Antifungal Drugs
Drugs for systemic antifungal treatment include amphotericin B (and its lipid
formulations), various azole derivatives, echinocandins, and flucytosine (see Table:
Some Drugs for Systemic Fungal Infections
). Amphotericin B, an effective but
relatively toxic drug, has long been the mainstay of antifungal therapy for invasive
and serious mycoses. However, newer potent and less toxic triazoles and
echinocandins are now often recommended as first-line drugs for many invasive
fungal infections. These drugs have markedly changed the approach to antifungal
therapy, sometimes even allowing oral treatment of chronic mycoses.
Some Drugs for Systemic Fungal Infections
Drug
Uses
Dose
Some Adverse Effects
Amphotericin
B
Most fungal infections
(Not for
Pseudallescheria sp)
Conventional
(deoxycholate)
formulation: 0.5–
1.0 mg/kg IV
once/day
Conventional formulation:
Acute infusion reactions,
neuropathy, GI upset, renal
failure, anemia,
thrombophlebitis, hearing
.loss, rash, hypokalemia,
hypomagnesemia
Various lipid
formulations: 3–5
mg/kg IV
once/day
Lipid formulations:
Infusion reactions*, renal
failure*
Anidulafungin
Candidiasis, including
candidemia
200 mg IV on day
1, then 100 mg IV
once/day
For esophageal
candidiasis, half
of this dose
Hepatitis, diarrhea,
hypokalemia, infusion
reactions
Caspofungin
Aspergillosis
Candidiasis, including
candidemia
70 mg IV on day
1, then 50 mg IV
once/day
Phlebitis, headache, GI
upset, rash,
Fluconazole
Mucosal and systemic
candidiasis
Cryptococcal
meningitis
Coccidioidal
meningitis
100–800 mg po or
IV once/day
(loading dose may
be given)
Children: 3–12
mg/kg po or IV
once/day
GI upset, hepatitis, QT
prolongation
Flucytosine
Candidiasis (systemic)
Cryptococcosis
12.5–37.5 mg/kg
po qid
Pancytopenia due to bone
marrow toxicity,
neuropathy, nausea,

Drug
Uses
Dose
Some Adverse Effects
vomiting, hepatic and renal
injury, colitis
Itraconazole
Dermatomycosis
Histoplasmosis,
blastomycosis,
coccidioidomycosis,
sporotrichosis
100 mg po
once/day to 200
mg po bid
Hepatitis, GI upset, rash,
headache, dizziness,
hypokalemia,
hypertension, edema, QT
prolongation
Micafungin
Candidiasis, including
candidemia
100 mg IV
once/day (dose
150 mg for
esophageal
candidiasis)
Phlebitis, hepatitis, rash,
headache, nausea
Posaconazole
Prophylaxis for
invasive aspergillosis
and candidiasis
200 mg po tid
Hepatitis, GI upset, rash,
QT prolongation
Oral candidiasis
100 mg po bid on
day 1, then 100
mg once/day for
13 days
Oral candidiasis
refractory to
itraconazole
400 mg po bid
Voriconazole
Invasive aspergillosis
Fusariosis
Scedosporiosis
6 mg/kg IV for 2
loading doses,
then 200 mg po q
12 h
or
3 to 6 mg/kg IV q
12 h
GI upset, transient visual
disturbances, peripheral
edema, rash, hepatitis, QT
prolongation
*This adverse effect is less common with lipid formulations than with the
conventional formulation.
Clinical Calculator: QT Interval Correction (EKG)
Amphotericin B
Amphotericin B has been the mainstay of antifungal therapy for invasive and serious
mycoses, but other antifungals (eg, fluconazole, voriconazole, posaconazole, the
echinocandins) are now considered first-line drugs for many of these infections.
Although amphotericin B does not have good CSF penetration, it is still effective for
certain mycoses such as cryptococcal meningitis.

For chronic mycoses , amphotericin B deoxycholate is usually started at ≥ 0.3 mg/kg
IV once/day, increased as tolerated to the desired dose (0.4 to 1.0 mg/kg; generally
not > 50 mg/day); many patients tolerate the target dose on the first day.
For acute, life-threatening mycoses , amphotericin B deoxycholate may be started at
0.6 to 1.0 mg/kg IV once/day.
Formulations
There are 2 formulations of amphotericin:
Deoxycholate (standard)
Lipid-based
The standard formulation , amphotericin B deoxycholate, must always be given in
5% D/W because salts can precipitate the drug. It is usually given over 2 to 3 h,
although more rapid infusions over 20 to 60 min can be used in selected patients.
However, more rapid infusions usually have no advantage. Many patients experience
chills, fever, nausea, vomiting, anorexia, headache, and, occasionally, hypotension
during and for several hours after an infusion. Amphotericin B may also cause
chemical thrombophlebitis when given via peripheral veins; a central venous catheter
may be preferable. Pretreatment with acetaminophen or NSAIDs is often used; if
these drugs are ineffective, hydrocortisone 25 to 50 mg or diphenhydramine 25 mg is
sometimes added to the infusion or given as a separate IV bolus. Often,
hydrocortisone can be tapered and omitted during extended therapy. Severe chills and
rigors can be relieved or prevented by meperidine 50 to 75 mg IV.
Several lipid vehicles reduce the toxicity of amphotericin B (particularly
nephrotoxicity and infusion-related symptoms). Two preparations are available:
Amphotericin B lipid complex
Liposomal amphotericin B
Lipid formulations are preferred over conventional amphotericin B because they
cause fewer infusion-related symptoms and less nephrotoxicity.
