heart enlargement (right ventricular and right atrial); Increased pulmonary vessel markings.• ECG—right-axis deviation and RVH. In the right precordial leads, an rsR ′ pattern is usually present.
• Echocardiogram gives definitive diagnosis.
• Treatment:
• Surgical or Catheterization closure is generally recommended for secundum ASD with a Qp:Qs ratio >2:1 or in symptomatic children.• • Closure is performed electively between ages 1-3 years to avoid late complications.
• • Surgical correction is done earlier in children with CHF or significant pulmonary hypertension.
Course & Prognosis
Patients usually tolerate an ASD well in the first two decades of life, and the defect often goes unnoticed until middle or late adulthood.Pulmonary hypertension and reversal of the shunt (Eisenmenger syndrome) are rare late complications.
Infective endocarditis is uncommon.
Spontaneous closure can occur, most frequently in children with a defect less than 4 mm in diameter.
Mortality of surgical closure is < 1%.
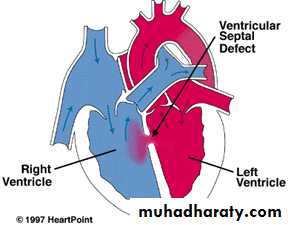
Ventricular Septal Defect
VSD – is an abnormal opening in the ventricular septum, which allows free communication between the Rt & Lt ventricles. Accounts for 25% of CHD. Most VSDs are perimembranous.• Clinical Signs & Symptoms
• • Small (restrictive {near normal RV pressure} )VSD, < 5mm, are usually asymptomatic and 50% will close spontaneously by age 2 years.• • Moderate – large VSDs (nonrestrictive VSDs), almost always have symptoms(dyspnea, feeding difficulties, poor growth, sweating, recurrent pulmonary infections, heart failure) and will mostly require surgical repair
Examination
Small (restrictive)VSDNo lifts, heaves, or thrills are present.
The first sound at the apex is usually covered by the murmur, and the second sound at the pulmonary area is physiologically split.
A medium to high-pitched, harsh pansystolic murmur is heard best at the left sternal border in the third and fourth intercostal spaces. The murmur radiates over the entire precordium.
Large Ventricular Septal Defects With Pulmonary Hypertension
The precordium is prominent, the sternum bulges.Both LV and RV heaves are palpable.
S2 is palpable in the pulmonary area.
A thrill may be present at the lower left sternal border.
S2 is usually single or narrowly split, with accentuation of the pulmonary component. The murmur ranges from grade I to IV/VI and is usually harsh and pansystolic.
A diastolic flow murmur may be heard in the mitral area indicating large size defect.
Imaging Studies
CX-ray findings depend on the size of the VSD.
• Small VSD: usually have normal studies.
• Larger VSD: cardiomegaly, increased pulmonary blood flow, main pulmonary artery segment may be dilated.
ECG :
• normal in small left-to-right shunts.
• Left ventricular hypertrophy (LVH) usually occurs in patients with large left-to-right shunts. Combined ventricular enlargement occurs in patients with pulmonary hypertension caused by increased flow, increased resistance, or both.
Echocardiography
Two-dimensional echocardiography can reveal the size of a VSD and identify its anatomic location.Cardiac Catheterization and Angiocardiography
Catheterization is indicated in those patients with increased pulmonary vascular resistance.• Treatment
• Small muscular VSDs are more likely to close (up to 80%) than membranous VSDs (up to 35%).• • Small VSD - no surgical intervention, no physical restrictions, just reassurance and periodic follow-up and endocarditis prophylaxis if required.
• • Symptomatic VSD - Medical treatment initially with afterload reducers & diuretics.
• • Prophylaxis against infective endocarditis if required.
Indications for surgical closure of a VSD include
1. Patients at any age with large defects in whom clinical symptoms and failure to thrive cannot be controlled medically.
2.Infants between 6 and 12 mo of age with moderate to large defects associated with pulmonary hypertension, even if the symptoms are controlled by medication.
3.Patients older than 24 mo with a Qp:Qs ratio greater than 2 : 1.
4.Supracristal VSD of any size are usually referred for surgery because of their higher risk for developing aortic valve regurgitation .Severe pulmonary vascular disease nonresponsive to pulmonary vasodilators is a contraindication to closure of VSD.
Complications
Large defects lead to heart failure, failure to thrive.Endocarditis
Pulmonary hypertension and Eisenminger syndrome
Patent Ductus Arteriosus
Persistence of the normal fetal vessel that joins the PA to the Aorta.• Normally the duct should close in the 1st week of life.
• Accounts for 10% of all CHD, may be seen in association with other congenital heart lesions and can often play a critical role in some lesions.
• Female : Male ratio of 2:1
• Can be caused by Congenital Rubella.
• The frequency of PDA in preterm infants weighing less than 1500 g
ranges from 20% to 60%.
• Clinical Signs & Symptoms
• Small PDA is usually asymptomatic. Normal peripheral pulses. Murmur.• Large PDA can result in symptoms of CHF and FTT.
• Collapsing arterial pulses.
• Widened pulse pressure .
• Enlarged heart, prominent apical impulse. A thrill may be present.
• The murmur is characteristic. It is a rough machinery murmur maximal at the second left intercostal space.
• Mid-diastolic flow murmur may be heard at the apex.
Imaging Studies
• ECG and Chest x-ray findings are normal with small PDAs• moderate to large shunts may result in a full pulmonary artery silhouette and increased pulmonary vascularity.
• ECG findings vary from normal to evidence of LVH. If pulmonary HTN is present, there is also RVH.
• Echocardiography
• Cardiac Catheterization and Angiocardiography
• Pulse oximetry/arterial blood gas (ABG) analysis usually demonstrate normal saturation
• Treatment:
• Indomethacin, inhibitor of prostaglandin synthesis can be used in premature infants. slow iv infusion (0.1-0.2 mg/kg/dose over 30 min) every 12-24 hr for 3 doses. Iboprufen is also effective and causes less side effects .Both have no effect on duct closure in full term neonates and in children.
• PDA requires surgical or catheter closure.
• Closure is required for heart failure & to prevent pulmonary vascular disease and endocarditis.
• Usually done by ligation & division or intra -vascular coil.
• Prophylaxis against infective endocarditis if indicated.
