
1
Pharmacology (Lec.1)
Dr. Nahla
Introduction
Definitions:
1. Pharmacology:
science dealing with → interactions between
chemicals (drugs) and living systems.
2. Drug:
chemical substances, when introduced into the body, alters
the body's function, producing → biological effects . Can be:
Stimulatory.
Inhibitory.
3. Prodrug:
chemical, is readily absorbed and distributed and then
converted to → active drug by → biologic process inside the body.
4. Medical pharmacology:
science of materials used to:
Prevent.
Diagnose.
Treat.
5. Toxicology:
deals with the → undesirable effects of chemicals in
biological system.
6. Pharmacogenomics (pharmacogenetics):
study the → genetic
variations that cause individual differences in drug response. Aren't
found in general population (allergies), but due to→ an inherited
trait that produces a diminished or enhanced response to a drug.
*Differences in enzyme activity:
1. Acetylation polymorphism.
2. Butyl-cholin-esterase alterations.
3. Cytochrome P450 aberration.
General concept of Pharmacology:
1. Pharmaceutical Phase:
when medications → enters the body in one
form and changes into another form.
2. Pharmacokinetics:
what the body does to the drug.
The pharmacological effect of drugs depends on → its concentration
at the site of action:
Absorption.
Distribution.
Metabolism.
Elimination.
Inas Waleed
3rd Stage of Dentistry

2
3. Pharmacodynamics:
what the drug does to the body.
Interaction of drugs with → cellular proteins (receptors/enzymes),
to → control changes in physiological function of particular organs:
Drug receptor interaction → binding.
Dose-response → effect.
Signal transduction → mechanism of action.
4. Pharmacotherapeutics:
proper selection of an agent whose
biological effect is most appropriate to treat particular disease
state.
Routes of administration:
its determined by:
Properties of the drug (water/lipid soluble).
Therapeutic objective (desirability of a rapid onset of action).
1. Enteral (GI route) → systemic:
Oral: by the → mouth (most common).
Sublingual: drugs subject to → high degree of first-pass
metabolism.
Rectal (high vascular): excellent site of → absorption (it's also
used to → administer antiemetic agents)
2. Parenteral (Injections) route → local:
they're three:
Intravascular (I.V.).
Intramuscular (I.M.).
Subcutaneous (S.C.).
3. Others:
Inhalation.
Intrathecal/Intraventricular.
Topical (skin and mucous membrane).
Trans-dermal.
Intranasal.
3
1.Pharmacokinetic Phase
(What does the body to the drug)
*Absorption:
the movement of drug from its site of application into the
blood / lymphatic system without being chemically altered.
Rate of absorption depend on → route of administration.
I.V. route → absorption is complete, that's mean; the total dose of
drug reaches the systemic circulation.
Other routes → absorption is partial → lower bio-availability.
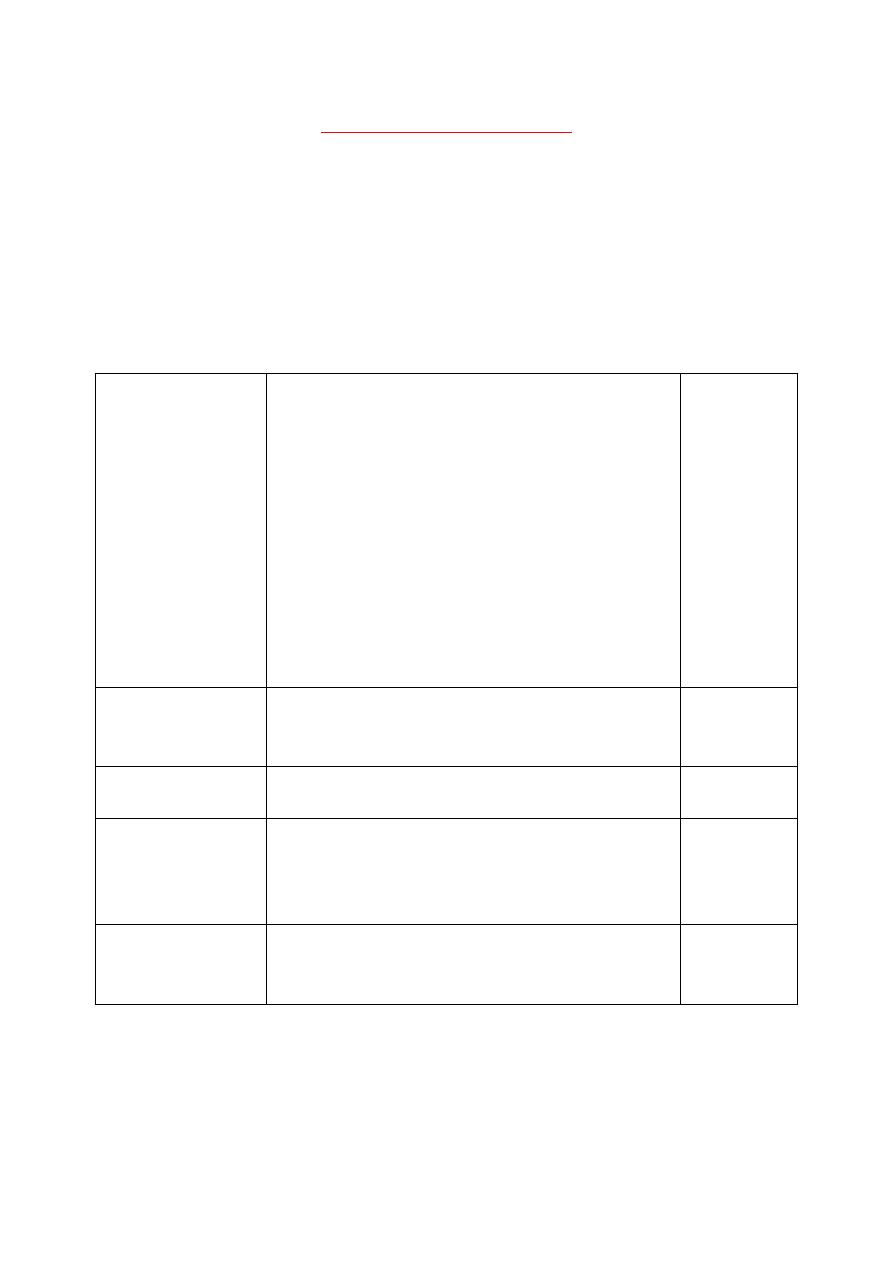
Mechanisms of drug transport across membranes (absorption):
1. Passive
(simple) diffusion
(Glomerular
filtration)
The drug moves from a region of ↑ conc.
to one of ↓ conc. (directly proportional to
the conc. gradient).
Doesn't involve a carrier.
Not saturable.
Low structural specifity.
Rapid for → lipophilic, non-ionic and small
molecules.
*Lipid-soluble drugs: moves across most
biological membranes.
*Water-soluble drugs: penetrate the cell
membrane through aqueous channels.
No energy.
2. Pore transport
(aqueous
channels)
Small hydrophilic drugs (water-soluble):
diffuse by → passing through pores
(aqueous channels).
No energy.
3. Facilitated
diffusion
Bind to carrier → non-covalently.
No energy.
4. Active
transport
Identical to facilitated diffusion.
The drug moves from a region of ↓ conc.
to one of ↑ conc. (against conc. gradient).
Energy is
needed
(ATP).
5. Pinocytosis
and phagocytosis
Engulfing of drug for large substance to →
enter the cells (iron).
Appropriate binding proteins.
-

4
Factors affecting drug absorption:
1. Effect of pH on drug absorption:
Most drugs are → week acidic / weak bases:
*Acidic drugs (HA) release H
+
, producing a charged ion A
-
[ HA ↔ H
+
+ A
-
] HA: can penetrate.
*Basic drug (BH
+
) release H
+
, producing an uncharged ion B
[ HB
+
↔ H
+
+ B
] B: can penetrate.
Passage of uncharged drug through a membrane:
Drug passes through membrane more readily if it's uncharged.
The effective conc. of permeable form is determined by → the
relative conc. of the charged and uncharged forms.
The ratio between the two forms (charged and uncharged) is
determined by → pH at the site of absorption.
→ pKa: the strength of weak acid/base.
Lower pKa → stronger acid. Higher pKa → stronger base.
2. Physical factors influencing absorption:
1. Blood flow to the absorption site:
Decrease in blood flow → decrease in absorption.
Blood flow in the intestine is much greater than the flow of
stomach.
2. Total surface area available for absorption:
Absorption in the intestine is more efficient, because it has a
surface rich in microvilli.
3. Contact time at the absorption surface:
If drug moves through GIT very quickly (diarrhea), it's not
well absorbed.
Anything that delays the transport of drug from the stomach
to the intestine, delays the retention of drug.
Presence of food in stomach → dilutes the drug.
→ shows gastric emptying.
Drugs taken with meal is generally absorbed more slowly.
4. Expression of P-glycoprotien.
5
3. Distribution:
when the drug leaves the blood and enters the
interstitium and/or the cells of the tissue.
1. Blood flow: rate of B.F varies widely, due to → unequal
distribution of C.O.P. to the various organs.
B.F. to the brain, liver and kidney is → greater than B.F. to
skeletal muscle.
Adipose tissue has a → low rate of B.F.
2. Capillary permeability: is determined by:
a. Capillary structure: varies widely, due to → the fraction of
the basement membrane that's exposed by → slit (tight)
junctions between endothelial cells.
In the brain: the capillary structure is continuous → there
are no slit junctions.
In contrast, the liver and spleen: large part of their
basement membrane it's exposed, due to → large
discontinuous capillaries, through which plasma proteins
can pass.
*Blood-Brain Barrier (BBB):
Drugs must pass through the endothelial cells of
CNS.
Lipid-soluble drugs readily penetrate into the CNS,
since they → can dissolve in the membrane of
endothelial cells.
Ionized/polar drugs, generally, fail to enter the CNS,
since they are → unable to pass through the
endothelial cells which have no slit junction.
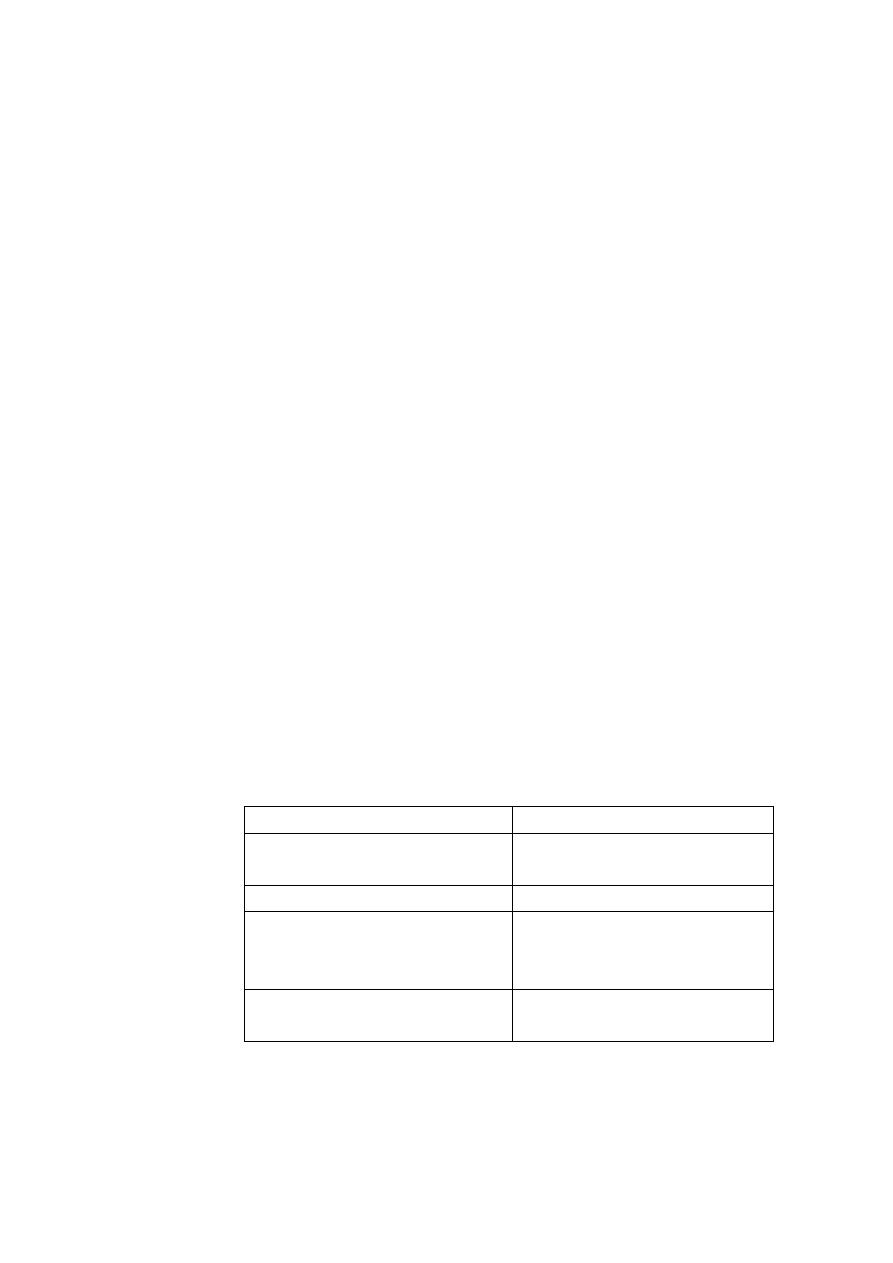
b. Drug structure (chemical nature of the drug):
1. Hydrophobic drug
2. Hydrophilic drug
Uniform distribution of
electrons.
Non-uniform distribution of
electrons.
No charge.
Positive/negative charge.
Readily penetrate most
biological membranes.
Don't readily penetrate
biological membranes, must
go through the slit junctions.
Can dissolve in the lipid
membranes.
-
6
3. Binding to proteins:
Plasma protein:
1. Sequesters drugs in a non-diffusible form.
2. Slows their transfer out of the vascular compartment.
Binding is → non selective, takes place at → sites on the
protein to which endogenous compounds attach (bilirubin).
*Plasma albumin: is the major drug binding protein, and may
act as drug reservoir.
As the conc. of free drug ↓, due to → elimination by
metabolism/excretion, the bound drug dissociates from the
protien.
This maintain the free drug conc. as a constant fraction.
*Binding of drugs to plasma proteins:
Bound drug are pharmacologically inactive.
Only the free unbound drug can:
1. Act on target sites in the tissue
2. Elicit a biological response.
By binding to plasma proteins, drugs become → trapped,
and, in effect, → inactive.
*Clinical importance of drug displacement:
1. Class I drug → Tolbut-amide (95% bound, 5% free), that's
mean; most of the drug is:
Sequestered on albumin (bound).
Pharmacologically inert.
2. Class II drug → Sulfon-amide antibiotic: if is
administrated, it displaced Tolbut-amide from albumin,
leading to → rapid ↑ in the conc. of free Tolbut-amide in
plasma (100% free).

7
4. Drug metabolism:
Drugs are eliminated by:
1. Biotransformation.
2. Excretion into the urine/bile.
Liver → major site of drug metabolism.
Specific drugs may undergo → biotransformation in other tissues
Kidney → cannot eliminate lipophilic drugs, that readily cross cell
membranes and are reabsorbed in the distal tubules.
*Lipid-soluble agents: must be first metabolized in the liver, using
two phases:
1. Phase I: convert lipophilic molecule into → more polar
molecules, by introducing → polar functional group.
May ↑, ↓ or leave unaltered the drugs pharmacologic
activity.
Are catalyzed by → Cytochrome P-450 system.
Takes place → smooth (no ribosome) E.R. in hepatocytes.
Smooth microsomes are rich in enzymes responsible for
oxidative drugs metabolism.
The activity of these enzymes require:
1. Reducing agent.
2. NADPH.
3. Molecular O
2
.
*Cytochrome P-450: iso-enzymes located in → cells of liver
and intestinal tract.
1. Cytochrome P-450 enzyme induction: stimulation of
hepatic drug metabolism.
Some → stimulate their own metabolism.
→ accelerate the metabolism of other drugs.
E.g. → Pheno-barbital.
2. Cytochrome P-450 enzyme inhibition: ↓ the
stimulation of hepatic drug metabolism enzyme, which
lead to → ↑ levels of active drug in the body.
E.g. → Alcohol.
2. Phase II: coupling the drug with an endogenous substrate.
Endogenous substrate originate in → the diet, so
nutrition plays an important role in the regulation of drug
coupling.
More water-soluble compounds are → most often
therapeutically inactive
E.g. → Glucuronidation.

