Gas Transport & pH in the Lung
By phrmacistMaha A. Hamdi
partial pressure gradients for O2 and CO2are the key to gas movement and that O2 "flows downhill" from the air through the alveoli and blood into the tissues, whereas CO2 "flows downhill" from the tissues to the alveoli.
Consider air, which has an approximate composition of 79 % N2 &21% O2.
The total pressure of this mixture at sea level averages 760 mm Hg.each gas contributes to the total pressure in direct proportion to its concentration.
Therefore, 79% of the 760 mm Hg is caused by nitrogen (600 mm Hg) and 21 % by oxygen (160 mm Hg).
, the "partial pressure" of N2 in the mixture is 600 mm Hg, and the "partial pressure" of O2 is 160 mm Hg; the total pressure is 760 mm Hg, the sum of the individual partial pressures.
.
Rate of diffusion
several factors affect the rate of gas diffusion in a fluid:(1) the solubility of the gas in the fluid
(2) the cross-sectional area of the fluid
(3) the distance through which the gas must diffuse,
4) the molecular weight of the gas
(5) the temperature of the fluid.
about 99% of the O2 that dissolves in the blood combines with the O2-carrying protein hemoglobin.
about 94.5% of the CO2 that dissolves enters into a series of reversible chemical reactions that convert it into other compounds.
the presence of hemoglobin ↑ the O2-carrying capacity of the blood 70-fold, and the reactions of CO2 increase the blood CO2 content 17-fold.
Oxygen TransportOxygen Delivery to the Tissues
O2 delivery to a particular tissue depends on :1-amount of O2 entering the lungs,
2- adequacy of pulmonary gas exchange
3- blood flow to the tissue
A- the degree of constriction of the vascular bed in the tissue
B-cardiac output
4- the capacity of the blood to carry O2
amount of O2 in the blood is determined by :
1-amount of dissolved O22- amount of hemoglobin in the blood
3- affinity of the hemoglobin for O2
Reaction of Hemoglobin & Oxygen
Combination of the1st heme in the Hb molecule with O2 increases the affinity of the 2nd heme for O2, and oxygenation of the second increases the affinity of the3ed , and so on, so that the affinity of Hb for the fourth O2 molecule is many times that for the first.When blood is equilibrated with 100% O2 (PO2 = 760 mm Hg), the normal hemoglobin becomes 100% saturated
In vivo, the hemoglobin in the blood at the ends of the pulmonary capillaries is about 97.5% saturated with O2 (PO2 = 97 mm Hg).
Because of a slight admixture with venous blood that bypasses the pulmonary capillaries (physiologic shunt), the hemoglobin in systemic arterial blood is only 97% saturated.
In venous blood at rest, the hemoglobin is 75% saturated .
(Table 36–1); ganongFactors Affecting the Affinity of Hemoglobin for Oxygen
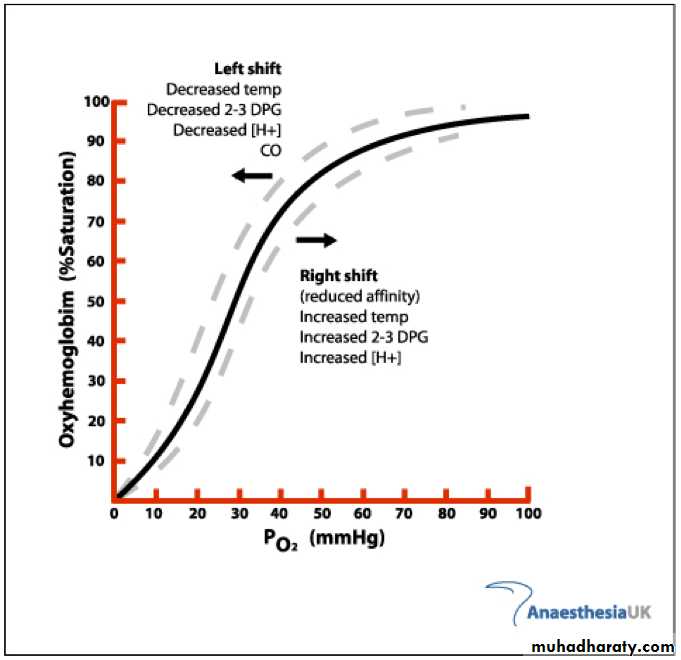
the pH, the temperature, and the concentration of 2,3-biphosphoglycerate (BPG; 2,3-BPG).A ↑ in temperature or a ↓ in pH shifts the curve to the right.
When the curve is shifted in this direction, a higher PO2 is required for hemoglobin to bind a given amount of O2.
a fall in temperature or a rise in pH shifts the curve to the left, and a lower PO2 is required to bind a given amount of O2.
