1
L7
Peripheral neuropathy
D. Hazim
Peripheral Neuropathy:
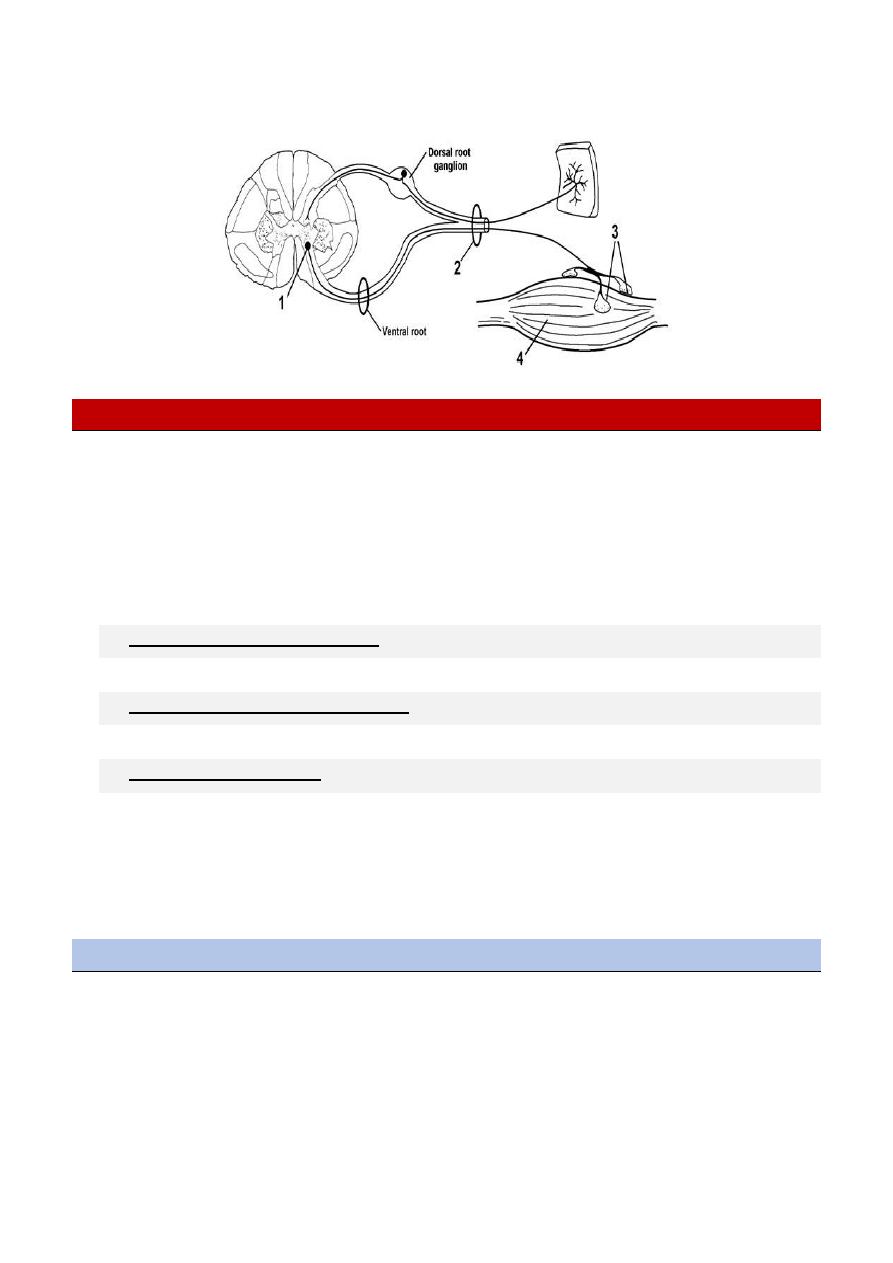
Peripheral nerves are composed of sensory, motor, and autonomic elements.
Diseases can affect the cell body of a neuron or its peripheral processes, namely the axons or the
encasing myelin sheaths.
Most peripheral nerves are mixed and contain sensory and motor as well as autonomic fibers.
Thus, peripheral neuropathies can impair sensory, motor, or autonomic function, either singly
or in combination.
MONONEURITIS SIMPLEX
This term signifies involvement of a single peripheral nerve.
MONONEURITIS MULTIPLEX
Several individual nerves are affected, usually at random and noncontiguous.