8
5. Drug elimination (excretion):
removal of drug from the body.
1. Major organs:
Kidney: into the urine.
Liver.
GIT and lung.
2. Minor organs:
Milk: nursing mothers.
Salivary glands.
Sweat.
*Mechanism of renal elimination of a drug:
1.Glomerular filtration (passive diffusion):
Drugs enter the kidney through → renal arteries, which divide
to form → glomerular capillary plexus.
Lipid solubility and pH don't influence the passage of drugs.
Small molecules, water soluble and free drugs → pass more
rapidly.
Drugs bound to plasma protein → don't pass through G.F.
2.Tubular secretion:
Drugs bind to carriers are → transported.
Drugs was not transferred into G.F., leaves the → glomeruli,
through → efferent arteriole, which divide to form → capillary
plexus surrounding the → nephric lumen in the proximal
tubule.
3.Tubular reabsorption:
If the drug is → uncharged, may diffuse out of → the nephric
lumen, and back into → systemic circulation.
Manipulating the pH of urine to:
↑ the ionized form of the drug in the lumen, to →
↓ the amount of back diffusion, and to →
↑ the clearance of an undesirable drug.
Small non-ionic drugs → pass more rapidly.

9
2.Pharmacodynamic Phase
(What does the drug to the body)
Occurs when medication reaches the → target cell, tissue, organ and
a therapeutic effect occurs.
Mechanism of drug action:
1. Physical action:
alter the environment of the cell through physical
action (Kaolin adsorbs toxins in → diarrhea).
2. Chemical action:
alter the environment of the cell through chemical
action (NaHCO
3
in → hyperacidity).
3. Cytotoxic action:
stop cell division (anti-cancer drugs)
4. Interfere with selective passage of ions
(Ca
+2
and Na
+
entry → local
anesthetics drugs)
5. Interference with normal metabolic pathway
(Sulphon-amides
competes with PABA → essential for bacterial growth).
6. Action on enzyme stimulation/inhibition:
enzyme inhibition could
be:
Reversible: short-term (Neostigmine → Cholin-esterase
inhibitor).
Irreversible: long-term for new enzyme synthesis (irreversible
Anti-cholin-esterase).
7. Action on specific receptors (drug receptor interaction):
Receptors: are macromolecular protein structures, present on →
cell membrane / within the cell.
React with a ligand (drug, hormone or neurotransmitter) to →
produce a biological response.
Receptors translate the signal from → ligand, to → several
subcellular elements (enzymes), to produce a biological
response.
10
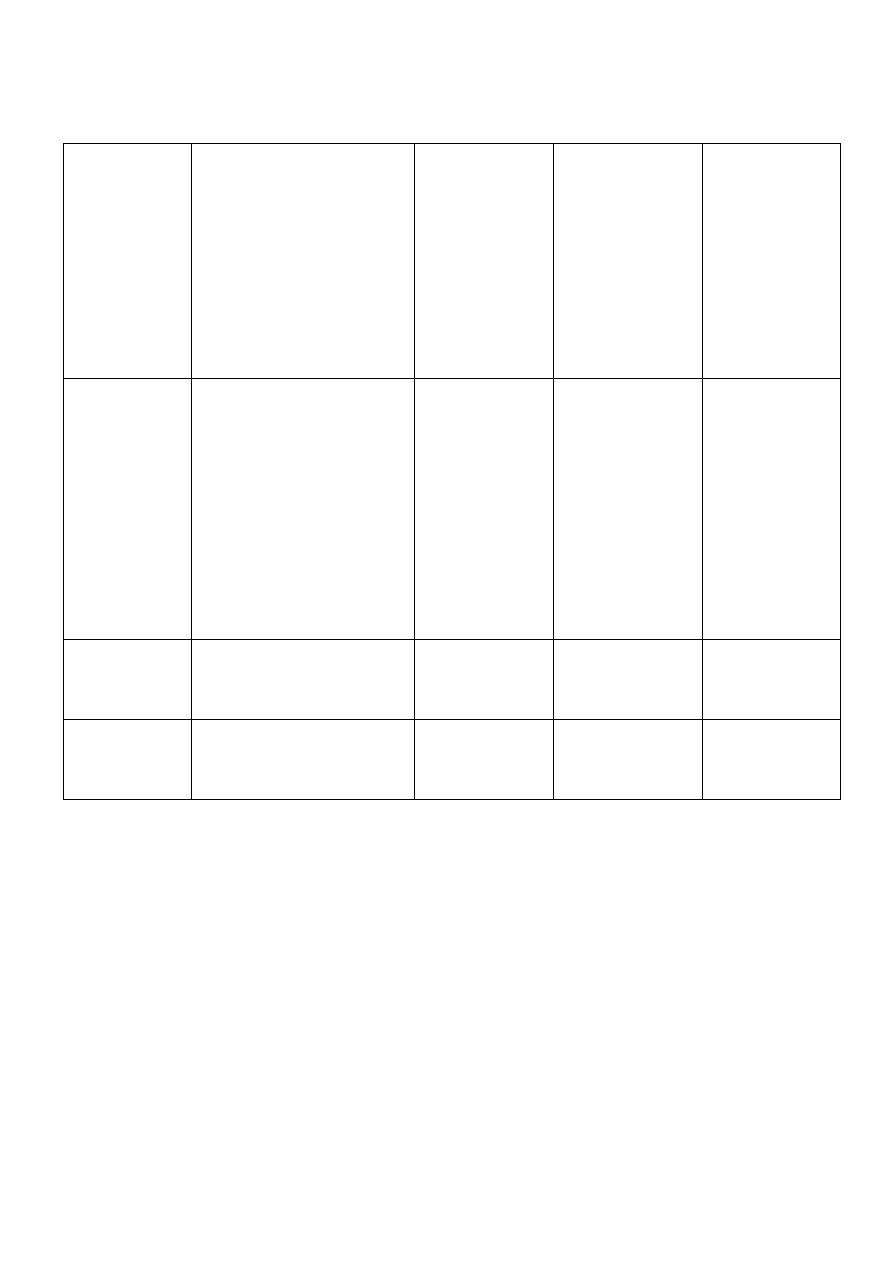
Drugs can be categorized into:
Agonists
Drugs which →
stimulate receptors:
initiate changes in cell
function producing
effects.
Potency depends on:
1. Affinity.
2. Efficacy.
E.g → Diazepam
Affinity:
tendency to
bind
receptors.
Efficacy: ability
to initiate
changes, which
lead → effect.
Rapid
dissociation
rate.
Antagonists
Drugs which → block
receptors; they bind
to receptors without
initiating change in
receptors.
Have no effect in the
absence of agonist.
Prevent the action of
agonists.
E.g → Flumazenil.
Affinity.
No efficacy.
Slow
dissociation
rate.
Partial
agonists
Stimulate and block
receptors.
E.g →Buprenorphine.
Affinity.
Efficacy.
Moderate
dissociation
rate.
Inverse
agonists
Produce effects
opposite to agonist.
E.g → Carbolines.
Affinity
Efficacy
Rapid
dissociation
rate.

11
Types of antagonists:
1. Pharmacological Antagonists:
A. Competitive
B. Non-Competitive
Compete for the binding site.
Bind anywhere in the receptor.
Reversible.
Irreversible.
Surmountable.
Un-surmountable.
The effect can be overcome by
more agonist (drug).
Higher the conc. of antagonist
used → more drug you need to
get the same effect.
The effect cannot be overcome
by more agonist (drug).
Causes → parallel shift of the log
dose response curve to the right.
Causes → shift of the log dose
response curve to the right.
Slope of the curve and maximum
effect → not changed.
Slope of the curve → changed.
-
Inactivates the receptors.
2. Functional Antagonists:
1. Physiologic antagonist:
Binds to → non-related receptor.
Opposite effect to that produced by the drug.
Intrinsic activity = 1.
* Glucocorticoid hormones → ↑ blood sugar.
* Insulin → ↓ blood sugar.
2. Chemical antagonist:
It's a chelator (sequester).
Interacts directly with the drug, being → antagonized, to
remove it / prevent it from binding to its receptor.
Doesn't depend on → interaction with agonists receptors.
* Heparin (anti-coagulant, acidic): if there's → too much
bleeding and hemorrhage.
*Prot-amine sulfate (base): form a → stable inactive complex
with heparin, and inactivates it.

12
Receptors and signal transduction mechanism:
binding of agonist to
receptors → activates effectors/signaling mechanism.
1. Ion-channel linked receptors (Ligand-gated ion channel):
Ach + nicotinic receptors → Na
+
influx → depolarization.
GABA + GABAA receptors → Cl
-
influx → hyper-depolarization.
These responses takes → milliseconds.
2. G-protein linked receptors (glucagon):
Time elapsed between binding to receptor and cellular response
takes → seconds.
These receptors has → a seven trans-membrane, because →
receptor polypeptide across cell membrane 7 times.
When agonist bind to → the domain, G-protein will be →
activated, to transduce agonist-induced signal to a variety of
effectors elements located intracellular / in the cell membrane.
Effector element change → the conc. of an intracellular second
messenger.
*Second messenger → include:
1. cAMP (cyclic Adenosine Mono-Phosphate).
2. Ca
+2
ion.
3. Phospho-ino-sitides.
4. cGMP (cyclic Guanosine Mono-Phosphate).
3. Membrane-tyrosinase kinase linked receptors (insulin):
Receptors for → insulin, act on → membrane receptors, which can
phosphorilate → signal transducers and activators of
transcription, which → dimerize, and then → dissociate from the
receptor to:
1. Cross the nuclear membrane.
2. Modulate gene transcription.
4. DNA-linked receptors (intracellular receptor):
When agonist bind to → the domain, hsp90 domain is → released
leaving the DNA binding domain, which regulates:
1. Gene transcription.
2. Translation.
3. Protein synthesis.
Has a slow onset → long duration.

13
Variation in drug responsiveness
Idio-syncratic drug response: unusual individual reaction, caused
by: 1. Genetic differences in metabolism.
2. Hyper-sensitivity.
3. Tolerance.
Hypo-reactive: intensity of effect is ↓.
Hyper-reactive: intensity of effect is ↑.
Hyper-sensitivity: allergic / immunological response to drug.
Tolerance: responsiveness usually ↓ as a consequence of →
continuous drug administration. Need → greater doses /
substitute different drug.
Tachy-phylaxis: responsiveness ↓ rapidly, after administration of
a drug.
Four general mechanisms:
1. Patients may differ in the rate of:
Absorption of drug.
Distribution of drug through the body.
Elimination of drug from the body.
These will → alter the conc. of drug that reaches receptor.
Can be due to → age, weight, sex, disease state, liver and kidney
disease, genetic differences.
2. Patients may vary in their:
Conc. of endogenous receptor ligand.
Response to pharmacologic antagonist.
14
3. Patients may have differences in the:
Number of receptors sites.
Function of receptors.
Due to → efficiency of coupling receptor to effectors.
Antagonist
Agonist
When discontinued, elevated
number of receptors can
produce an exaggerated
response to physiologic conc. of
agonist.
When discontinued, elevated
number of receptors have been
down-regulation, is too low for
endogenous agonist to produce
effective stimulation.
E.g. → Clonidine (α-agonist):
1. ↓ blood pressure.
2. Produce → hyper-tensive crisis.
4. Patients vary in:
Functional integrity of biochemical processes in the responding
cell.
Physiologic regulation by → interacting organ systems.
Caused by → age, general health, severity and patho-physiologic
mechanism of the disease.
Drug therapy:
1. Correct diagnoses.
2. Accurately directed.

15
Pharmacology (Lec.2)
Dr. Nahla
Parasympathetic (Cholinergic) nervous system
Parasympathetic and it's division → maintains essential bodily
functions required for life:
1. Digestive processes.
2. Elimination of wastes.
Dominant over the sympathetic system in → "rest and digest"
situations.
Parasympathetic system is → not a functional entity and never
discharge a complete system. If it did, it would produce → massive,
undesirable and unpleasant symptoms.
Functions of the parasympathetic system:
1. Eye:
Meiosis (↓ pupil size):
Spasm of ciliary muscle so → the eye is accommodated for near
vision.
It cause → headache, convulsions, anxiety, respiratory failure...
Decrease intraocular pressure (↓ IOP):
Iris is pulled away → from the angle of anterior chamber.
Trabecular meshwork at the base of ciliary muscle is → open,
and facilitate → outflow of aqueous humor into → canal of
schlemm.
Canal of schlemm drain → anterior chamber of eye, so → ↓IOP.
2. Exocrine glands:
↑ the secretion of → salivary, lachrymal, bronchial
and sweat gland.
3. Heart:
Action of Ach on → heart mimic vagal stimulation: regulates
the heart by → the release of Ach at SA node.
Bradycardia (↓ heart rate).
A-V block.
Cardiac arrest.
4. Bronchi:
bronchoconstriction with ↑ secretion (Lead → symptoms
in asthmatic).
Inas Waleed
3rd Stage of Dentistry

16
5. Alimentary tract:
↑ motor activity.
↑ exocrine secretion.
Colicky pain may occur.
Reduce → sphincter tone: the patient may defecate
unconsciously.
Reduce → lowering esophageal sphincter tone: create a risk
of regurgitation and inhalation.
6. Bladder and uterus:
tone of detrusor muscle is ↑ and cause →
contraction and drug promote micturation.
*Human uterus is not sensitive to → muscarinic agonists.
7. N-M junction:
muscle fasciculation.
8. CNS:
stimulation followed by → depression, mental excitement,
confusion, restlessness, insomnia, tremors, convulsion...
9. Blood vessels:
Ach cause → vasodilatation and ↓ blood pressure.
*Cholinergic receptors on blood vessels cause → vasodilatation.
10. Death:
due to → actions on CNS.
→ paralysis of respiratory muscle.
→ excessive bronchial secretion and constriction.

