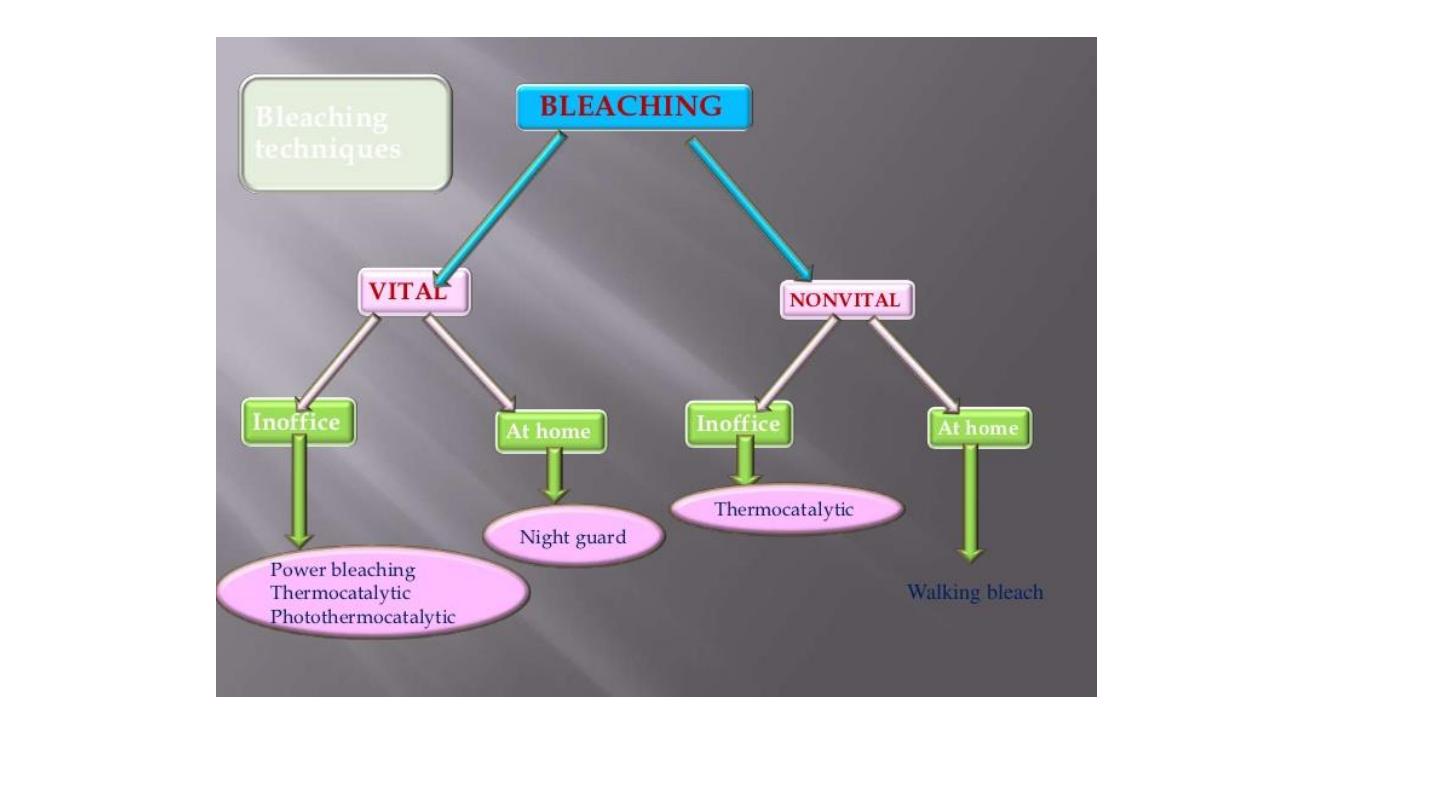
Bleaching of discolored teeth
By Balsam M. mirdan

Introduction
• The lightening of the color of the tooth through the application of chemical
agents to oxidize the organic pigmentation in the tooth is referred to as
bleaching.
• The teeth color is determined by the dentin, with translucent enamel,
which is playing a lesser role through scattering at wavelengths in the blue
range.
• shade selection is a crucial clinical step during prosthetic treatment .
During the visual teeth color determination it is suggested that the first
impression is frequently the best match, and shade matching trials should
be limited to 5 seconds at a time to prevent eye fatigue, because the vision
pigment is used up quickly in the mechanism of color perception.
recommendation to relax the eyes by observing a blue card between two
shade matching trials (because blue and yellow are complementary colors).

COLOUR
• Teeth made of many colours, with natural gradation from the darker
cervical to the lighter incisal third
• Variation affected by thickness of enamel and dentine, and
reflectance of different colours
• Blue, green and pink tints in enamel, yellow through to brown shades
of dentine beneath
• Canine teeth darker than lateral incisors
• Teeth become darker with age (secondary/tertiary dentine, tooth
wear/dentine exposure)

CAUSES OF DISCOLORATION
1. Extrinsic discoloration
2. Intrinsic discoloration
3. Internalized discoloration
Stains that occur subsequent to dental development, entering hard
tissues through enamel defects

Extrinsic Discoloration
• Discoloration present on the enamel or
acquired pellicle generally of metallic or
nonmetallic origin: They are found on the
outer surface of teeth and are usually
occurs when some agent literally stains or
damages the enamel surfaces of the
teeth. of local origin which can be
removed by oral prophylaxis.

Extrinsic Discoloration
• Cigarettes, cigars and pipes will produce a yellowish brown to black
discoloration, usually in the cervical portion of the teeth and primarily
on
the lingual surfaces.
• Chewing tobacco stains frequently penetrate the enamel producing a
deeper stain
• Coffee and tea cause severe tenacious discolorations, usually brown
to black stains.

Extrinsic Discoloration
•Plaque, chromogenenic bacteria
•Mouthwashes (chlorhexidine)
•Foods (curry, cooking oils and fried foods, foods with colorings,
berries, beetroot)
• Antibiotics (erythromycin, amoxicillin-clavulanic acid)
• Iron supplements
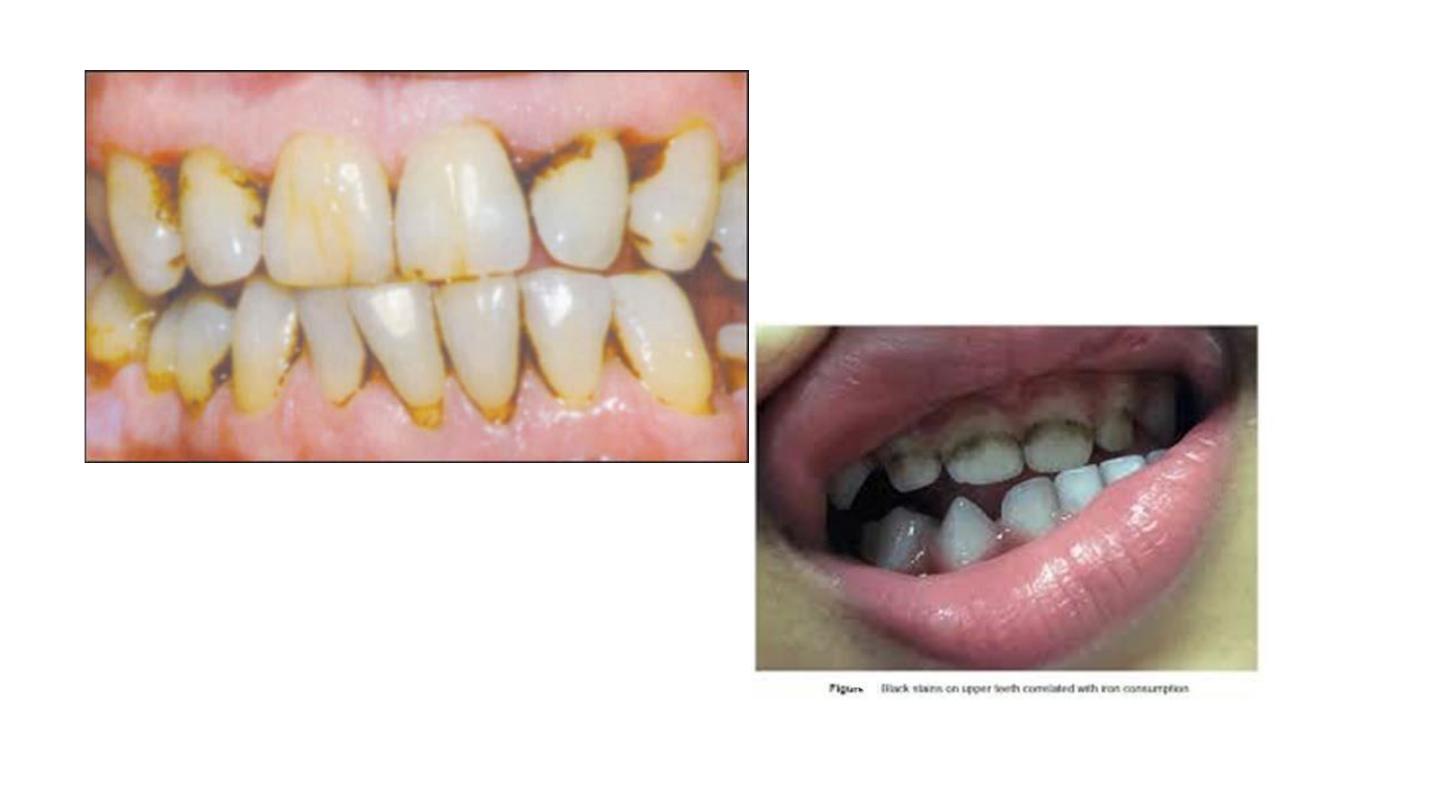

Intrinsic Discoloration
Discoloration is a result of change in the structural form or the
composition of dental hard tissues.
These are stains within the enamel and dentin caused by the
deposition or incorporation of substances within these structures,
such as tetracycline stains, dentinogenesis imperfecta and fluorosis by
products released into the dentinal tubules during
• illness (e.g. bilirubin involved with jaundice)
• trauma(primarily the breakdown of hemoglobin), or
• pigmentation escaped from the medicaments and materials used in
restorative dentistry.