POLYNEUROPATHY
The term “polyneuropathy” denotes a disorder in which the function of numerous
peripheral nerves is affected at the same time. This leads to a predominantly distal and
symmetric deficit, with loss of tendon reflexes except when small fibers are selectively
involved.
Approach to Neuropathic Disorders
Is it Motor, sensory, autonomic, or combinations?
Is it focal, symmetrical or asymmetrical?
Is it Acute (days to 4 weeks), Subacute (4 to 8 weeks) or Chronic (>8 weeks)?
Is there evidence for a hereditary neuropathy (family history)
Are there any associated medical conditions (DM, cancer, autoimmune, connective
tissue)?
Drug history.
2
Causes of peripheral neuropathy
Idiopathic inflammatory neuropathies
Acute idiopathic polyneuropathy (Guillain-Barré syndrome)
Chronic inflammatory demyelinating polyneuropathy.(CIDP)
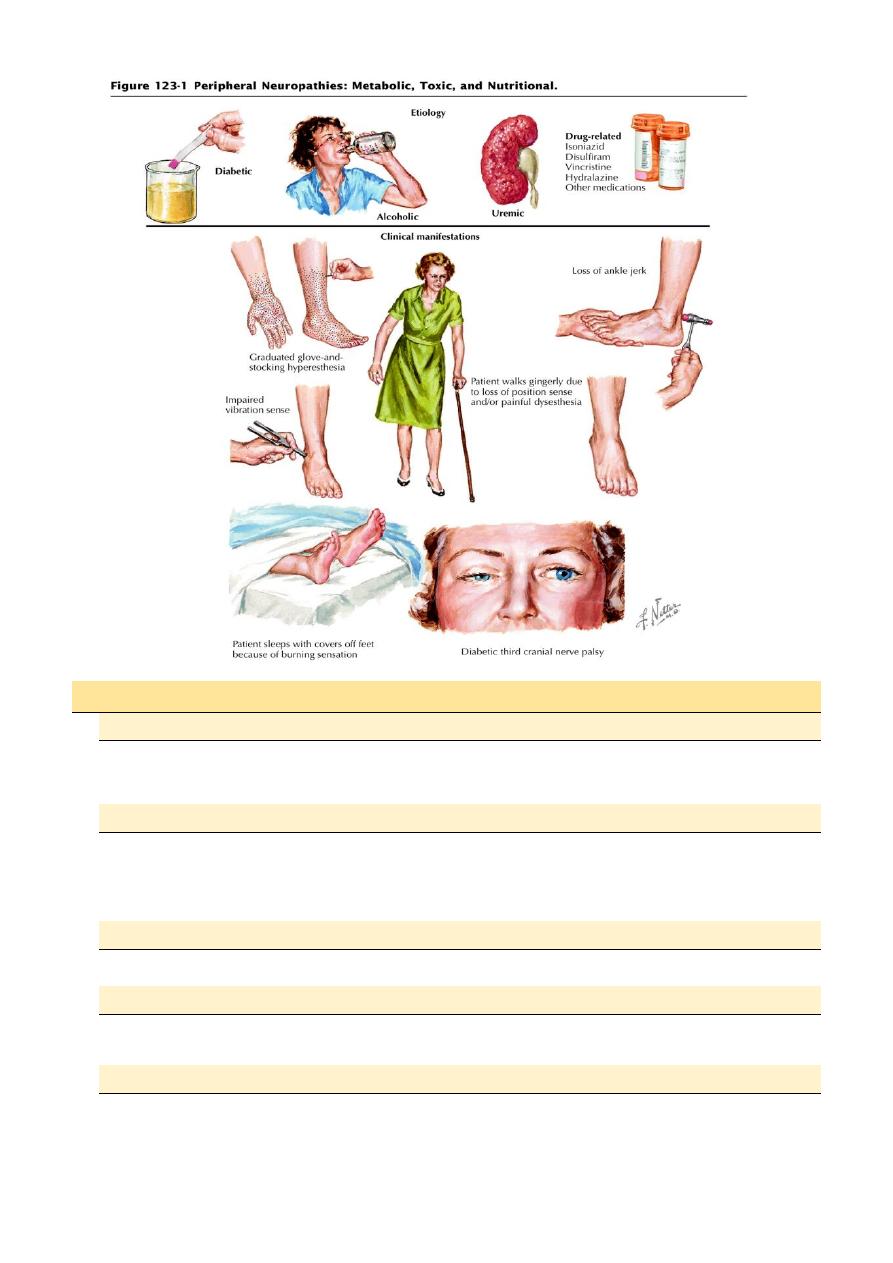
Metabolic and nutritional neuropathies
Diabetes
Hypothyroidism
Vitamin B
12
deficiency
Infective and granulomatous neuropathies
AIDS, leprosy, diphtheria, sarcoidosis and sepsis.