17
Therapeutic uses in dentistry:
Cholino-mimetic drugs:
have an affinity for → muscarinic sites, are
capable of → stimulating salivation.
Xerostemia:
is a common problem encountered by the dentists in
patients with → Sjorgren's syndrome: patients who have head and
neck radiation, they undergoing treatment involving drugs that
produce → dry mouth.
Muscarinic receptor agonists:
May be useful in → stimulating salivary flow, when there's:
1. Functional salivary gland tissue present.
2. No contraindication for their use.
Should not be administrated if they will → compromise other
therapy.
Are contraindicated in:
1. Urinary tract obstruction.
2. Hyper-active airway disease.
3. Chronic obstructive pulmonary disease.
4. Acute heart failure.
5. GI spasm.
6. Hyper-thyroidism.
7. Acute iritis.
Anti-muscarinic therapy:
for → overactive bladder, would tend to
be compromised by the → administration of muscarinic receptor
agonists.
Pilo-carpine:
is usually taken at doses of → 5-10mg, 3 times daily,
30 min before each meal.
Cevi-meline:
is usually taken at doses of → 30 mg, 3 times daily.
Physos-tigmine:
for → I.V. sedation.
18
Cholinergic Agents, Cholino-mimetic Drugs,
Parasympathomimetic Drugs
*Definition:
are drugs that → directly and indirectly → promote the
function of the neurotransmitter Ach (agonists).
*Mechanism of action of cholinergic drugs:
mimic action of Ach.
1. Binds with receptors on → cell membrane of the target organ.
2. Changes → the permeability of the cell membrane, and →
permitting Ca and Na to flow into → the cells.
3. This depolarization in cell membrane cause → response.
Classification of cholinergic drugs:
a) Direct acting cholinergic drugs:
activate → muscarinic / nicotinic
receptors.
a) Choline ester:
1.Acetylcholine (Ach)
2.Betha-nechol
3.Carbacol
Quaternary ammonium
compound.
Structurally related to Ach.
Not hydrolyzed by → Ach-
esterase. It's inactivated
through hydrolysis by →
other esterase.
Major actions are on:
1. Smooth musculature of
bladder.
2. GIT.
High potency.
-
Duration of action → 1 hour.
Duration of action → long.
Has → muscarinic and
nicotinic activity.
Has → strong muscarinic
activity, and little or no
nicotinic activity.
Has → muscarinic and
nicotinic activity.
Therapeutic applications:
of no importance, because:
1. Multiplicity of action.
2. Rapid inactivation by
→ Ach-esterase.
Therapeutic applications: in
urologic treatment, to
stimulate → the atonic
bladder in:
1. Post-partum.
2. Post-operative non-
obstructive urinary
retention.
Therapeutic applications:
rarely, except in → the eye:
is used as → meiotic agent
to cause:
1. Contraction of the
pupil.
2. ↓ I.O.P.
19
b) Alkaloid:
*Cevimeline: it's used to → treat the symptoms of → xerostomia.
Well absorbed after oral administration.
Peak blood conc. occur in → 1.5-2 hours.
Most of drug is → metabolized to:
1. Sulfoxides.
2. Glucuronic acid conjugates.
Elimination half-life is of about → 5 hours
1.Muscarine
2.Nicotine
3.Pilo-carpine
Present in small
amount in → fungus
Amanita muscaria.
Present as → gum and
patches.
Tertiary amine.
Stable to →hydrolysis.
Less potent than → Ach.
Has→ muscarinic activity, used in →
ophthalmology.
No therapeutic
applications
Therapeutic applications:
as adjunct (acid) to →
stop tobacco smoking.
Therapeutic applications: is effective
in → opening the trabecular
meshwork around schlemm's canal,
causing → an immediate drop in IOP
as a result of the ↑ drainage of
aqueous humor.
1. In glaucoma: is the drug of choice
in the emergency of ↓ IOP of
narrow-angle and wide-angle
glaucoma.
2. Xerostomia (dry mouth):
a) After irradiation of head and
neck.
b) Sjogren's syndrome.

20
b) Indirect acting cholinergic drugs (Cholinesterase inhibitors /
Anticholinesterase):
1. Reversible:
2. Irreversible:
Iso-fluro-phate and Echo-thio-phate
(very long acting)
Used in → the eye for → chronic treatment of open-angle
glaucoma.
A number of → synthetic organophosphate compound, which
are used in:
1. Agriculture.
2. Pesticides.
3. Insecticides.
Are extremely → toxic and volatile liquids.
Used by → the militaries as → nerve gases:
1. GA → tabun.
2. GB → sarin.
3. GD → soman.
1.Short acting: 10-
20 minutes.
*Endro-phonium: they're give → parentrally (by injection)
Clinical uses:
1. Diagnosing → Myasthenia gravis (M.G.)
2. Differentiate between:
*M.G: weakness due → severe disease / inadequate anti-
cholin-esterase treatment.
*Cholinergic crisis: weakness due → over treatment with anti-
cholin-esterase.
2. Intermediate -
long term acting
*Tubo-cura-nine: are non-
depolarizing neuromuscular
blocking drug (muscle
relaxant), used in →
neuromuscular blockade with
surgical anesthesia.
*Neos-tigmine and Pyridos-tigmine:
used to → reverse the
pharmacologic muscle paralysis
rapidly after the surgical procedure.
They're give → I.V / I.M for → rapid
effect.

21
Poisoning with cholinesterase inhibitors (organophosphorous
poisoning):
*Clinical features:
1. Excessive → salivation, nausea, vomiting, abdominal pains,
diarrhea, lacrimation...
2. Excessive → bronchial secretion, bronchoconstriction, cough,
wheezing, dyspnea.
3. Bradycardia.
4. Involuntary micturation.
5. On CNS → irritability, anxiety, fear, seizures.
*Treatment:
1. Maintenance of vital respiratory signs.
2. Decontamination: to → prevent more absorption by:
Removal of clothes.
Washing of skin.
3. Atropine (anti-muscarinic drug): administrated → parentrally in
large doses until sing of effect appear.
4. Atropine eye drops: relieve → headache caused by → meiosis.
5. Mechanical ventilation: is needed to → assist respiratory
muscle, because Atropine → has only muscarinic effect, so not
block n-m junction which has nicotinic receptors.
6. Diazepam: for → convulsions.
7. Reactivation of Ach-esterase:
Prali-doxime (PAM): can reactive the inhibited Achesterase,
because it has → very high affinity for the phosphorous
atom, that allows it to:
1. Displace the organophosphate.
2. Regenerates the Achesterase enzyme.
These substances → hasten the destruction of →
accumulated Ach and unlike Atropine.
They have → anti-muscarinic and anti-nicotinic effects.
Best result is obtained if received → within 12 hours of the
poisoning, but muscle power may improve → within 30
minutes.

22
Clinical indications of the cholinergic agents:
1. Reduce IOP
→ in patients with glaucoma .
→ during ocular surgery.
2. Treatment of atony:
of → GIT and bladder.
3. Diagnose and treatment
→ myasthenia gravis (Indirect acting
cholinergic drug → Reversible → Endro-phonium).
4. Minor uses
→ antidotes to n-m blocking agents.
→ tricyclic antidepressant.
→ belladona alkaloid.
*Tacrine:
newest anti-cholin-esterase, used for → treatment of mild-
moderate dementia associated with Alzheimer's disease.
Adverse reactions of the cholinergic agents:
1. On eye:
blurred vision, ↓ accommodation, meiosis.
2. On skin:
↑ sweating.
3. On GIT:
↑ salivation, belching, nausea, vomiting, intestinal cramps
(abdominal colic), diarrhea.
4. On CVS:
vasodilatation, ↓ heart rate (-ve chrono-tropic), ↓ cardiac
contraction (-ve ino-tropic), this result in → hypo-tension (↓ BP).
5. At motor end plate:
hyper-polarization of skeletal muscle, which will
→ reduce contraction.

23
Anti-cholinergic Agents, Cholinergic Antagonists,
Cholinergic Blockers, Parasympatholyc Drugs
*Definition:
bind to → cholinoceptors, but don't evoke the usual
receptor mediated intracellular effects.
Are beneficial in a → variety of clinical situations.
Classification of anticholinergic drugs:
1. Anti-muscarinic drugs (Muscarinic Antagonists):
I. Tertiary amine:
alkaloid esters of tropic acid.
1. Atropine (hyoscyamine): found → plant (Atropa Belladonna).
2. Hemo-Atropine: semi-synthetic.
3. Scopol-amine.
*Mechanism of action of Atropine:
Has → high affinity for muscarinic receptors.
It binds → competitively and reversibly, preventing Ach
from binding to that site.
It's stimulating / depressing, depend on → target organs,
dose and disease.
It's a central and peripheral muscarinic blocker.
In general actions, last about → 4 hours, except when
placed topically in → the eye, last for → days.
*Actions of Atropine:
1. CNS:
Normal dose has → minimal effect and slow sedation.
Oppose cholinergic effect at → post-ganglionic
cholinergic N-endings and on peripheral blood vessels.
Doesn't oppose cholinergic effect at → N-m junction /
autonomic ganglia.
a. Mydriasis (dilated pupil) by → blocking mitral
regurgitation in → papillary m.
b. Cycloplegia by → weakening the contraction of
ciliary m. and loss of accommodation for near vision.
c. Reduction of lachrymal secretion (dry/sandy eye).
d. ↑IOP: due to → dilated iris blocking drainage of IO
fluid from angle of anterior chamber.

24
2. CVS: depends on the → dose:
Low doses
High doses
↓ cardiac rate (bradycardia), due
to → central vagal nerve activation.
↑ cardiac rate (tachycardia).
Cardiac receptors on SA-node are blocked
Atropine → has no significant effect on peripheral
blood vessel in therapeutic dose, but with poisoning →
vasodilatation.
3. Respiratory → Bronchial dilatation, ↓ secretion.
4. GIT:
a. Anti-spasmodic → reduce the activity of GIT.
b. Block salivary gland → xerostomia.
5. GUT → smooth muscle of ureter.
→ bladder walls are relaxed.
→ urination is slowed.
6. Exocrine gland secretions: all glands ↓ (except milk),
causing → dry mouth, eyes and skin (inhibit sweating).
→ ↓ bronchial secretion, and become → viscid.
*Poisoning with Atropine:
1. Dry mouth with dysphasia.
2. Mydriasis (dilated pupil) and blurred vision.
3. Hot flushes.
4. Dry skin with hyper-thermia.
5. Restlessness.
6. Anxiety.
7. Excitement hallucinations.
8. Delirium.
9. Mania.
10. Cerebral excitation followed by → depression and coma.
*Treatment:
1. Activated charcoal → to absorb the drug.
2. Diazepam → excitement.

25
II. Quaternary amine:
semi-synthetic and synthetic. They have
been develop to produce → more peripheral effects.
→ ↓ CNS effects.
1.Clidinium bromide
2.Propan-theline (Duspataline)
3.Ipra-tropium
Uses:
Treatment of → gastric
disorders.
Uses:
Relief symptoms in patients
with:
1. Irritable bowel syndrome.
2. Pancreatitis.
3. Gastritis.
4. Diverticulitis.
5. Colitis.
Anti-spasmodic for → ureter
and urinary bladder.
Uses:
Treatment of → asthma
and COAD in patients
unable to take
adrenergic drugs.
Derived from → Belladonna
Alkaloid.
Sometimes, is combined
with → Chlor-dia-zepoxide
(librax).
*Clinical uses of anti-muscarinic drugs:
1. On CNS:
a. Benz-hexol,
Orphe-radrine
b. Hyos-cine ,
Pro-methazine
c. Hyos-cine
Against rigidity.
Tremor in
parkinsonism.
Anti-emetic.
Prevent / reduce
motion sickness.
2. Ophthalmologic disorders: in cases that need → mydriasis with
cycloplagia / prolonged action.
*Tropic-amide → short-acting mydriatic drug.

26
3. Respiratory disorder:
a. Pre-operative
b. Ipra-tropium
c. Tio-tropium
↓ bronchial
secretion and spasm
by → pre-anesthetic
injection of:
1. Atropine.
2. Scopolamine
Treatment of →
asthma and COAD in
patients unable to
take adrenergic
drugs.
Long acting
quaternary aerosol,
by → inhalation.
4. CVS disorders: Atropine used → parentrally in:
a. Myocardial infarction (MI): because → it block reflex vagal
stimulation.
b. Treat → sinus bradycardia, when arrhythmias result from →
anesthetic, cholinesters, succinylcholine.
5. GIT disturbances:
Pro-pantheline
Hyosine-butyl
bromide
(buscopan)
Piren-zepine
Telen-zepine
Pre-anesthesia
Hyos-cine,
Pro-methazine
Anti-spasmodic:
for treatment →
spastic condition
of GIT, because it
lead to →
relaxation of
smooth m.
Relaxant for
smooth m.
Peptic ulcer.
Reduce
salivation.
Reduce gastric
secretion
Antiemetic.
6. Urinary disorders:
a. Fla-voxate
b. Pro-pantheline
c. Oxy-butynin
All are used to → relieve m.
spasm in cystitis and detrusor
instability.

27
7. Atropine used as antidotes to cholinergic and anti-cholin-esterase
agent: used to → treat poisoning from organophosphates.
→ block muscarinic effects, due → cholinergic drugs
(neostigmine).
28
*Adverse effect of anti-muscarinic drugs:
In infants: ordinary dose could result in → anti-muscarinic fever.
In adults: depend on the dose:
Small dose
Medium dose
Large dose
Over dose
↓ salivation.
Pupil dilatation.
Inhibition urination. (S,M,L, dose)
↓ bronchial
secretion.
Visual
accommodation ↓.
Inhibition intestinal
motility.
CNS excitation.
↓ sweating.
Heart rate ↑.
↓ in gastric section. Restlessness.
-
-
↓ motility.
Irritability.
-
-
-
Hallucinations.
*Contraindications of anti-muscarinic drugs:
1. Glaucoma.
2. Elderly men: should be → used in caution, and avoided → in
patients which have history of prostatic hyperplasia.
3. Atropine slow gastric empty: ↑ symptoms in patient with peptic
ulcer.
*Therapeutic uses of anti-muscarinic drugs in dentistry:
↓ the flow of saliva during dental procedures.
Small doses given → orally / parentrally, may produce side effects.
Diminish salivary flow in heavy metal poisoning.
Atropine is often used, because → it's well absorbed from GIT.
Atropine and Glyco-pyrrolate are used in → oral surgery as → intra-
operative anti-sialogogues (substances ↓ the production of saliva).
They are given → I.V.
Glyco-pyrrolate is a → quaternary amine, so has → fewer CNS effect
than Belladonna alkaloids.
Compared with Atropine, Glyco-pyrrolate is:
1. More selective anti-sialogogues.
2. Less likely to promote → tachycardia in conventional doses.
During → general anesthesia:
1. ↓ secretions in the respiratory tract.
2. Reduce the probability of laryngo-spasm.
3. Help in prevent reflex vagal slowing of the heart.
29
2. Anti-nicotinic drugs (Nicotinic Antagonists):
I. Ganglion blocking agents:
Hexa-methionin
Meca-mylamine
Trime-thaphan
(the only ganglion-blocker still clinical use)
Used in → treatment of hypertension.
Used IV to → treat severe accelerate
hypertension (malignant hypertension).
Adverse effect of ganglion blocking agents
are so → severe: patients are unable to
tolerate long-term treatment with them,
because → sympathetic and
parasympathetic are blocked.
Ganglion blocking agents interrupts
sympathetic control of venous-pooling →
postural hypotension is result.
-
Poorly lipid soluble.
Inactive orally.
Short half-life.
II. Neuromuscular (N-M) blocking drugs:
Are important for → producing complete skeletal m.
relaxation in → surgery, by → specific blockade of the N-M
junction.
Enable → light level of anesthesia to be employed with →
adequate relaxation of the muscles of the → abdomen and
diaphragm.
Relax → vocal cords, and allow the → passage of tracheal
tube.
*Patients who have received → m. relaxant, should always
have their → respiration controlled until the drug have been
→ inactivated / antagonized.

