
Lecture 2
Dr. Janan Alrefaee
Factors may affect GFR
1-Glomerular capillary filtration coefficient (kf): is the product of the permeability and
filtering surface area of the capillaries and it is 400 times higher than the Kf of most other
capillary systems of the body.
Kf = GFR/Net filtration pressure
Normal Kf = 125 ml/min/ 10mmHg = 12.5 ml/min/mmHg of filtration pressure.
Kf is decreased by increasing the thickness and decreasing the number of functional
glomerular capillaries by disease.
Although
Kf
GFR and
Kf
GFR but it is not a primary factor regulating GFR.
2- Bowman’s capsule hydrostatic pressure
Increasing the hydrostatic pressure in Bowman’s capsule (urinary tract obstruction) reduces
GFR and vice versa but it is not a primary factor regulating GFR.
3- Glomerular capillary colloid osmotic pressure
Normally, as blood passes through the glomerular capillaries, the fluid is filtered and the
plasma proteins are not filtered and concentrated lead to increase colloid pressure. An
increase in the glomerular capillary colloid osmotic pressure decreases GFR and vice versa.
4- Glomerular capillary hydrostatic pressure
The glomerular hydrostatic pressure is the primary factor for regulation of GFR. Increases in
glomerular hydrostatic pressure raise GFR and vice versa.
Glomerular hydrostatic pressure is determined by three variables: (1) arterial pressure
(positive relationship).
(2) Afferent arteriolar resistance (
resistance of afferent arterioles leads to
glomerular
hydrostatic pressure and
GFR. Conversely, dilation of the afferent arterioles leads to
both glomerular hydrostatic pressure and GFR).
(3) Efferent arteriolar resistance. Constriction of the efferent arterioles increases the
resistance to outflow from the glomerular capillaries. This raises the glomerular hydrostatic
pressure.
The effect of efferent arteriolar constriction on GFR is a biphasic depends on the severity of
the constriction; modest efferent constriction raises GFR slightly, but severe efferent
constriction (more than a threefold increase in resistance) tends to reduce GFR (the rise in
colloid osmotic pressure exceeds the increase in glomerular capillary hydrostatic pressure
caused by efferent arteriolar constriction).
Renal Blood Flow
In an average 70-kilogram man, the combined blood flow through both kidneys is about
1100 ml/min, or about 22 % of the cardiac output. The two kidneys are about 0.4 % of the
total body weight, so they receive an extremely high blood flow compared with other organs.

This Blood flow supplies the kidneys with nutrients and removes waste products and
additional blood flow is for the high rates of glomerular filtration.
Renal blood flow and oxygen consumption
The kidneys normally consume oxygen twice time than the brain but their blood flow almost
seven times than the brain. The fourth of normal renal oxygen consumption is for the renal
cells basic metabolic needs while the rest of oxygen is used for renal tubular sodium
reabsorption with positive proportion.
Determinants of renal blood flow
Renal blood flow is determined by the hydrostatic pressure difference between renal artery
and renal vein divided by the total renal vascular resistance.
Blood flow in the renal medulla
Blood flow in the vasa recta of the renal medulla is very low compared with flow in the renal
cortex (renal medulla Blood flow is only 1 to 2 % of the total renal blood flow); the vasa
recta play an important role in allowing the kidneys to form concentrated urine.
Physiologic control of glomerular filtration and renal blood flow
GFR is mainly determined by glomerular hydrostatic pressure and the glomerular capillary
colloid osmotic pressure (as above). These variables, in turn, are influenced by:
1- Sympathetic nervous system activation decreases GFR
The afferent and the efferent renal arterioles are innervated by sympathetic nerve fibers.
Strong stimulation of the renal sympathetic nerves can constrict the renal arterioles and
decrease renal blood flow and GFR (severe hemorrhage). Moderate or mild sympathetic
stimulation has little influence on renal blood flow and GFR.