A convenient index for comparison of such shifts is the P50, the PO2 at which hemoglobin is half saturated with O2.
The higher the P50, the lower the affinity of hemoglobin for O2
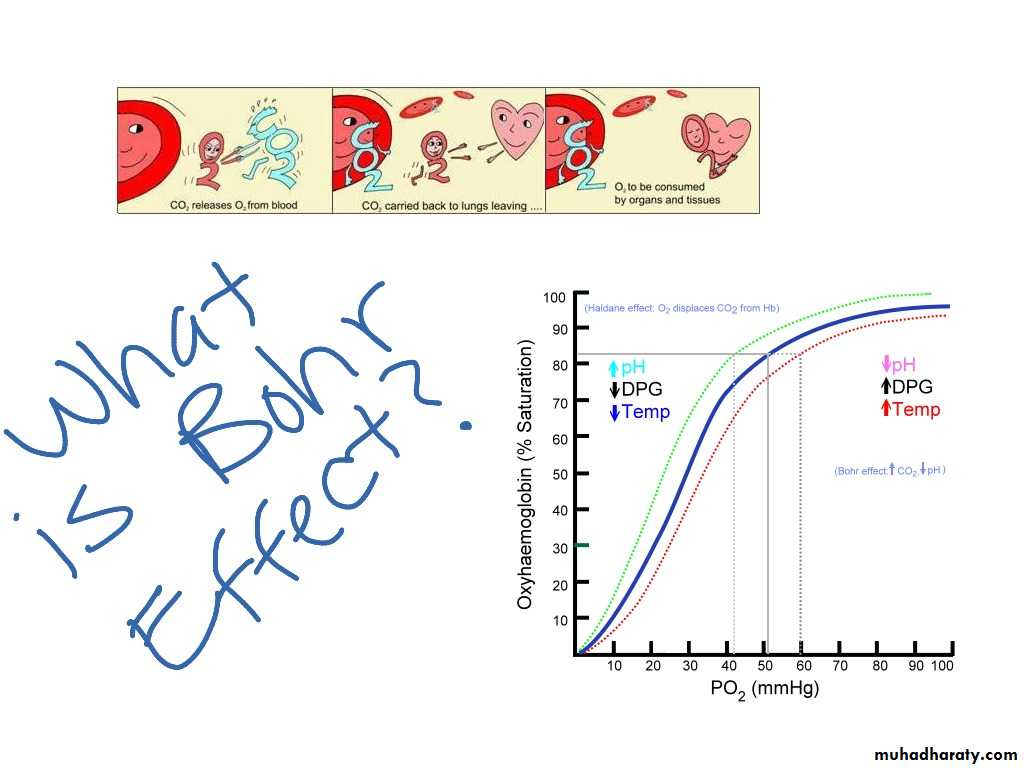
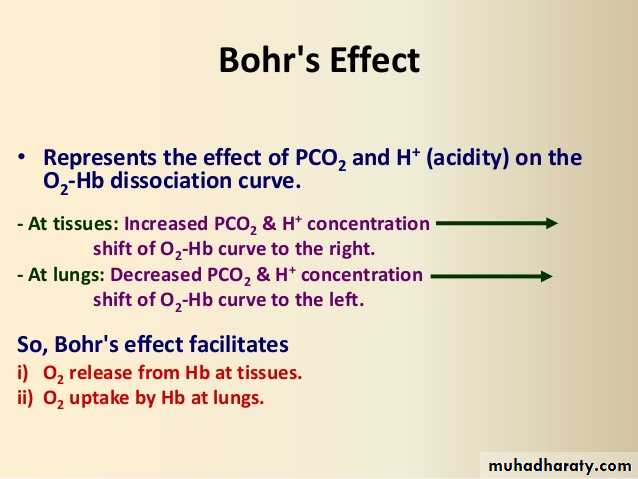
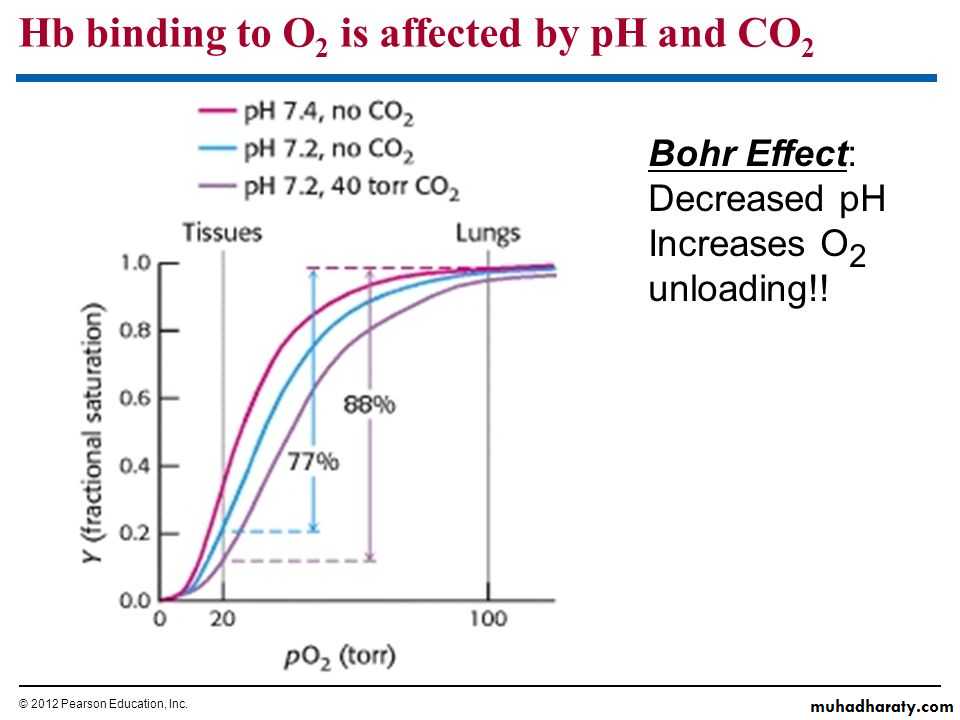
Bohr effect
Is decrease in O2 affinity of hemoglobin when the pH of blood falls .