Obstructive Heart Lesions
• Pulmonary Stenosis• Aortic Stenosis
• Coarctation of the Aorta
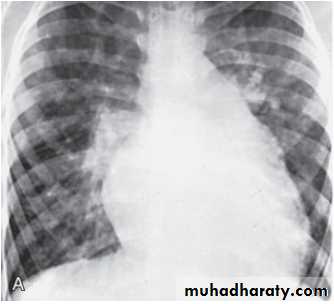
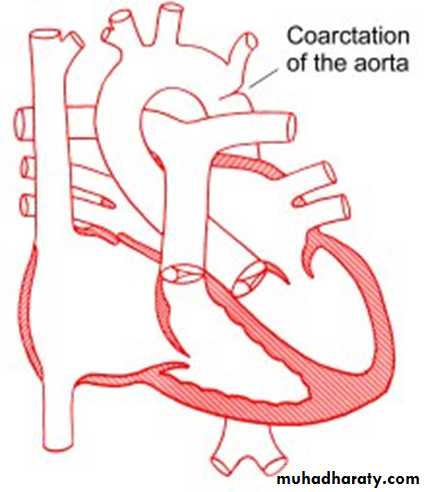
Coarctation of the Aorta
. Coarctation of the aorta is a narrowing in the aortic arch that usually occurs in the proximal descending aorta near the takeoff of the left subclavian artery near the ductus arteriosus (juxtaductal). It is commonly associated with bicuspid valve. Abdominal aorta is rarely involved.More common in Turner’s syndrome.
• Male: Female ratio 3:1.
• Accounts for 7 % of all CHD
• The obstruction to blood flow will lead to LVH.
• Clinical Signs & Symptoms
• Classic signs of coarctation are diminution or absence of femoral pulses. There is delay in femoral pulse compared with radial pulse. Normally, the femoral pulse occurs slightly before the radial pulse. A radial femoral• delay occurs when blood flow to the descending aorta is dependent on
• collaterals, in which case the femoral pulse is felt after the radial pulse
• In normal persons (except neonates), systolic BP in the legs obtained by the cuff
• method is 10-20 mm Hg higher than that in the arms. In coarctation of the aorta,
• BP in the legs is lower than that in the arms (> 15 mm Hg).
• 90% have systolic hypertension of the upper extremities.
• With severe coarctation, heart failure and shock may occur.
• The systolic murmur of coarctation is heard in the left axilla and the left back.
• Investigations
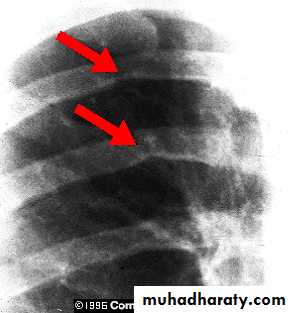
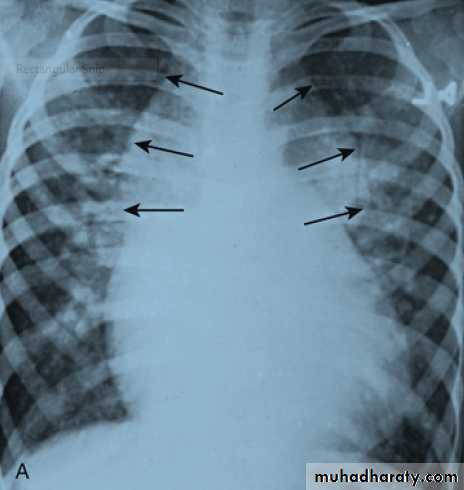
• Cardiomegaly, rib notching on X-ray.• ECG in older children may be normal or may show LVH.
• Echocardiography
• Color Doppler is useful for demonstrating the specific site of the obstruction. Pulsed and continuous wave Doppler studies determine the pressure gradient directly at the area of coarctation.
• MRI and CT when Echo is equivocal
• Catheterization is useful for associated lesions.
Notching of the ribs caused by marked enlargement
of the intercostal collaterals .
• Treatment
• With severe coarctation maintaining the ductus with (prostaglandin E) is essential.• Surgical intervention, to prevent LV dysfunction.
• Angioplasty is used in some centers.
• Re-coarctation can occur, balloon angioplasty is the procedure of choice.
• Prophylaxis against infective endocarditis if indicated.
Cyanotic congenital heart diseases
Congenital heart disease (CHD) produces cyanosis when obstruction to right ventricular inflow or outflow causes intracardiac right-to-left shunting or when complex anatomic defects cause an admixture of pulmonary (deoxygenated) and systemic (oxygenated) venous return in the heart.Cyanosis may be caused also by persistence of fetal pathways, such as right-to-left shunting across the foramen ovale and ductus arteriosus in the presence of pulmonary outflow tract obstruction or persistent pulmonary hypertension of the newborn (PPHN)
Cyanosis from pulmonary edema may also develop in patients with heart failure caused by left-to-right shunts, although the degree is usually less severe
Tetralogy of Fallot
It is the most common cyanotic congenital heart disease
Components:
• Pulmonary stenosis and infundibular stenosis (obstruction to right ventricular outflow)• VSD
• Right- sided (Dextroposition) overriding aorta (overrides the VSD)
• Right ventricular hypertrophy
Hemodynamics
Pulmonary stenosis plus hypertrophy of subpulmonic muscle (crista supraventricularis) → Varying degrees of right ventricular outflow obstruction → Blood shunted right-to-left across the VSD with varying degrees of arterial desaturation and cyanosis.Clinical Picture
• Cyanosis may present at any time but usually not in the first few weeks ( in contrast to TGA )• Paroxysmal attacks of Anoxic Spells (=Tet spells = hypercyanotic spells)
Most commonly start at age 4–6 months
• Predominantly after waking up or after crying.
• Child is irritable and cries.
• Dyspnea& deepening of cyanosis. Decrease or disappearance of the systolic murmur.
• Altered consciousness.
• There may be convulsions due to brain anoxia.
• Frequency varies from once a few days to many attack everyday.
Examination
Generally: growth failure.
Varying degrees of cyanosis .