Adverse effects
The main adverse effects are
Nephrotoxicity (most common)
Hypokalemia
Hypomagnesemia
Bone marrow suppression
Renal impairment is the major toxic risk of amphotericin B therapy. Serum creatinine
and BUN should be monitored before treatment and at regular intervals during
treatment: several times/wk for the first 2 to 3 wk, then 1 to 4 times/mo as clinically

indicated. Amphotericin B is unique among nephrotoxic antimicrobial drugs because
it is not eliminated appreciably via the kidneys and does not accumulate as renal
failure worsens. Nevertheless, dosages should be lowered, or a lipid formulation
should be used instead if serum creatinine rises to > 2.0 to 2.5 mg/dL (> 177 to 221
μmol/L) or BUN rises to > 50 mg/dL (> 18 mmol urea/L). Acute nephrotoxicity can
be reduced by aggressive IV hydration with saline before amphotericin B infusion; at
least 1 L of normal saline should be given before amphotericin infusion. Mild to
moderate renal function abnormalities induced by amphotericin B usually resolve
gradually after therapy is completed. Permanent damage occurs primarily after
prolonged treatment; after > 4 g total dose, about 75% of patients have persistent renal
insufficiency.
Amphotericin B also frequently suppresses bone marrow function, manifested
primarily by anemia. Hepatotoxicity or other untoward effects are unusual.
Azole Antifungals
Azoles block the synthesis of ergosterol, an important component of the fungal
cell membrane. They can be given orally to treat chronic mycoses. The first such
oral drug, ketoconazole, has been supplanted by more effective, less toxic triazole
derivatives, such as fluconazole, itraconazole, posaconazole, and voriconazole.
Drug interactions can occur with all azoles but are less likely with fluconazole.
The drug interactions mentioned below are not intended as a complete listing;
clinicians should r
Fluconazole
This water-soluble drug is absorbed almost completely after an oral dose. It is
excreted largely unchanged in urine and has a half-life of > 24 h, allowing single daily
doses. It has high penetration into CSF (≥ 70% of serum levels) and has been
especially useful in treating cryptococcal and coccidioidal meningitis. It is also one of
the first-line drugs for treatment of candidemia in nonneutropenic patients. Doses
range from 200 to 400 mg po once/day to as high as 800 mg once/day in some
seriously ill patients and in patients infected with Candida glabrata or other Candida
sp (not C. albicans or C. krusei); daily doses of ≥ 1000 mg have been given and had
acceptable toxicity.
Adverse effects that occur most commonly are GI discomfort and rash. More severe
toxicity is unusual, but the following have occurred: hepatic necrosis, Stevens-
Johnson syndrome, anaphylaxis, alopecia, and, when taken for long periods of time
during the 1st trimester of pregnancy, congenital fetal anomalies.
Drug interactions occur less often with fluconazole than with other azoles. However,
fluconazole sometimes elevates serum levels of Ca channel blockers, cyclosporine,
rifabutin, phenytoin, tacrolimus, warfarin-type oral anticoagulants, sulfonylurea drugs
(eg, tolbutamide), and zidovudine. Rifampin may lower fluconazole blood levels.

Itraconazole
This drug has become the standard treatment for lymphocutaneous sporotrichosis as
well as for mild or moderately severe histoplasmosis, blastomycosis, and
paracoccidioidomycosis. It is also effective in mild cases of invasive aspergillosis,
some cases of coccidioidomycosis, and certain types of chromoblastomycosis.
Despite poor CSF penetration, itraconazole can be used to treat some types of fungal
meningitis, but it is not the drug of choice. Because of its high lipid solubility and
protein binding, itraconazole blood levels tend to be low, but tissue levels are
typically high. Drug levels are negligible in urine and CSF. Use of itraconazole has
declined as use of voriconazole and posaconazole has increased.
Adverse effects with doses of up to 400 mg/day most commonly are GI, but a few
men have reported erectile dysfunction, and higher doses may cause hypokalemia,
hypertension, and edema. Other reported adverse effects include allergic rash,
hepatitis, and hallucinations. An FDA black box warning for heart failure has been
issued, particularly with a total daily dose of 400 mg.
Drug and food interactions can be significant. When the capsule form is used, acidic
drinks (eg, cola, acidic fruit juices) or foods (especially high-fat foods) improve
absorption of itraconazole from the GI tract. However, absorption may be reduced if
itraconazole is taken with prescription or OTC drugs used to lower gastric acidity.
Several drugs, including rifampin, rifabutin, didanosine, phenytoin, and
carbamazepine, may decrease serum itraconazole levels. Itraconazole also inhibits
metabolic degradation of other drugs, elevating blood levels with potentially serious
consequences. Serious, even fatal cardiac arrhythmias may occur if itraconazole is
used with cisapride (not available in the US) or some antihistamines (eg, terfenadine,
astemizole, perhaps loratadine). Rhabdomyolysis has been associated with
itraconazole-induced elevations in blood levels of cyclosporine or statins. Blood
levels of some drugs (eg, digoxin, tacrolimus, oral anticoagulants, sulfonylureas) may
increase when these drugs are used with itraconazole.
Posaconazole
The triazole posaconazole is available as an oral suspension and a tablet. An IV
formulation will probably be available soon. This drug is highly active against yeasts
and molds and effectively treats various opportunistic mold infections, such as those
due to dematiaceous (dark-walled) fungi (eg, Cladophialophora sp). It is the only oral
azole effective against many of the species that cause mucormycosis. Posaconazole
can also be used as fungal prophylaxis in neutropenic patients with various cancers
and in bone marrow transplant recipients.
Adverse effects for posaconazole, as for other triazoles, include a prolonged QT
interval and hepatitis.
Drug interactions occur with many drugs, including rifabutin, rifampin, statins,
various immunosuppressants, and barbiturates.

Voriconazole
This broad-spectrum triazole is available as a tablet and an IV formulation. It is
considered the treatment of choice for Aspergillus infections in immunocompetent
and immunocompromised hosts. Voriconazole can also be used to treat Scedosporium
apiospermum and Fusarium infections. Additionally, the drug is effective in candidal
esophagitis and invasive candidiasis, although it is not usually considered a first-line
treatment; it has activity against a broader spectrum of Candida sp than does
fluconazole.
