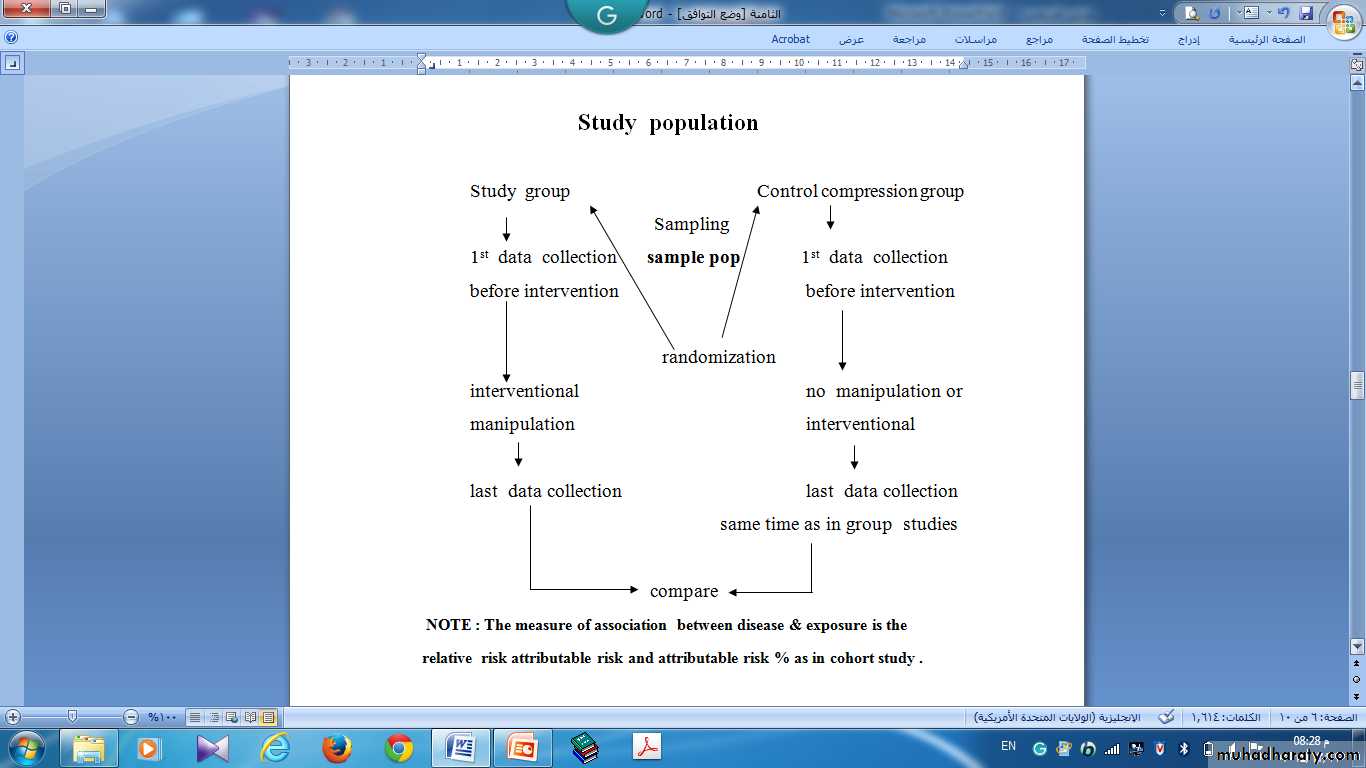
This type of study is similar to cohort studies, but the difference is that the investigator themselves will allocate the exposure i.e. Individuals are included on the basis of exposure, but the investigators allocate the exposure
Two types
بسم الله الرحمن الرحيم
Interventional studies
1
1-Randomized Controlled Clinical Trial (RCT): Individuals similar at the beginning are randomly allocated and exposed to a treatment group and a control group. The outcomes of the groups are compared after sufficient follow-up time. Properly executed, the RCT is the strongest evidence of the clinical efficacy of preventive and therapeutic procedures in the clinical setting.
2- Community trials : communities instead of individuals are allocated and exposed to an experiment. The experimental group a community exposed to an experimental factor and the control group a community that is not exposed to the experimental factor. The results are compared.
2
Randomized Controlled Clinical Trial (RCCT)
Two groups are selected.The 1st is the experimental group. The subjects are exposed to a factor as an experiment under medical supervision.
The 2nd is the control group. The subjects are not exposed to that factor.
3
These two groups are selected on random basis.
