Chemical fixatives are used to preserve tissue
from degradation, and to maintain the structure
of the cell and of sub-cellular components such
as cell organelles (e.g., nucleus,
, mitochondria).
The most common fixative for light microscopy is
10% neutral buffered formalin (4%
in
).
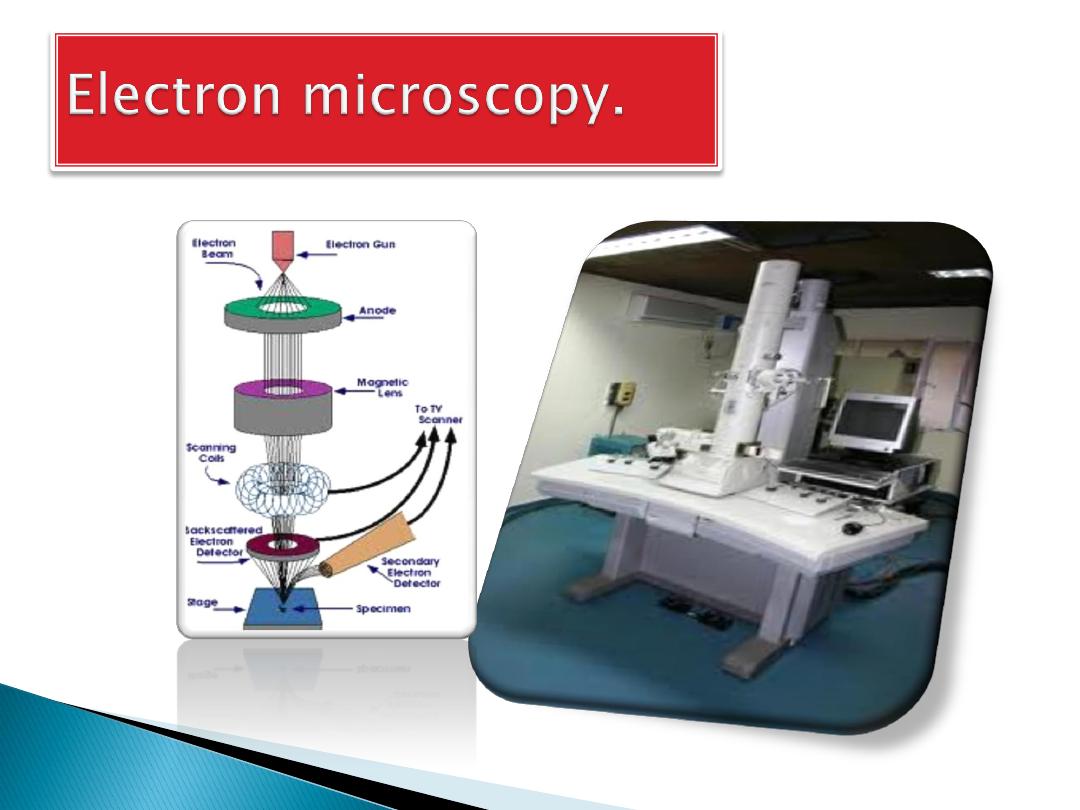
For electron microscopy, the most commonly
used fixative is
, usually as a 2.5%
solution in
.