Adverse effects that must be monitored for include hepatotoxicity, visual
disturbances (common), hallucinations, and dermatologic reactions. This drug can
prolong the QT interval.
Drug interactions are numerous, notably with certain immunosuppressants used after
organ transplantation.
Echinocandins
Echinocandins are water-soluble lipopeptides that inhibit glucan synthase. They are
available only for IV administration. Their mechanism of action is unique among
antifungal drugs; echinocandins target the fungal cell wall, making them attractive
because they lack cross-resistance with other drugs and their target is fungal and has
no mammalian counterpart. Drug levels in urine and CSF are not significant.
Echinocandins available in the US are anidulafungin, caspofungin, and micafungin.
There is little evidence to suggest that one is better than the other, but anidulafungin
appears to interact with fewer drugs than the other two.
These drugs are potently fungicidal against most clinically important Candida sp but
are considered fungistatic against Aspergillus.
Adverse effects include hepatitis and rash.
Flucytosine
Flucytosine, a nucleic acid analog, is water soluble and well-absorbed after oral
administration. Preexisting or emerging resistance is common, so it is almost always
used with another antifungal, usually amphotericin B. Flucytosine plus amphotericin
B is used primarily to treat cryptococcosis but is also valuable for some cases of
disseminated candidiasis (including endocarditis), other yeast infections, and severe
invasive aspergillosis. Flucytosine plus antifungal azoles may be beneficial in treating
cryptococcal meningitis and some other mycoses.
The usual dose (12.5 to 37.5 mg/kg po qid) leads to high drug levels in serum, urine,
and CSF.

Major adverse effects are bone marrow suppression (thrombocytopenia and
leukopenia), hepatotoxicity, and enterocolitis; only degree of bone marrow
suppression is proportional to serum levels.
Because flucytosine is cleared primarily by the kidneys, blood levels rise if
nephrotoxicity develops during concomitant use with amphotericin B, particularly
when amphotericin B is used in doses > 0.4 mg/kg/day. Flucytosine serum levels
should be monitored, and the dosage should be adjusted to keep levels between 40
and 90 μg/mL. CBC and renal and liver function tests should be done twice/wk. If
blood levels are unavailable, therapy is begun at 25 mg/kg qid, and dosage is
decreased if renal function deteriorates.
efer to a specific drug interaction reference before using azole antifungal drugs.
Blastomycosis
lastomycosis is a pulmonary disease caused by inhaling spores of the dimorphic
fungus Blastomyces dermatitidis; occasionally, the fungi spread hematogenously,
causing extrapulmonary disease. Symptoms result from pneumonia or from
dissemination to multiple organs, most commonly the skin. Diagnosis is clinical, by
chest x-ray, or both and is confirmed by laboratory identification of the fungi.
Treatment is with itraconazole, fluconazole, or amphotericin B.
(See also the Infectious Diseases Society of America’s
management of patients with blastomycosis
In North America, the endemic area for blastomycosis includes
Ohio–Mississippi River valleys (extending into the middle Atlantic and
southeastern states)
Northern Midwest
Upstate New York
Southern Canada
Rarely, the infection occurs in the Middle East and Africa.
Immunocompetent people can contract this infection. Although blastomycosis may be
more common and more severe in immunocompromised patients, it is a less common
opportunistic infection than histoplasmosis or coccidioidomycosis.
B. dermatitidis grows as a mold at room temperature in soil enriched with animal
excreta and in moist, decaying, acidic organic material, often near rivers. In the lungs,
inhaled spores convert into large (15 to 20 μm) invasive yeasts, which form
characteristic broad-based buds.
Once in the lungs, infection may
Remain localized in the lungs
Disseminate hematogenously
Hematogenous dissemination can cause focal infection in numerous organs, including
the skin, prostate, epididymides, testes, kidneys, vertebrae, ends of long bones,
subcutaneous tissues, brain, oral or nasal mucosa, thyroid, lymph nodes, and bone
marrow.
Symptoms and Signs
Pulmonary
Pulmonary blastomycosis may be asymptomatic or cause an acute, self-limited
disease that often goes unrecognized. It can also begin insidiously and develop into a
chronic, progressive infection. Symptoms include a productive or dry hacking cough,
chest pain, dyspnea, fever, chills, and drenching sweats.
Pleural effusion occurs occasionally. Some patients have rapidly progressive
infections, and acute respiratory distress syndrome may develop.
Extrapulmonary
In extrapulmonary disseminated blastomycosis, symptoms depend on the organ
involved.