1/Tetracycline Discoloration
Teeth are most susceptible to tetracycline discoloration during their
formation, i.e. during the second trimerster in utero to roughly 8 years
after birth.
The tetracycline molecules appear to chelate with calcium and become
incorporated into the hydroxyapatite crystals.
• The tetracycline involves predominantly the dentin.
• Severity of the stains depends on the time and duration, the dosage
of the drug administration, and the type of tetracycline.
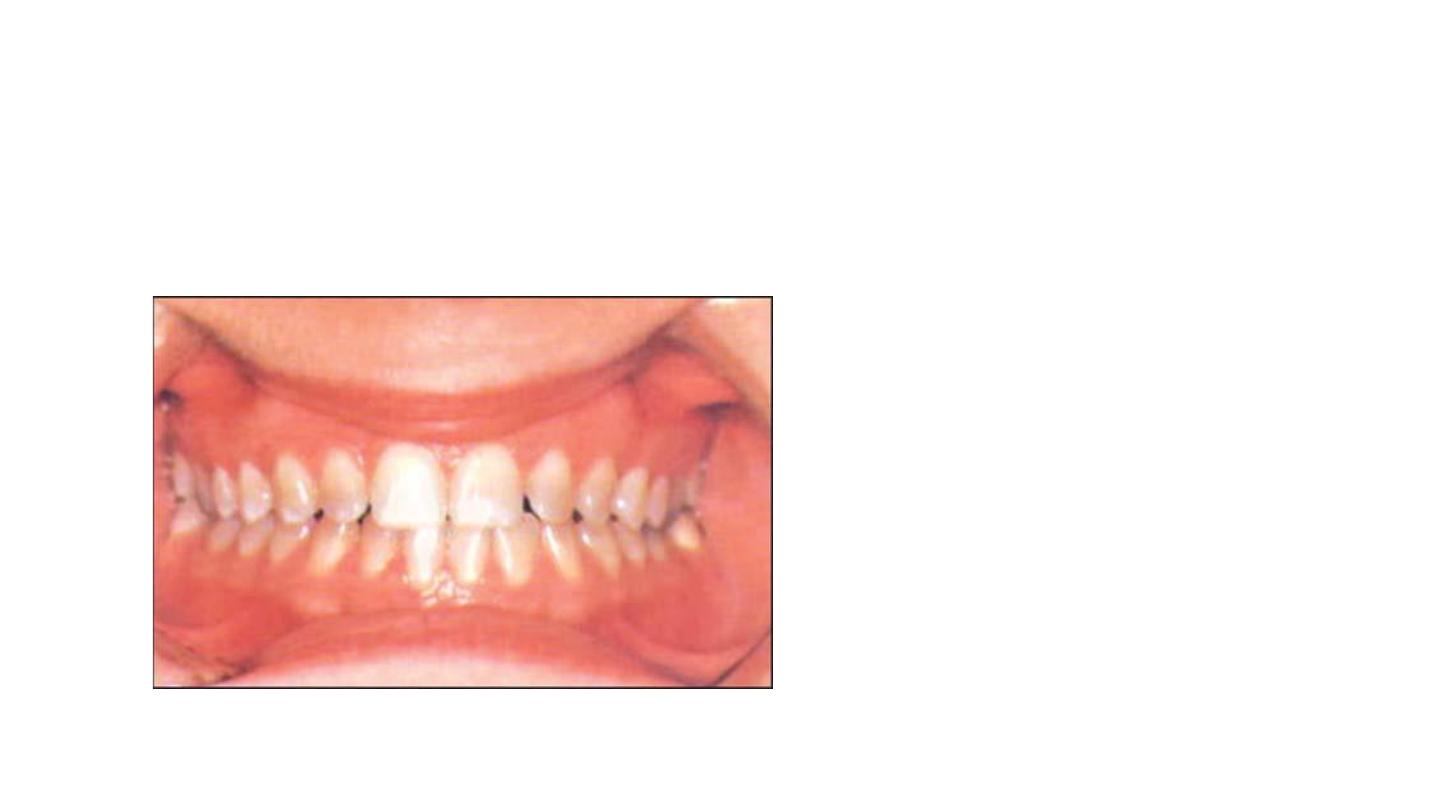
First degree tetracycline staining:
• Is a light yellow, brown or gray
staining.
• Uniformly distributed throughout
the crown
Responds well to bleaching in two or
three sessions.
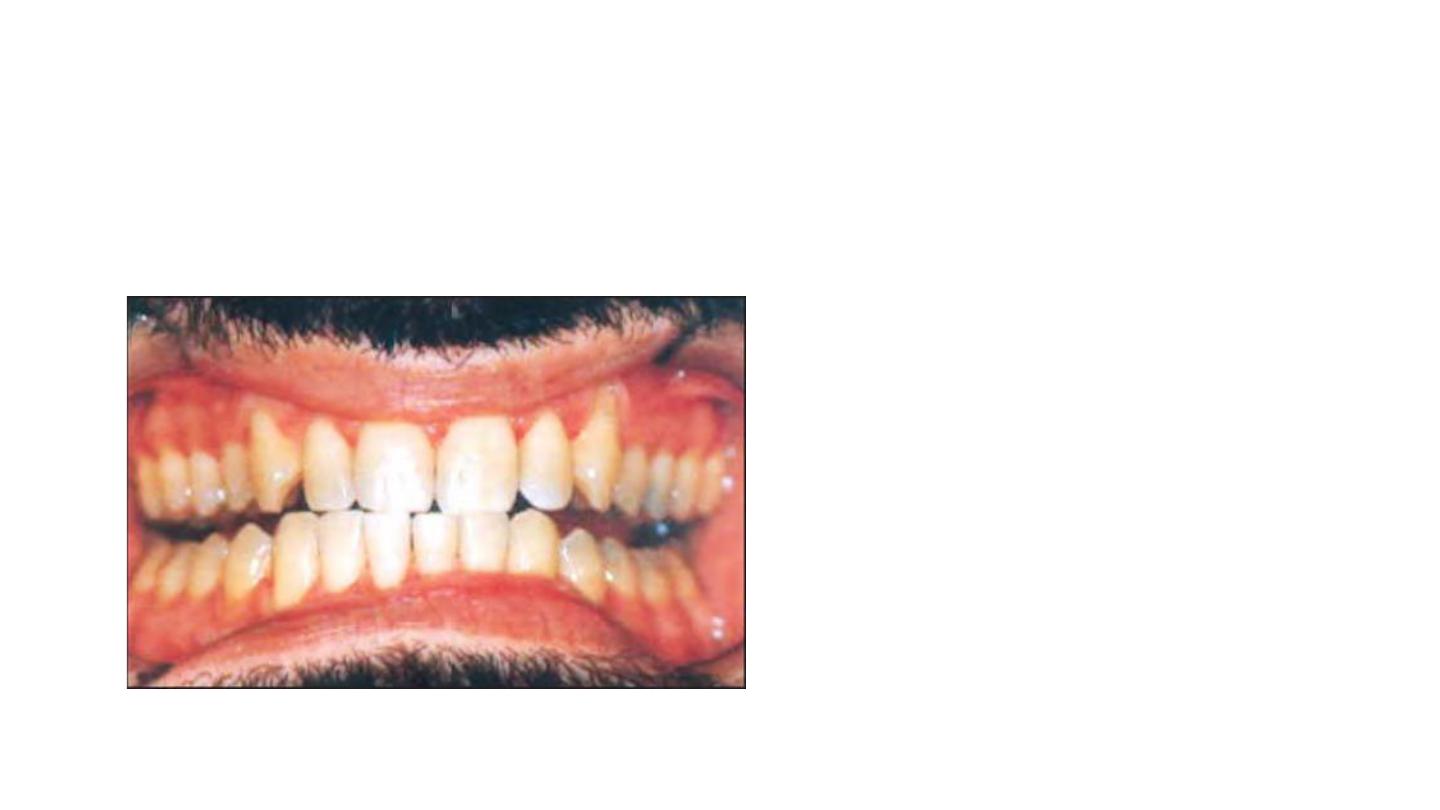
Second degree tetracycline staining:
• Dark or gray staining.
• More extensive than first degree with no
banding.
Responds well to bleaching in 4 to 6
sessions.
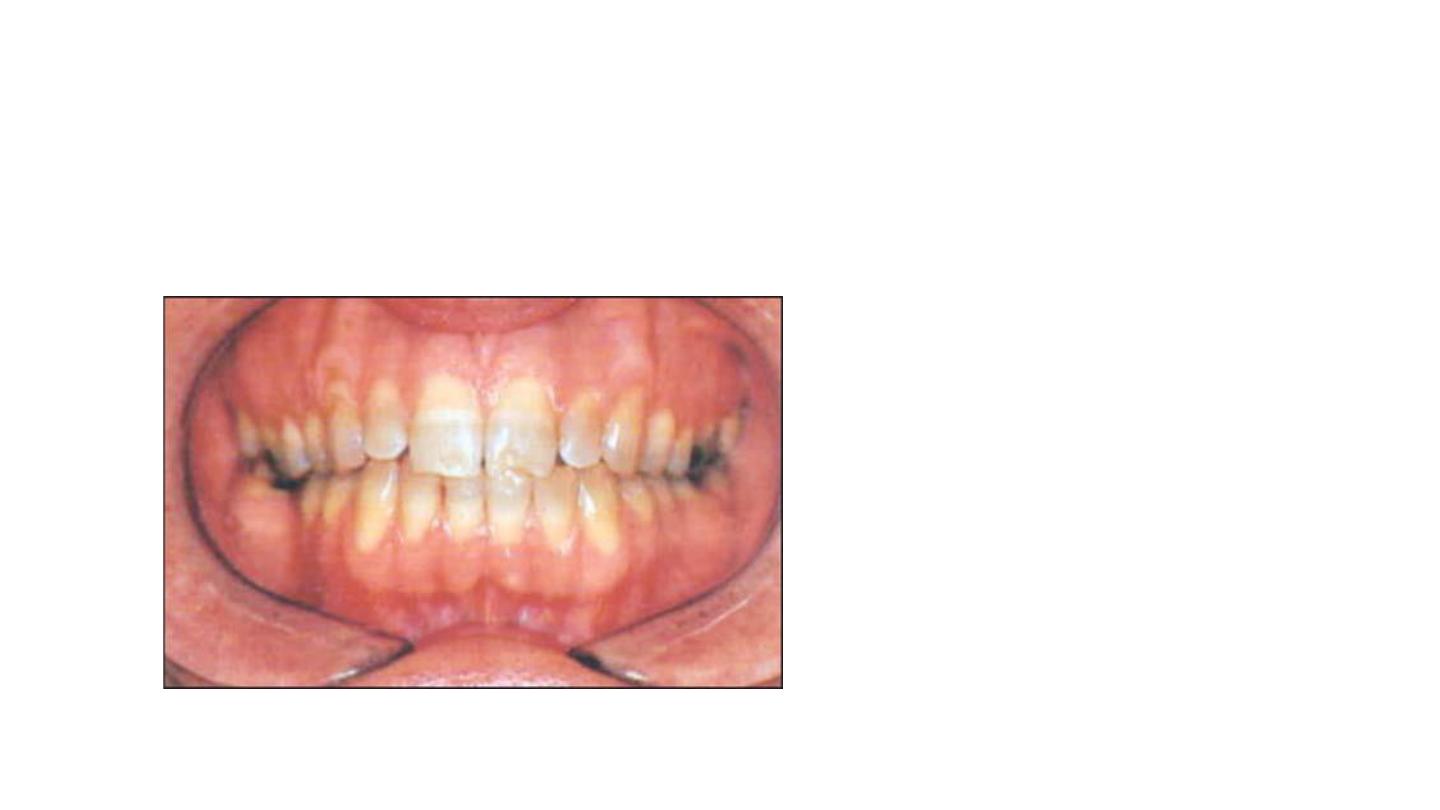
Third degree tetracycline staining:
• Dark gray or blue with marked
banding.
Responds to bleaching but bands
usually evident following extensive
treatment.
It may be removed with some
veneering technique.
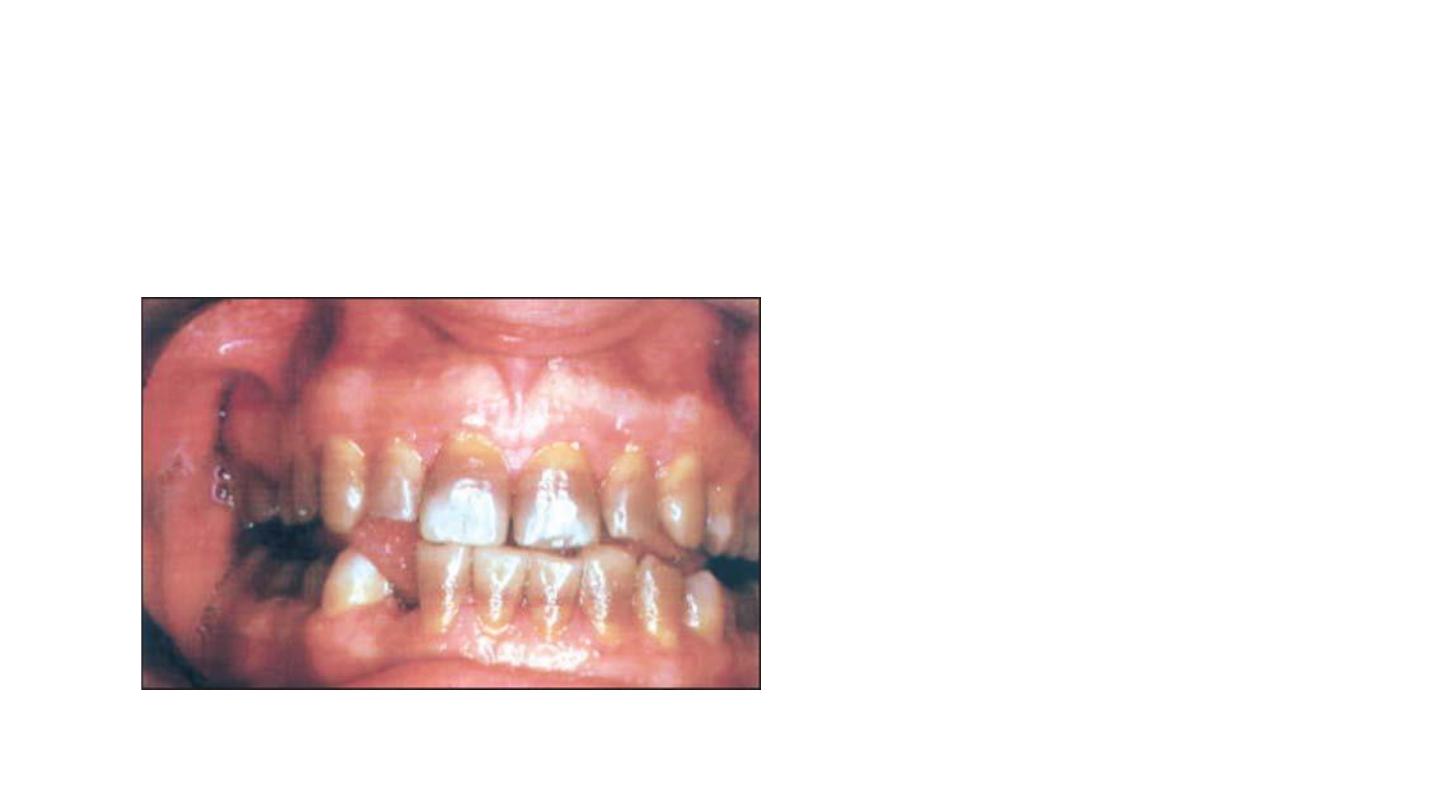
Fourth degree tetracycline
staining:
• Includes those stains that are
too dark to attempt vital
bleaching.

