TETRACYCLINES
Presented by:Professor Dr. Imad A-J Thanoon
Bacterial Protein Synthesis Inhibitors
TetracyclinesMacrolides
Clindamycin
Chloramphenicol
Classification:
According to source:Naturally-occurring
Tetracycline
Chlortetracycline
Oxytetracycline
Demeclocycline
Semi-synthetic
Doxycycline
Lymecycline
Meclocycline
Methacycline
Minocycline
Rolitetracycline
Tetracyclines
Inhibit bacterial protein synthesis by binding to and interfering with ribosomes
1) Short-acting (6-8 hours), low lipid solubility
Chlortetracycline, Tetracycline, Oxytetracycline
2) Intermediate-acting (12 hours)
Demeclocycline and Methacycline
3) Long-acting (16-18 hours), high lipid solubility
Doxycycline , Minocycline and Tigecycline
First Generation:(1948 to 1963):
ChlortetracyclineOxytetracycline
Tetracycline
Demeclocycline
Rolitetracycline
Limecycline
Clomocycline
Second-generation (1965 to 1972):
MethacyclineDoxycycline
Minocycline
Third-generation (glycylcycline) : Tigecycline (2005)
Antimicrobial Activity
Broad-Spectrum Bacteriostatic AntibioticsActive against many gram-positive and gram-negative bacteria, including
Anaerobes
Rickettsiae
Chlamydiae
Mycoplasmas
Protozoa, e.g. amebas, also effective in acne vugaris, anthrax,H. pylori,plague, malaria and syphilis if penicillin is C/I
Pharmacodynamics(MOA)
The Tetracyclines bind to the 30S subunit and prevent binding of the incoming charged tRNA unit (Inhibit step 1 in bacterial protein synthesis).Tetracyclines enter microorganisms
Susceptible cells concentrate the drug intracellularly
Tetracyclines bind to 30S subunit of the bacterial ribosomeBlocking the binding of tRNA to the acceptor site on the mRNA- ribosome complex
This prevents addition of amino acids to the growing peptide
PHARMACOKINETICS
Absorption60-70% tetracycline, oxytetracycline, demeclocycline, and methacycline
95-100% doxycycline and minocycline
Tigecycline is poorly absorbed orally and must be administered intravenously.
Absorption occurs in upper small intestine and is impaired by
Food (except doxycycline and minocycline)
Divalent cations (Ca2+, Mg2+, Fe2+) or Al3+
Antacids
Minocycline best CSF penetration
PHARMACOKINETICS
40-80% bound by serum proteinsDistributed widely to tissues and body fluids except for CSF(10-25%)
Tetracyclines cross the placenta to reach the fetus and are also excreted in milk Chelation with calcium, damage growing bones and teeth
10 – 50 % excreted into the urine
10 - 40 % excreted in feces
Doxycycline and Tigecycline eliminated by nonrenal mechanisms do not accumulate in renal insufficiency
DRUG INTERACTION
Antacid Impaired absorption
CarbamazepinePhenytoin
Barbiturates
Chronic alcohol ingestion
Diuretics Nitrogen retention
Decreases the half-life of Doxycycline
INDICATIONS ( Clinical uses)Tetracycline
Drug of choice in infection with
Mycoplasma pneumoniae
Chlamydiae
Rickettsiae
Some spirochetes
Used in PEPTIC ULCER caused by H.pylori
Vibrio infections( Cholera)
Chlamydial infections, including sexually transmitted diseases
In combination with an aminoglycoside, indicated for plague, tularemia, and brucellosis
Treatment of acne
Exacerbations of bronchitis
Community-acquired pneumonia
Lyme disease
Relapsing fever
Leptospirosis
Nontuberculous mycobacterial infections (e.g., Mycobacterium marinum)
Minocycline Meningococcal carrier state
Demeclocycline Inhibits the action of ADH So used in inappropriate secretion of ADH
Tigecycline
Tetracycline-resistant strains are susceptible to Tigecycline.Methicillin& Vancomycin-resistant Staphylococci
Penicillin-susceptible and – resistant streptococci
Vancomycin-resistant enterococci
Gram-positive rods
Enterobacteriaceae
Gram-positive and gram-negative anaerobes
Rickettsiae, chlamydia, and legionella
Rapidly growing mycobacteria
Proteus and P aeruginosa, are intrinsically resistant.