30
a. Non-depolarizing Competitive N-M blocking agents:
*Action:
Cause N-M block by → competing with Ach at the
receptor site at the → N-M junction, so it:
1. Prevent depolarization of muscle cell
membrane.
2. Inhibit muscle contraction.
Causing → complete skeletal m. relaxation
pK = 20 minutes to several hours.
Give → parentrally (by injection).
*Tubocuranine:
Produce → competitive block at → end plate, causing
→ flaccid paralysis lasts 30-60 minutes.
Blocks → autonomic ganglia, causing:
1. Initial transient drop in blood pressure.
2. Histamine release, which may → induce
broncho-spasm.
*Action of competitive N-M blocking agents is →
antagonized by:
1. Anti-cholin-esterase.
2. Neostigmine (give IV), preceded by → atropine:
to prevent the parasympathetic autonomic effect
of Neostigmine (bradycardia and salivation).
31
b. Depolarizing Non-competitive N-M blocking agents:
*Action:
Attached to → nicotinic receptors, act like → Ach:
depolarize the N-M junction.
Unlike Ach, which is instantly destroyed by →
Achesterase, the depolarizing agent persists at → high
conc. in synaptic cleft:
1. Remaining attached to the receptor for long time.
2. Providing a constant stimulation of the receptor.
Initially → produces short-lasting muscle fasciculation,
followed by → paralysis.
Drug → doesn't produce ganglionic block (except in
high doses).
→ have weak histamine-releasing factor.
The duration of action of Succinylcholine is → short.
Useful used in → brief procedures:
1. Tracheal intubations.
2.Electroconvulsive therapy (ECT)
*PK of Succinyl-choline (Suxamethonium):
Injected IV.
It's action cannot be → reversed (unlike non-
depolarizing).
Short duration of action.
Hydrolyzed by → plasma pseudo-cholinesterase.
It's persistence in the body ↑ by:
1. Neostigmine → inactivate the enzyme.
2. Patients with → hepatic disease and severe
malnutrition → plasma conc. of enzyme is lower than
the normal.
3. Procaine and amethocaine are → destroyed by this
enzyme
4. Persons with → hereditary defect in amount/kind of
enzyme, who → cannot destroy the drug → paralysis.
*Treatment → ventilation until recovery.
→ fresh blood transfusion.
32
*Adverse effects of Succinyl-choline (Suxamethonium):
1. Hyperthermia: when Halothane is used as →
anesthetic, the administration of Succinylcholine cause
→ malignant hyperthermia (muscular rigidity and
hyperpyrexia) in genetically susceptible people.
*Treatment → rapidly cooling the paitent.
→ administration of Dantrolene.
2. Apnea → genetically related deficiency of plasma
cholinesterase OR presence of an atypical form of the
enzyme. Can lead to apnea due to → paralysis of the
diaphragm.
3. Cardiovascular:
1. Repeated injections of Succinylcholine can cause →
bradycardia and cardiac arrest, due to → activation
of cholinoceptors in the → heart. This can be
prevented by → Atropine.
2. Release of K from muscle, which can be enough to
cause → cardiac arrest in patients with already
hyper-thermia.
4. Muscle pain: lasting 1-3 days due to → muscle
fasciculation preceded the → paralysis by
Suxamethonium.
*Treatment → preceding Succinylcholine with a small
dose of a competitive N-M blocking agent →
Tubocuranine.
5. High dose: stimulate → uterus, and cause →
premature labor in pregnant.
*Contraindication of Succinyl-choline (Suxamethonium):
1. Hyper-sensitivity to Succinylcholine.
2. Severe liver disease.
3. Burned patient.
4. Pregnancy.

33
*Uses of Neuromuscular (N-M) blocking drugs:
1. Main use→ producing complete skeletal muscle relaxation
in surgery.
2. Other uses:
Control ventilation: in respiratory failure, due to →
obstructive airway disease.
Treatment of convulsions: by → ↓ peripheral
manifestation of convulsion (as these drug not cross
→ BBB, has → no effect on central processes)
*Applications in dentistry of Neuromuscular (N-M) blocking
drugs:
Dentist has → few indications for the use of N-M blocking
agents.
Used in:
1. Mandible fractures, when muscle relaxation is needed
to permit manipulation of bone fragments.
2. Trismus, when no more conservative means exist to
permit → mouth opening for diagnosis and treatment.
Succinylcholine OR relatively short-acting non-depolarizing
blocking drug is used to → aid the insertion of an
endotracheal tube, when the use of general anesthesia
makes intubation appropriate.
Where general anesthesia is used, Succinylcholine should
always be available to treat → intractable laryngo-spasm.
34
Pharmacology (Lec.3)
Dr. Nahla
Adrenergic Drugs
Introduction:
CNS receives diverse → internal and external stimuli.
These stimuli are → integrated and expressed subconsciously
through → ANS, to → modulate the involuntary functions of the
body.
ANS consist of two large divisions:
1. Sympathetic outflow → thoraco-lumbar.
2. Parasympathetic outflow → cranio-sacral.
Which are defined by their → anatomic origin.
Biochemistry:
1. Catechol-amines
2. Non-catechol-amines
Sympathomimetic amines that
contain → 3,4-dihydroxybenzene
group.
Compound lack → catechol
hydroxyl group.
High potency: directly activating α
or β receptors.
-
Rapid inactivation:
Parentrally → brief period of
action.
Orally → ineffective, due to
inactivation.
Longer half-lives, because they
aren't inactivated by → COMT.
Poor penetration into the CNS,
because are → polar.
Have clinical effects → anxiety,
tremor, headaches... That are
attributable to action of the CNS.
↑ lipid solubility, due to → lack of
polar hydroxyl groups, which
permits → greater access to the
CNS.
Epinephrine
Nor-epinephrine
Iso-proterenol
Dop-amine
Phenyl-ephrine
Ephidrine
Amphet-amine
*COMT: Catechol-O-Methyl Transferase.
Inas Waleed
3rd Stage of Dentistry

35
1. Adrenergic Agonists
Direct
Indirect
Mixed
Epi-nephrine
Amphet-amine
Ephidrine
Nor-epi-nephrine
Tyr-amine
Pseudo-ephedrine
Iso-proterenol
Cocaine
Metar-aminol
Dop-amine
Dobut-amine
Oxy-metazoline
Phenyl-ephrine
Methox-amine
Clo-nidine
Meta-proterenol
Al-buterol, pir-buterol
and ter-buterol
Sal-meterol and
for-meterol
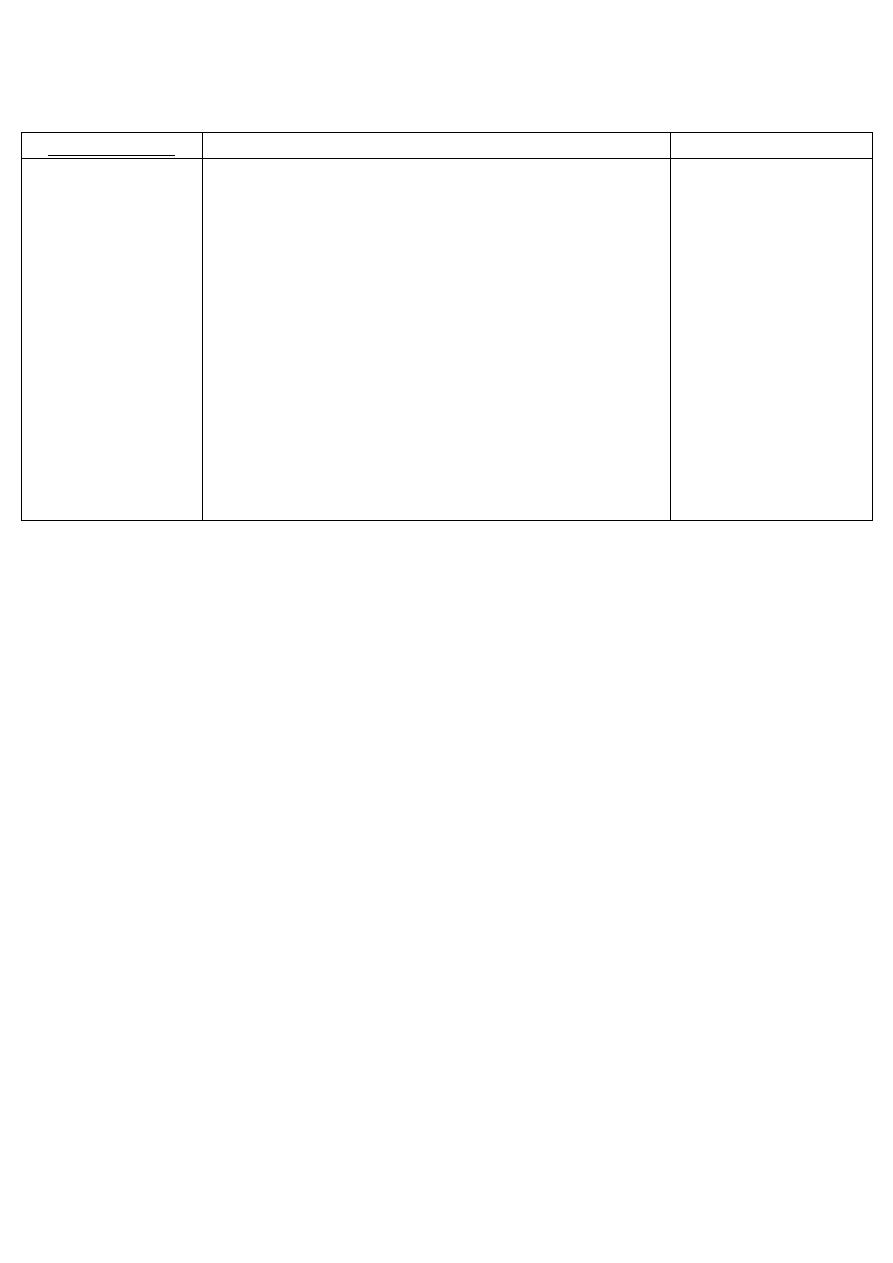
Mechanism of action of adrenergic agonists:
1. Direct-acting:
2. Indirect-acting:
3. Mixed-acting:
Act directly on α or β
adrenergic receptors,
producing → effects
similar to those that
occur following:
1. Stimulation of
sympathetic nerves
2. Release of the
hormone
epinephrine from
the adrenal medulla.
May block the uptake
of nor-epinephrine or
are taken up into the
pre-synaptic neuron,
and cause → the
release of nor-
epinephrine from the
adrenergic neuron.
As in neuronal
stimulation: the nor-
epinephrine traverses
the synapse and binds
to the α or β receptors.
Act directly on α or β
receptors (adrenergic
receptors) and release
of nor-epinephrine
from the adrenergic
neuron.
36
1. Direct-acting:
1. Epinephrine
Therapeutic uses
Adverse effects
One or four
catechol-amines.
Stimulate α and β
adrenergic
receptors.
Low dose: β
effects →
vasodilatation.
High dose: α
effects →
vasoconstriction.
1. Treatment of → asthma and anaphylactic shock,
few minutes after S.C. administration.
2. Glaucoma:
2% topically, to ↓ IOP in open-angle glaucoma.
↓ the production of aqueous humor by →
vasoconstriction of the ciliary body b.v.
3. Anaphylactic shock: treatment of → type I hyper-
sensitivity reactions in response to → allergens.
4.Cardiac arrest: restore cardiac rhythm in patients
with cardiac arrest.
5. Anesthetics: L.A. sol.
6. Very weak solutions: can be used → topically to
vasoconstrict m.m. to control oozing of capillary
blood.
CNS disturbances:
anxiety, fear,
tension, headache,
tremor.
Hemorrhage
(cerebral): due to
marked→
elevation of B.P.
Cardiac
arrhythmias: if the
patient is receiving
digitalis.
Pulmonary edema.

37
Indications (Epinephrine):
1. Hyperthyroidism:
Epinephrine have enhanced cardio-vascular actions in patients
with → hyperthyroidism.
The dose must be → reduced.
Mechanism: ↑ the production of → adrenergic receptors on the
vasculature of the hyperthyroid individual, leading to →
hypersensitive response.
2. Cocaine:
Epinephrine produces → exaggerated cardio-vascular actions,
due to → the ability of cocaine to prevent reuptake of catechol-
amines into the → adrenergic neuron.
It remains at the → receptor site for longer periods of time.
3. Diabetes:
Epinephrine ↑ the release of → endogenous store of glucose.
In diabetic, insulin dosage may have to be ↑.
4. β-blockers:
They prevent → Epinephrine's effect on β-receptors, leaving α-
receptor stimulation un-opposed.
This lead to → an ↑ in peripheral resistance and an ↑ in B.P.
5. Inhalation anesthetics:
Inhalational anesthetics → sensitize the heart to the effects of
Epinephrine, which may led to → tachycardia.

38
3. Iso-proterenol
Therapeutic uses
Adverse effects
Stimulate → α and β adrenergic
receptors.
Non-selectively, so its → rarely
used.
It's action on α-receptor is →
insignificant.
Rarely used as → broncho-dilator
in asthma.
Can be employed to → stimulate
the heart in emergency
situations.
Similar to Epin-
ephrine.
4. Dop-amine
Therapeutic uses
Adverse effects
Immediate metabolic
precursor of → Nor-epin-
ephrine.
Occurs → naturally in the
CNS in the basal ganglia,
where it act as a →
neurotransmitter, like in
the adrenal medulla.
Stimulate → α and β
adrenergic receptors.
Low dose: activating β
1
cardiac receptors.
High dose: activating α
1
receptor →
vasoconstriction.
Drug of choice for → shock, is
given by → continuous infusion.
↑ B.P. by:
1. Stimulation β-receptors on the
heart to ↑ COP
2. Stimulation α-receptors on
blood vessels to ↑ peripheral
resistance.
Enhance perfusion to the kidney
and splanchnic areas.
An ↑ blood flow to the kidney
enhances → the GFR and causes
→ sodium dieresis.
An overdose produce →
same effect as
sympathetic
stimulation.
Rapidly metabolized to
→ Homovanillic acid by
MAO or COMT.
It's adverse effects are
→ short-lived (nausea,
hyper-tension,
arrhythmia).
*MAO: Mono-Amine Oxidase.
2. Nor-epinephrine (NE)
Therapeutic uses
Adverse effects
Is a neuromediator of
adrenergic nerves, so
when the drug is given in
therapeutic doses to
humans →the α
adrenergic receptors is
most affected.
Shock: it ↑ the vascular resistance, so it
↑ B.P. (Metar-aminol is favored, because
it doesn't ↓the blood flow to the kidney,
as Nor-epinephrine).
Other actions: are not clinically significant.
Potent vasoconstrictor: will cause →
extravasations (discharge of blood from
vessel into tissues) along the → injection
site.
Similar to →
Epinephrine.
May cause →
blanching and
sloughing of skin
along injected
vein, due to →
extreme
vasoconstriction.