2/Fluorosis Staining
Mottled enamel that occurs when children ingest excessive fluoride
during development of enamel and dentin.
• Damage occurs during development usually from third month of
gestation through eighth year of life.
• High concentration of fluoride in excess of 1ppm (more than 4 ppm -
moderate to severe discoloration) is believed to cause a metabolic
alteration in the ameloblasts resulting in defective matrix and
improper calcification.
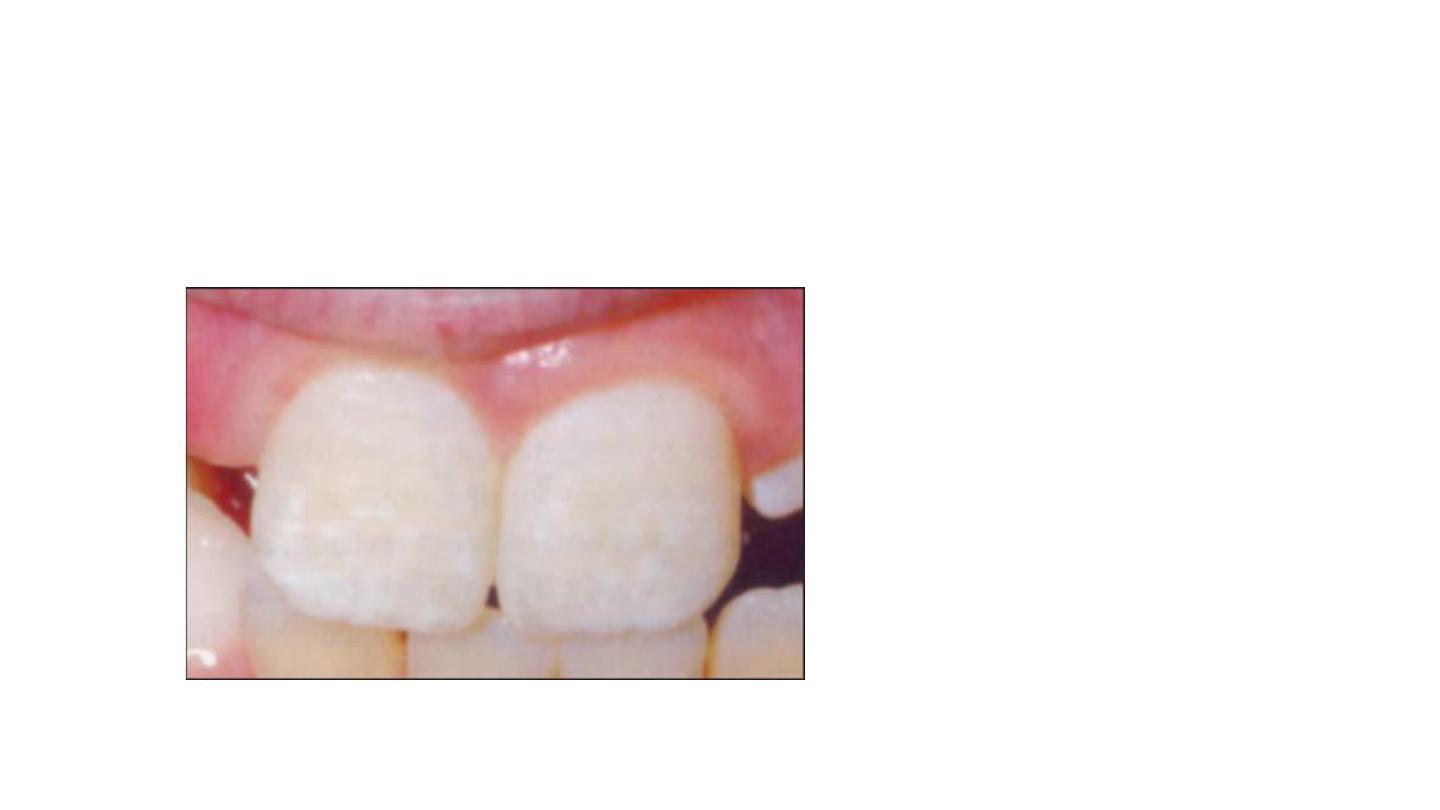
Simple fluorosis staining appears
as brown pigmentation on a
smooth enamel surface
Responds well to bleaching
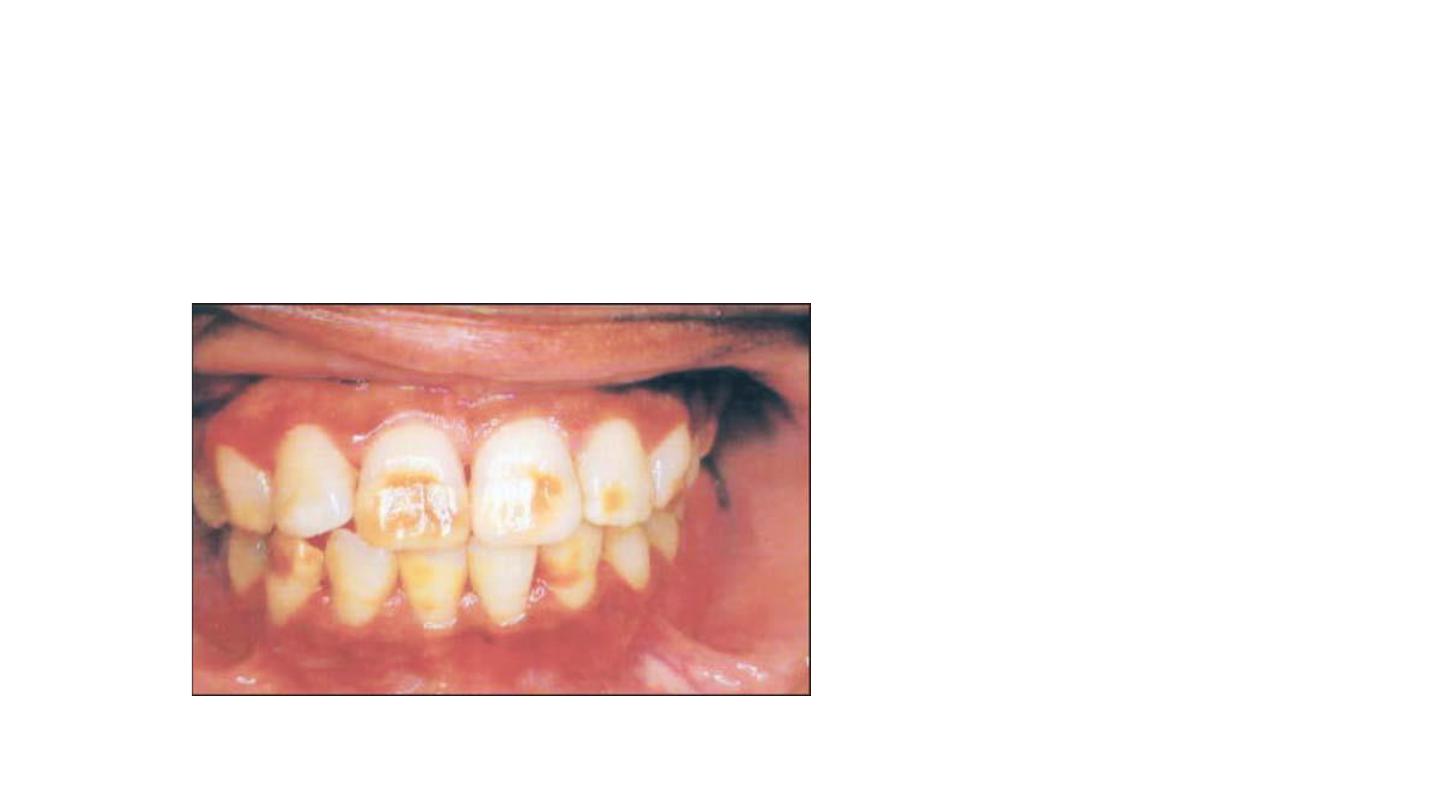
Opaque fluorosis appears as flat
gray or white fleeks on enamel
surface.
Responds poorly to bleaching
because tooth cannot be bought to