Uses Intra-abdominal Infections
Tigecycline is used for the treatment of complicated intra-abdominal infections
including appendicitis, cholecystitis, diverticulitis, gastric/duodenal perforation, intra-abdominal abscess, intestinal perforation, and peritonitis
Skin and Skin Structure Infections
Complicated deep soft tissue infections, including wound infections and cellulitis (10 cm or larger, requiring surgery or drainage, or with complicated underlying disease), major abscesses, infected ulcers, and burns.ADVERSE EFFECTS
• GASTROINTESTINAL ADVERSE EFFECTS• BONE STRUCTURES AND TEETH
• When a tetracycline is given during pregnancy
• Deposited in the fetal Teeth& Bones
• Fluorescence, Discoloration, and Enamel Dysplasia; Bone deformity or Growth inhibition
,
3) LIVER TOXICITY, rare but fatal occur with high IV doses in pregnant or patient with hepatic or renal dysfunction
Impair hepatic function
Hepatic necrosis (4 g)
4) KIDNEY TOXICITY
Administration of outdated tetracycline
Damage to renal proximal tubule
Renal tubular acidosis(Fanconi-like syndrome)
Converted to Epitetracycline & Anhydrotetracycline highly toxic compounds to renal tubules5) LOCAL TISSUE TOXICITY
I/V injection Venous ThrombosisI/M injection Painful local irritation
6) PHOTOSENSITIZATION
Demeclocycline Sensitivity to sunlight or ultraviolet light
7) VESTIBULAR REACTIONS, especially with minocyclineDizziness
Vertigo
Nausea
Vomiting
Sulfonamides and Cotrimoxazole
Presented by:
Professor Dr. Imad A-J Thanoon
Sulfonamides - Classification
Short acting: Sulfadiazine, Sulfadimidine, SulfacetamideIntermediate acting: Sulfamethoxazole
Long acting: Sulfamethoxypyrazine, Sulfadoxine, Sulfadimethoxine etc.
Topically used: Mafenide, Silver sulfadiazine and Sulfacetamide
Ulcerative colitis: Sulfasalazine
Sulfonamides – Antibacterial Property
Bacteriostatic against gm +ve and gm –ve bacteriaBactericidal in urine
Susceptible organisms: S. pyogens, H. influenzae, H. ducreyi, Callymatobacterium grannulomatosis, V. cholerae, Chlamydia, Actinomyces etc.
Few strains of Staph aureus, gonococci, meningococci, pneumococci, E. coli and Shigella
Protozoa:
Plasmodium (Sulfadoxine + Pyrimethamine)
Toxoplasmosis (Sulfadiazine + Pyrimethamine)
Sulfonamides – Kinetics
Rapidly and completely absorbed from GITExtend of plasma protein binding differs (10 – 95%)
Longer acting ones are highly plasma protein bound
Widely distributed – enters in serous cavity easily
Metabolized by non microsomal acetyl transferase in liver – slow and fast acetylators
Acetylated product excreted in urine (more toxic than parent) – crystalluria
Reabsorbed in tubule
Sulfonamides
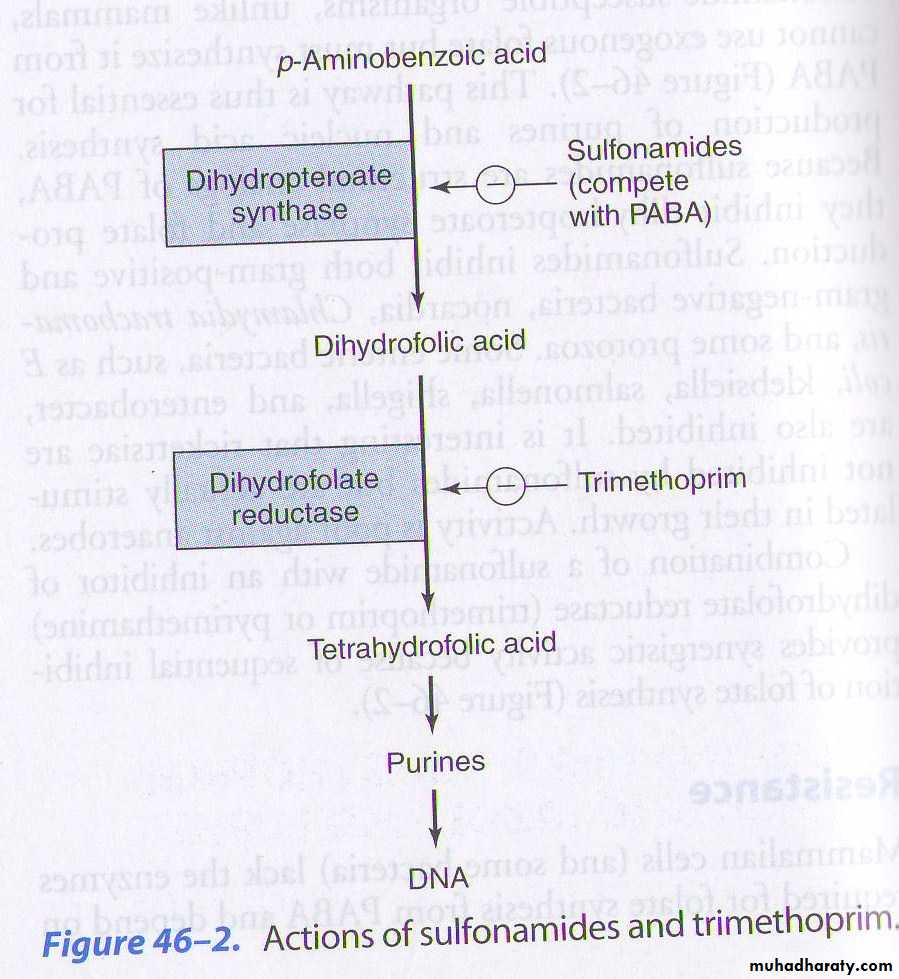
Mechanism of action :
Competitive inhibitor to dihydropteroate synthase enzyme .