39
5. Dobut-amine
Therapeutic uses
Adverse effects
Is a β
1
-receptor
agonist.
↑ cardiac rate and
output, with few
vascular effects.
↑ COP in → congestive heart
failure.
Inotropic support after cardiac
surgery.
↑ COP with little change in heart
rate, doesn't significantly elevate
O
2
demands of the myocardium
(major advantage).
Should be used with caution in
→ atrial fibrillation, because it ↑
atrio-ventricular conduction.
Other effects are → similar to
epinephrine,
Tolerance may → developed on
prolonged use.
6. Oxy-metazoline
Stimulate → α
1
and α
2
adrenergic receptors.
Used locally in → the eye or the nose as a → vasoconstrictor.
Is found as
1. Short-term nasal spray.
2. Ophthalmic drops for the relief of redness of the eye and in swimming, cold or contact
lenses.
Mechanism of action: direct stimulation of→ α-receptors on blood vessels, supplying →
the nasal mucosa and the conjunctiva to: ↓ blood flow and ↓ congestion.
Is absorbed in → the systemic circulation (regardless of the route of administration), may
produce → nervousness, headaches and trouble sleeping.
When administrated in the nose
1. Burning of the nasal mucosa.
2. Sneezing may occur.
Rebound congestion is observed with → long-term used.
7. Phenyl-ephrine
Binds primarily to → α-receptors, and favors α
1
over α
2
receptors.
Non-catechol derivative, therefore is not a substrate for → COMT.
Used topically in → the nasal m.m and in ophthalmic solutions for mydriasis.
Act as a → nasal decongestant, and produces → prolonged vasoconstriction.
Is used to:
1. ↑ B.P.
2. Terminate episodes of supra-ventricular tachycardia (rapid heart action arising from
both: atrio-ventricular and atria).
Large doses can cause: 1. Hypertensive headache.
2. Cardiac irregularities.
40
8. Methox-amine
Adverse effects
Binds primarily to → α-receptors, and favors α
1
over α
2
-receptors.
Used clinically to:
1. Relieve attacks of paroxysmal supra-ventricular tachycardia.
2. Overcome hypotension during surgery, involving halothane
anesthetics.
1. Hypertensive
headache.
2. Vomiting.
9.Clonidine
Is an α
2
agonist.
Used in → essential hypertension to ↓ B.P, because of it's action in the CNS.
Acts → centrally to produce inhibition of sympathetic vasomotor centers, ↓ sympathetic
outflow to the periphery.
10. Meta-proterenol
Is not a catecholamine, it's resistant to methylation by → COMT.
It can be administrated → orally or by inhalation.
Acts primarily at β
2
-receptors, producing little effect on the heart.
Produces dilatation of → the bronchioles, and improve → airway function.
Is useful as → broncho-dilator, in the treatment of → asthma.
→ reverse broncho-spasm.
11. Al-buterol, Pir-buterol and Ter-buterol
Short-acting → β
2
agonists.
Used as → broncho-dilators.
Administrated → metered-dose inhaler.
Compared with → non-selective β-adrenergic agonists (meta-proterenol): these drugs can
produce equivalent broncho-dilation with less cardiac stimulation.
12. Sal-meterol and For-moterol
β
2
-adrenergic selective.
Long-acting broncho-dilators.
Single dose by → metered-dose inhaler device (dry powder inhaler), provides → sustained
broncho-dilation over 12 h, compared with less than 3 h for albuterol.
Unlike formoterol, salmeterol has → somewhat delayed onset of action.
Are not recommended as → mono-therapy.
Highly efficacious, when combined with a → corticosteroid.
Agents of choice for treating → nocturnal asthma in symptomatic patients taking other
asthma medications.
2. Indirect-acting:

41
1. Amphet-amine
Cause → nor-epinephrine (NE) release only.
CNS stimulant effects: have lead to their use for → treating hyperactivity in children,
narcolepsy and appetite control.
↑ B.P by → α effect on vasculature.
→ β effect on heat.
It's use in pregnancy should be → avoided, because has adverse effects on the development
of the fetus.
2. Tyr-amine
Can enter the nerve terminal.
Cause → NE release only.
Clinically → not useful.
It's important → because it's found in fermented food (rip cheese and Chianti wine).
Normally, it's oxidized by → MAO in GIT.
If patient is taking → MAO inhibitors, it can precipitate serous vasopressor episodes.
3. Cocaine
Local anesthetic having the ability to → block Na
+
/ K
+
- activated ATPase, required for →
cellular uptake of NE on the cell membrane of the adrenergic neuron.
Cause → NE accumulates in the synaptic space.
Magnified effects of NE and epinephrine (E).
↑ duration of action of NE and E.
Can ↑ B.P → α agonist actions, and β stimulatory effects.
CNS stimulant.
Drug of abuse (cause addiction).

42
3. Mixed-acting:
1. Ephedrine
Causes → NE release and stimulates receptor.
α and β stimulant.
Use → asthma as nasal sprays.
Slower action.
Is eliminated largely unchanged in the urine.
↑ systolic and diastolic B.P. by → vasoconstriction and cardiac stimulation.
Produces → broncho-dilation.
Enhanced → contractility, and improves → motor function in myasthenia gravis (MG), when
used in conjunction with → anti-cholin-esterase.
Produce → mild stimulation of CNS, this ↑ alertness, ↓ fatigue and prevents sleep.
Improve → athletic performance.
Used to → treat chronic asthma (rather than acute asthma, to prevent attacks), as a nasal
decongestant, due to its' → local vasoconstrictor action.
2. Pseudo-ephedrine
Used to → treat nasal and sinus congestion or congestion of the Eustachian tubes.
Clinical use: is declining, due to → the availability of better and more potent agents that
cause fewer adverse effects. It containing → herbal supplements (ephedra) were prohibited
by → the US food and drug administration, because of → life-threatening cardiovascular
reactions.
Has been illegally converted to → metha-amphetamine.
Thus, products containing pseudo-ephedrine have certain restrictions and should be kept
behind the sales counter.
Fewer CNS effects.
Undergoes → incomplete hepatic metabolism before elimination in the urine.
*Ephedrine and Pseudo-ephedrine
Plant alkaloids (made synthetically).
Non-catechols, are poor substrates for → COMT and MAO.
Long duration of action.
Excellent absorption orally.
Penetrate into → CNS.

43
2.Adrenergic Antagonists (Blockers / Sympatholytic)
These drugs are → reversible and irreversible.
1. α-Adrenergic Blocking Agents
Blockade of these receptors ↓ the
sympathetic tone of the blood vessels,
resulting in ↓ peripheral vascular
resistance, lowering B.P.
This induces a → reflex tachycardia
resulting from the lowered B.P.
2. β-Adrenergic Blocking Agents
All the clinically available are →
competitive antagonists.
Non-selective act at both: β
1
and β
2
receptors.
Cardiovascular-selective act at: β
1
.
↓ B.P in hyper-tension, they don't
induce postural hypo-tension, because
→ α-receptors remain functional.
All end in -olol, except: Labetalol and
Carvedilol.
Phenoxy-benzamina
Propranolol
Phen-tolamine
Timolol and Nadolol
Prazo-sin, Terazo-sin, Doxazo-sin, Alfuzo-
sin and Tamsulo-sin
Atenolol, Metoprolol, Bisoprolol,
Betaxolol, Nebivolol and Acebutolol
Yohimbine
Esmolol
-
Pindolol and Acebutolol
-
Labetalol and Carvedilol
44
1. α-Adrenergic Blocking Agents:
1. Phenoxy-
benzamine
Actions
(cardio-vascular)
Therapeutic uses
Adverse effects
Non-selective.
Linking covalently
to → α
1
post-
synaptic and α
2
pre-synaptic
receptors.
Irreversible and
non-competitive.
Last about 24h
after a single
administration.
After the drug is
injected, a delay of
a few hours occurs
before a blockade
develops, because
→ the molecule
must undergo bio-
transformation to
the active form.
By blocking α-receptors →
prevents vasoconstriction of
peripheral blood vessels by →
endogenous catecholamines.
The ↓ peripheral resistance
provokes → reflex tachycardia.
Block α
2
pre-synaptic inhibitory
receptors in the heart can → ↑
COP. These receptors when
blocked will → release more NE,
which → stimulates β-receptors on
the heart to ↑ COP.
Epinephrine reversal: all α-
blockers reverse the α-agonist
actions of E (the vaso-constrictive
action of E is interrupted, but
vaso-dilation of other vascular
beds caused by stimulation of β
2
-
receptors is not blocked).
Therefore, the systemic B.P ↓ in
response to E given in the
presence of phenoxy-benzamine.
Treatment of →
pheo-chromo-
cytoma (prior to
surgical removal
of the tumors)
Useful in the
chronic
management of
these tumors
(inoperable)
Treating
Reynaud's
disease.
Autonomic
hyper-reflexia,
which →
predisposes
paraplegics to
strokes.
Postural
hypotension.
Nasal stuffiness.
Nausea and
vomiting.
Reflex
tachycardia:
mediated by →
the
baroreceptor
reflex, and is
contraindicated
in patients with
↓ coronary
perfusion.
2. Phen-tolamine
Competitive block of → α
1
and α
2
receptors.
Last about 4h after a single administration.
Produces → postural hypo-tension, and causes → epinephrine reversal.
Induced → reflex cardiac stimulation and tachycardia which are mediated by:
1. The baroreceptor reflex.
2. Blocking the α
2
-receptors of the cardiac sympathetic nerves.
Can trigger → arrhythmias and anginal pain, it's contraindicated in patients with ↓
coronary perfusion.
Is used for → short-term management of pheo-chromo-cytoma.
45
3. Prazo-sin, Terazo-
sin, Doxazo-sin,
Alfuzo-sin and
Tamsulo-sin
Cardiovascular
effects
Therapeutic uses
Adverse
effects
Prazo-sin, Terazo-
sin, Doxazo-sin are
useful in → the
treatment of
hypertension.
Alfuzo-sin and
Tamsulo-sin are
useful in → the
treatment of
benign prostatic
hypertrophy (BPH).
Metabolism → lead
to inactive
products that are
excreted in the
urine, except for
those of doxazo-
sin, which appear
in feces.
Doxazo-sin is the
longest acting.
All ↓ peripheral
vascular resistance
and ↓ arterial B.P
by causing the
relaxation of →
arterial and venous
smooth m.
Tamsulo-sin has
the least effect of
B.P.
Cause minimal
changes in COP,
renal blood flow
and GFR (unlike
phenoxy-
benzamine and
phen-tolamine).
Individuals with ↑ B.P who have
been treated with one of those
drugs → don't become tolerant to
its action.
1st dose effect of these drug
produces → exaggerated
orthostatic hypertensive response
that can result in syncope
(fainting). Can be minimized by →
adjusting the 1st dose to 1/3rd or
1/4th of the normal dose and
giving the drug at bedtime.
Mono-therapy in → hypertension.
Alternative to surgery, in patients
with symptomatic BPH.
Tamsulo-sin is → more potent
inhibitor of α
1A
-receptor found on
the smooth m. of the prostate (this
cause tamsulosin's minimal effect
on B.P).
Dizziness.
Lack of
energy.
Nasal
congestion.
Headache.
Drowsiness.
Orthostatic
hypotension
(lesser
degree).
4. Yohimbine
Selective α
2
competitive blocker.
Found as a component of → the bark of the Yohimbine tree.
Works at → the level of CNS, to ↑ sympathetic outflow to the periphery.
Directly block α
2
-receptors, and has been used to → relieve vasoconstriction
associated with Reynaud's disease.
Is contraindicated in → CNS and cardiovascular conditions, because → it's a CNS and
cardiovascular stimulant.
46
2. β-Adrenergic Blocking Agents:
1.Propranolol
Therapeutic uses
Adverse effects
Drug interactions
Non-selective.
Is the prototype
β-adrenergic
antagonist.
Blocks both β
1
and β
2
receptors.
Sustained-
release
preparations for
once-a-day
dosing are
available.
1. Hypertension:
↓ COP.
Inhibition of rennin release from the kidney.
↓ sympathetic outflow from the CNS.
2. Glaucoma:
Topically applied timolol, occurs by ↓ the secretion of aqueous
humor by the ciliary body.
Is the drug of choice in an acute attack of glaucoma.
β-blockers are only used to treat this disease chronically.
3. Migraine (prophylactically): the mechanism depends on the
blockade of catecholamine-induced vasodilation in the brain
vasculature.
4. Hyperthyroidism:
Are effective in blunting the widespread sympathetic
stimulation.
In acute thyroid storm: β-blockers may be lifesaving in
protecting against serious cardiac arrhythmias.
Broncho-constriction: must
never used in treating any
individual with COPD or
asthma.
Arrhythmias: must be
trapped off gradually for 1
week.
Long-term treatment with
β-antagonist leads to up-
regulation of the β-
receptor.
Disturbances in
metabolism: fasting
hypoglycemia may occur.
*Cardio-selective β-blockers
are preferred in treating
asthmatic patients who use
insulin.
Drugs that
interfere with the
it's metabolism
(cimetidine,
fluoxetine,
paroxetine and
ritonavir) can →
potentiate it's
anti-hypertensive
effects.
Conversely, drugs
that stimulate it's
metabolism
(barbiturates,
phenytoin and
rifampin) can ↓ its
effects.
47
1.Propranolol
Therapeutic uses
Adverse effects
Drug interactions
-
5. Angina pectoris:
Useful in chronic treatment, but not for acute.
↓ O
2
requirement of heart m., therefore, is effective in ↓ the chest
pain on exertion that's common in angina.
Tolerance to moderate exercise is ↑.
6. Myocardial infarction:
Patients who had one myocardial infarction appear to be protected
against a 2nd heart attack by prophylactic use of β-blockers.
Mechanism: blocking of the actions of circulating catecholamine, which
would ↑ the O
2
demand in an already ischemic heart muscle.
↓ the incidence of sudden arrhythmic death after myocardial
infarction.
-
-
*COPD: Chronic Obstructive Pulmonary Disease.

48
2. Timolol and Nadolol (NAH-doh-lole)
Blocks both β
1
and β
2
receptors.
More potent than propranolol.
Nadolol: has very long duration of action.
Timolol: ↓ the production of aqueous humor in the eye.
It's used → topically in the:
1. Treatment of chronic open-angle glaucoma
2. Systemic treatment of hypertension.
3. Atenolol, Metoprolol, Bisoprolol,
Betaxolol, Nebivolol and Acebutolol
Actions
Preferentially block β
1
-receptor at doses
50-100 fold less than those required for
block β
2
-receptor.
Cardio-selective blockers (acebutolol,
atenolol and metoprolol).
Cardio-selectivity is → most pronounced
at low doses, and is → lost at high doses.
*Has some intrinsic agonist activity.
In hypertension: cardio-selective β-
blockers are useful in → hypertensive
patients with impaired pulmonary
function.
Cardio-selective β-blockers are useful in
→ diabetic hypertensive patients who
are receiving insulin or oral hypoglycemic
agents.
↑ exercise tolerance in angina.
4. Esmolol
Side effect
Very short life time, due to →
metabolism of an ester linkage.
It's only given → I.V. if required during
surgery or diagnostic procedures
(cystoscopy).
Have relatively little effect on:
1. Pulmonary function.
2. Peripheral resistance.
3. Carbohydrate metabolism.
Coldness of the extremities, because
have less effect on peripheral resistance.
Less frequent → side effects.

49
5. Pindolol and Acetubolol
Cardio-vascular actions
Therapeutic use
Are not pure antagonists (partial agonist): stimulate
β-receptor to which they are bound, but inhibit
stimulation by → the more potent endogenous
catecholamines, E and NE.
These opposing ↓ effects on cardiac rate an COP
compared to that of β-blockers without ISA.
↓ metabolic effects: blockers with ISA minimize the
disturbance of → lipid and carbohydrate
metabolism that are seen with other β-blockers.
In hypertensive patients with
moderate bradycardia,
because → further ↓ in heart
rate is less pronounced with
these drugs.
Carbohydrate metabolism is
less affected making them
valuable in the treatment of
diabetics.
*ISA: Intrinsic Sympatho-mimetic Activity.
6. Labetalol and Carvedilol
Therapeutic use
(In hypertension)
Adverse effect
Blocks α and β receptors.
Reversible β-blockers with concurrent
α
1
blocking actions that produce →
peripheral vasodilatation, then ↓ B.P.
Contrast with the other β-blockers
that produce → peripheral
vasoconstriction, and they are
therefore useful → in treating
hypertensive patients for whom ↑
peripheral vascular resistance is
undesirable.
Don't alter → serum lipid or blood
glucose levels.
Carvedilol: ↓ lipid peroxidation and
vascular wall thickening, effects are →
benefit in heart failure
Labetalol: useful for →
treating the elderly or
black hypertensive
patient in whom ↑
peripheral vascular
resistance is undesirable.
Labetalol: can be
employed as →
alternative to methyldopa
in the treatment of →
pregnancy-induced
hypertension.
I.V. labetalol: used to
treat → hypertensive
emergencies, because it
can rapidly ↓ B.P.
Orthostatic
hypotension.
Dizziness.
*(with α
1
-
blockade)
50
Drugs Affecting Neurotransmitter Release Or Uptake
*Some agents act on the adrenergic neuron:
1. To interfere with neurotransmitter release.
OR
2. To alter the uptake of the neurotransmitter into the adrenergic nerve.
1. Reserpine
2. Guanethidine
3. Cocaine
Is a plant alkaloid.
Blocks the Mg
+2
/ATP
dependent transport of
biogenic amines: NE,
dopamine and serotonin from
the cytoplasm into → storage
vesicles in the adrenergic
nerves of all body tissues.
This causes → the ultimate
depletion of biogenic amines.
Sympathetic function, in
general, is impaired because of
↓ the release of NE.
Has slow onset, long duration.
Effects persist for many days
after discontinuation.
Blocks the release of → stored
NE and displaces NE from
storage vesicles (producing a
transient ↑ in B.P).
This leads to → gradual
depletion of NE in nerve
endings, except for those in
the CNS.
Cause
1. Orthostatic hypotension.
2. Interferes with male sexual
function.
Super-sensitivity to NE, due to
→ depletion of amine, which
can result in hypersensitivity
crisis in patients with pheo-
chromo-cytoma.
Although
cocaine inhibits
NE uptake, it's
an adrenergic
agonist.
51
Pharmacology (Lec.4)
Dr. Nahla
Analgesic drug
*Analgesics: medications that → relieve the pain without causing loss
of consciousness.
1. Non-opioid
2. Opioid
NSAIDS
Agonists (strong, moderate, weak)
COX-2 Inhibitors
Mixed agonist-antagonists and
partial agonist
Acetaminophen (para-amino-
phenol group)
Antagonists
1.Non-opioid
1. NSAIDs (Non-Steroidal Anti-Inflammatory Drugs):
1. Salicylate group
(Aspirin,
Sodium salicylate, Coline
salicylate, Diflunisal, Salicylic
acid, Methyl salicylate)
2. Propionic acid derivatives group
(Ibuprofen, Kenoprofen,
Ketoprofen, Naproxen,
Oxaprozin)
3. Indo-leacetic acid group
(Indo-
methacin)
4. Oxicam derivatives group
(Piro-
xicam and meloxicam)
5. Fenamates group
(Mefenamic
acid and Meclofenamic acid)
6. Diclofenac group
7. Ketorolac
8. Phenyl-butazone group
Inas Waleed
3rd Stage of Dentistry