lightness in area.
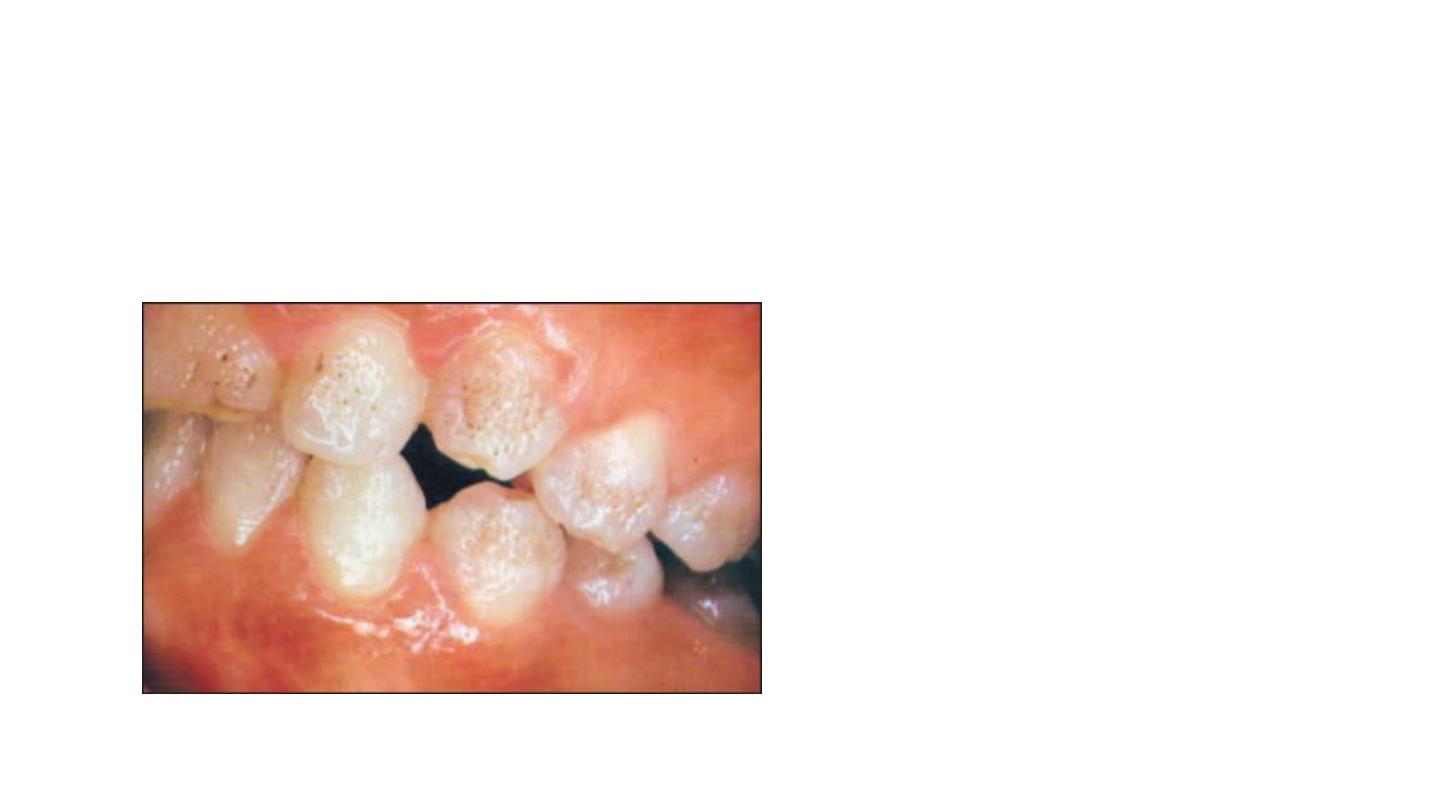
Fluoride staining with pitting has dark
pigmentation with surface defects,
necessitates bleaching
followed by composite resin bonding
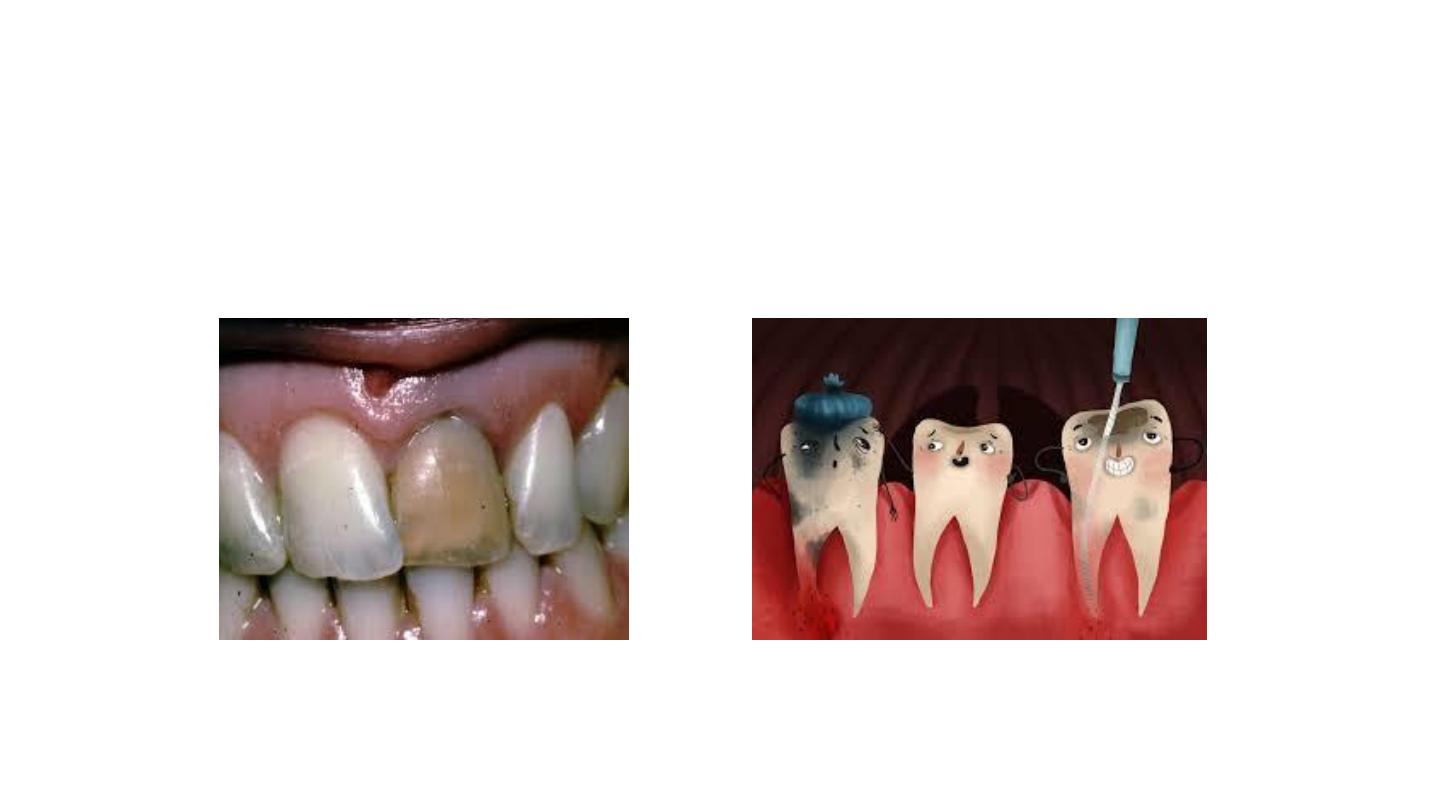
3/Discoloration from Pulp Necrosis
Trauma can cause hemorrhage as blood vessels
rupture in the pulp chamber.
– Blood is hydraulically driven into the dentinal
tubules, where the RBC undergo hemolysis liberating
hemoglobin. Hemoglobin is degraded
releasing iron that forms a black compound by
combining with hydrogen sulfide to become iron
sulfide.
– Immediately after injury, crown remains pink
as blood breaks down. The tooth becomes orange,
then blue, then brown or black.
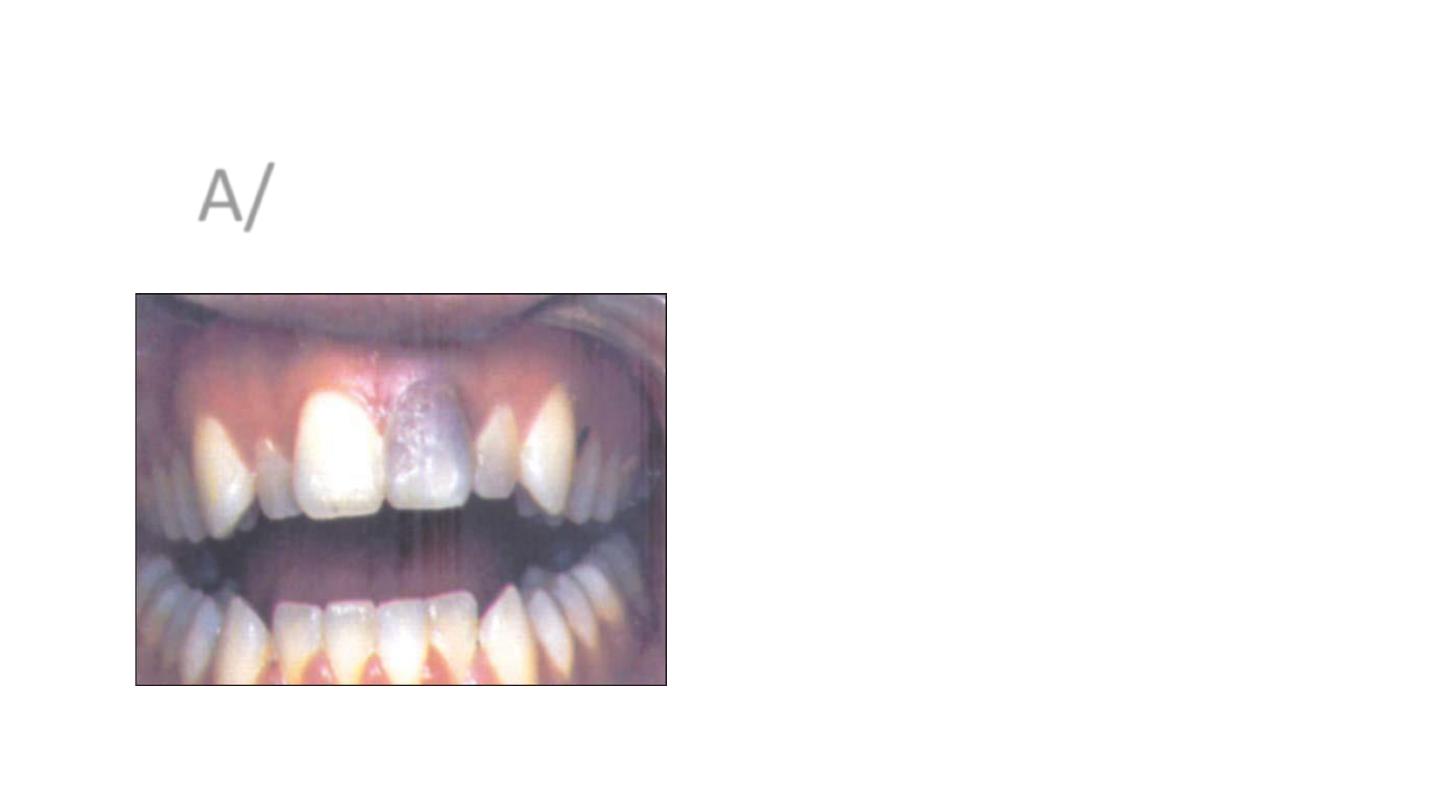
A/ Trauma-related discoloration

