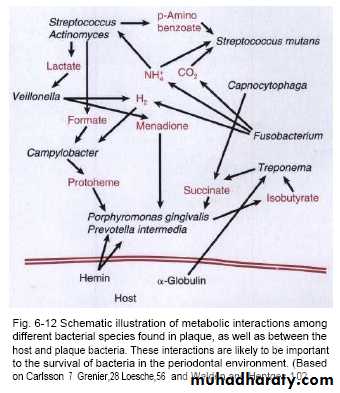
Bacterial interaction
Microbes in the oral environment can interact with each other both in promoting and suppressing the neighbouring bacteria.Mechanisms that accomplish this include:
1-competition for receptors for adhesion by prior occupation of colonizing sites and prevention of attachment of 'late-comers'2-production of toxins, such as bacteriocins, that kill cells of the same or other bacterial species; e.g. Streptococcus salivarius produces an inhibitor (enocin) that inhibits S. pyogenes
3-use of metabolic end-products of other bacteria for nutritional purposes (e.g. Veillonella spp. use acids produced by Streptococcus mutans)
4-coaggregation with the same species (homotypic) or different species (heterotypic) of bacteria, e.g. corn-cob formation .
Nutrition of oral bacteria
Oral bacteria obtain their food from a number of sources.These include Host resources:
• remnants of the host diet always present in the oral cavity
(e.g. sucrose, starch)
• salivary compositions (e.g. glycoproteins, minerals, vitamins)
• crevicular exudate (e.g. proteins)
• gaseous environment
Iron (which released by RBC breakdown) is used in metabolism of Porphmonas gingivalis.
Steroid hormone is used as nutrient for Prevotella intermedia.
Microbial resources:
• extracellular microbial products of the neighbouring bacteria,especially in dense communities such as plaque.
• intracellular food storage (glycogen) granules.
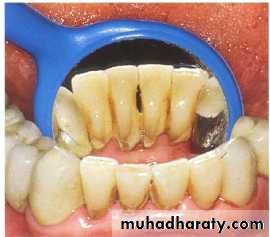
Dental calculus
Calcified deposits on teeth & other solid structures in oral cavity
Its divided into supra & subgingivalIts represented mineralized dental plaque .
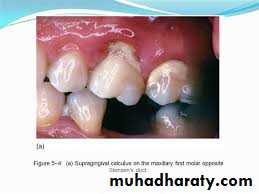
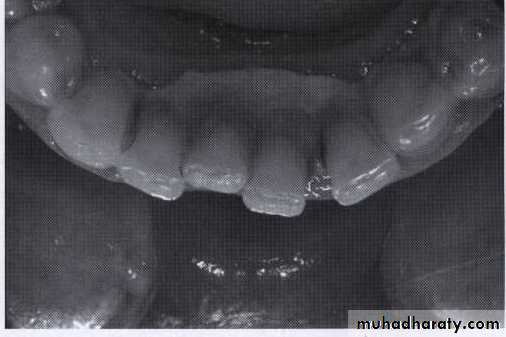
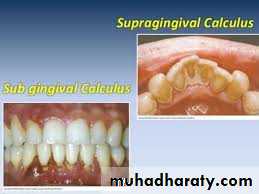
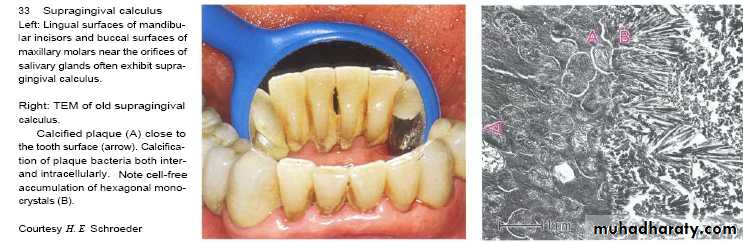
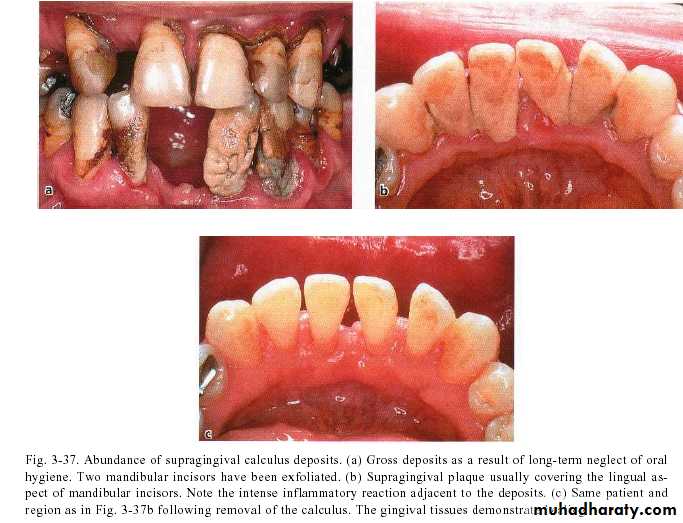
Supragingival calculus
It is yellow-white in colour ,located along gingival margins.