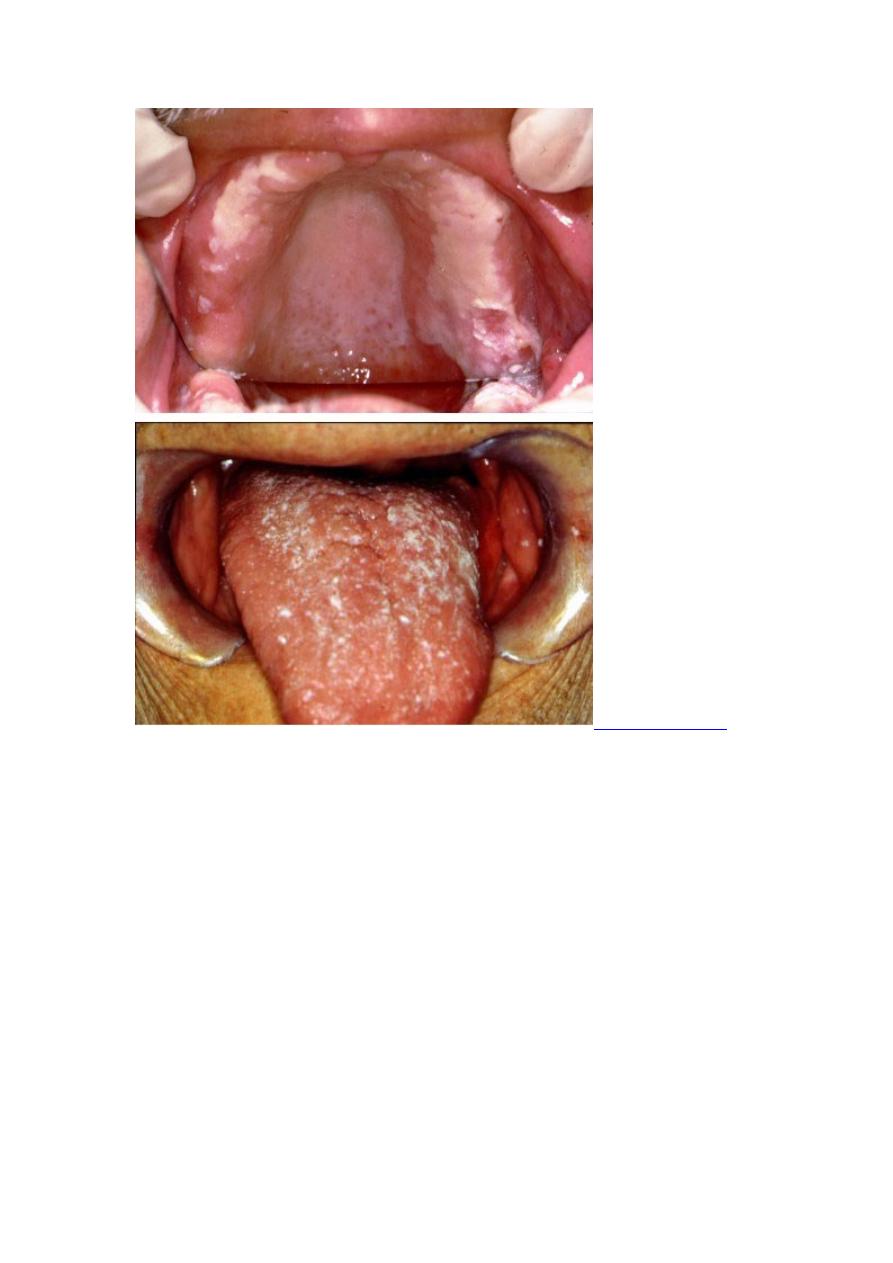
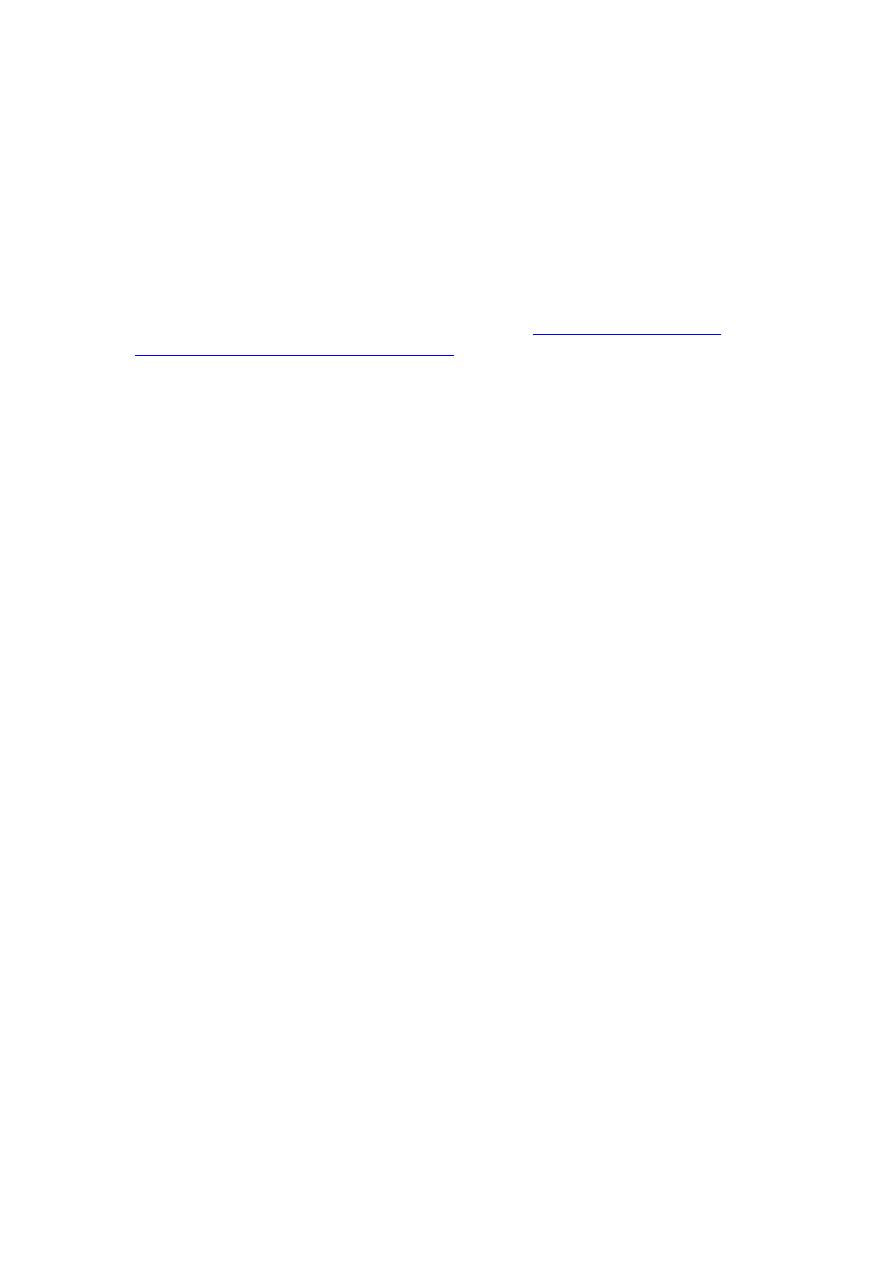
Skin lesions are by far the most common; they may be single or multiple and may
occur with or without clinically apparent pulmonary involvement. Papules or
papulopustules usually appear on exposed surfaces and spread slowly. Painless
miliary abscesses, varying from pinpoint to 1 mm in diameter, develop on the
advancing borders. Irregular, wartlike papillae may form on surfaces. Sometimes
bullae develop. As lesions enlarge, the centers heal, forming atrophic scars. When
fully developed, an individual lesion appears as an elevated verrucous patch, usually ≥
2 cm wide with an abruptly sloping, purplish red, abscess-studded border. Ulceration
may occur if bacterial superinfection is present.