2-Hormonal control of GFR and renal circulation
a- Norepinephrine, epinephrine, and endothelin constrict renal blood vessels and
decrease GFR and renal blood flow. norepinephrine and epinephrine influence on GFR
is little except under severe conditions (severe hemorrhage).
b- Angiotensin II: increase angiotensin II levels (as in decreased arterial pressure or
volume depletion) constricts efferent arterioles leading to 1-raise glomerular
hydrostatic pressure, so it prevent decreases in glomerular hydrostatic pressure and
GFR and maintain normal excretion of metabolic waste products. 2-decrease
peritubular capillaries blood flow which increases reabsorption of sodium and water
and restore blood volume and blood pressure.
c- Endothelial-derived nitric oxide: normally it maintains vasodilation of the kidneys,
decreases renal vascular resistance and increases GFR, so allows the kidneys to
excrete normal amounts of sodium and water (impaired nitric oxide production
blood pressure).
d- Prostaglandins and bradykinin: they cause vasodilation, increased renal blood flow
and GFR. Its importance appears by opposing vasoconstriction of afferent arterioles
(by the sympathetic nerves) and prevents excessive reductions in GFR and renal blood
flow. (So in volume depletion as severe hemorrhage or after surgery, the
administration of non steroidal anti-inflammatory agents, such as aspirin, that inhibit
prostaglandin synthesis may cause significant reductions in GFR).
Note: There are 2 defence lines buffer the effects of spontaneous changes in GFR on urine
output are: The first line is the renal autoregulatory mechanisms, especially