deoxygenated Hb (deoxyhemoglobin) binds H+ more actively than does oxygenated Hb (oxyhemoglobin).
The pH of blood falls as its CO2 content increases, so that when the PCO2 rises, the curve shifts to the right and the P50 rises.
Most of the unsaturation of hemoglobin that occurs in the tissues is secondary to the decline in the PO2, but an extra 1–2% unsaturation is due to the rise in PCO2 and consequent shift of the dissociation curve to the right.
2,3-BPG is very plentiful in red cells, which is a product of glycolysis .
an ↑ of 2,3-BPG shifts the reaction to the right, causing more O2 to be liberated.Because acidosis inhibits red cell glycolysis, the 2,3-BPG concentration falls when the pH is low.
Conversely, thyroid hormones, growth hormones, and androgens can all increase the concentration of 2,3-BPG and the P50.
Exercise produce an increase in 2,3-BPG within 60 min, although the rise may not occur in trained athletes.
The P50 is also increased during exercise, because the temperature rises in active tissues and CO2 and metabolites accumulate, lowering the pH
Myoglobin
Myoglobin is an iron-containing pigment found in skeletal muscle.
it resembles hemoglobin but binds 1 rather than 4 mol of O2 per mole.
it takes up O2 from hemoglobin in the blood.
Myoglobin
It releases O2 only at low PO2 values, but the PO2 in exercising muscle is close to zero.The myoglobin content is greatest in muscles specialized for sustained contraction.
The muscle blood supply is compressed during such contractions, and myoglobin may provide O2 when blood flow is cut off.
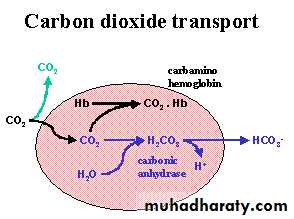
Carbon Dioxide TransportFate of Carbon Dioxide in Blood
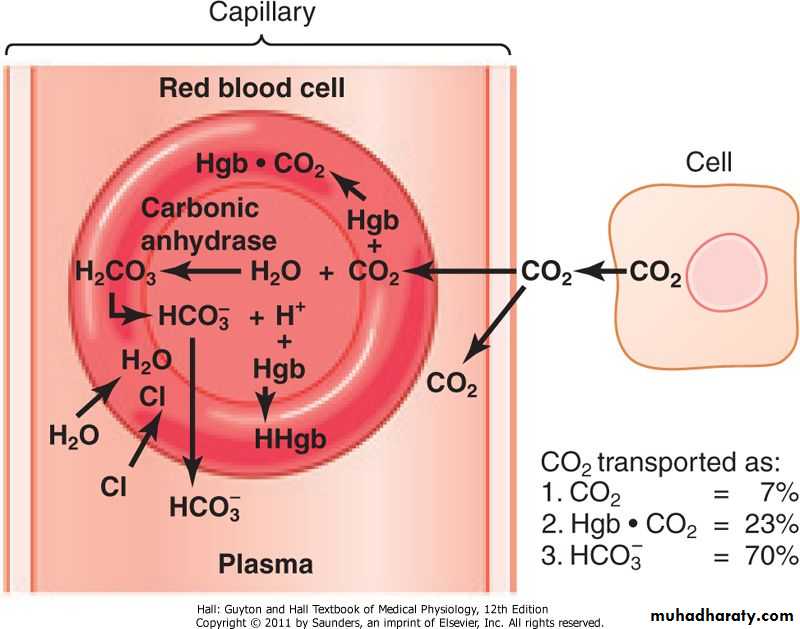
The solubility of CO2 in blood is about 20 times that of O2; therefore, considerably more CO2 than O2 is present in simple solution at equal partial pressures.The CO2 that diffuses into red blood cells is rapidly hydrated to H2CO3 because of the presence of carbonic anhydrase.