52
Mechanism of action:
1. Anti-inflammatory activity:
The major anti-inflammatory mechanism is by → inhibition of
prostaglandin (PG) synthesis.
NSAIDs competitively → inhibit cyclo-oxygenase (COXs): the
enzyme that catalyze the synthesis of cyclic endoperoxides from
arachidonic acid to form prostaglandins.
*COX enzyme: there are two types:
1. COX-1: is synthesized continuously and present in → all
tissues and cell types (expressed constitutively).
Is important for the production of PGs:
1. Platelet aggregation.
2. Regulation of blood flow in the → kidney and stomach.
3. Regulation of gastric acid secretion.
2. COX-2: is considered an inducible iso-enzyme.
Is expressed constitutively in the → kidney, brain, bone,
female reproductive system, neoplasias and GIT.
Plays an important role in → pain and inflammatory
processes.
2. Analgesic activity: NSAIDs are mainly effective against the type of
pain in which PGs sensitize → pain receptors (inflammation and
tissues) including:
Pain of arthritis.
Bursitis.
Pain of muscular and vascular origin.
Dysmenorrhea.
The effectiveness of these agents against: headache my result from
→ their ability to inhibit PG-mediated cerebral vascular
vasodilatation.
3. Antipyretic activity: inhibition of PG-E2 synthesis in circum-
ventricular organ in and near the preoptic hypothalamic area.
Infections, tissue damage, inflammation, graft rejection and
malignancies → enhance the formation of cytokines that ↑ PG-E2
production.
PG-E2 triggers the hypothalamus to → promote the:
1. ↑ in heat generation.
2. ↓ in heat loss.
53
1. Salicylates group (Aspirin, Sodium salicylate, Coline salicylate,
Diflunisal, Salicylic acid, Methyl salicylate):
Mechanism of action:
Inhibits the enzyme COX-1: irreversibly inactivating of cyclo-
oxygenase lead to → inhibit PG synthesis.
Aspirin have 3 major therapeutic actions:
1. Analgesic.
2. Anti-inflammatory.
3. Antipyretic.
Clinical uses (therapeutic uses):
1. Anti-inflammatory, antipyretic and analgesic uses:
Salicylic acid derivatives are used in the treatment of →
rheumatic fever, osteoarthritis and rheumatoid arthritis.
These treated conditions need analgesia, include →
headache, arthralgia and myalgia.
2. External applications:
Salicylic acid is used topically to treat → corns, calluses and
warts.
Methyl salicylate (oil of wintergreen) is used externally as →
cutaneous counterirritant in liniments.
3. Cardiovascular applications:
Aspirin is used to → inhibit platelet aggregation.
Low doses (75-375mg) are used prophylactically to:
a. ↓ the risk of recurring transient ischemic attacks (TIA)
and stroke or death in those having → single or multiple
episodes of TIA or stroke.
b. ↓ the risk of death in those having an → acute
myocardial infarction.
c. ↓ the risk of recurrent non-fatal myocardial infarction
and/or death in patients with → previous myocardial
infarction or unstable angina pectoris.
d. ↓ the risk of myocardial infarction and sudden death in
patients with → chronic stable angina pectoris.
e. ↓ the risk in patients undergoing → certain
revascularization procedures.

54
4. Patients with anti-phospholipids syndrome who has recurrent
miscarriages: can be treated, but not with warfarin because it
has the potential to cause → fetal abnormality if given during
pregnancy.
Treatment is with → aspirin (75mg) or another type of
anticoagulant (heparin). Sometimes, both are used.
5. Chronic use in colorectal cancer.
6. Facilitates closure of the paten ductus arteriosus.
Adverse effects:
1. GIT effect.
2. Prolong bleeding time.
3. Allergic reaction.
4. Reye's syndrome: salicylates must be avoided in children and
teenagers (< 15 years old) with varicella or influenza to prevent
Reye's syndrome. There's 20-30% of mortality (occurs rarely).
5. Over dose toxicity (Salicylism): is characterized by → nausea,
vomiting, headache, dizziness, tinnitus, mental confusion,
electrolyte imbalance, hyper-thermia, hyper-ventilation,
convulsions, respiratory and metabolic acidosis and death.
2. Propionic acid derivatives group (Ibuprofen, Kenoprofen,
Ketoprofen, Naproxen, Oxaprozin):
all these drugs have → anti-
inflammatory, analgesic and antipyretic effects.
Therapeutic uses
Side effect
1. Chronic treatment of rheumatoid arthritis and
osteoarthritis (less GIT effects than aspirin).
2. Headache pain.
3. Dental pain.
1. GIT disturbance.
2. CNS effect.
55
3. Indoleacetic acid group (Indo-methacin):
Therapeutic uses
Side effect
1. Potent anti-inflammatory (acute
gouty arthritis, osteoarthritis
and ankylosing spondylitis).
2. Control of pain.
3. Antipyretic effect (not use to
lower fever).
4. Delay labor.
5. Treatment of patent ductus
arteriosus.
1. GIT disturbance: nausea,
vomiting, diarrhea, anorexia,
abdominal pain, ulceration,
perforation and hemorrhage.
2. CNS effect.
3. Chronic use ↓ anti-hypertensive
drugs (furosamide, thiazid, β-
blocker, ACE inhibitors).
*ACE: Angiotensin Converting Enzyme.
4. Oxicam derivatives group (Piroxicam and meloxicam):
Therapeutic uses
Side effect
1. Rheumatoid arthritis.
2. Osteoarthritis.
GIT disturbances in 20% of patients.
5. Fenamates group (Mefenamic acid and Meclofenamic acid):
Therapeutic uses
Side effect
1. Dental pain.
2. Painful menstruation
(dysmenorrhea).
1. Diarrhea.
2. Cases of hemolytic (not used
more than 1 week).
6. Diclofenac group:
Therapeutic uses
Side effect
1. Rheumatoid arthritis.
2. Ankylosing spondilitis.
3. Osteoarthritis.
1. GIT disturbances.
2. ↑ liver enzyme level.
7. Phenylbutazone group:
Therapeutic uses
Side effect
1. Acute gout.
2. Acute rheumatoid arthritis, when
others not affected.
1. GIT disturbances.
2. Fluid retention.
3. Skin rash.
4. CNS effect.
56
2. COX-2 Inhibitors:
group of drugs that inhibit → COX-2, which is
responsible for → anti-inflammatory action.
*Celecobix:
Therapeutic uses
Side effect
1. Rheumatoid arthritis.
2. Osteoarthritis.
3. Pain.
Doesn't inhibit platelet aggregation
and doesn't ↑ bleeding time
(unlike aspirin).
1. GIT reduce to 50%: less bleeding
and ulcer.
2. Minimal renal and
cardiovascular side effect.
3. No effects on bleeding time (not
inhibit platelet).
3. Acetaminophen (paraaminophenol group):
Mechanism of action:
They act by → inhibiting PG synthesis in CNS.
Have → antipyretic, analgesic and weak anti-inflammatory effect,
because → they have less effect on COX in peripheral tissues.
No anti-platelet effect.
Lack many side effect of aspirin.
Pharmacological action:
1. Anti-pyretic.
2. Analgesic.
Therapeutic uses:
1. Substitute for the → analgesic and antipyretic effects of aspirin,
for patients with → gastric complaints, those in who prolongation
of bleeding time would be a disadvantage, or those who don't
need the anti-inflammatory action of aspirin.
2. Analgesic/antipyretic of choice for children with viral infection
chickenpox (aspirin ↑ the risk of Reye's syndrome).
3. It doesn't antagonize the → uricosuric agents (probenecid or
sulfinpyrazone), therefore, may be used in → patients with gout
who are taking these drugs.
Adverse effects:
No side effect with therapeutic dose.
Large dose (10-20g → 20-30 tablet) causes → hepatic necrosis
(toxicity).

57
Symptoms:
Start in the first 2 days: nausea, vomiting, anorexia, abdominal
pain and liver toxicity start in 3rd day, which lead to →
encephalopathy, coma and death.
Treatment:
Gastric lavage.
Activated charcoal, sodium or magnesium solution.
Oral N-acetyl-cysten (antidote).
58
Pharmacology (Lec.5)
Dr. Nahla
Analgesic drug
*Analgesics: medications that → relieve the pain without causing loss
of consciousness.
3. Non-opioid
4. Opioid
NSAIDS
Agonists (strong, moderate, weak)
COX-2 Inhibitors
Mixed agonist-antagonists and
partial agonist
Acetaminophen (para-amino-
phenol group)
Antagonists
2. Opioid
Opioid Analgesics:
Include → opiates and semi-synthetic alkaloids.
They are derived from:
1. Opium poppy.
2. Pharmacologically similar synthetic surrogates.
3. Endogenous peptides.
Depend on their interaction with opioid receptors, the drugs are
classified as → agonists, mixed agonist-antagonists and antagonists.
1. Agonist
2. Mixed Agonist-Antagonist
and partial agonist
3. Antagonists
Strong → morphine, meperidine,
methadone, fentanyl
Pentazocine.
Bupre-norphine.
Naloxone.
Naltrexone.
Nalmefene.
Moderate → oxycodone ,codeine.
Weak → propoxyphene.
Mechanism of action:
Opioid analgesics bind to → opioid receptors on inhibitory fibers,
stimulating them, then prevent → stimulation of the GATE.
Prevent pain impulse transmission to the brain.
Inas Waleed
3rd Stage of Dentistry

59
Strong agonists:
1. Morphine:
is the major analgesic drug, and is the prototype strong
agonist.
Contained in → crude opium.
Show a high affinity for µ-receptors, and varying affinities for K
and δ receptors.
*Miosis: the pinpoint pupil → characteristic of morphine use.
Therapeutic uses:
1. Analgesia: treatment of constant moderate to severe pain
(duration of action is 4-6h)
2. Treatment of acute pulmonary edema: IV edema associated
with left ventricular failure, by its → vasodilator effect.
Tolerance:
Tolerance to the → respiratory depressant, analgesic, euphoric
and sedative effects of morphine.
No tolerance to the → pupil-constricting and constipation.
Physical and physiological dependence:
Occur with morphine and with some of other agonists.
Withdrawal produces a → series of autonomic ,motor and
physiological responses that:
1. Incapacitate the individual.
2. Cause unbearable-symptoms.
Its' very rare that the effects are so profound as to cause death.
Opiate withdrawal syndrome:
Manifested as → anxiety, irritability, chills, hot flashes, joint
pain, lacrimation, rhinorrhea, diaphoresis, nausea, vomiting,
abdominal cramps and diarrhea.
Adverse effects:
1. Nausea.
2. Constipation.
3. Severe respiratory depression, which can result in death.
4. Allergy-enhanced hypotensive effects.
60
2. Meperidine
Therapeutic uses
Adverse effects
Synthetic.
Structurally
unrelated to
morphine.
Binds to µ and K
opioid receptors.
Causes →
dilatation of
pupil, due to →
atropine-like
action.
Analgesia: for any
type of severe pain.
Employed in
obstetrics (analgesia
during labor), due to
its shorter action
and different route
of metabolism. It
has less effects on
uterine smooth
muscle than
morphine.
Anxiety.
Tremors.
Muscle fasciculation.
Convulsions (rarely).
Severe hypotension.
Dry mouth and blurred vision, due to its
antimuscarinic action.
Can cause dependence: can substitute for
morphine or heroin in opiate-dependent
persons.
Partial-cross tolerance with the other
opioids.
3. Methadone
Therapeutic uses
Adverse effects
Synthetic.
Orally effective opioid.
Approximately equal in
potency to morphine, but
induces less euphoria and
has longer duration of
action.
It's actions are mediated
by → µ-receptors.
Analgesic.
In the controlled withdrawal of
dependant abusers from heroin and
morphine: Orally administrated,
methadone is substituted for the
injected opioid. The patient is then
slowly weaned from methadone.
Methadone causes → withdrawal
syndrome that's milder but more
prolonged (days-weeks) than other
opiods.
Physical
dependence (like
morphine).
61
4. Fentanyl
Therapeutic uses
Adverse effects
Chemically related to
meperidine.
Has 100-fold the
analgesic potency of
morphine, and is used
in → anesthesia.
Highly lipophilic.
Rapid onset and short
duration of action (15-
30 min).
Injected → IV,
epidurally or
intrathecally.
General anesthesia → during
cardiac surgery, because of its
insignificant effects on myocardial
contractility.
Analgesia for chronic pain:
1. Epidural: used post-operatively
and during labor.
2. Transdermal patch: used with
caution, because can cause
hypoventilation and lead to
death.
3. Oral transmucosal preparation:
used in the treatment of cancer
patients with breakthrough pain
who are tolerant to opioids.
Similar to those of µ-
receptor agonists.
Fentanyl patch is
contraindicated in →
the management of
acute and
postoperative pain or
pain that can be
ameliorated with other
analgesics, because of
life-threatening
hypoventilation.
Pupillary constriction
(unlike morphine).
Moderate agonists:
1. Oxycodone
Semi-synthetic, derived from → morphine.
Orally active, and sometimes is formulated with → aspirin or acetaminophen.
Used to treat → moderate to severe pain, and has many properties common with
morphine.
Is metabolized to → products with lower analgesic activity, and is excreted via → kidney.
Abuse of the sustained-release preparation (ingestion of crushed tablets) lead to death.
The higher-dosage forms of the latter preparations, can be used only by → patients who
are tolerant to opioids.
2. Codeine
Therapeutic uses
Present in → crude opium in lower conc.
Inherently less potent.
Show a high affinity for µ-receptors, and
varying affinities for K and δ receptors
(like morphine).
Analgesic: for → mild-moderate pain.
Often is used in combination with →
NSAIDs and acetaminophen.
Good antitussive activity.