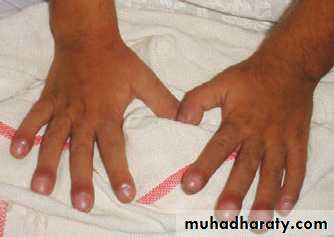
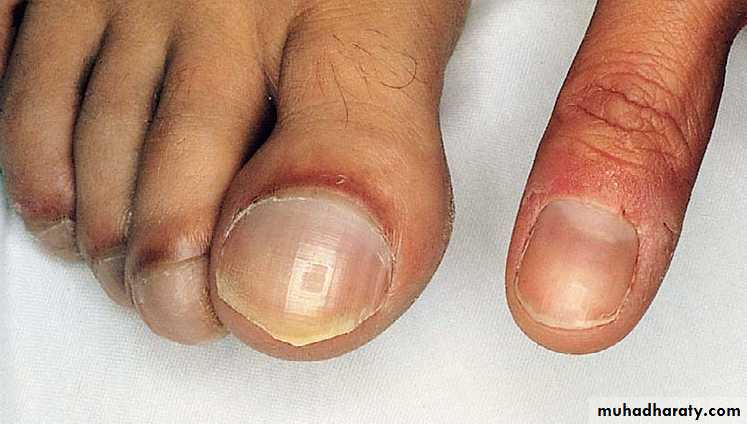
Clubbing of fingers & toes.
Right ventricular impulse at the left sternal border.
A systolic thrill may be felt along the left sternal border in the 3rd and 4th parasternal spaces.
Single S2
Murmur: usually ejection systolic heard best over the pulmonary area.
Squatting increase systemic vascular resistance . This decreases the amount of right-to-left shunt, forcing blood through the pulmonary circuit, and would help ward off cyanotic spells.
Squatting may be seen in older children during exercises.
Investigations
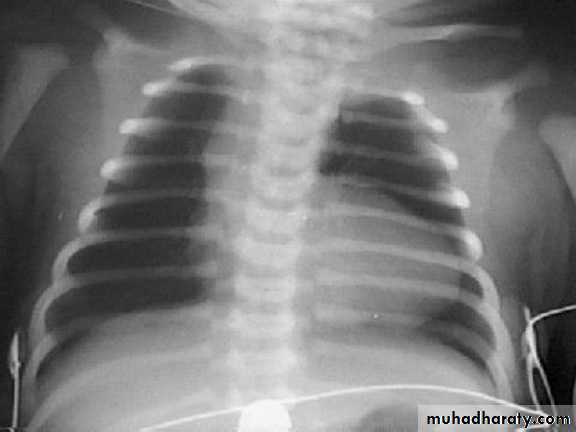
CXR : Boot-shaped normalsize heart
ECG : RAD & RVH
Echocardiography
Cardiac Catheterization
Angiogram
CBC: secondary polycythemia
Complications:
• Each anoxic spell is potentially fatal
• Polycythemia may lead to Cerebrovascular thrombosis especially in patients with iron deficiency.
• Anoxic infarction of CNS
• Brain Abcess
• Infective endocarditis
• Fatal arrhythmias
• Growth failure
Management of anoxic spell:
Knee chest positionHumified O2
Be careful not to provoke the child
Morphine 0.1 -0.2 mg/kg subcutaneously.
Correct acidosis : sodium bicarbonate iv 1 mmol/kg slowly
Propranolol start (0.1mg/kg/iv) slowly during spells followed by (0.5 to 1.0) mg/kg/6hourly orally (prophylaxis) until surgery is performed.
Vasopressors: Methoxamine or Phenylephrine iv drip
The onset of Tet spells usually prompts surgical intervention
Surgical repair for tOF
Palliative procedure: Blalock Taussig shuntSubclavian a. to Pulmonary a. anastomosis
Corrective surgery: complete surgical repair with VSD closure and removal of the pulmonary stenosis can be performed electively at 4-6 mo of age.
Transposition of Great Areries (TGA)
Aorta originating from the right ventricle, and pulmonary artery originating from the left ventricle.Accounts for 5-7% of all congenital heart disease.
Survival is dependent on the presence of mixing between the pulmonary and systemic circulation( associated ASD, VSD, or PDA).
50% of patients have a VSD.
Usually presents in the first day of life with profound cyanosis without respiratory distress.
DTGA is more common in infants of diabetic mothers and in males (3 : 1).
Examination :
Cyanosis in an otherwise healthy looking babyLoud S2
Loud VSD murmur if it is present
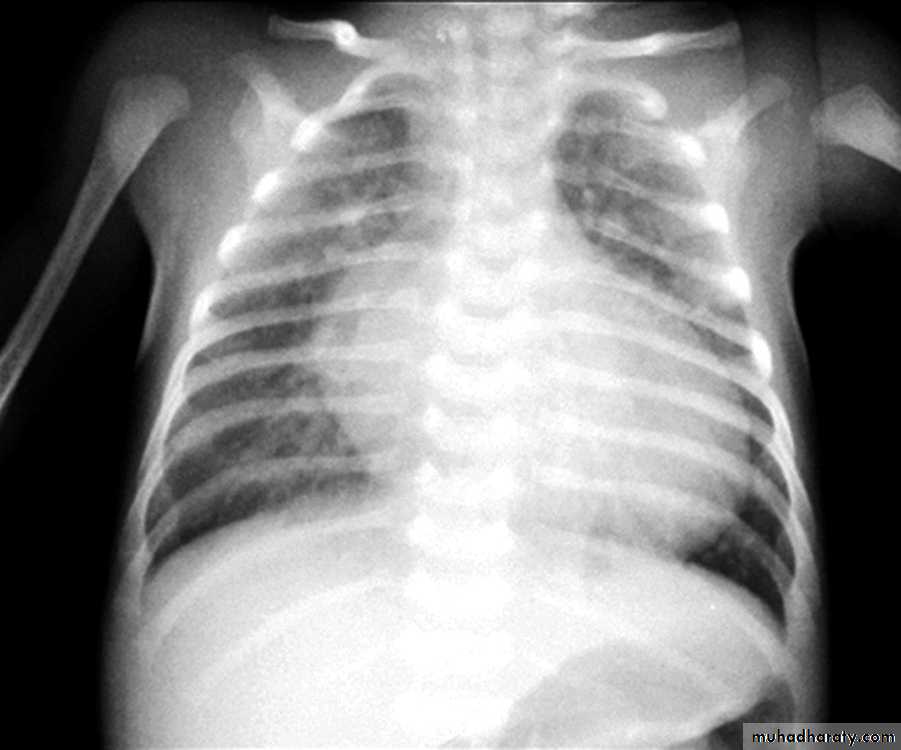
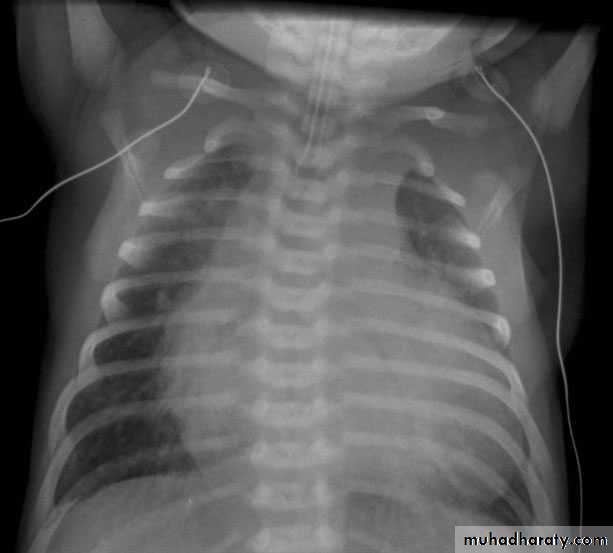
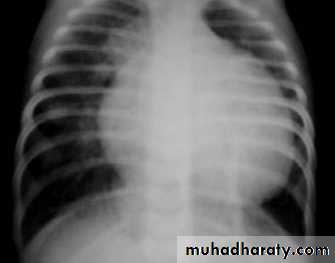
CXR : Egg on side & narrow mediastinum
ECG : frequently looks normal
Echocardiography
Cardiac Catheterization and Angiocardiography
Acute (Emergency) management in newborn baby
When transposition is suspected, an infusion of prostaglandin E1 (PGE1 ; 0.01- 0.20 μg/kg/min) should be initiated immediately to maintain patency of the ductus arteriosus and improve oxygenation. If significant hypoxia persists on prostaglandin therapy, Rashkind balloon atrial septostomy should be done.
Surgical repair Aterial switch (old style).
Arterial switch (ASO) : performed at age 4–7 days
TRICUSPID ATRESIA:
The absence of the tricuspid valve results in a hypoplastic right ventricle. All systemic venous return must cross the atrial septum into the left atrium.A PDA or VSD is necessary for pulmonary blood flow and
survival.
Clinical Manifestations
Usually severely cyanosed since birth.Single S2.
If a VSD is present, there may be a murmur.
ECG: LVH & LAD with right atrial enlargement .
Echocardiography &Cardiac Catheterization : for definite diagnosis
Treatment:PGE-1, and minimal O2 to maintain ductal patency
Palliative procedure: Blalock-Taussig procedure
Definitive: bidirectional cavopulmonary shunt (bidirectional Glenn) and Fontan procedure.
The combination of cyanosis and left axis deviation on the ECG is highly suggestive of tricuspid atresia
Ebstein anomaly