• If trauma is the cause of pulpal necrosis, the discoloration
becomes more severe over time. However, if not, the
discoloration can be reversed, and the tooth can regain its
original shade) . It has be shown that the pinkish hue seen
initially after trauma might disappear in 2-3 months if the
tooth becomes revascularized

B/Pulp degeneration without hemorrhage
• Necrotic tissue contains various protein degradation products which
create a grayish brown discoloration of the crown.
• This responds well to non-vital bleaching technique.

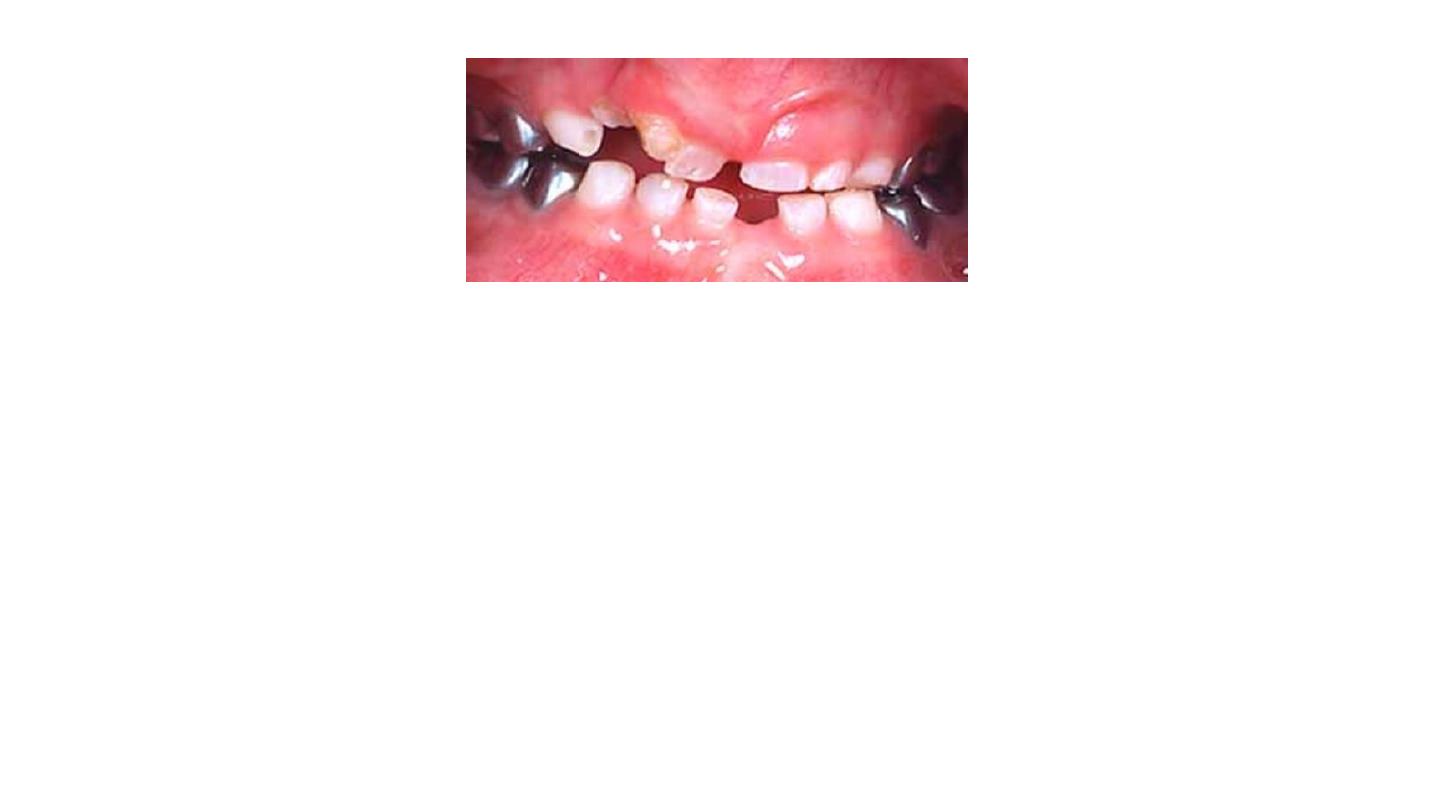
4/ Iatrogenic Discoloration
• Considered intrinsic because it affects inner structure of the tooth.
a. Trauma during pulp extirpation “hemorrhage”.
b. Failure to remove all pulpal remnants. Responds well to non-vital
bleaching
technique.
c. Medications and materials used in dental restorations if they leak.
d. Metals like amalgams-reflect as a discoloration through the enamel

e. Breakdown of restorations such as acrylics, silicate cements or
composite resins can cause the tooth to look grayer and discolored.
f. Silver nitrates—cause black or bluish black discolorations.
g. Volatile oils—cause yellowish brown stains.
h. Iodine-creates brown, yellow or orange stains.
i. Root canal sealers containing silver cause black stains.
j. Pins cause blue grayish stains.
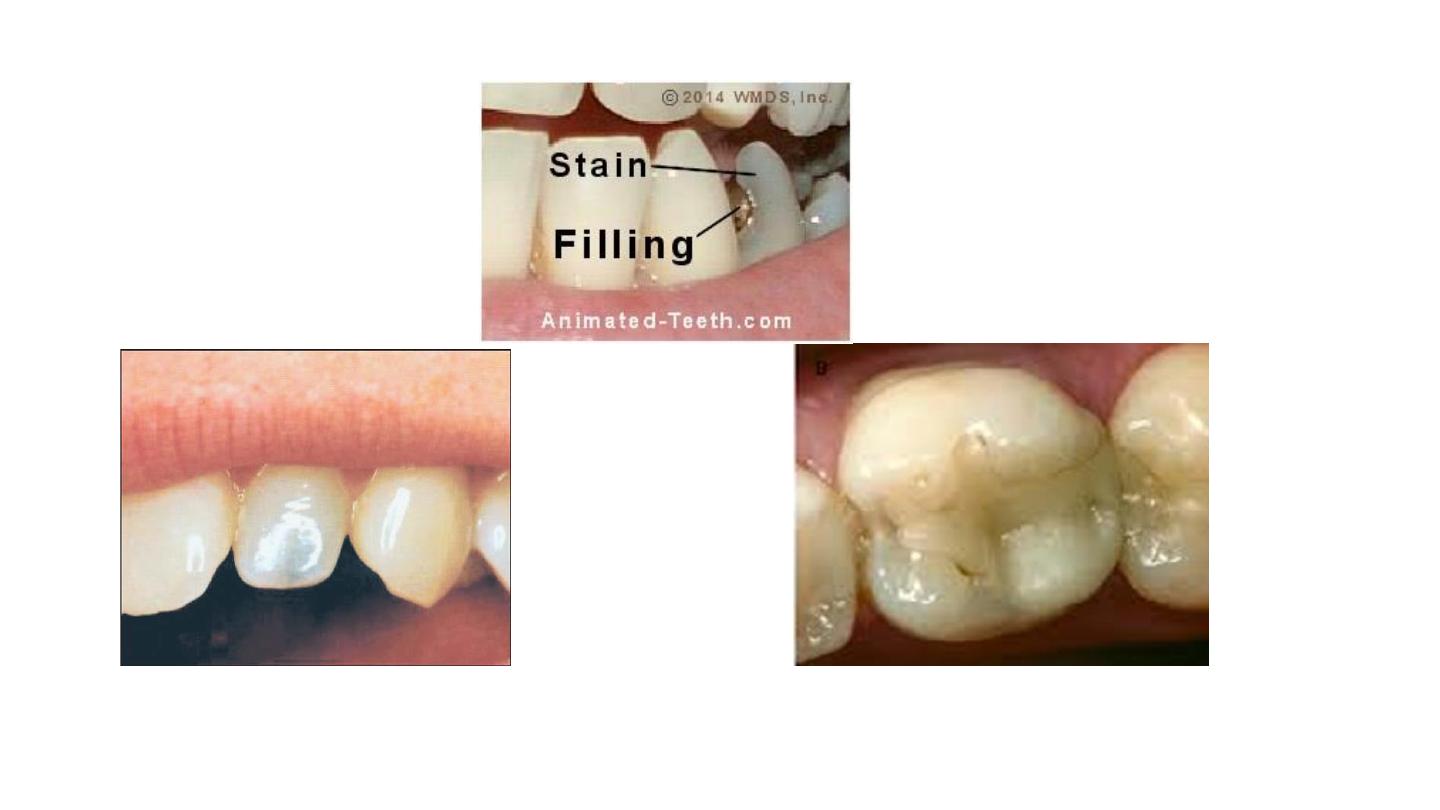
Discoloration due to pin restoration
Discoloration due to composite resin

