The Inflammatory Response
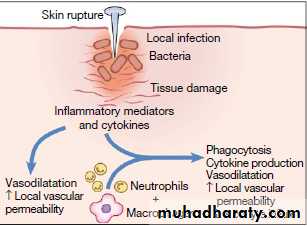
definitionInflammation is the response of tissues to injury or infection, and is necessary for normal repair and healing.
Acute inflammation:
-is the result of rapid and complex interplay between the cells and soluble molecules of the innate immune system
The effects are:
Vasodilatationincreased local vascular permeability, which increases flow of fluid and cells to the affected tissue.
pro-inflammatory cytokines produced at the site of injury have profound systemic effects. IL-1, TNF-α and IL-6 act on the hypothalamus to raise the temperature set-point, causing fever,
and also stimulate the production of acute phase proteins by the liver.
The classical external signs include heat, redness, pain and swelling
Acute phase proteins:
They are produced by the liver in response to inflammatory stimuli and have a wide range of activities.C-reactive protein (CRP) and serum amyloid A may be increased 1000-fold, contributing to host defence and stimulating repair and regeneration.
Fibrinogen plays an essential role in wound healing,
α1-antitrypsin and α1-antichymotrypsin control the pro-inflammatory cascade by neutralising the enzymes produced by activated neutrophils, preventing widespread tissue destruction.
antioxidants such as haptoglobin and manganese superoxide dismutase scavenge for oxygen free radicals,
iron-binding proteins such as transferrin, ferritin and lactoferrin decrease the iron available for uptake by bacteria.
Resolution of inflammation:
Extravasated neutrophils undergo apoptosis and are phagocytosed by macrophages, along with the remains of microorganisms.Macrophages synthesise collagenase and elastase, which break down local connective tissue and aid in the removal of debris.
Macrophage-derived cytokines, including transforming growth factor (TGF)-β and platelet-derived growth factor, attract fibroblasts and promote the synthesis of new collagen, while angiogenic factors stimulate new vessel formation.
Sepsis and septic shock:
Failure of normal inhibitory mechanisms results in excessive production of pro-inflammatory cytokines by macrophages, causing hypotension, hypovolaemia, decreased perfusion and tissue oedema resulting in septic shock.uncontrolled neutrophil activation causes release of proteases and oxygen free radicals within blood vessels, damaging the vascular endothelium and further increasing capillary permeability.
Direct activation of the coagulation pathway combines with endothelial cell disruption to form clots within the damaged vessels.
The clinical consequences include cardiovascular collapse, acute respiratory distress syndrome, disseminated intravascular coagulation, multi-organ failure and often death.
Septic shock most frequently results from infection with Gram-negative bacteria, because lipopolysaccharide is particularly effective at activating the inflammatory cascade.
Chronic inflammation:
Failure of elimination may result in chronic inflammation. Persisting microorganisms stimulate the ongoing accumulation of neutrophils, macrophages and activated T lymphocytes. If this is associated with local deposition of fibrous connective tissue, a granuloma may form.
Granulomas are characteristic of infections such as tuberculosis and leprosy, in which the microorganism is protected by a robust cell wall which shields it from killing, despite phagocytosis.
Investigations in inflammation:
CBP: Leucocytosis is common, The platelet count may also be increased. Chronic inflammation is frequently associated with a normocytic normochromic anaemia of chronic diseaseThe CRP is the most widely used clinical measure of acute inflammation, but increased levels of fibrinogen, ferritin and complement components are also associated with the acute phase response, while albumin levels are reduced.
C-reactive protein(CRP):
is an acute phase reactant synthesized by the liver, which opsonises invading pathogens.Levels of CRP increase within 6 hours of an inflammatory stimulus and may rise up to 1000-fold.
the plasma half-life of CRP is 19 hours,
For reasons which remain unclear, some diseases are associated with only minor elevations of CRP concentration despite unequivocal evidence of active inflammation. These include SLE, systemic sclerosis,ulcerative colitis and leukaemia. An important practical point is that intercurrent infection does provoke a significant CRP response in these conditions.
Erythrocyte sedimentation rate (ESR):
It is an indirect measure of the acute phase response. It measures the rate of fall of erythrocytes through plasma, and a major determinant of this is aggregation of red cells. causing them to stack together like tyres, or rouleaux.Thus the ESR is a composite measure of erythrocyte morphology and of plasma protein composition and concentration.
The most common cause of an increased ESR is an acute phase response, which causes an increase in plasma protein concentration. This is accompanied by a corresponding increase in circulating CRP levels.