The H2CO3 dissociates to H+ and HCO3–, and the H+ is buffered, primarily by hemoglobin, while the HCO3– enters the plasma.
Some of the CO2 in the red cells reacts with the amino groups of hemoglobin and other proteins (R), forming carbamino compounds:
binding of O2 to hemoglobin reduces its affinity for CO2.
venous blood carries more CO2 than arterial blood, CO2 uptake is facilitated in the tissues, and CO2 release is facilitated in the lungs.About 11% of the CO2 added to the blood in the systemic capillaries is carried to the lungs as carbamino-CO2.
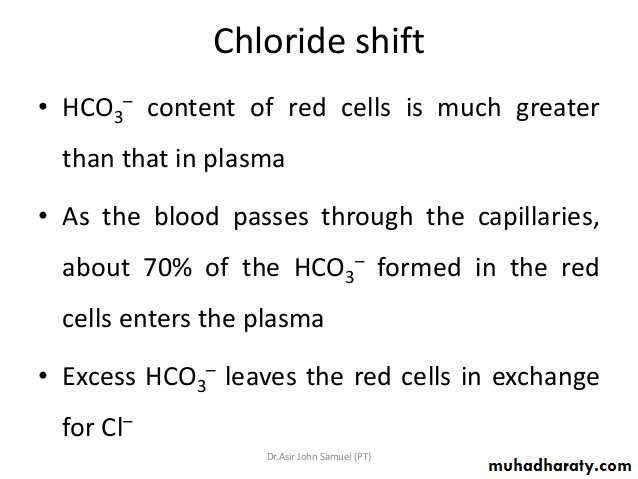
Chloride Shift
Because the rise in the HCO3– content of red cells is much greater than that in plasma as the blood passes through the capillaries, about 70% of the HCO3– formed in the red cells enters the plasma.
The excess HCO3– leaves the red cells in exchange for Cl–.
Chloride Shift
Because of this chloride shift, the Cl– content of the red cells in venous blood is significantly greater than that in arterial blood.The chloride shift occurs rapidly and is essentially complete within 1 s.
Summary of Carbon Dioxide Transport
Table 36–2 Fate of CO2 in Blood.In plasma
1. Dissolved2. Formation of carbamino compounds with plasma protein
3. Hydration, H+ buffered, HCO3– in plasma
In red blood cells
1. Dissolved
2. Formation of carbamino-Hb
3. Hydration, H+ buffered, 70% of HCO3– enters the plasma
4. Cl– shifts into cells; mOsm in cells increases
Acid–Base Balance & Gas Transport
The major source of acids in the blood under normal conditions is through cellular metabolism.
CO2 formed by metabolism in the tissues is in large part hydrated to H2CO3,
most of the CO2 is excreted in the lungs, and the small quantities of the remaining H+ are excreted by the kidneys
Fruits are the main dietary source of alkali.
They contain Na+ and K+ ,salts of weak organic acids, and the anions of these salts are metabolized to CO2, leaving NaHCO3 and KHCO3 in the body.Such ingestion contributes little to changes in pH and a more common cause of alkalosis is loss of acid from the body as a result of vomiting of gastric juice rich in HCl.
This is, of course, equivalent to adding alkali to the body.
Buffering in the Blood
Acid and base shifts in the blood are largely controlled by three main buffers in blood:(1) proteins,
(2) hemoglobin,
(3) the carbonic acid–bicarbonate system.
Buffering in the Blood
1-Plasma proteins are effective buffers because both their free carboxyl and their free amino groups dissociate.2-The second buffer system is provided by the dissociation of the imidazole groups of the histidine residues in hemoglobin
Buffering in the Blood
In the pH 7.0–7.7 range, the free carboxyl and amino groups of hemoglobin contribute relatively little to its buffering capacity.However, the hemoglobin molecule contains 38 histidine residues, and on this basis—plus the fact that hemoglobin is present in large amounts—the Hb in blood has 6 times the buffering capacity of the plasma proteins.
In addition, the action of hemoglobin is unique because the imidazole groups of deoxyhemoglobin (Hb) dissociate less than those of oxyhemoglobin (HbO2), making Hb a weaker acid and therefore a better buffer than HbO2.
Buffering in the Blood
3-The third and major buffer system in blood is the carbonic acid–bicarbonate system:
H2CO3↔