Downward displacement of abnormal tricuspid valve into right ventricle; the right ventricle gets divided into two parts: an atrialized portion, (which is thin-walled), and a smaller normal ventricular myocardium (the really functioning RV).• Right atrium is huge; tricuspid valve regurgitant
• Right ventricular output is decreased because:
− Poorly functioning, small right ventricle
− Tricuspid regurgitation
− Variable right ventricular outflow obstruction—abnormal anterior tricuspid valve leaflet. Therefore, increased right atrial volume shunts blood through foramen Ovale or ASD → cyanosis.
Clinical presentation
− Severity and presentation depend upon degree of displacement of valve and degree of right ventricular outflow obstruction° May not present until adolescence or adulthood
° If severe in newborn → marked cyanosis, huge heart
− Pansystolic murmur of tricuspid insufficiency over most of anterior left chest (most characteristic finding)
Chest x-ray: heart size varies from normal to massive .
pulmonary oligemia in severe cases.ECG: tall and broad P waves, RBBB, WPW (delta wave) may be
associated.
Echocardiography
• Treatment:
− PGE1− Systemic-to-pulmonary shunt
− Then staged surgery
Total Anomalous Pulmonary Venous Return:
Total anomalous pulmonary venous return (TAPVR) occurs when all the pulmonary veins connect to the systemic veins or right atrium instead of draining into the left atrium.The anomalous drainage may be at the supracardiac (e.g. innominate vein or superior vena cava), cardiac (right atrium directly or coronary sinus) or infracardiac (e.g. portal vein, inferior vena cava) level or may be of a mixed type with more than one anomalous site.
Presentation
Congestive heart failure and varying degrees of cyanosis.A prominent left parasternal heave (RV volume overload).
The second heart sound may be widely split and fixed with respiration(due to associated ASD)
There is a flow murmur across the pulmonary and mitral valve.
ECG shows right ventricular hypertrophy .
CXR will show increased pulmonary vascular markings. The ‘snowman’ sign (enlarged superior mediastinum) is seen in supracardiac TAPVR to the left innominate vein in older infants.
Echocardiogram defines the anatomy sufficiently.
Treatment: Cardiac surgery
• Approach to neonatal cyanosis
• Central cyanosis is a bluish discoloration of the skin, mucus membranes and tongue that is observed when deoxygenated hemoglobin is > 3g/dl in arterial blood or > 5g/dl) in capillary blood.Another type of cyanosis, called peripheral cyanosis, involves a bluish discoloration of the skin but sparing of the mucus membranes & tongue. In this type, a normal PaO2 value is detected. Vasomotor instability, and vasoconstriction caused by cold, low cardiac output and polycythemia can all slow movement through the capillaries and lead to peripheral cyanosis.
Peripheral cyanosis is often a normal finding in newborns, especially when only the extremities are affected (acrocyanosis) due to vasoconstriction as a result of transient hypothermia; however, it is important to rule out serious causes of peripheral cyanosis, such as .sepsis
Pregnancy & labor HX Associated causes GDM TTN, RDS, CHD, Low BSOligohydramnios Pulmonary hypoplasis.Hypertension IUGR, polycythemia, hypoglycemia
Maternal age Down’s S.Lithium(1st trimister) Epstein anomalyPROM, fever, GBS +ve SepsisSedatives/anesthetics ApneaC-section TTN, RDS, PPHNPreterm infant RDS, ApneaMeconium MAS
Differential Diagnosis : 1.CNS (associated with other CNS manifestation)2.Respiratory(associated with dyspnea and respiratory findings)3.Right to Left shunt(little or no dyspnea , possibility of murmurs)4.Methemoglobinemia
Hyperoxia Test This test is usually performed using a hood rather than nasal cannula or face mask, to best guarantee delivery of almost 100% oxygen to the patient. If the PaO2 rises above 150 mm Hg during 100% oxygen administration, an intracardiac right-to-left shunt can usually be excluded.