If bone lesions develop, overlying areas are sometimes swollen, warm, and tender.
Genital lesions cause painful epididymal swelling, deep perineal discomfort, or
prostatic tenderness detected during rectal examination.
CNS involvement can manifest as brain abscess, epidural abscess, or meningitis.
Diagnosis
Fungal cultures and smear
Blastomyces urine antigen
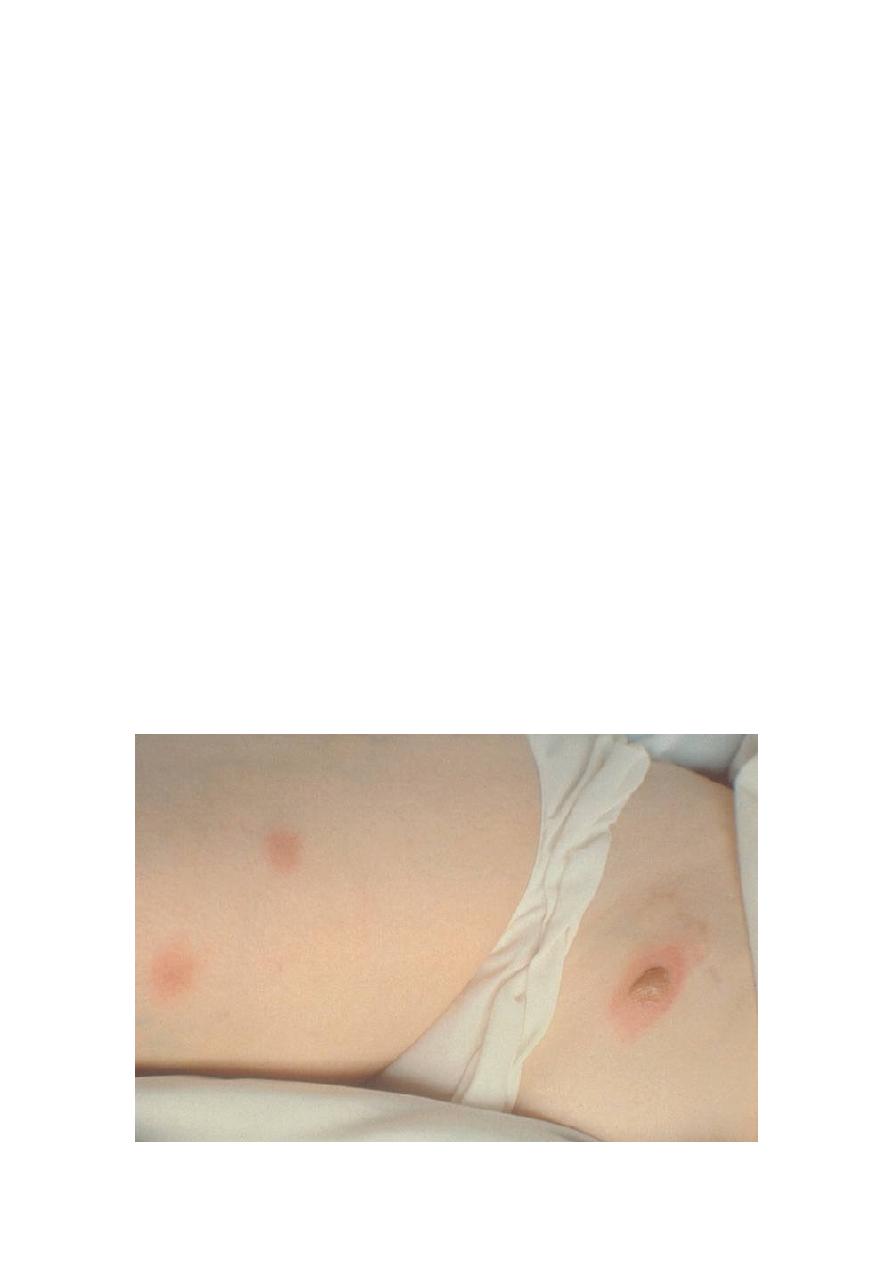
A chest x-ray should be taken. Focal or diffuse infiltrates may be present, sometimes
as patchy bronchopneumonia fanning out from the hilum. These findings must be
distinguished from other causes of pneumonia (eg, other mycoses, TB, tumors). Skin
lesions can be mistaken for sporotrichosis, TB, iodism, or basal cell carcinoma.
Genital involvement may mimic TB.
Cultures of infected material are done; they are definitive when positive. Because
culturing Blastomyces can pose a severe biohazard to laboratory personnel, the
laboratory should be notified of the suspected diagnosis. The organism’s

characteristic appearance, seen during microscopic examination of tissues or sputum,
is also frequently diagnostic. Serologic testing is not sensitive but is useful if positive.
A urine antigen test is useful, but cross-reactivity with Histoplasma is high.
Treatment
For mild to moderate disease, itraconazole
For severe, life-threatening infection, amphotericin B
Untreated blastomycosis is usually slowly progressive and is rarely ultimately fatal.