Large amount located at buccal aspect of upper 6 & lingual aspect of lower ant.teeth (next to stenson, warton & bartholin ducts).
Its easily detached, rapidly recur after removal .
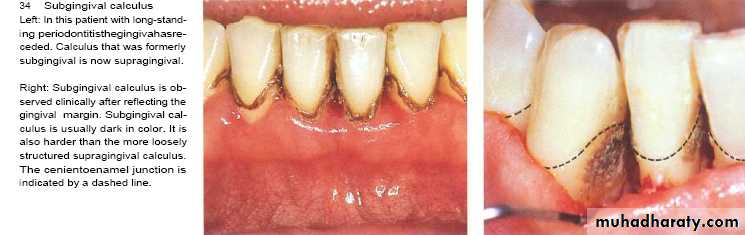
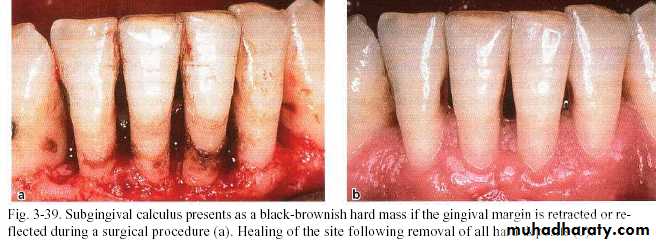
Subgingival calculus
It is usually harder & more tenaciously adhere than supragingival calculus.
It has a brown to black color due to iron pericpetation.
It almost extend to base of pocket but don’t reach juncitional epithelium.
Following gum recession or gingival shrinkage ,it could become supragingival.
Diagnosis of dental calculus
• Supragingival calculus• It can be detected by inspection or probing.
• Subgingival calculus
• It is dark color calculus that shines through thin gingival margin.
• It can be detached by airblast.
• It can be probed in deep pocket.
• It can be seen by reflection of flap during periodental surgery.
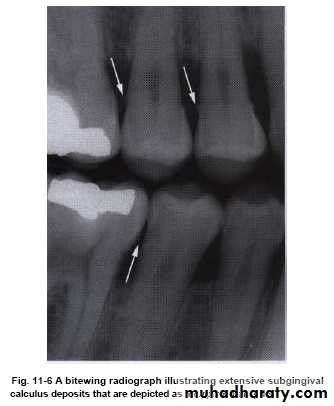
• It can be seen in radiograph (radiopaque projections that protrude into interdental space).
Bacteria and calculus
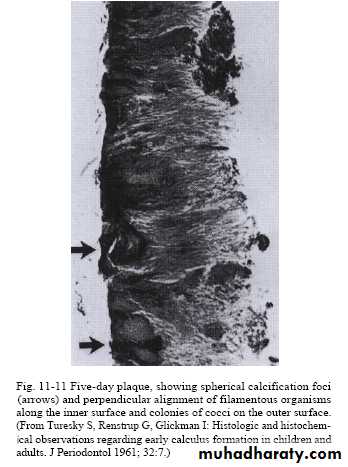
Predominant flora in calculus are cocci, bacilli and filaments (especially in the outer layers), and occasionally spiral organisms.The bacteria near the enamel surface tend to have a reduced cytoplasm to cell wall ratio, suggesting that they are metabolically inactive.
