
Experimental Epidemiology
Dr. Sijal Fadhil Farhood AL-joborae
F.I.C.M.S M.Sc. M.B.Ch.B.

•In the 1920s,experimental epidemiology meant a
study of epidemics among colonies of experimental
animals such as rats and mites, in modern usage,
experimental studies are often equated with
RANDOMIZED CONTROLLED TRIAL.

• Experimental or interventional studies are similar in approach to
cohort studies except that the conditions in which study is carried
out are under the direct control of the investigator.
• Thus the experimental studies involve some action, intervention or
manipulation such as deliberate application or withdrawal of the
suspected cause or changing one variable in the causative chain in
the experimental group while making no change in the control
group, observing and comparing the outcome of the experiment in
both the groups.

• In contrast with observational studies(case-control
and cohort studies) in which the epidemiologist takes
no action but only observes the natural course of
events or outcome.

Aims of experimental studies
• 1-To provide scientific proof of etiological or risk factors which
may permit the modification or control of those diseases.
• 2-To provide a method of measuring the effectiveness and
efficiency of health services for the prevention ,control and
treatment of disease and improve the health of the
community.

Experimental studies have all the advantages and
disadvantages of the usual prospective cohort studies plus
three additional problems,(cost,ethics,and feasibility)

Animal studies
• Throughout history animals have played an important role in
man’s quest for knowledge about himself and his
environment.
• Animal studies have contributed to our knowledge of
anatomy,physiology,pathology,microbiology,immunoloygene
tics,chemotherapy and so many others
.

Important applications of animal experiments
1-Experimental reproduction of human disease in animals to
confirm etiological hypothesis and to study the pathogenic
phenomena or mechanisms.
2-Testing the efficacy of preventive and therapeutic measures
such as vaccines and drugs.
3-Completing the natural history of the disease.

Advantages and limitations of animal studies
• Advantages: The experimental animals can be bred in the laboratories
and manipulated easily according to the wishes of the investigator.
• Limitations:
• Not all human disease can be reproduced in animals.
• All the conclusions derived from animal experiments may not be
strictly applicable to human beings.

Human experiments
Human experiments will always be needed to investigate
disease etiology and to evaluate the preventive and
therapeutic measures, These studies are even more essential in
the investigation of diseases that can not be introduced in
animals.
Although the experimental method is unquestionably the most
incisive approach to scientific problem, ethical and logistic
considerations often prevent it’s application to the study of disease in
human.
Therefore, before doing human experiments, the benefits of the
experiments have to be weighed against risks involved.
The volunteers should be made fully aware of all possible
consequences of the experiment.

Types of experimental studies
1-Randomized controlled trials (i.e those involving a process of
random allocation).
2-Non-randomized or non-experimental trial(i.e. those
departing from strict randomization for practical purposes, but
in such manner that non-randomization does not seriously
affect the theoretical basis of the conclusions)