Random selection (Randomization) means: every subject in the sample has the an equal chance to be chosen in the experimental group or in the control group.The main difference between experimental and analytic cohort studies, is that the investigator in the experimental studies, allocates by himself the study groups into: experimental group and the control group. While in the analytic cohort studies the participants are naturally allocated into exposed and non exposed.
4
Strengths or advantages :-
It's regarded as the gold standard of the epidemiological studies. It is the strongest & the most direct epidemiology evidence to judge the causal association . " like an experiment in the lab where the exposure is under the investigators control , who controls all the factors except the risk factor ".5
• it can detect mild to moderate difference (10-20%) which is difficult in cohort studies .
• e.g. conducting a study on which medication should be given after a myocardial infarction MI, should it be B-blockers or Ca++ channel blockers for the prognosis of MI ? Here the difference will be very small (2-5%) but very important to find it out to save patients lives .
• It can control & manipulate many confounders .
• It can demonstrate the temporal relationship between the exposure & outcome with the highest degree of confidence .
6
• Limitations or disadvantages :-
Expensive & time consumingIt doesn't represent the real life situation " because we control all the other factors except the exposure .
Ethical problems : for certain factors , there is some doubt about the benefit or harm to the study subject.
Feasibility problems: were it is difficult to find the control" non exposed: group.
e.g. a study in a city about Vitamin C supplement & disease , here it was hard to find a control group because the majority of the population used vitamin C supplement .
7
Types :
1. Therapeutic or secondary prevention trials :-
* the study groups are "Diseased"
it is conducted on patients to evaluate the effect of certain drugs or procedures in minimizing symptoms, complications or death.
e.g. to study the best treatment for coronary heart disease, is it medical treatment or surgical treatment.
So :
Group 1 : patients with CHD treated by Medication on.
Group 2 : patients with CHD treated Surgically on .
& then we studied CHD mortality rate between both groups .
8
2. Preventive or primary prevention trials :-
Conducted on healthy people who are at normal risk or high risk to develop an outcome .e.g. polio vaccine to prevent poliomyelitis ..
Group 1 : took a full vaccination
Group 2 : took a placebo.
Result " vaccination " group 1 was successful
9
Selection of study groups :-
I- Reference population :
Represents the group on which the results will be applicable
e.g. a study to prevent MI in males at 45 y of age, the reference population will be males at 45y of age.
II-Experimental population.
Represents the group on whom the study " trials " will be conducted upon .
*Volunteer Bias:-
The volunteers do not represent the true community population on which we will generalize the study result " because not everyone will accept to participate in the study".10
There should be :-
• Sufficient outcome :-There must be good No. of people in the study having to outcome . e.g. recovery or death
• complete & accurate information :
Especially information about follow-upThe participants should be informed about :
The aim of the study, possible benefits, side effects & the possibility of having a placebo during the study period .11
12
Masking (Blinding)
Masking involves several components:
First, we would like the subjects not to know which group they are assigned to. This is of particular importance when the outcome is a subjective measure, such as headache or low back pain. If the patient knows that he or she is receiving a new therapy, enthusiasm and certain psychological factors may operate to elicit an improved response.
13
How can subjects be masked?
One way is by using a placebo ; an inert substance that looks, tastes, and smells like the active agent. However, use of a placebo does not automatically guarantee that the patients are masked (blinded).
In addition to blinding the subjects, we also want to mask (or blind) the observers or data collectors in regard to which group a patient is in. This is called “double blinding.”
• Placebo: A placebo is the non treatment material used in a control group in place of the actual treatment. If a drug is being evaluated, the inactive vehicle or carrier is used alone so it is as similar as possible in appearance and in administration to the active drug. Placebos are used to blind observers and, for human trials, the patients to which group the patient is allocated.
Limitation of placebo :
There is a tendency of the patient to report a good result of any treatment.• Tendency to report side effects with the Rx or placebo
14
• A Diagram Of Conducting The Study
• ( Accepting Group )•
• Screening For ( Inclusion & Exclusion )
•
• Accepting & Eligible
•
• Randomization Into Rx ( Exposure Groups ) Groups
15
• Causes of exclusion :-
Definite history of out come under study.Definite need for the study treatment " like someone has Diabetes Mellitus we must give him a treatment & not placebo.
Contraindication to the study treatment. e.g. giving aspirin to someone with peptic ulcer.
16
17
• Efficacy ;The risks of death or of developing a disease or complication in each group can be calculated, and the reduction in risk (efficacy) can then be calculated.
• Ratio of the risks in the two treatment groups (the relative risk).