Vasculitis neuropathies
Polyarteritis nodosa
Rheumatoid arthritis
SLE
Neoplastic and paraproteinemic neuropathies
Compression and infiltration by tumor
Paraneoplastic syndromes

3
Drug, alcohol and toxin induced neuropathies
Examples of drugs
Pyridoxine
Metronidazole
Dapsone
Isoniazid
Example of toxin:
Organophosphates
Heavy metal
Lead, Thallium, arsenic
Hereditary neuropathies
Friedreich ataxia, CMT (Charcot-Marie-Tooth).
Compressive neuropathy:
E.g.: carpel tunnel syndrome
Idiopathic Inflammatory Neuropathies
Acute Idiopathic Polyneuropathy (Guillain-Barré Syndrome)
GBS is an acute or subacute polyneuropathy that can follow minor infective illnesses,
,vaccination ,surgical procedures, or may occur without obvious precipitants
Clinical and epidemiologic evidence suggests an association with preceding infection
such as Campylobacter jejuni, Cytomegalovirus, Mycoplasma pneumonia, Epstein–
Barr virus etc.
Its precise cause is unclear, but it appears to have an immunologic basis. Both
demyelinating and axonal forms have been recognized, with distinctive clinical and
electrophysiological features, the demyelinated form is more common.
Clinical Features
Diagnostic criteria for Guillain-Barré syndrome
Required for diagnosis
Progressive weakness of more than one limb
Distal areflexia with proximal areflexia or hyporeflexia
Supportive of diagnosis
Progression for up to 4 weeks
Recovery beginning within 4 weeks after progression stops
Relatively symmetric deficits
Mild sensory involvement
Cranial nerve (especially VII) involvement
Autonomic dysfunction
No fever at onset
Increased CSF protein after 1 weeks

4
CSF white blood cell count ≤10/mL
Nerve conduction study show slowing or block by several weeks
Against diagnosis
Markedly asymmetric weakness
Bowel or bladder dysfunction (at onset or persistent)
CSF white blood cell count >50 .
Well-demarcated sensory level.
Excluding diagnosis
Isolated sensory involvement
Investigative studies
• The cerebrospinal fluid (CSF) often shows a characteristic abnormality, with increased
protein concentration but a normal cell count (cytoalbumino dissociation); abnormalities
may not be found in the first week.
• Electrophysiological studies may reveal marked slowing of motor and sensory
conduction velocity.
Treatment
• Plasmapheresis appears to reduce the time required for recovery and may decrease the
likelihood of residual neurologic deficits and need for ventilation a course of
plasmapheresis usually consists of 40–50 mL/kg plasma exchange (PE) four to five times.
• It is best instituted early, and it is indicated especially in patients with a severe or rapidly
progressive deficit or respiratory compromise.
• Intravenous immunoglobulin (400 mg/kg/d for 5 days) appears to be equally effective
and should be used in preference to plasmapheresis in adults with cardiovascular
instability and in children;
The two therapies are not additive.
No role for steroid
.
In the worsening phase of GBS, most patients require monitoring in a critical care setting, with
particular attention to
Vital capacity
Heart rhythm
Blood pressure
Deep vein thrombosis prophylaxis like heparin and compressive stokes
Cardiovascular status monitoring, and chest physiotherapy.
As noted, 30% of patients with GBS require ventilator assistance.
Prognosis and Recovery
Approximately 70-75% of patients recover completely, 25% are left with mild neurologic
deficits, and 5% die from respiratory and autonomic dysfunction.

5
Chronic Inflammatory Demyelinating Polyneuropathy
CIDP is distinguished from GBS by its chronic course.
Onset is usually gradual over a few months or longer, but in a few cases the initial attack is
indistinguishable from that of GBS.
An acute-onset form of CIDP should be considered when GBS deteriorates >9 weeks after onset
or relapses at least three times.
Symptoms are both motor and sensory in most cases, in other respects, this neuropathy shares
many features with the common demyelinating form of GBS.
Types of Diabetic Neuropathy
Peripheral Neuropathy.
Proximal Neuropathy (diabetic amyotrophy).
Autonomic Neuropathy.
Focal Neuropathy.
Mubark A. Wilkins