Randomized controlled trials(RCT)
The basic steps in conducting the RCT include the following:
1-Drawing up a protocol.
2-Selecting reference and experimental populations.
3-Randomization.
4-Manipulation or intervention.
5-Follow-up.
6-Assessment of outcome.
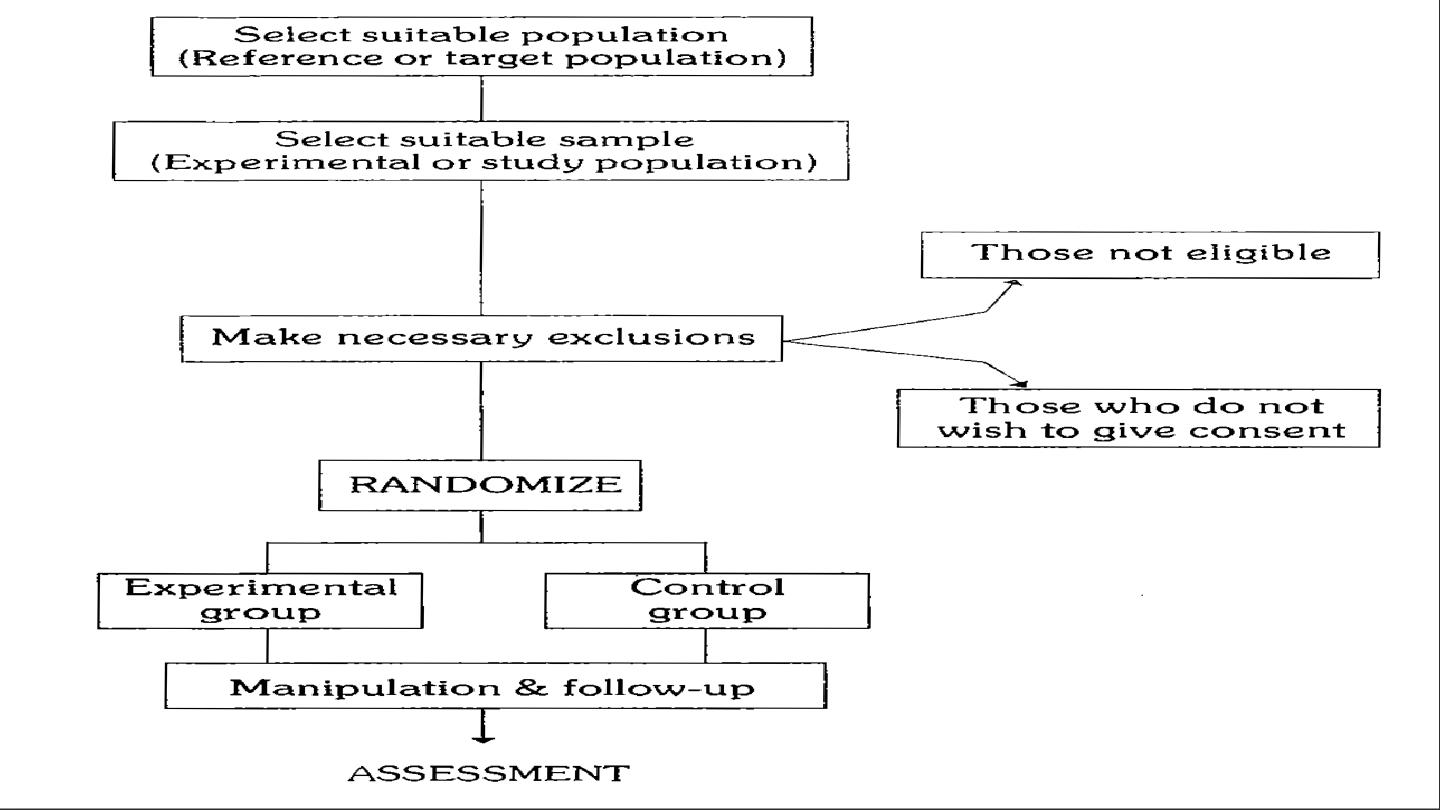

1-Drawing up a protocol: It specifies aims and objectives of the study,
question to be answered, criteria for the selection of study and
control groups, size of the sample, the procedure for allocation of
subjects into study and control groups, treatment to be applied….etc.
2-Selecting reference and experimental populations:
A: Reference or target population: it is the population to which the
finding of the trial ,if found successful, are expected to be applicable
(e.g. drug, vaccine),a reference population may be as broad as
mankind or it may be geographically limited or limited to person in
specific age, sex, occupation, thus it may comprise the population of a
whole city or a population of school children ,industrial workers ..etc
according to the nature of the study.
• B- Experimental or study population:
• It is derived from reference population ,it is the actual population that
participates in the experimental study ,it should be randomly chosen from the
reference population so that it has the same characteristics as the reference
population because if not, we can not generalize the finding of the study to the
reference population.
• It is important to choose a stable population whose cooperation is assured (to
avoid loss to follow up)
• *compliance:
is important in intervention studies for the whole period of
the study, so non compliance will decrease the statistical power of a trial to
detect any true effect of the study treatment.