tubuloglomerular feedback. The second line is the glomerulotubular balance (discussed
later).
Autoregulation of GFR and renal blood flow
Autoregulation is the intrinsic feedback mechanisms of the kidneys normally keep the renal
blood flow and GFR relatively constant, despite marked changes in arterial blood pressure
which is independent of systemic influences (For instance, a decrease in arterial pressure to
as low as 75 mm Hg or an increase to as high as 160 mm Hg changes GFR only a few
percentage points). These autoregulation mechanisms are:
1-Tubuloglomerular feedback
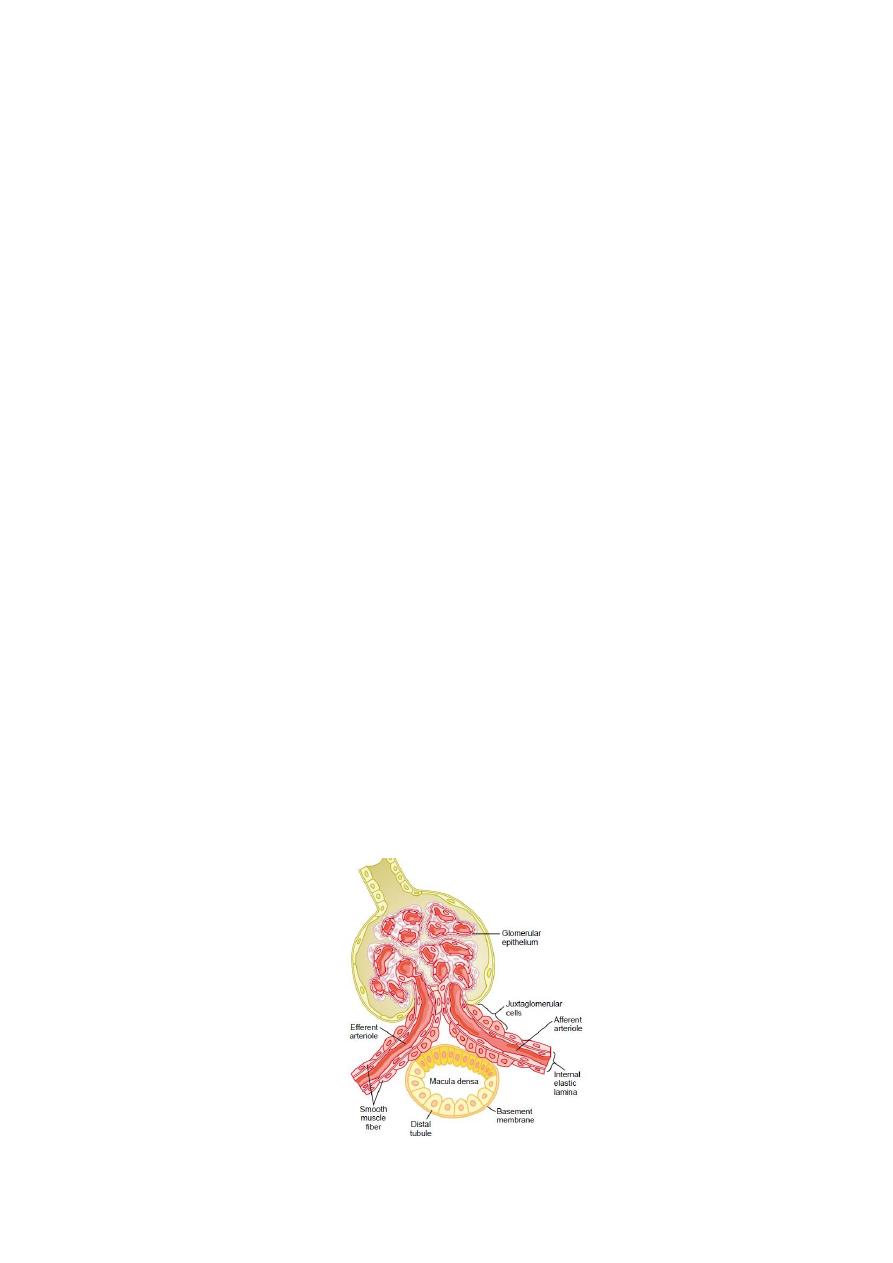
This feedback depend on special anatomical arrangements of the juxtaglomerular complex
(Fig. 2.1) that consists of macula densa cells which is a specialized group of epithelial cells
in the initial portion of the distal tubule that comes in close contact with the afferent and
efferent arterioles that have juxtaglomerular cells in their walls. The macula densa cells
contain Golgi apparatus, which are intracellular secretory organelles directed toward the
arterioles.
Figure 2.1 Structure of the juxtaglomerular apparatus
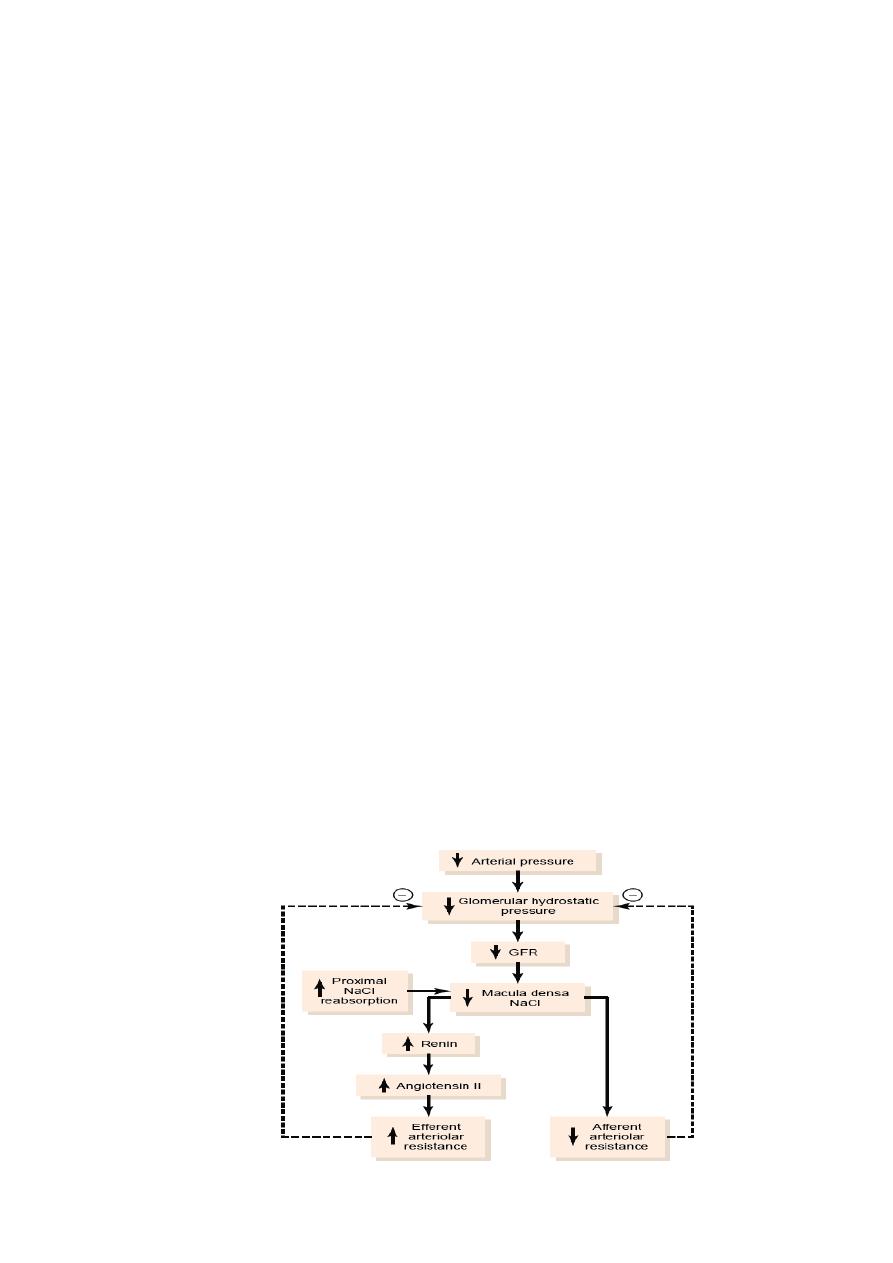
The tubuloglomerular feedback mechanism starts as GFR decreases lead to slow the flow
rate in the loop of Henle and increased reabsorption of sodium and chloride ions in the
ascending loop of Henle and reducing the concentration of sodium chloride at the macula
densa cells which initiates a signal from the macula densa that has two effects that act
together to control GFR (Fig. 2.2):
(1) An afferent arteriolar vasodilator feedback mechanism: the signal dilates the afferent