Periodic fever syndromes
Familial Mediterranean fever (FMF)Hyper-IgD syndrome (HIDS)
TNF receptor-associated periodic syndrome (TRAPS)
Familial Mediterranean fever (FMF)
predominantly affecting Mediterranean people, including Arabs, Turks, Sephardic Jews and Armenians.
It results from mutations of the MEFV gene, which encodes a protein called pyrin. Pyrin regulates neutrophil mediated inflammation by indirectly suppressing the production of IL-1.
FMF is characterised by painful attacks of fever associated with peritonitis, pleuritis and arthritis, and lasts from a few hours to 4 days. During acute episodes, CRP levels are markedly increased. The majority of individuals have their first attack before the age of 20.
The major complication of FMF is AA amyloidosis. Colchicine significantly reduces the number of febrile episodes in 90% of patients but is ineffective during acute attacks.
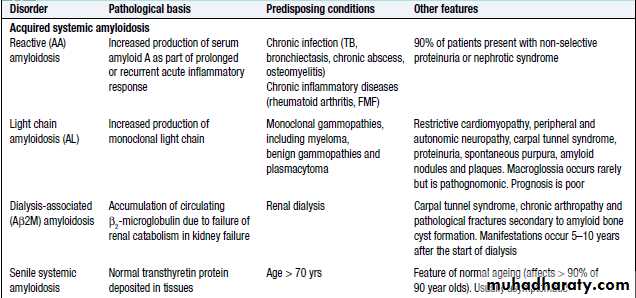
Amyloidosis
Amyloidosischaracterized by the extracellular deposition of insoluble proteins. These complex deposits consist of fibrils of the specific protein involved linked to glycosaminoglycans, proteoglycans and serum amyloid P (SAP).
Protein accumulation may be localised or systemic, and the clinical manifestations depend upon the organ(s) affected.
The diagnosis of amyloidosis should be considered in all cases of unexplained nephrotic syndrome, cardiomyopathy and peripheral neuropathy.
Amyloid diseases are classified by aetiology and type of protein deposite
Diagnosis:
The diagnosis is established by biopsy, which may be of an affected organ, rectum or subcutaneous fat. The pathognomonic histological feature is apple-green birefringence of amyloid deposits when stained with Congo red dye and viewed under polarised light.Immunohistochemical staining can identify the type of amyloid fibril present.
Quantitative scintigraphy with radiolabelled serum amyloid P is a valuable tool in determining the overall load and distribution of amyloid deposits.
Management:
The aims of treatment are to support the function of affected organs and, in acquired amyloidosis, to prevent further amyloid deposition through treatment of the primary cause.Liver transplantation may provide definitive treatment in selected patients with hereditary transthyretin amyloidosis.
Autoimmune Disease
Autoimmunity can be defined as the presence of immune responses against self tissue, It is often a harmless phenomenon, identified only by the presence of low titre autoantibodies or autoreactive T cells.autoimmune diseases occur if these responses cause significant organ damage. These are a major cause of chronic morbidity and disability, affecting up to 1 in 30 adults at some time and it may be :
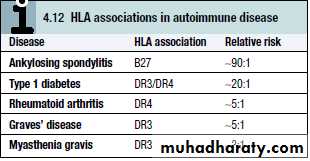
organ specific like: Graves’ disease, Hashimoto’s thyroiditis, Addison’s disease, Pernicious anaemia and Type 1 diabetes
multisystemic disease like systemic lupus erythromatosis.
Factors predisposing to autoimmune disease:
Both genetic and environmental factors contribute. Autoimmune diseases are much more common in women than in men, for reasons which remain unclear.The most important genetic determinants of autoimmune susceptibility are the HLA genes, reflecting their importance in shaping lymphocyte responses. Other susceptibility genes include those determining cytokine activity, co-stimulation and cell death.
Several environmental factors can trigger autoimmunity in genetically predisposed individuals. The most widely studied of these is infection, as occurs in acute rheumatic fever following streptococcal infection or reactive arthritis following bacterial infection.
A number of mechanisms have been postulated, such as cross-reactivity between the infectious pathogen and self determinants (molecular mimicry), and release of sequestered antigens, which are not usually visible to the immune system, from damaged tissue.
Occasionally, the development of autoimmune disease is a side-effect of drug treatment. For example, the metabolic products of the anaesthetic agent halothane bind to liver enzymes, resulting in a structurally novel protein. This is recognized as a new (foreign) antigen by the immune system, and the autoantibodies and activated T cells directed against it may cause hepatic necrosis.