Criteria of the participants or volunteers
1-They must give informed consent.
2-They should be representative of the population to which
they belong (reference population).
3-They should be eligible for the trial.

3-Randomization: (it is a heart of control trial)
is a statistical procedure by which a participants are allocated into study group
(in which intervention is given) and control group (in which intervention is not
given).
Randomization is an attempt to eliminate bias(selection bias) and and allow
comparability
4-Manipulation or intervention: This creates an independent variable(e.g. drug ,
vaccine, a new procedure) whose effect is then determined by measurement of
the final outcome ,which constitutes the dependent variable (e.g incidence of
disease, survival time, recovery period).

5-Follow-up: This implies examination of the experimental and
control groups at a defined interval in times, in a standard manner,
with equal intensity, in the same time frame till the final assessment
of outcome.
6-Assessment:
A-Positive results
B-Negative results
The incidence of positive/negative results is compared in both groups
and the differences are tested for statistical significance.

• Bias may arise from errors of the assessment of the outcome due to human elements.
• Sources of bias are:
• 1-There may be bias in the part of participants, who may subjectively feel better or report
improvement if they knew they were receiving a new form of treatment this is known as
subject variation.
• 2-there may be observer bias ,that is the investigator measuring the outcome of the
therapeutic trial may be influenced if he knows beforehand the particular procedure or
therapy to which the patient has been subjected, this is known as observer bias .
• 3-there may be bias in evaluation, that is the investigator may give a favorable report of the
outcome of the trial .
• Randomization can not guard against these sort of bias.
In order to reduce these problems ,a technique known as blinding is
adopted which will ensure that the outcome is assessed objectively.
Blinding can be done in three ways:
1-Single blind trial: the trial is so planned that the participants is not
aware whether he belongs to the study group or control group.
2-Double blind trial: the trial is so planned that neither the doctor nor
the participant is aware of the group allocation and the treatment
received.
3-Triple blind trial: The participants, the investigator and the person
analyzing the data are all blind.
double blind is most frequently used method when a blind trial is
conducted.