5/Discoloration as a Symptom
of Systemic Condition
• Erythroblastosis fetalis (Rh incompatibility) between mother and fetus
characterized by breakdown of an excessive number of erythrocytes -
degradation of these blood cells causes intrinsic staining of dentin of
developing teeth.
• Jaundice results in staining of dentin bluish green or brown by
bilirubin or biliverdin.
• Porphyria (rare condition) - excessive pigment production infuses
dentin and makes primary and permanent teeth purplish brown.
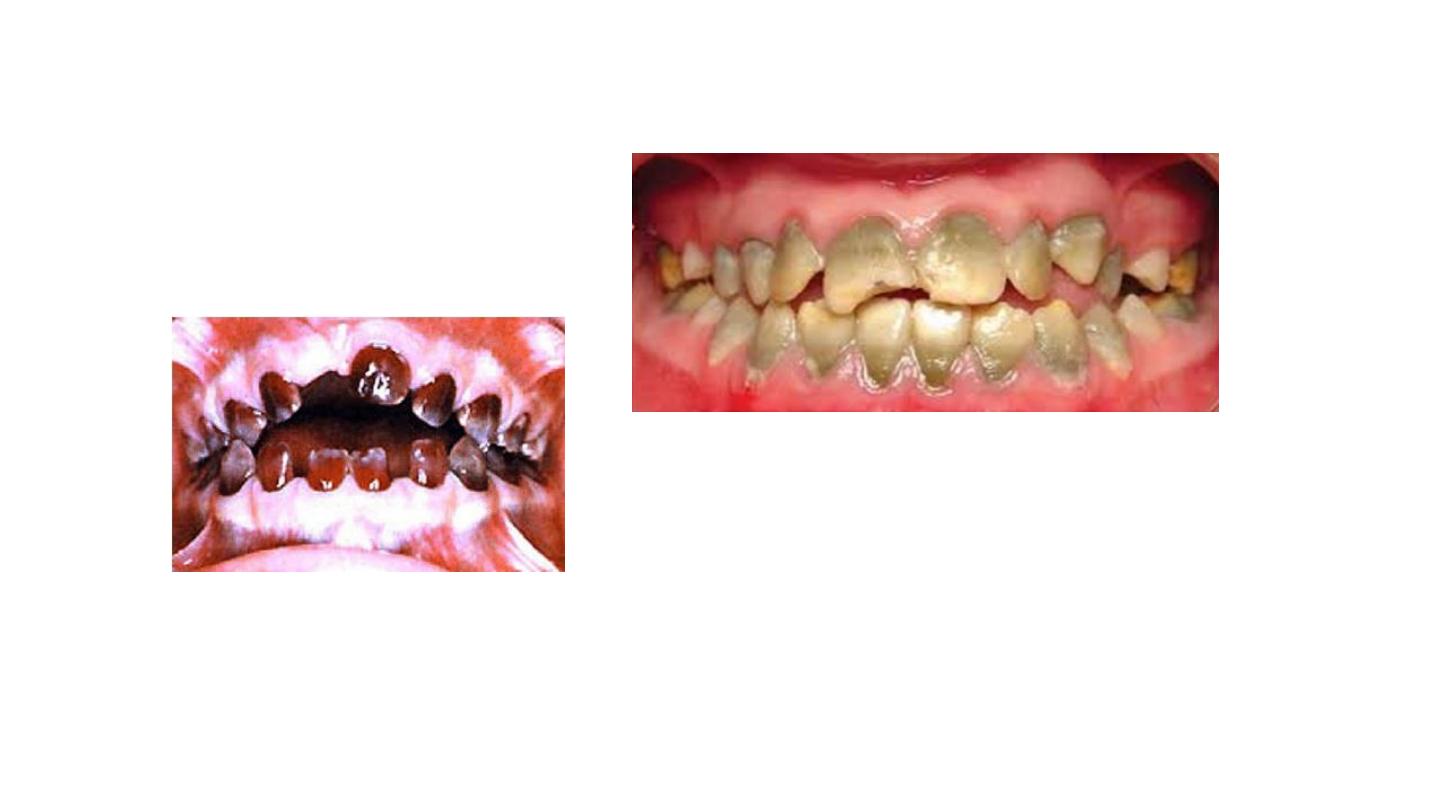
Porphyria
jaundice discoloration of teeth
• Genetic conditions, such as amelogenesis imperfecta, interfering with normal
enamel matrix formation.
• Acquired illnesses such as cerebral palsy, serious renal damage and severe allergies,
neurologic and other traumatic injuries can interfere with the normal development of
the enamel.
• Enamel hypoplasia caused by deficiencies of vitamins A, C, D and calcium and
phosphorous during the formative period.
If these conditions cause tooth deformity or white spots, they respond poorly to
bleaching

6/Discoloration due to Heredity and Dental
History
• Some people are genetically programmed to have lighter or darker
teeth.
• Dental caries may be seen as an opaque halo or as a gray
discoloration. Bleaching is not effective until the cause of discoloration
is removed.
• Deeper pigmentation as a result of bacterial degradation of food
debris in areas of tooth decay or decomposition. If breakdown is
repaired,
• bleaching may not be necessary.
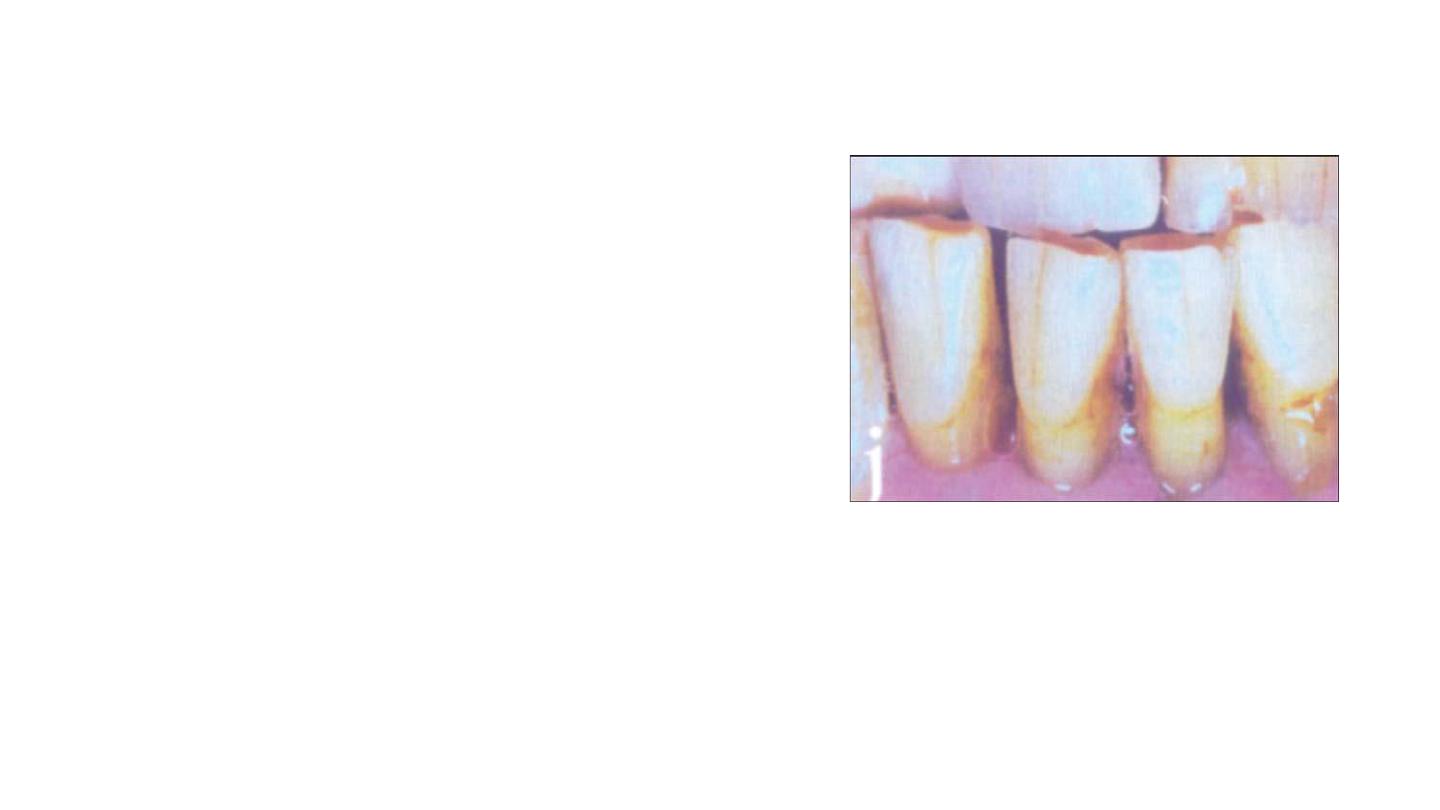
7/Discoloration due to Aging
Advantage in older patients pulp recession makes aging a boon in
terms of bleaching, since, it makes the patient less sensitive to
bleaching
During the natural aging process the
physiologic deposition of secondary dentin
affects the light-transmitting properties of
teeth, resulting in a gradual darkening based
on a narrowing of the pulp space, resulting in
an increase in tooth structure that in turn
affects opacity. In addition, the chemical
structure of teeth changes over time.

BLEACHING OF NON-VITAL TEETH
• 1. In office bleaching.
• 2. Out of office bleaching (walking bleach technique).
• 3. Other bleaching techniques.

General procedures:
Preparation of the affected non-vital
teeth:
• Isolation is done with a rubber dam.
• The tooth is meticulously cleaned internally.
• Establish a lingual opening of sufficient size to provide access to the
pulp chamber and orifice of the root canal.