Treatment depends on severity of the infection. For mild to moderate disease,
itraconazole 200 mg po tid for 3 days, followed by 200 mg po once/day or bid for 6 to
12 mo is used. Fluconazole appears less effective, but 400 to 800 mg po once/day
may be tried in itraconazole-intolerant patients with mild disease. For severe, life-
threatening infections, IV amphotericin B is usually effective; therapy is changed to
itraconazole once patients improve.
Voriconazole and posaconazole are highly active against B. dermatitidis, but their role
has not yet been defined.
Key Points
Inhaling spores of the dimorphic fungus Blastomyces can cause pulmonary
disease and, less commonly, disseminated infection (particularly to the skin).
In North America, blastomycosis is endemic in the regions around the Great
Lakes and in the mid-Atlantic and Southeast.
Diagnose using cultures of infected material; serologic testing is specific but
not very sensitive.
For mild to moderate disease, use itraconazole.
For severe disease, use amphotericin B.
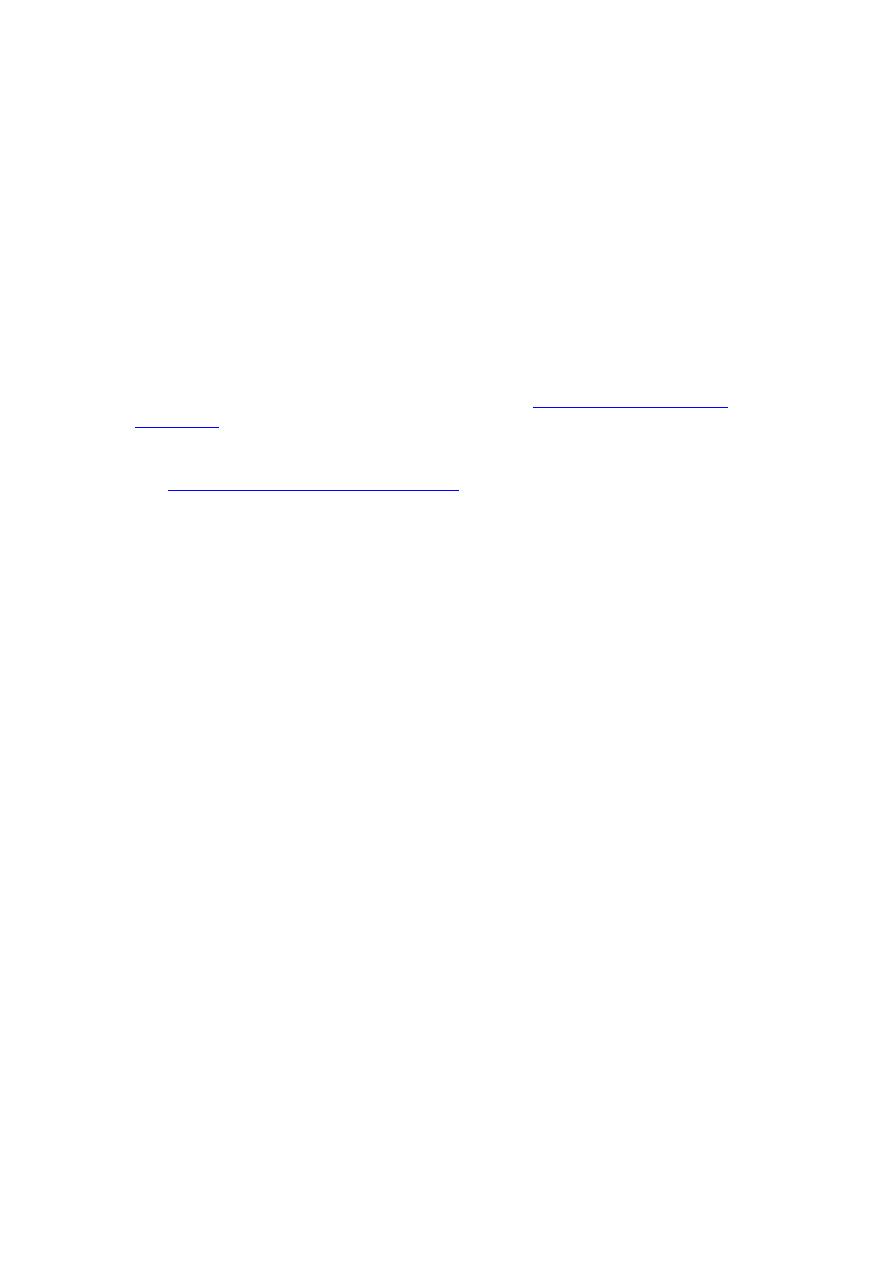
Candidiasis (Invasive)
(Candidosis; Moniliasis)
Candidiasis is infection by Candida sp (most often C. albicans), manifested by
mucocutaneous lesions, fungemia, and sometimes focal infection of multiple sites.
Symptoms depend on the site of infection and include dysphagia, skin and mucosal
lesions, blindness, vaginal symptoms (itching, burning, discharge), fever, shock,
oliguria, renal shutdown, and disseminated intravascular coagulation. Diagnosis is
confirmed by histopathology and cultures from normally sterile sites. Treatment is
with amphotericin B, fluconazole, echinocandins, voriconazole, or posaconazole.
(See also the Infectious Diseases Society of America’s
Candida sp are commensal organisms that inhabit the GI tract and sometimes the skin
(see
Candidiasis (Mucocutaneous) : Etiology
). Unlike other systemic mycoses,
candidiasis results from endogenous organisms. Most infections are caused by C.
albicans; however, C. glabrata (formerly Torulopsis glabrata) and other non-albicans
species are increasingly involved in fungemia, UTIs, and, occasionally, other focal
disease. C. glabrata is frequently less susceptible to fluconazole than other species; C.
kruseiis inherently resistant.