62
Weak agonists:
Propoxyphene
Adverse effects
Derivative of methadone.
Dextro isomer is used as →
analgesic to relieve mild to
moderate pain.
Weaker analgesic than
codeine
Often used in combination
with → acetaminophen.
Levo isomer is not analgesic,
but it has → antitussive
action.
Nausea, anorexia and constipation.
In toxic doses, can cause → respiratory depression,
convulsions hallucinations and confusion.
Cardio-toxicity and pulmonary edema.
Severe CNS depression is produced when used with
→ alcohol and sedatives.
Death by → respiratory depression and cardio-
toxicity.
Respiratory depression and sedation can be →
antagonized by naloxone, but the cardio-toxicity
cannot.
Mixed agonist-antagonist and partial agonist:
1. Pentazocine
Adverse effects
Agonist on K-receptors (promotes analgesia by activating
receptors in the spinal cord).
Weak antagonist on µ and δ receptors.
Used to → relieve moderate pain.
Administrated → orally or parentrally.
Tolerance and dependence develop on → repeated use.
Doesn't antagonize the respiratory depression of
morphine, but it can → precipitate a withdrawal syndrome
in a morphine abuser.
↑ B.P (in angina).
Hallucinations,
Nightmares.
Dysphoria.
Tachycardia.
Dizziness.
Respiratory depression.
↓ the activity of GIT.
↓ renal plasma flow.
2. Buprenorphine
Therapeutic uses
Adverse effects
Partial agonist.
Acting on µ-receptors.
Acts like morphine in
naive patients, but it can
also precipitate
withdrawal in morphine
uses.
Administrated →
sublingually or parentrally.
Long duration of action
Opiate detoxification, because
it has a → less severe and
shorter duration of
withdrawal symptoms,
compared to methadone.
Its approved for office-based
detoxification or
maintenance.
Injectable form is indicated
for → relieve moderate to
severe pain.
Respiratory
depression, that
cannot be easily
antagonized by
naloxone.
↓ ( or rarely ↑) B.P.
Nausea.
Dizziness.
63
Antagonists:
Produces → no profound effects in normal individuals.
In patients dependent of opioides, the antagonists:
1. Rapidly reverse (antagonize) the effect of agonists (heroin).
2. Precipitate the symptoms of opiate withdrawal.
1. Naloxone
2. Naltrexone
3. Nalmefene
Competitive antagonist to →
µ, K and δ receptors.
10-fold higher affinity for µ
than for K receptors. This may
explain why naloxone readily
→ reverses respiratory
depression with only minimal
reversal of the analgesia that
results from agonist
stimulation of K-receptors in
the spinal cord.
Reverse the → coma and
respiratory depression of
opioid overdose.
Within 30 sec of IV injection of
naloxone, coma and
respiratory depression
(characteristic of high doses of
heroin) are reversed, causing
the patient to be revived and
alert.
Has a half-life of 60-100 min.
Actions similar to →
naloxone.
Longer duration of action
than naloxone.
Hepato-toxic.
In combination with
clonidine, and sometimes
with buprenorphine, its
used for → rapid opioid
detoxification.
Beneficial in treating →
chronic alcoholism by an
unknown mechanism
(benzodiazepines and
clonidine are preferred).
Parenteral opioid
antagonist.
Actions similar to →
naloxone and
naltrexone.
Administrated → IV,
IM or SC.
Has a half-life of 8-
10h, longer than
naloxone and several
opioid agonists.
64
Other Analgesics
Tramadol
Adverse effects
Non-opioid centrally acting analgesic.
Binds to → µ-receptors.
Weakly inhibits reuptake of NE and
serotonin.
Used to → manage moderate to
moderately severe pain; adjunctive to
opioids in → chronic pain states.
Undergoes extensive metabolism (one
metabolite is → active).
Anaphylactic reactions.
Toxic in overdose (seizures), specially in
→ patients taking selective serotonin
reuptake inhibitors (tri-cyclic anti-
depressants).
Respiratory depressant activity is → less
than that of morphine.
Should be avoided in → patients taking
monoamine oxidase (MAO) inhibitors.

65
Pharmacology (Lec.6)
Dr. Nahla
Anesthesia
*Anesthesia:
means → no sensation.
*Types:
1. Local anesthesia: loss of sensation over a small area of the body.
2. Regional anesthesia: loss of sensation over a specific region of the
body (lower trunk).
3. General anesthesia: loss of sensation of the entire body
Local Anesthesia
Local anesthetic (LA):
is an agent that interrupts pain impulses in a specific
region of the body without loss of patient consciousness.
Normally, the process is completely → irreversible.
Don't produce any residual effect on the nerve fiber.
Are weak bases (pKa = 7.5-9.0).
Usually prepared as a → salt (HCL), to ↑ stability and water solubility.
When injected (5% - 40%) is converted to → non-ionized free base.
Mechanism of action:
L.A. binds directly to → intracellular voltage-dependent Na
+
channels.
Block primarily → open and inactive Na
+
channels at specific sites
within the channel.
L.A. interfere with → propagation of the action potential by →
blocking the ↑ in Na
+
permeability during depolarization.
L.A. provide → pain relief by → blocking nociceptive fibers.
Sensitivity to L.A. depends on:
1. Fiber diameter.
2. Fiber type.
3. Degree of myelination.
Order of sensory function block:
1. Pain.
2. Cold.
3. Warmth.
4. Touch.
5. Deep pressure.
6. Motor.
Recovery in reverse order.
Inas Waleed
3rd Stage of Dentistry
66
Chemistry:
*Chemical structure:
1. Amine group on one end (hydrophilic: soluble in water), connect to
an aromatic ring on the other end (lipophilic: soluble in lipids)
2. Amine group on the right side.
*Differences of Esters and Amides:
Esters
Amide
Link between intermediate chain and
aromatic ring.
Link between intermediate chain and
aromatic ring.
Metabolized in → plasma
pseudocholinesterases.
Metabolized in → liver.
Not stable in the solution.
Very soluble in the solution.
Cause allergic reactions.
Allergic reactions are rare (especially with
Lidocaine).
1. Cocaine.
2. Procaine.
3. Propoxycaine.
4. Tetracaine.
5. Benzocaine.
6. Dyclonine hydrochloride.
7. Chlorobutanol.
1. Lidocaine (Xylocaine ).
2. Prilocaine (Citanest).
3. Articaine (Septocaine).
4. Mepivacaine hydrochloride.
5. Bupivicaine (Marcaine).

67
Esters Local Anesthetics:
1. Cocaine (generic):
The 1st topical L.A.
Has sympathomimetic activity, it causes → vasoconstriction.
Powerful CNS stimulant.
Can induce psychic dependence with high rate of abuse.
Has no place in routine practice of dentistry.
2. Procaine (generic,
Novocarin)
Therapeutic uses
Adverse effects
Cause →
vasodilatation.
Short duration of
action.
Parenteral: 1, 2, 10%
for injection.
The drug of choice to reverse arterial
spasm.
Used for the treatment of → cardiac
arrhythmias (Procaine amide).
Combined with Penicillin G (Procaine
penicillin).
Used in dentistry with 2% of propoxycaine.
High potential
to cause allergic
reactions
(Ester).
3. Propoxycaine:
Rapid onset of action.
Long duration of action.
Used for → infiltration anesthesia.
Used in dentistry with 2% of procaine.
4. Tetracaine
Therapeutic uses
Slow onset of
action.
Long duration
of action.
No longer used in dentistry.
Parenteral: 1% for injection, 0.2,0.3% with 6% dextrose for spinal
anesthesia.
Topical: 1% ointment, 0.5% solution (ophthalmic), 1, 2 % cream,
2% solution for nose and throat, 2% gel.
5. Benzocaine
6. Dyclonine hydrochloride
7. Chlorobutanol
Derivative of → Procaine.
Poorly soluble in aqueous
fluids.
Low toxic potential.
Useful for → topical
anesthesia.
Cause:
1. Allergic reaction
(dermatitis, eczema)
2. Methemoglobinemia
Has ketone linkage between the
aromatic moiety and the rest of
the anesthetic molecule.
Tissue irritant, not available for
injection.
Lozenges form for the topical use.
Used in → patients allergic to
derivatives of p-aminobenzoic
acid.
Weak L.A.
Relieve → acute
pulpitis and post-
extraction pain.

68
Amide Local Anesthetics:
1. Lidocaine (Xylocaine):
1. The 1st modern L.A. agent.
2. Relieves pain during → dental surgeries.
3. Little allergenic reaction (Amide), it's → hypoallergenic.
4. Sets on quickly and anesthesia effect for several hours.
*Therapeutic uses:
1. Single L.A., parenteral:
For injection: 0.5 , 1 , 1.5 , 2 , 4 % .
0.5 , 1 , 1.5 , 2 % with 1:200.000 Epinephrine.
1 , 2 % with 1:100.000 Epinephrine.
2% with 1:50.000 Epinephrine.
2. Used in → management of ventricular arrhythmias after an
acute myocardial infarction.
3. Topical anesthetic:
2.5 , 5% ointments.
0.5 , 4% cream.
0.5 , 2.5% gel.
2 , 2.5 , 4% solutions.
23 , 46 mg/2 cm
2
patch.
4. Topical: Lidocaine (2.5%) and Etidocaine (2.5%) → eutectic
=mixture (EMLA cream).
*Clinical signs and symptoms of L.A. toxicity:
1. Numbness of tongue and mouth.
2. Behavioral and sensory disturbances.
3. Ringing in ears, metallic taste, tingling sensations.
4. Seizures (tonic clonic).
5. Depression / Loss of consciousness.
6. Respiratory failure, arrhythmias, cardiovascular (CV) collapse.
*Prevention of systemic toxicity:
Limit the amount of drug employed.
Use proper injection techniques.
*Local toxic reactions:
1. Selective destruction of → skeletal muscle fibers.
2. Epithelial damage from topical preparations.
3. Local necrosis from vasoconstrictor actions.
69
2. Prilocaine
(Citanest)
3. Articaine (Septocaine)
4. Mepivacaine
hydrochloride
Identical pKa and
same conc. with
Lidocaine.
Same duration as
Lidocaine.
Less toxic in higher
doses than Lidocaine
due to small
vasodilatory activity.
Can cause →
methemoglobinemia.
Newest L.A. drug approved by FDA.
Same pKa and toxicity as Lidocaine,
but it's half-life is less than about 1/4
of Lidocaine.
Enters blood barrier smoothly.
Is widely used.
Parenteral: 4% with 1:100.000
Epinephrine.
Development of → persistent
paresthesias, may be 3 times more
common with Articaine.
Similar in many
respects to Lidocaine.
Is marketed in as:
* 2% conc. with
1:20.000
Levonordefrin.
* 3% solution without
vasoconstrictor.
5. Bupivicaine (Marcaine):
Is a homologue of Mepivacaine.
Highly lipid soluble.
Slightly higher pKa and slower onset of action.
For dentistry: 0.5% Bupivicaine hydrochloride is available with
1:200.000 Epinephrine.
Bupivacaine with Epinephrine given for → nerve block
procedures operate anesthesia, which is several times longer
than that of other drugs.
Drug of choice for → epidural infusions, used for → post-
operative pain control and for labor analgesia.
Low conc. (≤ 0.25%) are used to → achieve prolonged peripheral
anesthesia and analgesia for postoperative pain control.
Is used as → infiltration anesthesia to control pain from a
surgical incision.
Most common toxic reaction (cardiotoxicity).
Parenteral:
*For injection 0.25 , 0.5 , 0.75%.
* 0.25% , 0.5 , 0.75% with 1:200.000 Epinephrine.
70
Pharmacology (Lec.7)
Dr. Nahla
Anesthesia
*Anesthesia:
means → no sensation.
*Types:
4. Local anesthesia: loss of sensation over a small area of the body.
5. Regional anesthesia: loss of sensation over a specific region of the
body (lower trunk).
6. General anesthesia: loss of sensation of the entire body
General Anesthesia
*General anesthesia (G.A.):
loss of sensation of the entire body.
Reversible inhibition of CN function, during which surgical procedures
can be carried out in the → absence of consciousness,
responsiveness to pain, defensive or involuntary movements and
insignificant autonomic reflex responses.
According to the mode of application, GA is divided into:
1. Inhalational Anesthetics (gaseous, volatile): administered in the
most part, and eliminated via → respired air. Serve to → maintain
anesthesia.
2. I.V. Anesthetics (injectable): employed for → induction; have
rapid onset of action. Are more agreeable to the patient than the
inhaled anesthetics.
Mechanism of action:
No receptor has been identified.
The focus is on the interactions of the inhaled anesthetics with
proteins compromising ion channels:
*For example: G.A ↑ the sensitivity of the ϒ-aminobutyric acid
(GABAA) receptors to the neurotransmitter (GABA), this causes a
prolongation of the inhibitory chloride ion current after a pulse of
GABA release. So postsynaptic neuronal excitability is ↓.
Other receptors are affected by → volatile (inhalational) anesthetics:
*For example:
a. Activity of the inhibitory glycine receptors in the spinal motor
neurons is ↑.
b. Block the excitatory postsynaptic current of the nicotinic
receptors.
The mechanism is not → understood.
Inas Waleed
3rd Stage of Dentistry

71
1. Inhaled Anesthetics
2. Intravenous Anesthetics
Volatile liquid:
1. Halothane.
2. En-flurane
3. Iso-flurane
4. Des-flurane
5. Sevo-flurane
Gas →
Nitrous oxide (N
2
O): laughing gas.
Barbiturates:
1. Thiopental
2. Methohexital
Dissociative →
Ketamine (Ketalar)
Miscellaneous:
1. Etomidate
2. Propofol (Diprovan)
3. Fos-propofol.
Opioids →
Fentanyl.
Benzodiazepines:
1. Midazolam (versed).
2. Lorazepam (ativan).
3. Diazepam (valium)
Inhaled anesthetics:
1. Halothane
(Therapeutic uses)
Side effects
Potent anesthetic.
Not hepato-toxic in pediatric patient.
It's pleasant odor, makes it → suitable in
children for inhalation conduction.
Used in → obstetric.
Weak analgesic.
Strong CV depressant.
Cardiac arrhythmias.
Strong respiratory depressant.
Hepatotoxic (Halothane Hepatitis).
Malignant hyperthermia (P
x
Dantrolene).
2. Enflurane
(Properties)
Side effects
Less potent than Halothane, but has rapid
induction and recovery.
Metabolism: one-tenth that of Halothane,
doesn't release hepatotoxic metabolites.
Fewer arrhythmias: less sensitization of the
heart to catecholamines.
Metabolism releases → fluoride ion-renal
toxicity.
CNS excitation at twice the MAC, can
induce → seizure.
Curare-like effect.

72
3. Isoflurane
4. Deflurane
5.
Sevoflurane
Very stable molecule.
Little metabolism
Doesn't induce cardiac
arrhythmias.
Doesn't sensitize the heart to
the action of cathecolamines.
Produces conc.-dependent
hypotension, due to →
peripheral vasodilation.
Dilates coronary vasculature, ↑
coronary blood flow and O
2
consumption by the →
myocardium. This property
make it beneficial in patients
with IHD.
Most rapid onset of action
and recovery of the
halogenated gas.
Widely used for →
outpatient surgery.
Irritating to the airway in
awake patients, causes →
coughing, salivation and
bronchospasm.
Poor induction agent.
Used for → maintenance of
anesthesia.
Rapid onset of action
and recovery.
Potent MAC = 2%.
Widely used for →
outpatient surgery.
Not irritating to the
airway.
Useful induction agent,
particularly in children.
*IHD: Ischemic Heart Disease.
*Nitrous oxide (N
2
O):
laughing gas
Therapeutic uses
Side effects
Potent analgesic, but
weak G.A.
Rapid onset of action
and recovery.
Doesn't depress
respiration and no
muscle relaxation.
No effect on CVS or
no ↑ cerebral blood
flow.
Dental surgery.
Entonox (N
2
O and O
2
in
equal volume): used as
→ analgesia in labor,
myocardial infarction,
post-operative
physiotherapy,
changing surgical
dressing and removal of
the drainage tubes.
Maintenance of
anesthesia.
The less → hepatotoxic, teratogenic,
bone marrow depression.
Second gas effect: N
2
O can
concentrate the halogenated
anesthetics in the alveoli when they
are concomitantly administrated,
because of its fast uptake from the
alveolar gas.
Diffusion hypoxia: speed of N
2
O
movement allows it to retard O
2
uptake during recovery.
Because it moves very rapidly into and
out of the body, N
2
O can ↑ the
volume (pneumothroax).
Inhibits methionine synthetase
(precursor to DNA synthesis).
Inhibits vitamin B12 metabolism.
*CVS: cardiovascular system.