In patients with pulmonary disease, PaO 2 generally increases significantly with 100% oxygen as ventilation-perfusion inequalities are overcome. In infants with cyanosis from a CNS disorder, the PaO 2 usually normalizes completely during artificial ventilation.
Although a significant heart murmur usually suggests a cardiac basis for the cyanosis, several of the more severe cardiac defects (e.g., TGA) may not initially be associated with a murmur. The CX-ray may be helpful in the differentiation of pulmonary and cardiac disease; in the latter, it indicates whether pulmonary blood flow is increased , normal, or decreased.
Two-dimensional echocardiography with doppler is the definitive noninvasive test to determine the presence of CHD. Cardiac catheterization is less often used for diagnostic purposes and is usually performed to examine structures that are sometime less well visualized by echocardiography,
If echocardiography is not immediately available to confirm a diagnosis of cyanotic CHD, the clinician caring for a newborn with possible cyanotic CHD should not hesitate to start a PGE1 infusion (for a possible ductal-dependent lesion).
CONGESTIVE HEART FAILURE
Heart failure occurs when the heart is unable to pump blood at a rate commensurate with metabolic needs (inadequate oxygen delivery). It may be due to a change in myocardial contractility that results in low cardiac output or to abnormal loading conditions being placed on the myocardium. The abnormal loading conditions may be after load (pressure overload, such as with aortic stenosis, pulmonary stenosis, or coarctation of the aorta) or preload(volume overload, such as in ventricular septal defect [VSD], patent ductus arteriosus (PDA), or valvular insufficiency). Volume overload is the most common cause of heart failure in children.Etiology:
CARDIACcongenital structural malformations
● excessive preload
● excessive afterload
non - congenital structural anomalies
● cardiomyopathy
● myocarditis
● myocardial infarction
● acquired valve disorders
● hypertension
● kawasaki syndrome
● arrhythmia (bradycardia or tachycardia)
NONCARDIAC
● Anemia
● Sepsis
● Hypoglycemia
● Diabetic ketoacidosis
● Hypothyroidism
● Other endocrinopathies
● Arteriovenous fistula
● Renal failure
History
Children do not present with the typical features of congestive heart failure as seen in adults.Age is very important when assessing child.
Infants:
Feeding difficulties
Easily fatigued
Sweating while feeding
Rapid respirations
Older children: (Like adults)
Shortness of breath
Dyspnea on exertion
Physical examination:
• Tachycardia
• Rapid respiration• Tender hepatomegaly
• Pulmonary rales
IMAGING STUDIES
The absence of cardiomegaly on a chest x-ray usually rules out the diagnosis of heart failure.Echocardiogram assesses the heart chamber sizes, measures myocardial Function, and diagnoses congenital heart defects when present. Also measures Ejection Fraction the normal range is 55–65%.
ECG : Can show different arrhythmias, chamber hypertrophy, myocaditis, pericarditis…
MRA is also very useful in quantifying left and right ventricular function, volume, and mass as well as coronary artery anatomy.
Other InvestigationsBlood gas and pHS Na+, K+ (specially in patients receiving diuretics)Serum B-type (brain) natriuretic peptide (BNP) a cardiac neurohormone released in response to increased ventricular wall tension, is elevated in patients with heart failure.
Treatment
The goals of medical therapy for congestive heart failure include the following:
Reducing the preload
Reducing the afterloadEnhancing cardiac contractility
Improving oxygen delivery
Enhancing nutrition
General measures:
Bed rest and limit activities.Nurse propped up or in semi-sitting position
Expressed breast milk for small infants
Increase dietary calories.
Fluid and salt restriction in volume overloaded
Correction of anemia ,acidosis, hypoglycemia and hypocalcaemia if present
Oxygen
Treatment: Phrmacological therapy
Preload reduction:1. Diuretics: (oral) or (IV) diuretics (furosemide, thiazide. metolazone).
2. Venous dilators (eg: nitroglycerin).
Contractility support:
1. Dopamin, dobutamin
2. Digoxin
Afterload reduction
1.Oral Angiotensin converting enzyme inhibitors (ACEI) e.g: captopril, enalapril
2. IV hydralazine, nitroprusside, or alprostadil
Doses:
Furosemide: 1 - 2mg/kg/dose PO or IV .Main : 1- 4 mg/kg/d
Chlorothiazide: 10 - 40 mg/kg/d PO divided bid
IV Dopamine : 5-10 mcg/kg/min IV (usual dosage; maximal dosage may be up to
28 mcg/kg/min)
Dobutamine: 5-10 mcg/kg/min iv
Captopril: 0.1- 6 mg/kg/d orally divided q8h
Enalapril: 0.1- 0.5 mg/kg/d orally divided doses, not to exceed 0.5 mg/kg/d
Carvidolol( β- blocker) : 0.2-0.4 mg/kg/dose bid.
Spironolactone: 1-3 mg/kg/day.
Milrinone (phosphodiesterase inhibitor) :is useful in treating patients with low cardiac output who are refractory to standard therapy IV infusion at 0.25-1 μg/kg/min .
Digoxin: start with rapid digitalization then shift to maintenance:
Rapid digitalization can be achieved by administration of “total digitalizing dose (TDD) as follow:Premature: 20 μg/kg (0.02 mg/kg).
Full-term neonate (up to 1 mo): 20-30 μg/kg
Infant or child: 25-40 μg/kg
Adolescent or adult: 0.5-1 mg in divided doses
NOTE: these doses are PO; IV dose is 75% of PO dose.
½ TDD is given initially followed by 1/4 TDD in 2 doses 12 hrs apart.
Maintenance digoxin : 5-10 μg/kg/day, divided q12h
Managing acute congestive heart failure (acute pulmonary edema) in children:
Admit to the ICU.
Head up position.Oxygen.
IV furosemide: 1-2 mg/kg.