General procedures:
• A slowly rotating bur is used to remove debris and a surface layer of
dentin within the pulp chamber.
• The root canal filling material should be removed to a depth of 2-3
mm apical to the cervical line.
• Zinc polycarboxylate cement, Cavit or zinc oxyphosphate cement can
be used to refill, 1-2 mm coronally to the CEJ.
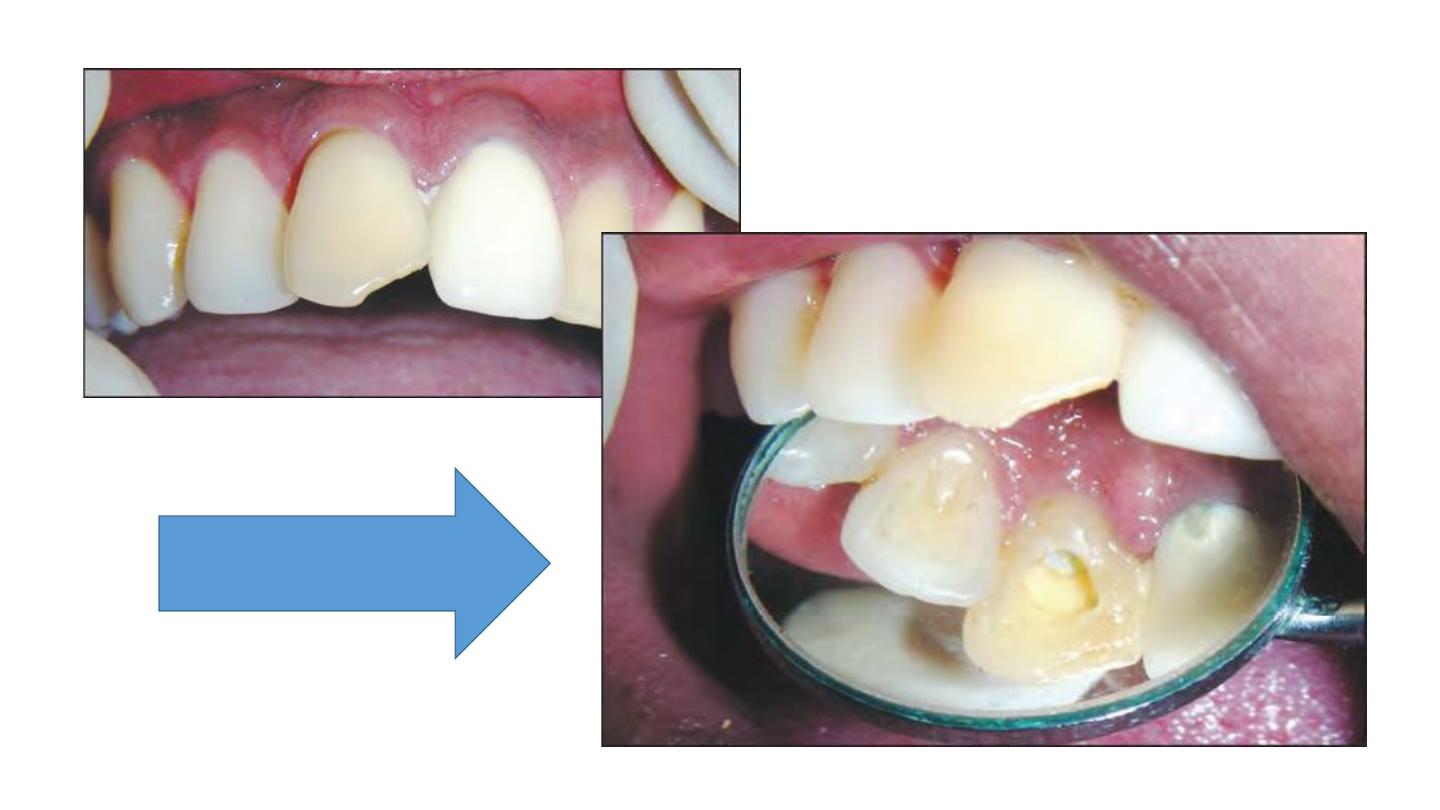
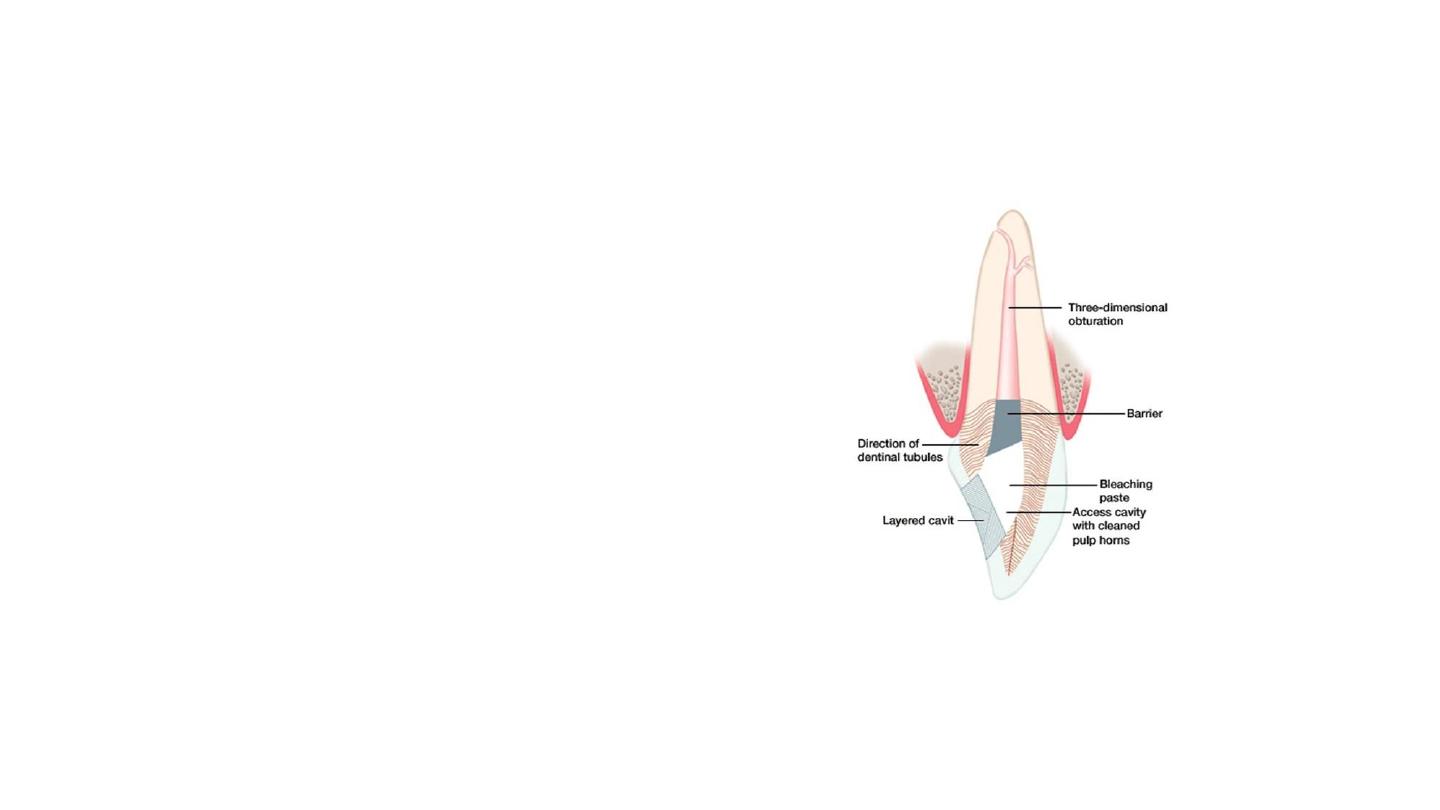
Removal of the temporary
restoration
Coronal barrier of GIC placed
Bleaching should never be attempted on
any tooth that does not have a complete
seal in the root canal.
The agent can escape through a porous
root canal filling and cause the patient
extreme discomfort as well as possible loss
of tooth.
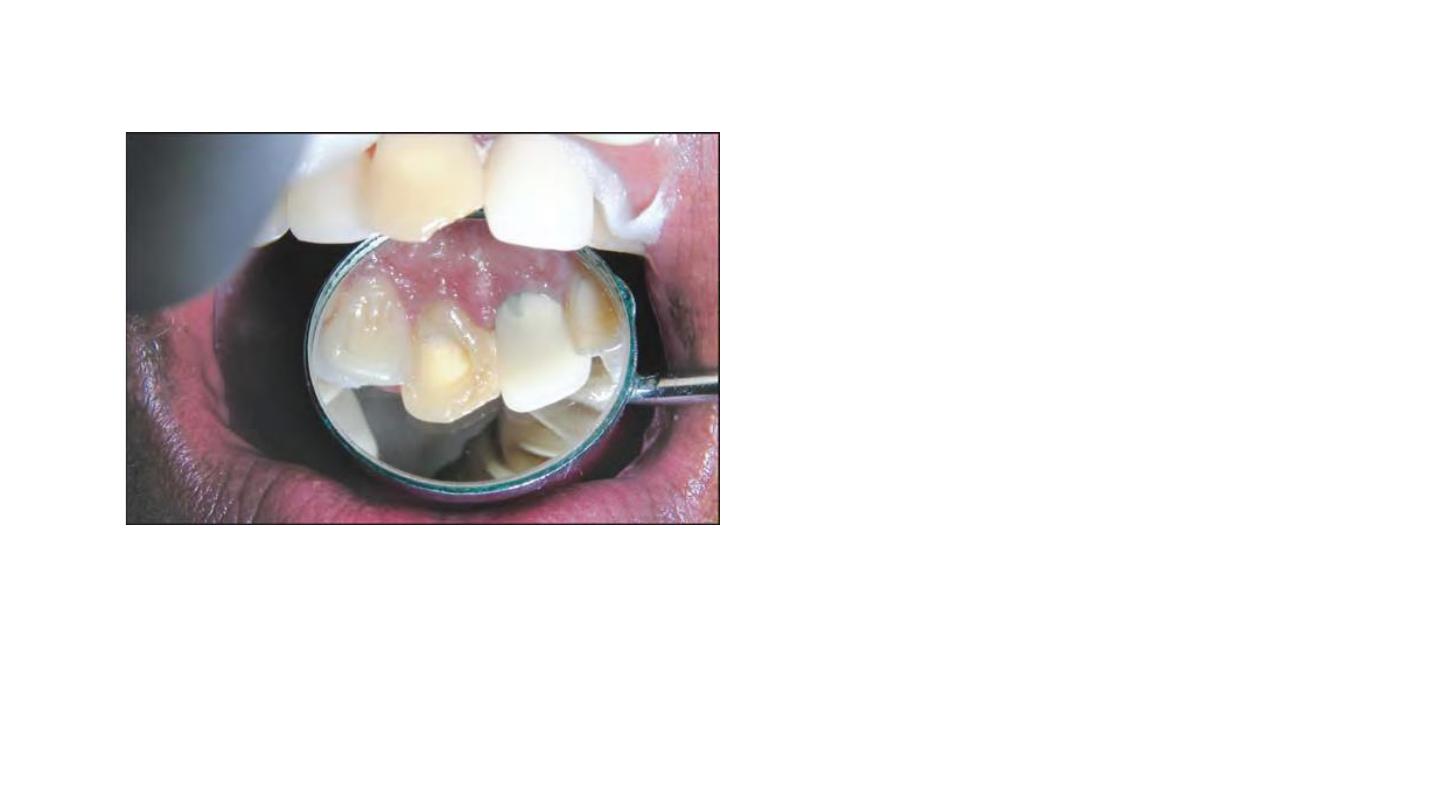
• Surface stains visible on the
inside of the preparation are
removed and the entire
preparation is swabbed with
chloroform or acetone to
dissolve any fatty material and
facilitate the penetraton of the
bleaching agent into the
tubules.

Bleaching Agents for Whitening of Root-filled Teeth
• .The bleaching agents that are most commonly used for whitening of
root-filled teeth are:
hydrogen peroxide ,
carbamide peroxide ,
And sodium perborate

The breakdown of hydrogen peroxide into active oxygen is accelerated by
application of heat the addition of sodium hydroxide, or light.
• Hydrogen peroxide–releasing bleaching agents are
therefore chemically unstable
. Only fresh preparations
should be used
, which must be stored in a dark cool
place
.
Carbamide peroxide is an organic white crystalline compound and is formed
by urea and hydrogen peroxide and used in different concentrations
. In a
hydrophilic environment it breaks down into approximately 3
%hydrogen
peroxide and 7
%urea

Sodium perborate is an oxidizing agent available as a powder. It
is stable when dry; however, in the presence of acid, warm air,
or water, it breaks down to form sodium metaborate
, hydrogen
peroxide, and nascent oxygen
.
The patient should be informed that the results of bleaching
therapy are not predictable, and that complete recovery of
color is not guaranteed in all cases.
It is very helpful to take pretreatment and post-treatment
photographs to show the patient the results obtained at
the end of the treatment
.

• Hydrogen might be applied directly or can be produced by a
chemical reaction from carbamide peroxide or sodium
perborate.
Hydrogen peroxide is used in dentistry as a whitening material at different
concentrations from 5
–35
%
Because of its low molecular weight, this substance can penetrate
dentin and can release oxygen that breaks the double bonds of the
organic and inorganic compounds inside the dentinal tubules

Before treatment a radiograph should be made to check the quality of the root filling.
The filling should not only prevent coronal-apical passage of microorganisms but also
prevent bleaching agents from reaching the apical tissues, potentially having
detrimental effects
Deficient restorations should be identified and replaced; carious lesions should be
restored

1. In-office Bleaching
(Thermocatalytic Techniques)
• The pulp chamber is filled loosely with cotton fibers and the labial
surface is covered with a few strands of cotton fiber to form a matrix
for retaining the bleaching solution.
• This is saturated with 35 percent H2O2 using a glass syringe fitted
with a stainless steel needle. The solution should be discharged slowly
to saturate the cotton inside the pulp chamber and on the labial
surface. Excess should be wiped immediately.