Candida sp account for about 80% of major systemic fungal infections and are the
most common cause of fungal infections in immunocompromised patients. Candidal
infections are one of the most common hospital-acquired infections.
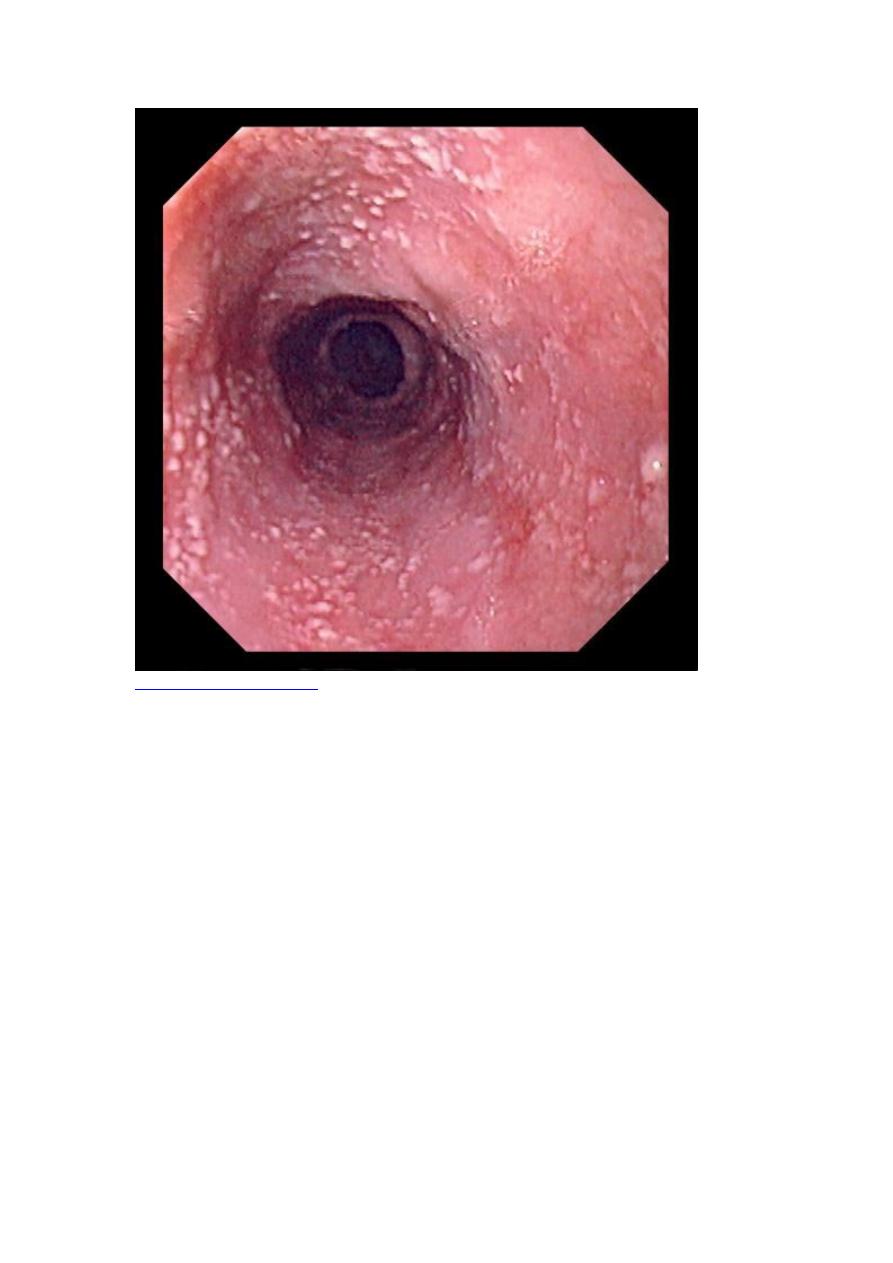
Candidiasis involving the mouth and esophagus is a defining opportunistic infection
in AIDS. Although mucocutaneous candidiasis is frequently present in HIV-infected
patients, hematogenous dissemination is unusual unless other specific risk factors are
present (see below). Neutropenic patients (eg, those receiving cancer chemotherapy)
are at high risk of developing life-threatening disseminated candidiasis.
Candidemia may occur in nonneutropenic patients during prolonged hospitalization.
This bloodstream infection is often related to one or more of the following:
Central venous catheters
Major surgery
Broad-spectrum antibacterial therapy
IV hyperalimentation
IV lines and the GI tract are the usual portals of entry. Candidemia often prolongs
hospitalization and increases mortality due to concurrent disorders. Prolonged or
untreated candidemia may lead to endocarditis or meningitis as well as to focal
involvement of skin, subcutaneous tissues, bones, joints, liver, spleen, kidneys, eyes,
and other tissues. Endocarditis is commonly related to IV drug abuse, valve
replacement, or intravascular trauma induced by indwelling IV catheters.

All forms of disseminated candidiasis should be considered serious, progressive, and
potentially fatal.
Symptoms and Signs
Esophageal candidiasis is most often manifested by dysphagia.
Candidemia usually causes fever, but no symptoms are specific. Some patients
develop a syndrome resembling bacterial sepsis, with a fulminating course that may
include shock, oliguria, renal shutdown, and disseminated intravascular coagulation.
Candidal endophthalmitis starts as white retinal lesions that are initially asymptomatic
but can progress, opacifying the vitreous and causing potentially irreversible scarring
and blindness. In neutropenic patients, retinal hemorrhages occasionally also occur,
but actual infection of the eye is rare.
Papulonodular skin lesions may also develop, especially in neutropenic patients, in
whom they indicate widespread hematogenous dissemination to other organs.
Symptoms of other focal infection depend on the organ involved.