Dopamine if there is associated hypotension: (5-10 mcg/kg/min) .
Nitrates ( nitroglycerin1–3 mcg/kg/min ) as venodilators if ↑ pulmonary capillary wedge pressure
???Digoxin (TDD):
Rheumatic fever:
Due to an immunologic reaction that is a delayed sequela of group A beta-hemolytic streptococcal infections of the pharynx.A family history of rheumatic fever and lower socioeconomic status are additional factors.
The infection often precedes the presentation of rheumatic fever by 2 to 6 weeks. Streptococcal antibody tests, such as the antistreptolysin O (ASOT) titer, are the most reliable laboratory evidence of prior infection.
Diagnosis:
= {2 Major or (1Major + 2Minor) Jones Criteria} + Evidence of antecedent Streptococcal infection(recent scarlet fever, positive throat culture, or elevated ASOT or other antistreptococcal antibodies).Major : Migratory polyarthritis, Carditis, Erythema marginatum, Chorea, Subcutaneous nodules.
Minor : Fever, Arthralgias, Previous rheumatic fever, leukocytosis, elevated ESR or CRP, and prolonged PR interval.
.
Changes in NEW REVISED JONES CRITERIA 2015
Carditis may be subclinical (Echo evidence of aortic and/or mitral regurgitation)Minor criteria = Fever (≥38 C) , Arthralgia, Elevated ESR( >30)(or
CRP >3), Prolonged PR interval.
Treatment
Bed restBenzathine Penicillin 1.2 million unit im. OR oral penicillins for 10 days
Salicylate: 50-70 mg/kg/day in 4 divided doses PO for 3-5 days, followed by 50 mg/kg/day in 4 divided doses PO for 3 wks and half that dose for another 2-4 wks
Prednisolone:for Patients with carditis and more than minimal cardiomegaly and/or congestive heart failure: 2mg/kg/day in 4 divided doses for 2-3 wk, followed by half the dose for 2-3 wk
and then tapering of the dose by 5 mg/24 hr every 2-3 days
Prevention:
Benzathine Penicillin: 600,000 IU for children weighing ≤27 kg ; 1.2 million IU for children weighing >27 kg every 4 wk.Duration of prophylaxis for the patient:
without carditis: 5years or until he is 21 years old.(the longer)
With carditis : 10 years or until age is 21 .(the longer)
With carditis and residual valve disease: 10 years or until age is 40 .(the longer)
Infective Endocarditis ;
Etiology/epidemiology
− Most common are Streptococcus viridans (alpha hemolytic) and Staphylococcus aureus
− Organism associations
° S. viridans—after dental procedures
° Group D streptococci—large bowel or genitourinary manipulation
° Pseudomonas aeruginosa and Serratia marcescens— intravenous drug users
° Fungi—after open heart surgery
° Coagulase-negative Staphylococcus—indwelling intravenous catheters
− Highest risk with prosthetic valve and uncorrected cyanotic heart lesions
• Clinical presentation
− Prolonged intermittent fever, weight loss, fatigue, myalgia, arthralgia, headache,nausea, vomiting, early finger clubbing.
− New or changing heart murmur
− Splenomegaly, petechiae, embolic stroke, CNS abscess, CNS hemorrhage, mycotic
aneurysm (all more with Staphylococcus), Glomerulonephritis (↓ C3).
− Skin findings—rare; late findings (uncommon in treated patients); represent vasculitis
from circulating Ag-Ab complexes; if present, are highly suggestive
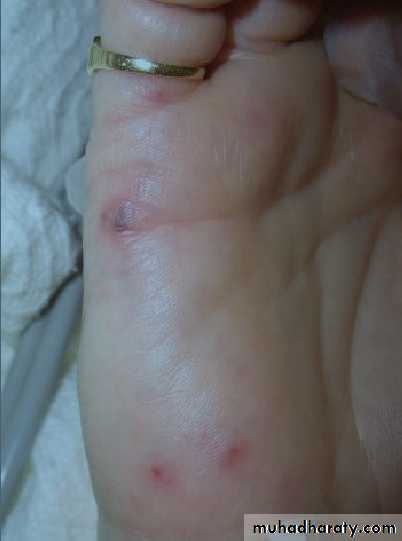
° Osler nodes—tender, pea-sized, intradermal nodules on pads of fingers and toes
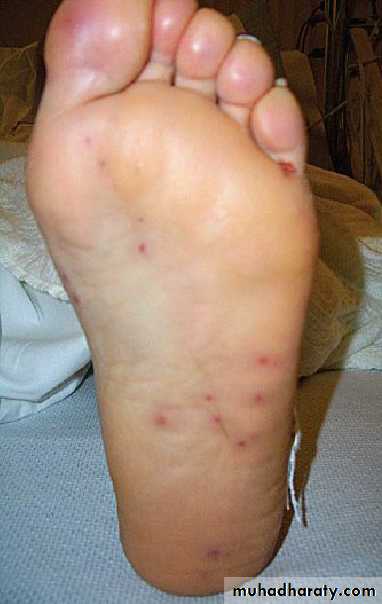
° Janeway lesions—painless, small erythematous or hemorrhagic lesions on
palms and soles
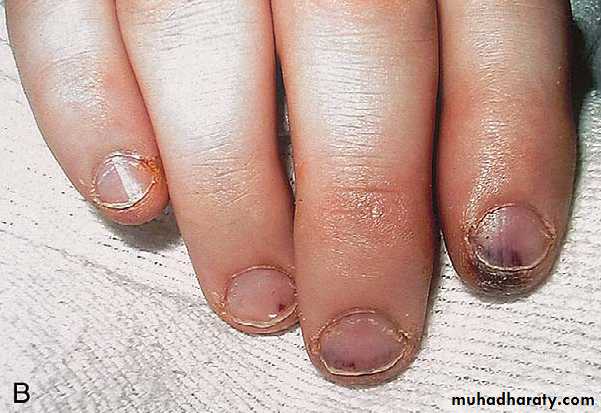
° Splinter hemorrhage—linear lesions beneath nail beds
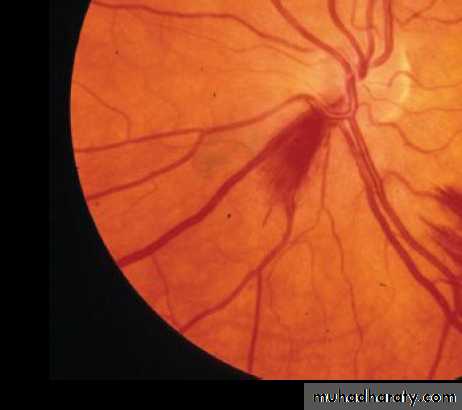
º Roth spots —retinal exudates
Diagnosis
The critical information for appropriate treatment of infective endocarditis is obtained from blood cultures . all other laboratory data are secondary inImportance.