Inhibit bacterial growth by blocking folic acid synthesis .
Sulfonamides - ADRs
Nausea, vomiting and epigastric painCrystalluria – alkanization of urine
Hypersensitivity (2 – 5%)
Hepatitis
Haemolysis – G-6-PD deficiency
Kernicterus
Sulfonamides - Uses
Rarely used now a days via systemic routeUTI: caused by E. coli and P. mirabilis: Sulfisoxazole – 1 gm 4 times daily
Malaria – sulfadoxine and pyrimethamine combination
Toxoplasmosis: sulfadiazine + pyrimethamine
Ulcerative colitis – Sulfasalazine – 1-4 gm initially and 500 mg 6 Hrly.
Locally:
Sodium sulfacetamide: 10-30% ophthalmic solution in bacterial conjunctivitis, trachoma etc.
Mafenide acetate (1% cream) and Silver sulfadiazine 1% cream): Burn dressing and chronic ulcers
Cotrimoxazole – In 1969
Fixed drug combination of Sulfamethoxazole and Trimethoprim
SYNERGISM
Trimethoprim
Trimethoprim (trimethyl benzyl pyrimidine) is a diaminopyrimidine, chemically related to PyrimethamineMOA: Sequential block of folate metabolism
Trimethoprim is 50,000 or more times more active against bacterial DHFRase enzyme than mammalian
So, no harm to human folate metabolism
Individually, both are bacteriostatic, but combination is –cidal
Maximum synergism if the organism is sensitive to both the agents
Tetrahydropteroic acid synthetase
Dihydrofolic acidDihyrofolate reductase
Tetrahydrofolic acidPurine synthesis
DNA synthesis
SulfonamidesTrimethoprim
MOA OF TRIMETHOPRIM-SULFAMETHOXAZOLE1.Sulfamethoxazole inhibits dihydrofolate synthase.
2.Trimethoprim inhibits dihydrofolate reductase.
PABACotrimoxazole – general points
Both drugs have almost similar half lives (10 Hrs)Optimal synergism is obtained at 20 (S) : 1 (T) concentration)
This ratio is obtained at 5:1 dose ratio ( e.g. 800 mg:160 mg)
Because TMP has large Vd and enters many tissues – plasma conc. Is low
But, TMP crosses BBB and placenta and SMZ not
TMP is more rapidly absorbed than SMZ
TMP is 45% plasma protein bound but SMZ is 65% bound
TMP is partly metabolized in liver
Cotrimoxazole – antibacterial spectrum
Similar to sulfonamides
Additional benefits: Salmonella typhi, Serratia, Klebsiella Enterobacter, Yersinia and Pneumocystis jiroveci
Sulfonamides resistance strains of S aureus, E coli, gonococci, meningococci and H influenzae
Cotrimoxazole - ADRs
All adverse effects of sulfonamides – nausea, vomiting, stomatitis, rash etcFolate deficiency (megaloblastic anaemia) – patients with marginal folate levels
Blood dyscrasias
Pregnancy: teratogenic risk, Neonatal haemolysis and methaemoglobinaemia
Patients with renal disease may develop uremia
Elderly – risk of bone marrow toxicity from cotrimoxazole
Diuretics given with cotrimoxazole have produced a higher incidence of thrombocytopenia
Bone marrow hypoplasia among AIDS patients with Pneumocystis infection
Cotrimoxazole - Uses
Uncomplicated infection of the lower urinary tract infectionCystitis
chronic and recurrent urinary tract infections
Respiratory tract infection – lower and upper, chronic bronchitis, facio-maxillary infections, otitis media due to gm+ve cocci and H influenzae etc
Typhoid
Bacterial diarrhoeas & dysentery: due to campylobacter, E coli, Shigella etc.
Pneumocystis jiroveci: Severe pneumonia - Prophylactic use in AIDS patients with neutropenia.
Chancroid – H. ducreyi
Alternative to penicillin in agrannulocytosis patients, septicaemia etc.