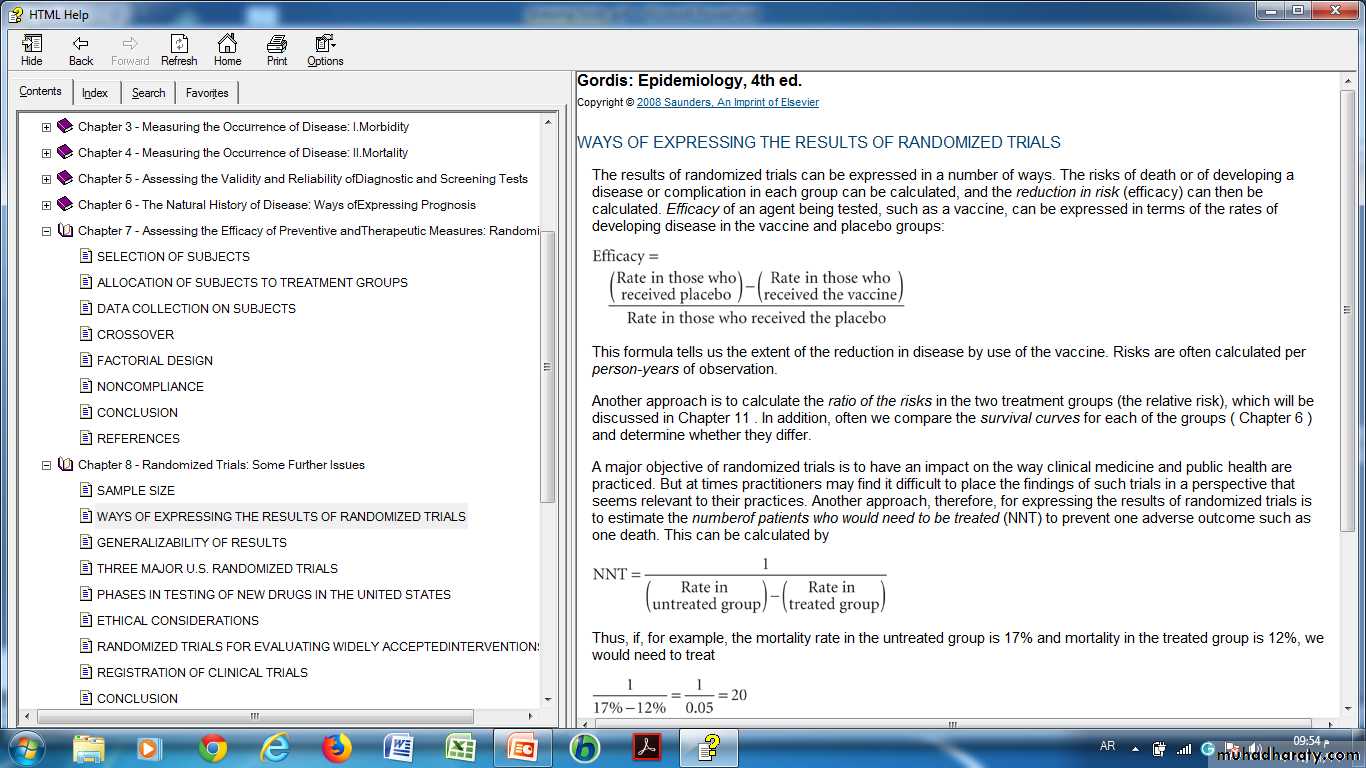
• Number of patients who would need to be treated (NNT)
18
WAYS OF EXPRESSING THE RESULTS OF RANDOMIZED TRIALS
19
20
Another approach, therefore, for expressing the results of randomized trials is to estimate the numberof patients who would need to be treated (NNT) to prevent one adverse outcome such as one death. This can be calculated by
• Maintenance of Assessment of compliance :
• Compliance : the commitment of the study participants by the treatment التزام المشارك .• The non- compliance is the major problem in the intervention. it's related to the complexity & the length of the follow-up .
• Causes of non -compliance :
• ( non –compliance decrease the statistical power of the study )
development of side effects .
forgetting to take the treatment.
withdrawal from trials.
choosing the alternative method.
the intervention becomes contraindicated .
21
Enhancement of compliance is by :
1. inclusion of interested & reliable group
2.frequent contact with participants
3. use of calendar pack of study treatment as in contraceptive pills each pill has a date on it .
4. provision of in centuries " حوافز "either financial or medical insurance "
How do check compliance:-
self report
pill count of non used medication
used of biochemical markers : e.g we give a drug that is secreted in urine & check the presence of the drug in urine .
22
• Stopping rules :
• These are criteria for early termination or modification of the trial when the appearance of extreme benefit or harm from early results .• This stopping should be :-
done by an external investigation
based on experience of adequate No. of subjects
The statistical difference should be high .
23