Three to 5 separate blood collections should be obtained after careful preparation of the phlebotomy site. Timing of culture is not important because bactremia is usually constant.
Two-dimensional Echocardiography can identify the size, shape, location, and mobility of the lesion and the presence of intracardiac vegetations(Mass).
The absence of vegetations does not exclude endocarditis, and vegetations are often not visualized in the early phases of the disease or in patients with complex congenital heart lesions.
Electrocardiography should be part of the evaluation and can demonstrate new rhythm disorders such as ventricular ectopy and conduction disorders such as complete heart block.
Diagnosis• 2 major criteria, or• 1 major criterion and 3 minor criteria, or• 5 minor criteria
Complications
− Most common: heart failure from aortic or mitral lesions− Others: systemic or pulmonary emboli, myocardial abscess, myocarditis, valve obstruction, heart block, meningitis, osteomyelitis, arthritis, renal abscess, immune complex−mediated glomerulonephritis.
TREATMENT
Antibiotic therapy must be started after blood cultures are obtained.Vancomycin+ gentamicin, for a 6-week course is the most common regimen( may be modified by the results of culture).
Prevention : According to (AHA 2017):
There is no evidence for IE prophylaxis in gastrointestinal procedures or genitourinary procedures.
Prophylaxis against IE is reasonable before dental procedures that involve manipulation of gingival tissue, manipulation of the periapical region of teeth, or perforation of the oral mucosa in patients with the following:
1. Prosthetic cardiac valves, including transcatheter-implanted prostheses 2. Prosthetic material used for cardiac valve repair.
3. Previous IE. 4. Unrepaired cyanotic congenital heart disease or repaired congenital heart disease, with residual shunts or valvular regurgitation at the site of or adjacent to the site of a prosthetic patch or prosthetic device. 5. Cardiac transplant with valve regurgitation due to a structurally abnormal valve.
6. Patients with permanently damaged valves from rheumatic heart disease should also be considered for prophylaxis.
Recommended propylaxis is
50 mg/kg of oral Amoxicillin for patients < 40 kg andor 2g for those > 40 kg.
This dose is to be given 1 hour prior to procedure.
If the patient is allergic to amoxicillin, then Cephalexin or
Clindamycin or Azithromycin or Clarithromycin are used.
Pediatric Viral Myocarditis
inflammatory disorder of the myocardium that is typically caused by a viral infection.particularly arises from adenovirus and enterovirus infections (eg, coxsackievirus), although many infectious organisms commonly seen in infants and children have been implicated in the disease. Occasionally, myocarditis may be a manifestation of drug hypersensitivity or toxicity.In mild forms, few or no symptoms are noted.Heart failure is the most common presenting picture in all ages.Chest pain may be due to myocardial ischemia or concurrent pericarditis.Dysrhythmias Parents of pediatric patients may refer to a recent, nonspecific, flulike illness , GI symptoms, poor feeding, or rapid breathing.
Investigations:1. General: viral studies , elevated ESR Elevated Creatinine kinase–MB isoenzymes (CK-MB) , Lactate dehydrogenase isoenzyme 1 and Troponin I.2. CX-ray : cardiomegaly and pulmonary edema .3. MRI4. Echo5. ECG: Low-voltage QRS (< 5 mm throughout the limb leads) is the classic pattern,prolonged PR interval, ST depression and T-inversion. Sinus tachycardia, arrythmias and AV block
TREATMENT
Bed rest and Monitoring.
• Control Heart Failure (these patints may have increased
• The use of immunosuppressive agents(including steroids) for the treatment of viral myocarditis is still controversial.
• Control Arrhythmias.
CARDIOMYOPATHIES:
-CONGESTIVE(DILATED)-HYPERTROPHIC-RESTRICTIVEDilated Cardiomyopathy
Pathophysiology- Extensive ventricular dilatation; mostly left ventricle.
- Vast majority are idiopathic (may be familial).
- Other causes-viral infection, endocrine (hypothyroidism), metabolic (storage disease), systemic disease (connective tissue), hereditary muscle or neurologic disease (muscular dystrophies), abnormality of coronary arteries.
Clinical presentation
- Initially nonspecific (respiratory symptoms, failure to thrive, abdominal complaints).- Then findings of failure:
- Tachycardia, decreased pulse pressure, cool and pale skin, decreased pulses, increased jugular venous pressure, hepatomegaly, edema, rales
- Cardiomegaly, mitral insufficiency, tricuspid insufficiency, gallop rhythm
Diagnosis
- ECG-atrial enlargement, left ventricular or right ventricular enlargement; nonspecific T-wave changes
-Chest x-ray--cardiomegaly, pulmonary congestion.
-Echocardiogram-dilatation of left atrium and left ventricle ± right ventricle and decreased contractility; decreased flow velocity across aortic valve with mitral regurgitation.
Prognosis : downward progression; relapse of heart failure; emboli; ventricular arrhythmias & sudden death.
Treatment
- Antifailure.- Antiarrhythmic agents
- May need an implantable cardioverter-defibrillator (ICD)
-Systemic anticoagulation
-Beta blocker (metoprolol, carvedilol)
- Trial of PO carnitine (for possibility of mitochondrial disorder)
-Referral to transplant center
- Pacemakers , including dual-chamber and biventricular pacing therapy, can improve patients with certain underlying electrical derangements.