arterioles, which raises glomerular hydrostatic pressure and return GFR toward normal.
(2) An efferent arteriolar
vasoconstrictor feedback mechanism: the signal increases
rennin release from the juxtaglomerular cells of the afferent and efferent arterioles (which are
the major storage sites for rennin). Renin acts as an enzyme to increase the formation of
angiotensin I, which is converted to angiotensin II. Finally, the angiotensin II constricts the
efferent arterioles and increasing glomerular hydrostatic pressure and returning GFR toward
normal.
2-Myogenic autoregulation of renal blood flow and GFR
Increase arterial pressure lead to stretch vessels wall which allows increased calcium ions
movement from the extracellular fluid into the cells, lead to contraction of the vascular
smooth muscle and raising vascular resistance which prevent excessive increases in renal
blood flow and GFR.
Note: A chronic high protein intake (large amounts of meat) as well as glucose intake
(high blood glucose levels in uncontrolled diabetes) both are known to increase renal blood
flow and GFR due to that the amino acids (which released from protein) and glucose are
reabsorbed along with sodium in the proximal tubule & decreases sodium delivery to the
macula densa, which elicits a tubuloglomerular feedback.
● An opposite occurs when proximal tubular reabsorption is reduced by diseases and
large amounts of sodium chloride are delivered to the distal tubule and, without appropriate
compensations (as a tubuloglomerular feedback– mediated renal vasoconstriction) would
quickly cause excessive volume depletion.
Figure 2.2 Macula densa feedback mechanism