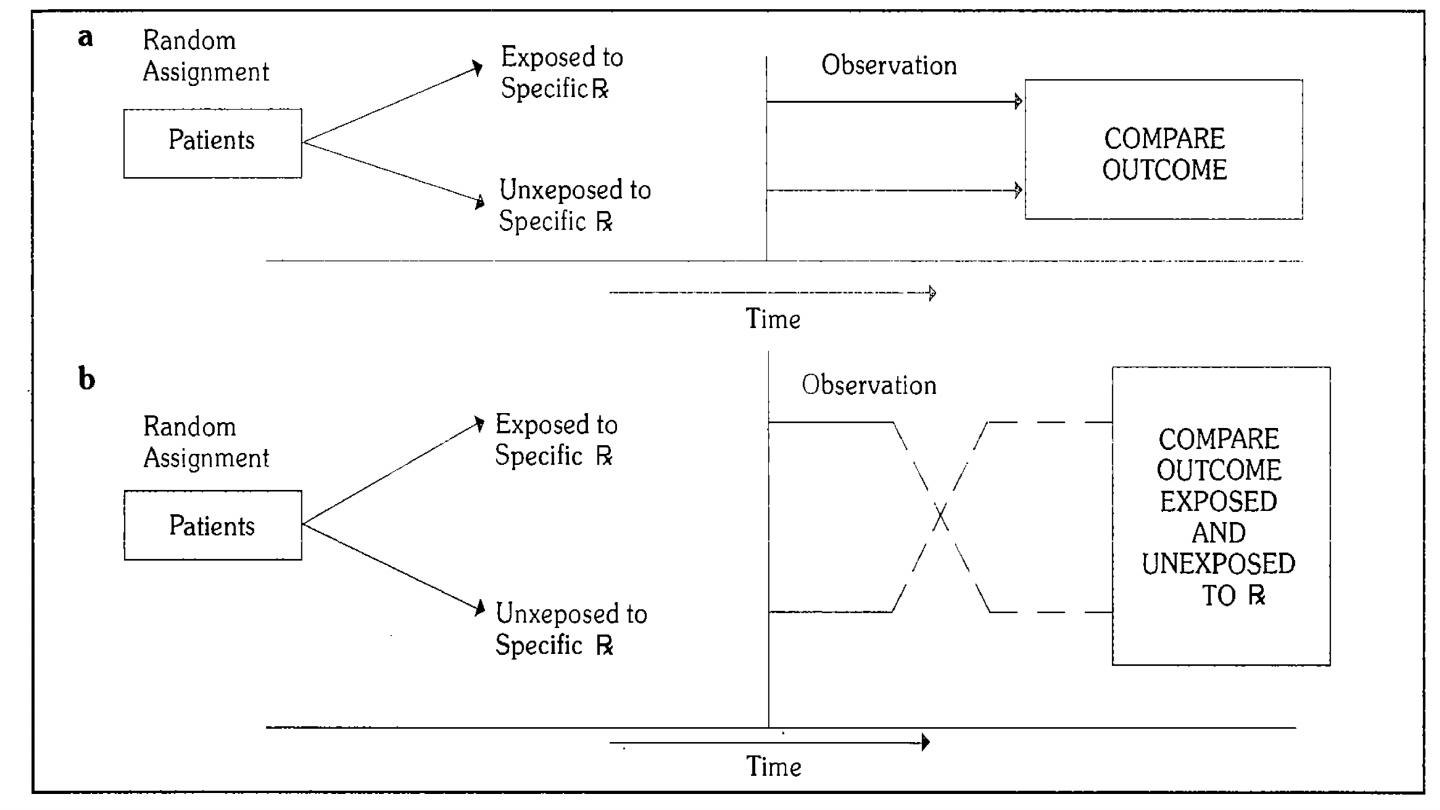
Study designs of controlled trial
1-Concurrent parallel study design.
Comparisons are made between 2 groups :
A: Experimental group (which is exposed to specific medication or intervention).
B:Reference group(which is not exposed to specific medication or intervention).
2-Cross-over types of study design: Comparisons are made between 2 groups :
A: Experimental group (which is exposed to specific medication or intervention).
B: Reference group(which is not exposed to specific medication or intervention).
Then the groups are crossed-over (exposed group now becomes non-exposed
and vise versa)

Types of randomized controlled trials
1-Clinical trials: it have been concerned with evaluating therapeutic agents, mainly
drugs.
Example: trials of folate treatment/supplementation before conception to prevent
recurrence of neural tube defects, trial of aspirin on cardiovascular mortality and beta
carotene on cancer incidence.
2-Preventive trials: it implies trials of primary preventive measures, these trial are
purported to prevent or eliminate disease on an experimental basis, e.g. trials of
vaccines.
3-Risk factors trials:
4-Cessation experiments:
5-Trial of etiological agents:
6-Evaluation of health services.

Types of randomized controlled trials
1-therapeutic(or secondary prevention)trial.
determines the ability of the agent or procedure to diminish
symptoms, prevent recurrence, or decrease death from the
disease.
2-preventive(or primary prevention) trial.
could be done on the whole community, called community trials
(prophylactic study)on healthy people like vaccine trial.

Non-RANDOMIZED TRIAL
• It is not always possible for ethical, administrative and other reasons to resort to a
randomizes controlled trial in human being, in such situation non-randomized trial is
used.
• As there is no randomization the degree of comparability will be lowered the chances
of spurious result is high.

Types of Non-RANDOMIZED TRIAL
1-Uncontrolled-trials:control groups is not used for example
effectiveness of pap test for cervical cancer.
2-Natural control(natural experiments):natures has separated the
population in two groups for example in respect to cigarette smoking
,people have separated themselves into two groups smokers and non
smokers.
3-Before and after comparison study(pre-post clinical trial):these
study center round comparing the incidence of disease before and
after introduction of a preventive measures.