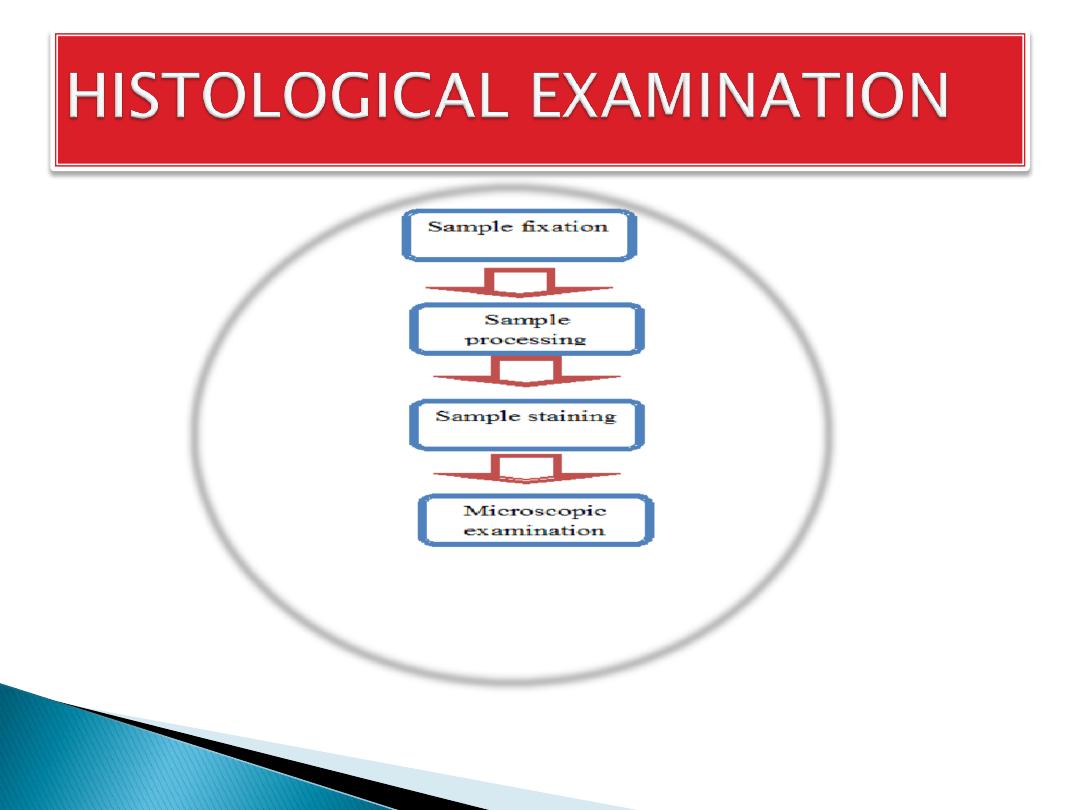
The aim of Tissue Processing:
1.
is to remove water
from tissues and
2.
replace with a medium that
solidifies to allow thin sections to be cut.
3.
Biological
tissue must be supported in a hard matrix to allow
sufficiently thin sections to be cut.
Typically 5 μm (micrometres; 1000 micrometres =
1 mm) thick for light microscopy and 80-100 nm
(nanometre; 1,000,000 nanometres = 1 mm) thick for
electron microscopy.
For light microscopy, paraffin wax is most frequently
used. Since it is immiscible with water, the main
constituent of biological tissue, water must first be
removed in the process of dehydration.

Steps of processing:
1.
Samples are transferred through baths of
progressively more concentrated
to
remove the water.
2.
This is followed by a hydrophobic clearing agent
(such as
) to remove the alcohol,
3.
finally molten
, the infiltration agent,
which replaces the xylene
Embedding:
After the tissues have been dehydrated, cleared, and
infiltrated with the embedding material, they are ready
for external embedding. During this process the tissue
samples are placed into molds along with liquid
embedding material (such as agar, gelatine, or wax)
which is then hardened. This is achieved by cooling in
the case of paraffin wax and heating (curing) in the case
of the epoxy resins. The acrylic resins are polymerised
by heat, ultraviolet light, or chemical catalysts. The
hardened blocks containing the tissue samples are then
ready to be sectioned.