A thin tapered tip from a single tooth bleaching instrument can
be inserted into the pulp chamber.
The heated tip is exposed for 5 minutes, in a sequence of 1
minute on and 15 seconds off.
• It has been established by Caldwell that a non-vital tooth can
be treated to a temperature of 73°C without causing the patient
any discomfort.
• Heat application is repeated 3 or 4 times at every appointment,
changing the pellet with “fresh” bleaching agent at each visit.
• At the end of each appointment the bleaching agent is sealed
into the pulp chamber for additional bleaching between
appointments as in the walking bleach technique.
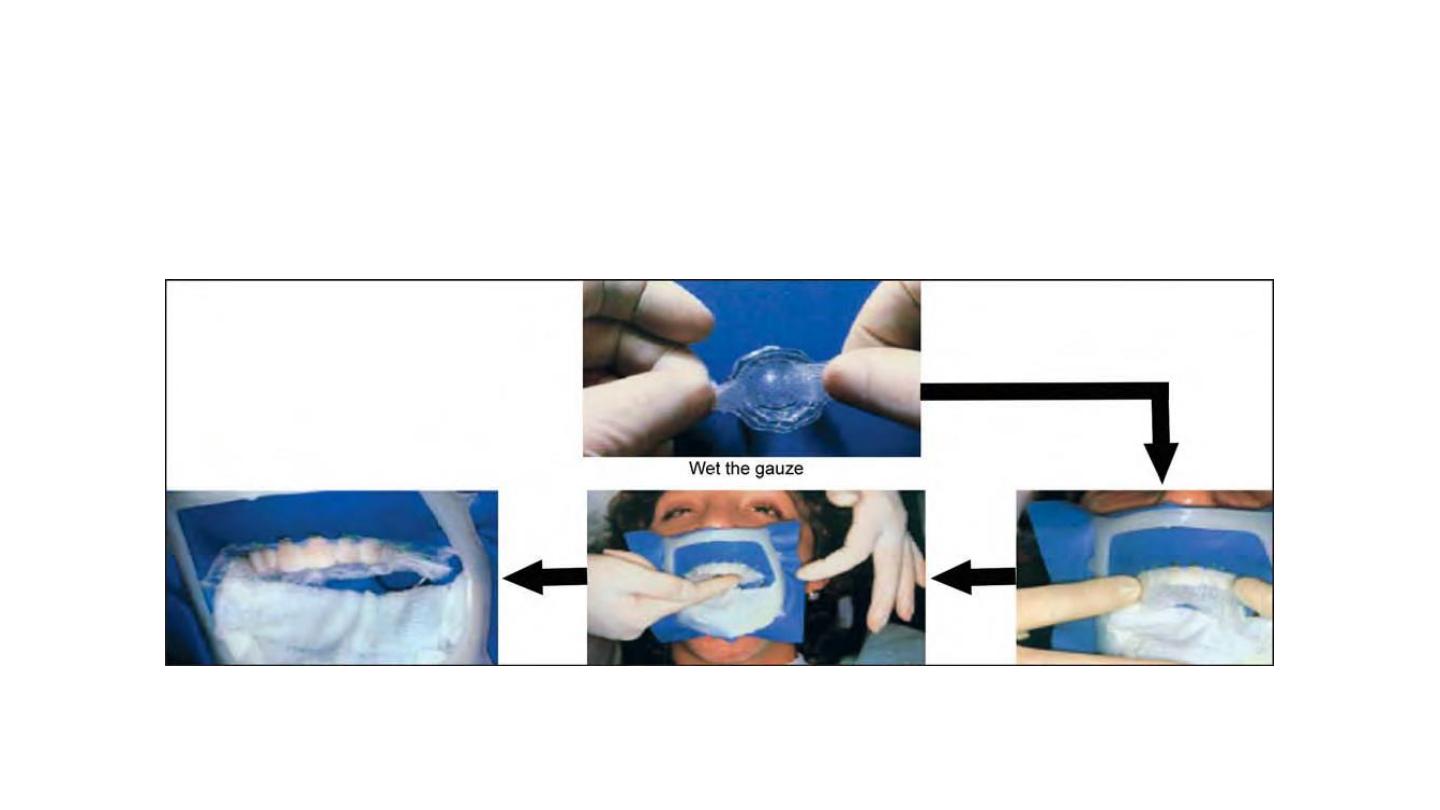
Patient Preparation

2-Out of Office Bleaching (Walking bleach)
First described by Nutting and Poe in 1963.
• This procedure consists of filling the prepared chamber (as described
previously) with a paste consisting of 35 percent H2O2 and sodium
perborate. (Their effect is thought to be synergistic).
• Sodium perborate is a white powder which decomposes into sodium
metaborate and H2O2 releasing oxygen. When mixed into a paste
withSuperoxol, this paste decomposes into sodium metaborate, water
and oxygen.

• The maximum bleaching is attained 24 hours after treatment.
• The patient should return in 3-7 days.
• If the shade is still dark, then procedure should be repeated.
• If adequately light, then permanent restoration with GIC or
composite resin is indicated.
• Generally two treatment sessions are required, although in some
cases one session is sufficient.

Other Methods of Non-vital Bleaching
• Fabrication of a study model.– Light cured composite is placed on the
model
of the tooth or teeth to be treated. This acts as a reservoir to be
created in a vacuum processed
• mouthguard whose thickness usually varies from 0.20 to 0.30 inch.
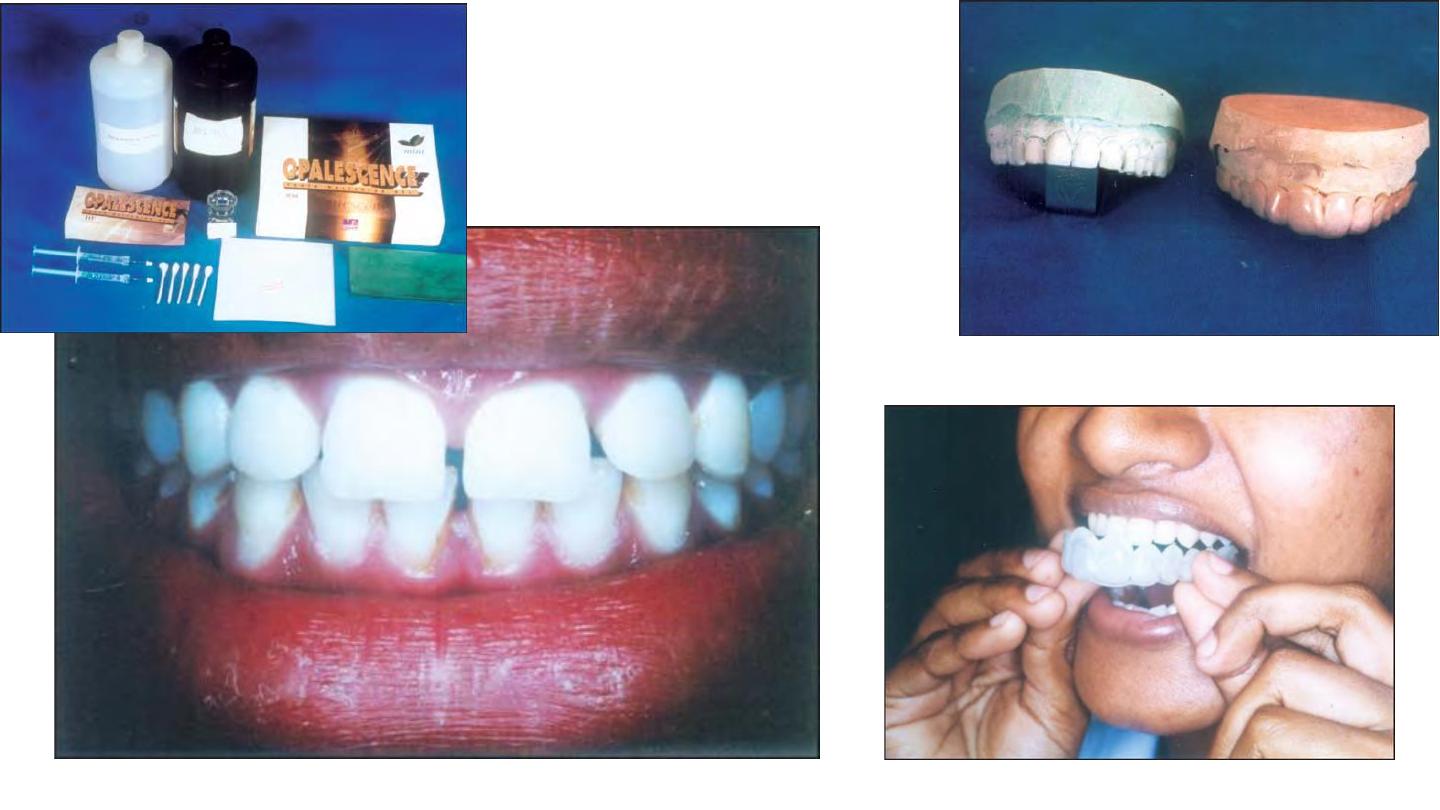

Mouth guard trimmed at the cervical margins on the labial and lingual portions and
tried in the patient's mouth.
– The gutta percha in the root canal is sealed off from the pulp chamber with GIC or
resinmodified GIC.
– Patient is taught how to inject 10 percent carbamide peroxide into the canal orifice
and into the mouth guard with a syringe.
Excess carbamide peroxide gel can be removed by brushing or using a paper tissue.
– The patient may either sleep with the gel or remove the mouthguard after 1 or 2
hours. If the patient prefers the latter, it will take a few
days longer.
– At the end of the daily treatment, patient rinses his or her mouth and then places a
cotton pellet to prevent food from getting into the opening

An explorer can be used by the patient to remove the cotton pellet
before the next procedure.
– The total treatment proceeds and concludes rapidly with the results
in as few as 3 or 4 days.

Complications of Internal Bleaching
1. Cervical resorption
Possible mechanism is that H2O2 percolates from the access cavity to
the root surface through the acid treated patent dentinal tubules.
– This stimulates an inflammatory response leading to dentin
resorption.
– Alternative theory - bacteria that have leaked into the pulp chamber
from the gingival crevice
via the dentinal tubules or directly from the access cavity may cause
resorption.
– Root resorption can be arrested by placing CaOH in the chamber.

– Oxidizing agents are safer to handle as a paste than in a solution.
• – Rubber dam application is a must.
• – Any spillage must be diluted immediately with copious volumes of
water.
2. Spillage of bleaching agents

3. Failure to bleach
Causes:
– Commonest is discoloration by metal ions in silver amalgam.
– Incomplete removal of composite resin or GIC, which prevents the
bleaching agents to penetrate into dentinal tubules.
– H2O2 which has passed its expiry date or improperly stored.

4. Over bleaching
– Recommended by certain authors as the tooth may darken with time
and assume desired shade.
– However, it is important not to over bleach. Therefore, ask the
patient to monitor color and return in case of over bleaching.

– Bleaching causes the coronal tooth structure to be brittle. This may
be caused due to removal of all the discolored dentin rather than using
the bleaching agents to discolor the dentin.
5. Brittleness of tooth crown