Diagnosis
Histopathology and fungal cultures
Blood cultures
Serum β-glucan testing
Because Candida spp are commensal, their culture from sputum, the mouth, the
vagina, urine, stool, or skin does not necessarily signify an invasive, progressive
infection. A characteristic clinical lesion must also be present, histopathologic
evidence of tissue invasion (eg, yeasts, pseudohyphae, or hyphae in tissue specimens)
must be documented, and other etiologies must be excluded. Positive cultures of
specimens taken from normally sterile sites, such as blood, CSF, pericardium,
pericardial fluid, or biopsied tissue, provide definitive evidence that systemic therapy
is needed.
Serum β-glucan is often positive in patients with invasive candidiasis; conversely, a
negative result indicates low likelihood of systemic infection.
Ophthalmologic examination to check for endophthalmitis is recommended for all
patients with candidemia.
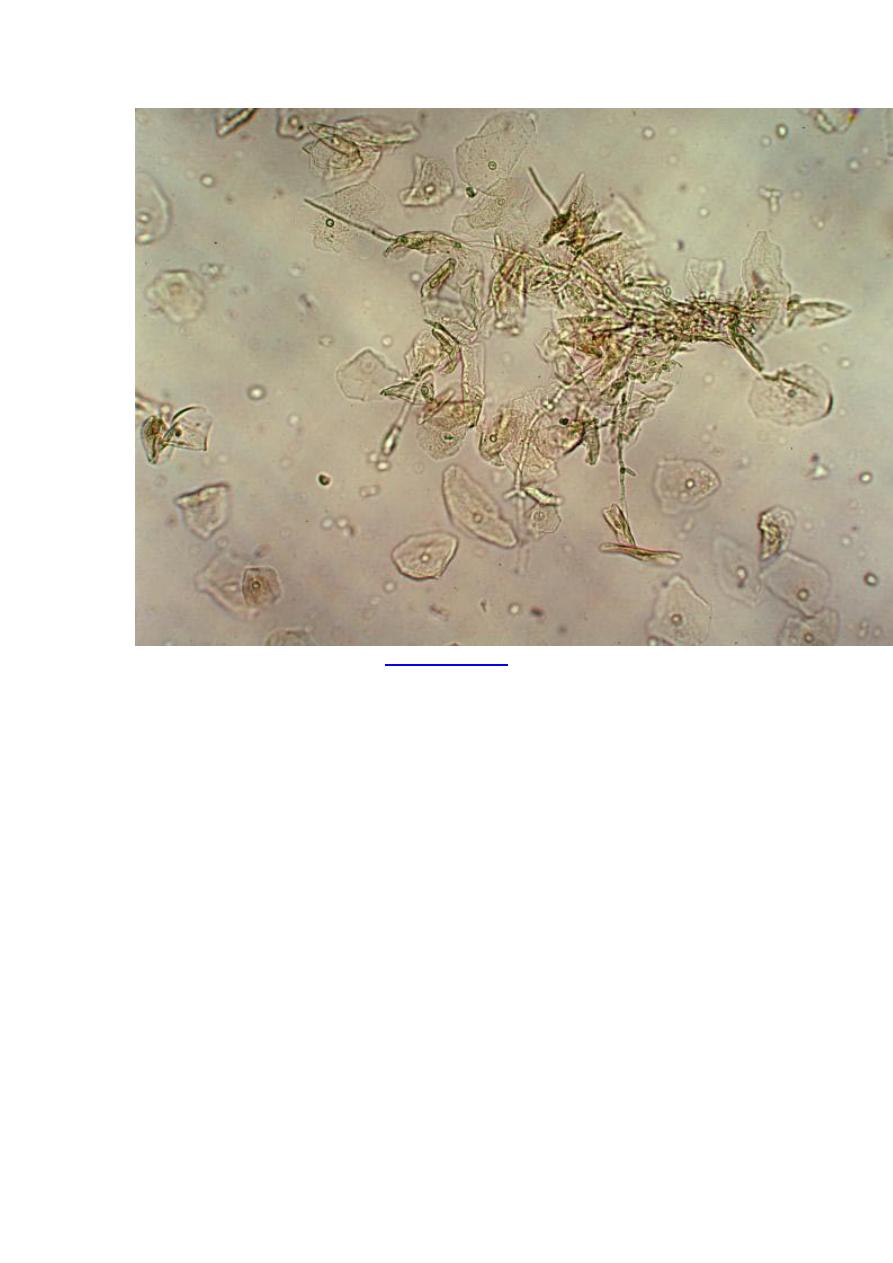
Treatment
An echinocandin if patients are severely or critically ill or if infection with C.
glabrata or C. krusei is suspected
Fluconazole if patients are clinically stable or if infection with C. albicans or
C. parapsilosis is suspected
Alternatively, voriconazole or amphotericin B
In patients with invasive candidiasis, predisposing conditions (eg, neutropenia,
immunosuppression, use of broad-spectrum antibacterial antibiotics,
hyperalimentation, presence of indwelling lines) should be reversed or controlled if
possible. In nonneutropenic patients, IV catheters should be removed.
When an echinocandin is indicated (if patients are moderately severely ill or critically
ill [most neutropenic patients] or if C. glabrata or C. krusei is suspected), one of the
following drugs can be used: caspofungin, loading dose 70 mg IV, then 50 mg IV
once/day; micafungin 100 mg IV once/day; or anidulafungin, loading dose 200 mg
IV, then 100 mg IV once/day.
If fluconazole is indicated (if patients are clinically stable or if C. albicans or C.
parapsilosis is suspected), loading dose is 800 mg (12 mg/kg) po or IV once, followed
by 400 mg (6 mg/kg) once/day.

Treatment is continued for 14 days after the last negative blood culture.
Esophageal candidiasis is treated with fluconazole 200 to 400 mg po or IV once/day
or itraconazole 200 mg po once/day. If these drugs are ineffective or if infection is
severe, voriconazole 4 mg/kg po or IV bid, posaconazole 400 mg po bid, or one of the
echinocandins may be used. Treatment is continued for 14 to 21 days.