Supragingival calculus contains more Gram-positive organisms, while subgingival calculus tends to contain more Gram negative species.
In some areas (especially the outer surface) cocci attach and
grow on the surface of filamentous microorganisms, giving a
'corn-cob' arrangement.
The filamentous bacteria tend to orient themselves at right angles to the enamel surface, producing a palisade effect (like books on a shelf).
Composition of dental calculus
70-90% inorganic salts (2/3 in crystalline form)Calcium 39%,phosphorus 19% (major element)
Organic portion;
protein-polysaccharide complex & lipid 0.2%,
desquamated epithilium,leukocytes and various type of microorganisms.
Supragingival calculus was derived from saliva while subgingival calculus was derived from gingival fluid.
Composition of dental calculus
• All plaque doesnt necessarily undergo calcification.• Early plaque contains small amount of inorganic materials that increase as plaque is developed into calculus.
• Plaque that is not developed into calculus reach a plateau of maximal mineral content within second day.
• Microorganisms not always essential in calculus formation.
Bacterial phosphatases and proteases can degrade some of the calcification inhibitors in saliva (statherin and proline-rich proteins).
Many toothpastes now contain pyrophosphate compounds which adsorb excess calcium ions, thus reducing intraplaque mineral deposition.
Structure of dental calculus
Small needle-shaped inorganic crystals.The surface is covered by layer of unmeniralized plaque.
The four main crystal forms and their percentages are as follows:
Hydroxyapatite, approximately 58%
Magnesium whitlockite, approximately 21%
Octacalcium phosphate, approximately 12%
Brushite, approximately 9%
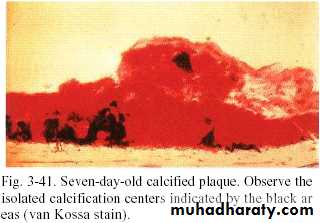
Formation of calculus
• 1-Dental plaque that has undergone meniralization.• 2- Then,plaque serve as organic matrix for subsequent mineralization.
• This occurs by binding of calcium ions to carbohydrate protein complex & precipitation of crystalline calcium & phosphate salts.
• 3- The first small crystal can be seen in inter microbial matrix then close apposition occur until matrix entirely calcified ,eventually microorganism also become mineralized.
Time in calculus formation
• The required time for supragingival calculus formation in some persons less than 2 weeks & start 4-8h after plaque accumulation.• After 48h.,50% of plaque become meniralized
• In one week,appear clinically in measurable quantity.
• In 12days, 60-90% of plaque become mineralized.
• Subgingival calculus usually takes many months to form.
Rate of calculus formation &accumulation
• It vary with persons,teeth and time in same individual.• So ,the persons were divided into heavy, moderate & slight calculus formers or non-former.
• Ten weeks is the time needed to reach maximum level of calculus accumulation (some said that it needs up to 6 months).
• The declination from maximum calculus accumulation usually occur (reversal phenomenon) by mechanical wear from food & movement of cheek, lips & tongue .
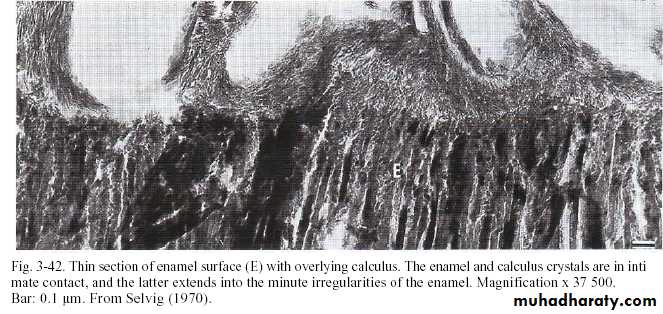
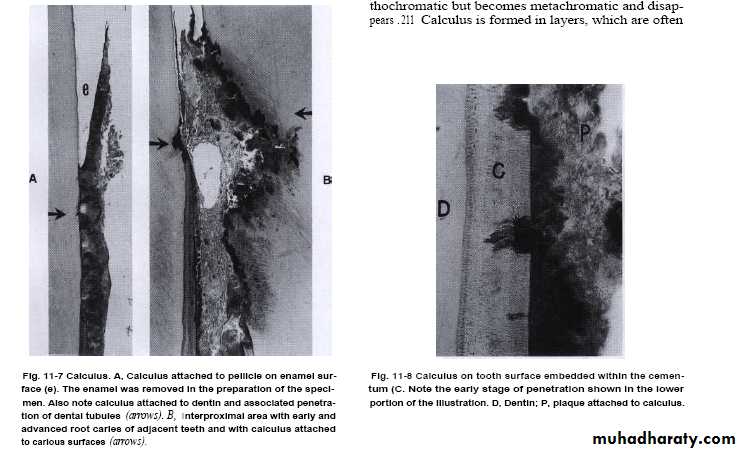
Attachment of calculus to the tooth
• Its tenaciously adhered especially subgingivally by 4 modes:-
• 1-Organic pellicle to enamel & dentin.