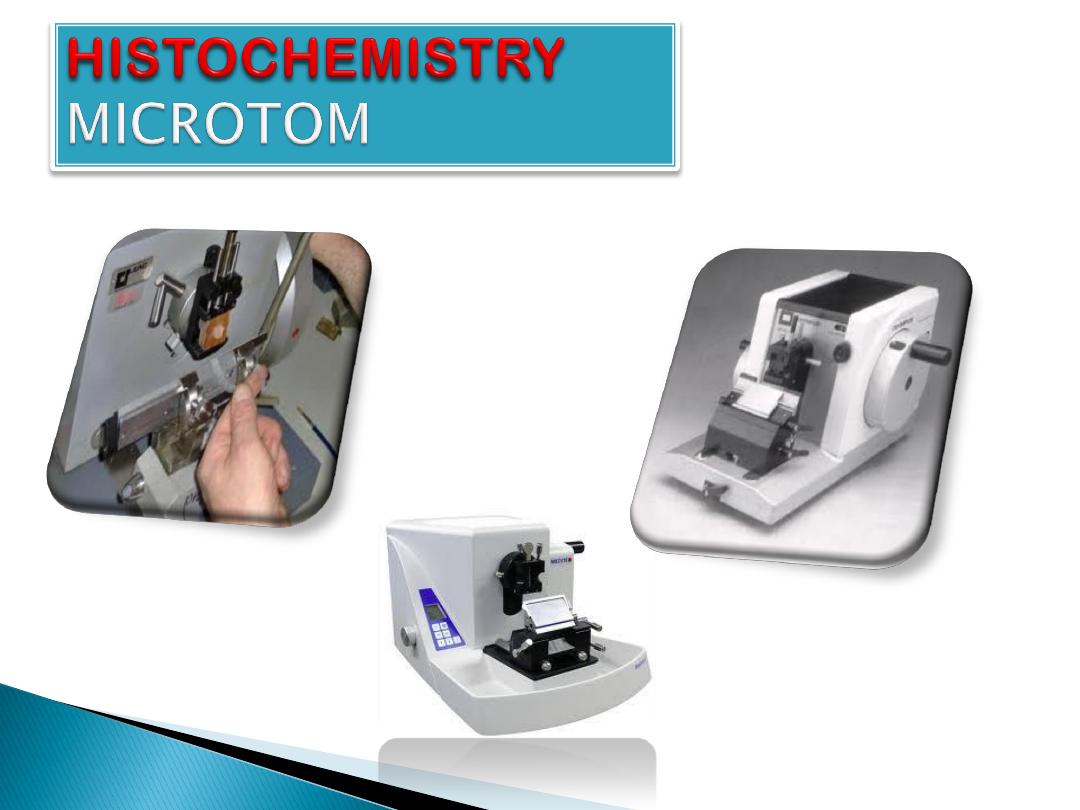
Sectioning:
Sectioning can be done in limited ways. Vertical
sectioning perpendicular to the surface of the tissue
is the usual method. Horizontal sectioning parallel to
the surface of the tissue.
For light microscopy, a steel knife mounted in a
microtome is used to cut 5-
-thick tissue
sections which are mounted on a glass
. For transmission electron microscopy, a
diamond knife mounted in an
is used
to cut 100-
-thick tissue sections which are
mounted on a 3-millimeter-diameter copper grid.
Staining:
Biological tissue has little inherent contrast in either
the light or electron microscope. Staining is employed
to give both contrast to the tissue as well as
highlighting particular features of interest. Where the
underlying mechanistic chemistry of staining is
understood, the term
is used.
and
(
) is the most
commonly used light microscopical stain in histology
and histopathology. Hematoxylin, a
dye, stains
blue due to an affinity to nucleic acids in the
cell nucleus; eosin, an
dye, stains the
pink. Uranyl acetate and lead citrate are
commonly used to impart contrast to tissue in the
electron microscope.


TYPES OF TISSUE SECTIONS:
1.
Soft tissue section.
2.
Bone decalcification.
3.
Ground section of bone.
4.
Frozen section processing.
1.
Soft tissue section.
2.
Bone decalcification: is the removal of
ions from the bone through
process thereby making the
bone flexible and easy for pathological
investigation.This is necessary in order to
obtain soft sections of the bone using the

3.
Ground section of bone: refers to a microscope
slide of bone that is prepared by taking a larger piece
of the bone and placing it between two pieces of
abrasive material--such as carbide paper. These are
rotated and "grind" the section down until the bone is
adequately thin to transmit incident light in a light
microscope, allowing for observation of the bone
structure. This section is then transferred to a glass
slide,
mounted,
and
cover
slipped.
In a ground section, there is no need to remove the
mineral, so that the bone in the section contains both
the mineral and the collagen that form most of the
bonestructure.
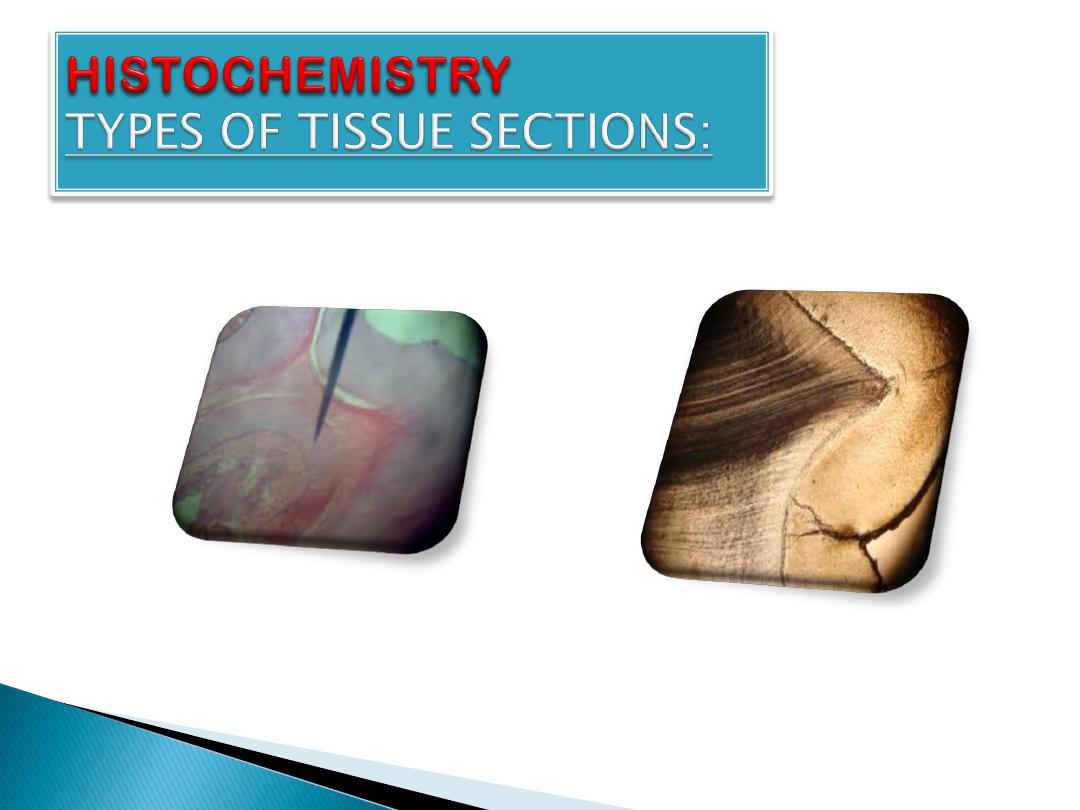
Decalcified section
Ground section