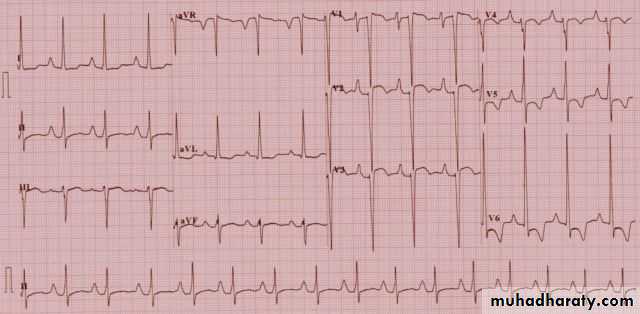
Supraventricular TachycardiThe mechanisms of tachycardia are generally divided into reentrant( either through accessory pathway or without) and automatic (ectopic) mechanisms and can be described by the location of tachycardia origination.Atrioventricular reciprocating tachycardia (AVRT) involves an accessory pathway and is the most common mechanism of SVT in infants
Symptoms and Signs
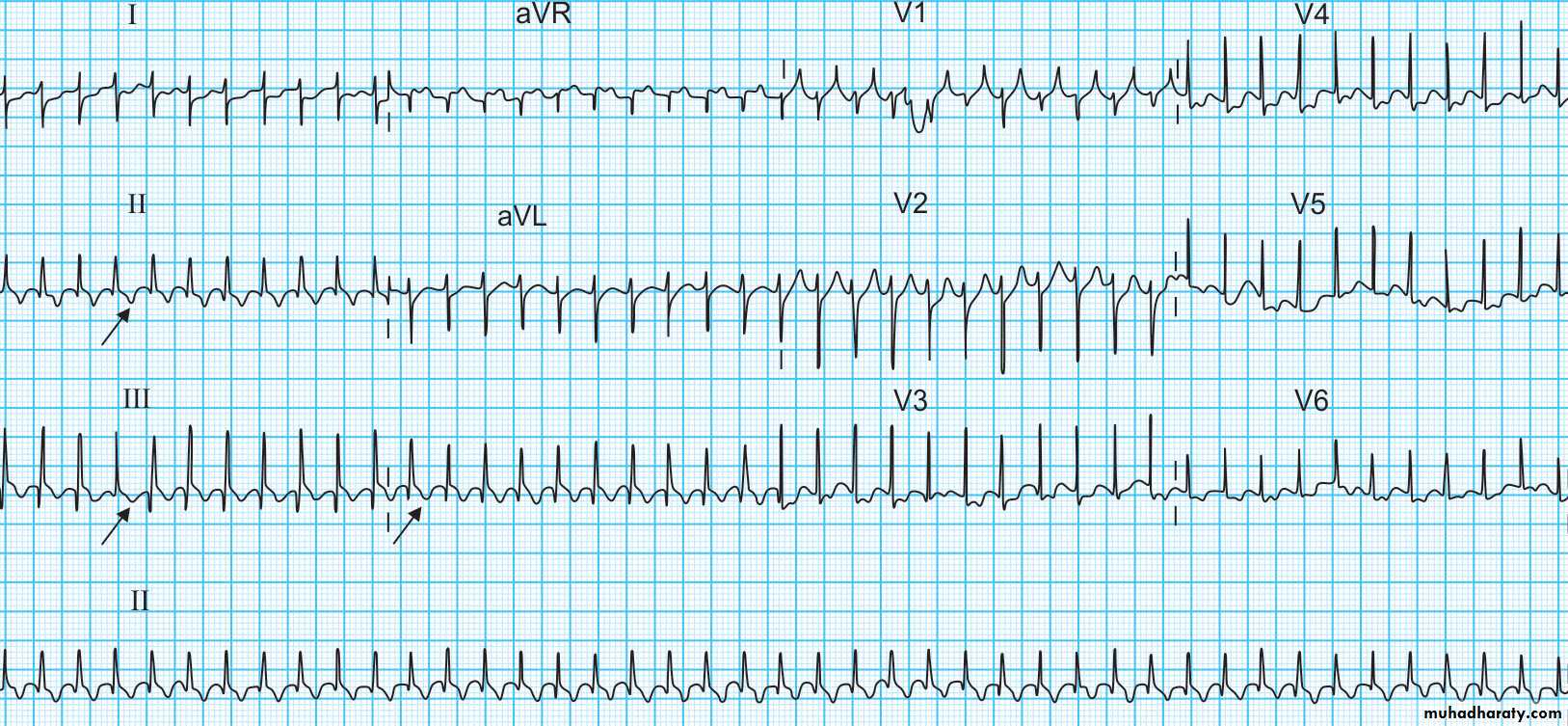
Presentation varies with age. Infants tend to turn pale and mottled with onset of tachycardia and may become irritable. With long duration of tachycardia, symptoms of HF develop. Older children complain of dizziness, palpitations, fatigue, and chest pain. Heart rates range from 240–300 beats/min . HF is less common in children than in infants. Tachycardia may be associated with either congenital heart defects or acquired conditions such as cardiomyopathies and myocarditis.Electrocardiography
ECG is the most important tool in the diagnosis of SVT and to define the precise tachycardia mechanism. Findings include a heart rate that is rapid and out of proportion to the patient’s physical status .. For reentrant mechanisms, the rhythm would be extremely regular with little variability. For automatic mechanisms, the rhythm would be less regular. The QRS complex is usually the same as during normal sinus rhythm. The presence of P waves and their association with the QRS are important in determining tachycardia mechanism. With automatic tachycardias, there is often a 1:1 or 2:1 A:V relationship with P waves preceding the QRS. With atrioventricular nodal reentrant tachycardia, P waves cannot be identified as they are occurring at the same time as the QRS.Acute Treatment
Close monitoring. Correction of acidosis and electrolyte abnormalities1. Vagal maneuvers: the “diving reflex” produced by placing an ice bag on the nasal bridge for 20 seconds (for infants) or by immersing the face in ice water (for children or adolescents) will increase parasympathetic tone and terminate some tachycardias. The valsalva maneuver, which can be performed by older compliant children, may also terminate reentrant tachycardias.
2. Adenosine: Adenosine transiently blocks AV conduction and terminates tachycardias that
incorporate the AV node.. The dose is100–250 mcg/kg by rapid intravenous bolus . It is antagonized by aminophylline and should be used
with caution in patients with sinus node dysfunction or asthma.
3. Transesophageal atrial pacing
4. Direct current cardioversion: (0.5–2 synchronized J/kg)
5. In older children Verapamil 0.1 – 0.2 mg / kg (contraindicated in infants)
Maintenance treatmentβ- blockers (propranolol 1-2 mg/ kg /ddd)Digoxin and verapamil can be used but not in patients with WPW syndrome.