• (pellicle under plaque also calcified so crystals comes in contact with the tooth surface).
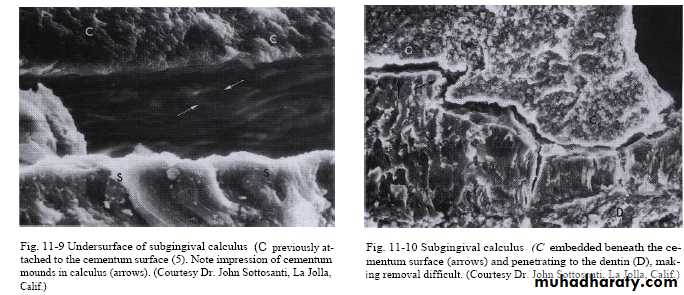
• 2-Penetration of calculus bacteria into cementum.
• 3-Mechanical locking into surface irregularities:-
• a- pits of previously inserted sharpeys fibers .
• b- resorped lacunae of cementocytes .
• c- root caries.
• 4-Close adaptation of calculus undersurface depression to gently sloping mounds of unaltered cemental surface .
Factors increasing rate of calculus formation
• Elevated saliva pH• Elevated saliva calcium conc.
• Elevated bacterial protein & lipid conc.
• Elevated conc.of protein & urea in submandibular salivary gland secretion
• Higher total salivary lipid level
Theories of calculus formation
Epitactic conceptseeding agent present in dental plaque (Leptotrichin buccalis bacteria or carbohydrate-protein complex)where it removes calcium ion from saliva(chelation) then bind with it to form nucleus that induce subsequent deposition of mineral
Theories of calculus formation
Extra alkaline condition of saliva:Adjacent to ducts, saliva most likely alkaline with high salivary film velocity so calcium & phosphores are less stable.
As in alkaline pH; calcium & phosphores deposites increase from saliva.
At acid pH; material deposits from saliva increase in carbohydrate& protein.
Theories of calculus formation
Buffer system control salivary pH (bicarbonate) that act through;
HCO3+H H2CO3 H2O +CO2
Decrease in pH of dental plaque associated with incraese H ion that bind with bicarbonate to produce carbonic acid
Buffer system resist changes in alkalinity or acidity of S.by moping up or releasing H ions
What is buffer?
It is a substance that has ability to bind or release H ion in a solution to keep the pH constant as carbonic acid move in both directions.Concentration of bicarbonate in saliva linked to flow rate (increasing in bicarbonate leads to increasing product of cell metabolism).
Saliva also contain carbonic anhydrase which is secreted from submandibular & parotid glands
It is enzyme derives the reaction converting carbonic acid to CO2 & H2O that mop up H.
Saliva pH around 6.8 (6.2-7.4),Increase with flow rate to reach during active secretion 8.0.
Faster flow rate associated with higher pH which in turn increase calculus formation.
PH above 6 would spontaneously precipitate calicium phosphate crystals.
Calculus Effects
Past concept said that the phosphorus & calcium were the causative factors of periodontal disease.Recently; the plaque was considered the causative factor & the calculus is the most important co-factor.
Its played a role by:-
1-Favors plaque growth & stabilize it & create areas where plaque removal is impossible.
2-Favors food retention & hinder dental cleaning.
3-It has endotoxin
4-Hinders periodental recovery