4.
Frozen section processing:
The other method of histology processing is called
processing.
In this method.
1. The tissue is frozen
2. Sliced thinly using a
mounted in a below-
freezing refrigeration device called the
.
3. The thin frozen sections are mounted on a glass
slide.
4. Fixed immediately & briefly in liquid fixative.
5. Stained using the similar staining techniques as
traditional wax embedded sections.


Advantages of this method:
is rapid processing
time, less equipment requirement, and less
need for ventilation in the laboratory.
Disadvantage:
is the poor quality of the final
slide.
Uses:
It is used in intra-operative pathology .
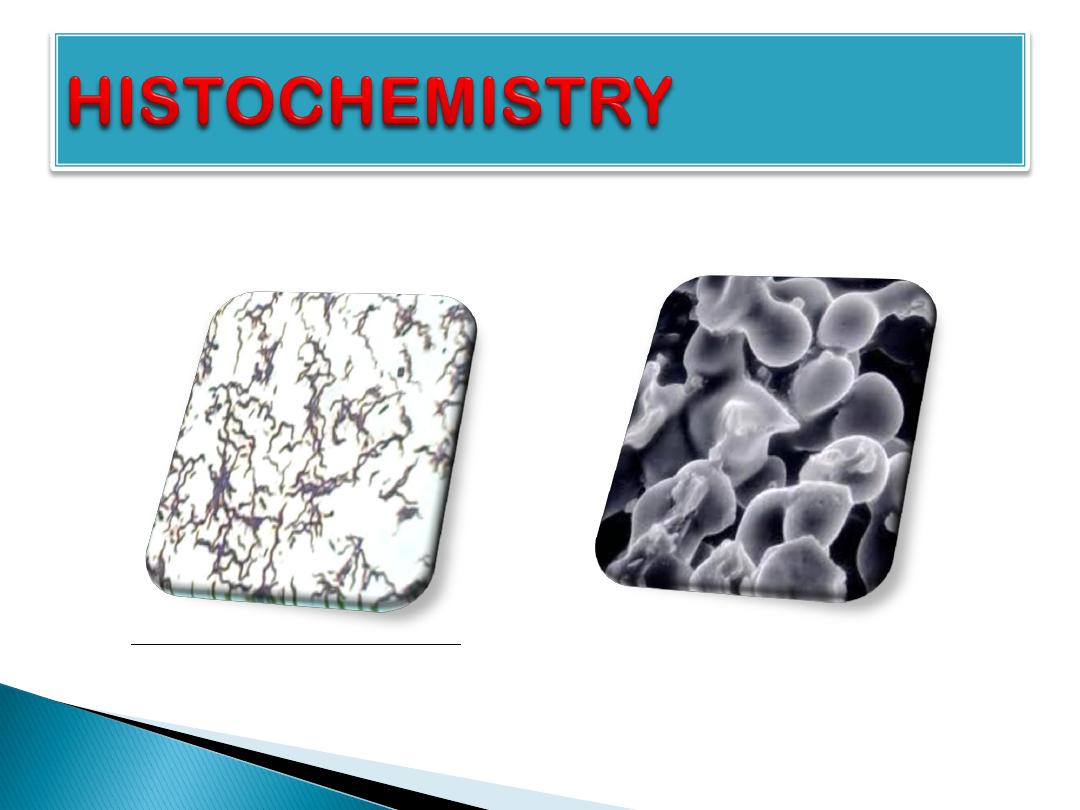
bacteria
of
view
microscop
Light
Electron microscop view of bacteria