73
Intravenous anesthetics:
1. Propofol (Diprovan)
Therapeutic uses
Side effects
Used in → operating
room and ICU.
IV sedative-hypnotic.
Onset is smooth and rapid
(40 sec).
Is accompanied by
excitatory phenomena,
such us: muscle twitching,
spontaneous movement
or hiccups.
Poor analgesia.
Administered IV for → induction
of anesthesia and maintenance.
First choice for anesthesia and
sedation, because it → produces
a euphoric feeling in the patient
and doesn't cause post-
anesthetic nausea and vomiting.
Used with → elderly, debilitated
and cardiac compromised
patients.
Pain on injection.
Generic formulation
contains → sodium
bisulfate (caution with
asthma).
Fatal syndrome
associated with → long-
term and high-dose
administration (egg
allergy).
2. Fospropofol:
Water-soluble prodrug of Propofol.
Recently approved.
May enhance some problems associated with administration of
Propofol.
3. Barbiturates
(Thiopental,
Methohexital)
Therapeutic uses
Side effects
Ultra-short acting.
High lipid solubility.
Quickly enter the CNS
and depress function in
less than 1 min.
Redistribution occur very
rapidly as well to other
body tissues, including
→ skeletal muscle and
adipose tissue (serve as
a reservoir).
Potent anesthetic,
but weak analgesic.
Used for →
induction of
anesthesia.
Used for → ECT,
dental procedures
and cardioversion.
Thiopental has minor effects on CVS,
but it may cause → severe hypotension
in hypovolemic or shock patient.
Cause → apnea, coughing, chest wall
spasm, laryngospasm and
bronchospasm.
Extravascular injection: painful and
tissue damage.
Dose-dependent histamine release.
Absolute contraindication → porphyria.
*ECT: Electro-Convulsive Therapy.

74
4. Etomidate
Therapeutic uses
Side effects
Hypnotic agent,
lacks analgesic
activity.
Induction is rapid.
Short acting.
Used to → induce anesthesia.
Only used for → patients with
coronary artery disease or CV
dysfunction.
No effect on heart and
circulation.
↓ in plasma cortisol and
aldosterone levels, which can
persist for up to 8h. Due to →
inhibition of 11-β-
hydroxylase Etomidate
(Amidate).
5. Benzodiazepines:
Used in → anesthesia practice.
Facilitate amnesia while causing sedation.
1. Midazolam (Versed).
2. Lorazepam (Ativan).
3. Diazepam (Valium).
6. Ketamina (Ketalar)
Therapeutic uses
Side effects
Phencyclidine derivative.
Interacts with NMDA receptor.
Shot acting anesthetic (15min).
Induces a dissociate state in
which the patient is
unconscious, but appear to be
awake and doesn't feel pain.
Profound analgesia.
Less vomiting.
Provides → sedation, amnesia
and immobility.
Short acting GA in:
1. Shock or CV instability.
2. Severe dehydration.
3. Bronchospasm.
4. Severe anemia.
5. Burn dressing changes.
6. OB-acute hemorrhage.
7. Poor rick patients
(trauma/elderly)
Is used when → cirulatory
depression is undesirable.
BP is often ↑.
Causes → nystagmus
(↑IOP).
↑ post-operative
nausea and vomiting.
↑ salivation and
respiratory secretions.
↑ cerebral blood flow.
Induces post-operative
hallucinations and
nightmares, particularly
in adults.
*NMDA: N-Methyl-D-Aspartate.
*OB: Obstetrical.
75
Pharmacology (Lec.8)
Dr.
Autacoids and Autacoids Antagonist
Histamine:
it's one of the most important Autacoids. An organic
nitrogen compound (formed from amino-acid histidine), involved in:
1. Immune responses (allergic and immune reactions).
2. Regulating physiological function in the → gut and sleep.
3. Acting as a → neurotransmitter.
Location:
Cells
Tissue
Mast cells (mainly).
Basophiles.
Neurons.
Enterochromaffin-like cells (ECL).
Skin.
Lung.
GIT.
Release:
occur by 2 processes:
1. Energy and Ca
+2
dependent degranulation
reaction
2. Energy and Ca
+2
independent
release (displacement)
When allergies enter into the body (by eat or
breath), result in → trigger immune cells to
produce large amounts of IgE, that recognize
that food.
Some IgE attaches to the → surfaces of mast
cells (sensitization).
On any subsequent exposure to the same
allergen, the allergens interact with the →
specific IgE on the surface of the mast cells.
In response, the activated mast cells rapidly
release → histamine.
Depending on the tissue in which they are
release, these chemicals will cause a person
to have → various symptoms of allergy.
Is induced by:
a) Drugs → morphine,
tubocuranine, etc.
b) Mast cell damage, which is
caused by → noxious
agents (venom) or
mechanical trauma, can
release → histamine
Inas Waleed
3rd Stage of Dentistry
76
Receptors:
H1
H2
H3
H4
CNS.
Smooth muscle cells of airways.
GIT.
CVS.
Endothelial cells.
Lymphocytes.
Parietal cells.
Vascular
smooth muscle
cells.
CNS.
PNS.
Bone marrow.
Immune cells.
Actions:
Vascular
Heart
GI system
Lung
CNS
H1 and H2:
vasodilatation.
H1: ↑ capillary
permeability,
which is occur
by →
contraction of
endothelial cells
of venules.
H1:↓ AV
conduction.
H2: ↑
chronotropy.
H2: ↑ inotropy.
H1: ↑ intestinal
motility and
secretions.
H2: acid, fluid
and pepsin
secretions.
H1: broncho-
constriction.
H1: ↑ mucous
viscosity.
H1: stimulation of
vagal sensory
nerve endings →
cough and
bronchospasm.
H1: pain and
itching.
H1 and H3:
neuro-
transmitters.
*To minimize Histamine reactions:
1. Physiological antagonism → epinephrine.
2. Inhibit the release of histamine → cromolyn.
3. Pharmacological antagonism → antihistamines.
77
1. H1-Receptors Antagonists (H1-RA)
Mechanism of action:
Act by competitive blockade of → H-receptors in the target
tissues, result in: ↓ the availability of these receptors for H.
This interaction is → reversible, and can be overcome by ↑ H-
release.
Generations:
1st generation (sedating):
2nd generation (non sedating):
3rd generation:
Diphen-hydramine.
Doxyl-amine.
Terfen-adine.
Chloro-phenir-amine.
Meclizine.
Hydroxine.
Cetirizine.
Loratadine.
Fexo-fenadine.
Levo-cetrizine.
Des-loratadine.
Therapeutic uses:
1. Allergic disease → nasal allergies, urticaria and common
cold.
2. Systemic anaphylaxis.
3. Anti-emetic: prohylactic for motion sickness →
promethazine.
4. Anti-vertigo → meclizine.
5. L.A. → diphen-hydramine and promethazine.
6. Anti-tussive → diphen-hydramine.
7. Ketotifen: oral form to prevent asthma attacks.
8. Other uses: ↓ of tremors and muscle rigidity in Parkinson's
disease.
Side effects (↓ in 2nd and 3rd generations):
Sedation (1st generation).
Dizziness.
Lack of coordination.
Euphoria.
Nervousness.
Blurred vision.
Urinary retention.
Dryness of mouth and respiratory passages.

78
Uses in dentistry:
Diphen-hydramine, hydroxyzine and promethazine are used
in → conscious sedation procedure and as premedication for
deep sedation and G.A.
Have L.A. activity, so it can be used for → patients who have
allergy to conventional L.A.
For management of → systemic anaphylactic reaction.
Treatment of → allergic lesions of oral mucosa.
2. H2-Receptors Antagonists (H2-RA):
Mechanism of action:
Competitively blockade the binding of histamine to H2-
receptors in the gastric parietal cells.
Clinical use:
Inhibitors of gastric acid secretion in the treatment of ulcers
and heartburn.
Examples:
1. Cime-tidine.
2. Rani-tidine.
3. Famo-tidine.
4. Niza-tidine
79
Serotonine:
is a monoamine neurotransmitter. One of the most
important autocoids, has many physiological roles and clinical
applications.
It's widely distributed in → plants and animal tissues.
In human: 90% → GIT, 8% → platelets and 2% → CNS.
Synthesis:
Tryptophan
a
5-hydroxytryptophan
b
serotonin (5HT)
* a = tryptophan hydroxylase.
* b = l-aromatic acid decarboxylase.
Pharmacological action:
1. Cardiovascular system:
Small +ve inotropic and +ve chronotropic effect on the heart.
Direct vasoconstriction (large arteries).
Indirect vasodilatation (NO and PGI2-mediated).
*PGI2: prostaglandin - I2.
2. Respiratory system:
Small direct stimulation to → bronchial smooth muscle in
normal person, but produce → bronchospasm in asthmatic
patients.
Hypoventilation, due to → stimulation of bronchial sensory
nerve endings.
3. GIT: potent stimulation on the → smooth muscle of the gut,
which:
↑ the tone.
Facilitate peristalsis.
Stimulate vomiting.
4. CNS: stimulation of → sensory nerve endings, which lead to →
pain and itching.
5. Glandular secretions: little inhibitory effects on exocrine glands.
6. Uterus: large doses → impairs placental blood supply, and may
lead to → fetal distress.

80
Agonists
Antagonists
1. Sumatriptan: 5-HT1D agonist, used
as → anti-migraine drug.
1. Cyproheptadine (periactin): H1 and 5-HT2
antagonists, used as → appetite stimulant.
*Other uses: allergic rhinitis and cold urticaria.
2. Fluoxetine: selective serotonin re-
uptake inhibitors, used as →
antidepressant drug.
2. Ondansetron: 5-HT3 antagonists, used in →
citotoxic induced nausea and vomiting.
3. Buspirone: 5-HT1D agonist, for →
anxiety.
3. Clozapine: 5-HT2A/2C antagonist, for →
schizophrenia.
Migraine:
syndrome of recurrent pulsatile (throbbing pain) unilateral
headache (few hours-few days in duration).
Types:
1. Migraine without aura (common).
2. Migraine with aura (classic).
Drugs used to treat Migraine headache:
1. Non-specific treatment (symptomatic)
2. Specific migraine therapy
Analgesics → NSAIDs.
Anti-emetic drugs → prochlor-perazine: to
control vomiting
Opioids: when other treatments are not
successful (rescue medication).
Triptans → Suma-triptan, Zolmi-triptan
and Almo-triptan.
Dihydro-ergotamine.
*Both are → 5-HT1D-receptor agonists.
Prophylaxis:
several drugs are effective in ↓ the frequency and
severity of migraine attacks:
β-blockers: propranolol and nadolol
Tri-cyclic anti-depressant: ami-triptyline
Anti-convulsant: dival-proex.
Calcium-channel blocker: verapamil

81
Pharmacology
Dr. Nahla
Resume
*Parasympathetic:
Cholinergic
Anti-muscarinic
Eye
Meiosis (↓ pupil size)
↓ IOP.
Mydriasis (↑pupil size → dilated pupil).
↑ IOP.
Exocrine
glands
↑
↓ (except milk).
CVS
Bradychardia (↓ heart rate).
Low dose → bradycardia (↓heart rate).
High dose → tachycardia (↑heart rate).
Bronchi
Bronchospasm (contraction).
Bronchial dilatation.
↓ secretion.
GIT
↑ motility.
↑ secretion.
↓ motility.
↓secretion.
Blood
vessels
Vasodilatation
Vasodilatation (poisoning)
Uterus
(female)
Contraction
-
Bladder
Relaxation
Relaxation
*Cholinergic agents, Cholinomimetic drugs, Parasympathomimetic drugs:
Direct
Indirect
a. Choline Ester:
1. Acetylcholine (Ach).
2. Bethanechol.
3. Carbacol.
b. Alkaloid:
1. Muscarine.
2. Nicotine.
3. Pilocarpine.
*Cevimeline.
a. Reversible:
1. Short-acting: (Endrophonium)
2. Long-intermediate acting:
Tubocuranine.
Neostigmine and
Pyridostigmine.
b. Irreversible:
Isoflurophate and Echothiophate.
Inas Waleed
3rd Stage of Dentistry

82
*Anti-cholinergic agents, Cholinergic antagonists, Cholinergic blockers,
Parasympatholyc drugs:
Anti-muscarinic drugs
(Muscarinic Antagonists)
Anti-nicotinic drugs
(Nicotinic Antagonists)
I. Tertiary amine:
1. Atropine.
2. Hemo-atropine.
3. Scopolamine.
II. Quaternary amine:
1. Clinidium bromide.
2. Propantheline (Duspataline).
3. Ipratropium.
I. Ganglion blocking agents:
1. Hexamethionin, Mecamylamine.
2. Trimethaphan.
II. Neuromuscular (N-M) blocking drugs:
1. Non-depolarizing competitive →
Tubocuranine.
2. Depolarizing non competitive →
Succinylcholine (Suxamethonium).
Clinical uses of Anti-muscarinic drugs:
1. On CNS:
Benzohexol,Orpheradrine.
Hyoscine, Promethazine.
Hyoscine.
2. Ophthalmologic disorders →
Tropicamide.
3. Respiratory disorder:
Preoperative (Atropine and Scopolamine).
Ipratropium.
Tiotropium.
4. CVS disorder →
Atropine.
5. GIT disturbances:
Propantheline.
Hyoscine-butyl brome (Buscopan).
Pirenzepine, Telenzepine.
Preanesthesia.
Hyoscine, Promethazine.
6. Urinary disorders
Flavoxate.
Propantheline.
Oxybutynin.
7. Antidotes to cholinergic and anticholinesterase agent →
Atropine.

83
*Adrenergic drugs:
Chemistry:
Catecholamines
Non-catecholamines
1. Epinephrine.
2. Nor-Epinephrine.
3. Isoproterenol.
4. Dopamine.
1. Phenylephrine.
2. Ephidrine.
3. Amphetamine.
1. Adrenergic agonists:
Direct
Indirect
Mixed
1. Epinephrine.
2. Nor-epinephrine.
3. Isoproterenol.
4. Dopamine.
5. Dobutamine.
6. Oxymetazoline.
7. Phenylephrine.
8. Methoxamine.
9. Clonidine.
10. Metaproterenol.
11. Albuterol, Pirbuterol and Terbuterol.
12. Salmeterol and Formeterol.
1. Amphetamine.
2. Tyramine.
3. Cocaine.
1. Ephidrine.
2. Pseudoephedrine.
3. Metaraminol.
2. Adrenergic antagonists:
3. α-Adrenergic Blocking Agents
4. β-Adrenergic Blocking Agents
1. Phenoxybenzamina.
2. Phentolamine.
3. Pra-zosin
Tera-zosin
Doxa-zosin
Alfu-zosin
Tamsu-losin.
4. Yohimbine.
1.Propranolol.
2.Timolol and Nadolol.
3.Atenolol
Metoprolol
Bisoprolol
Betaxolol
Nebivolol
Acebutolol.
4.Esmolol.
5.Pindolol and Acebutolol.
6.Labetalol and Carvedilol.
Drugs affecting neurotransmitter release or uptake:
1. Reserpine.
2. Guanethidine.
3. Cocaine.

84
*Analgesic drugs:
A. Non-opioid:
1. NSAIDs:
Salicylate group:
1. Aspirin.
2. Sodium salicylate.
3. Coline salicylate.
4. Diflunisal.
5. Salicylic acid.
6. Methyl salicylate.
Propionic acid derivatives group (Ibuprofen, Kenoprofen,
Ketoprofen, Naproxen, Oxaprozin).
Indo-leacetic acid group (Indo-methacin).
Oxicam derivatives group (Piroxicam and Meloxicam).
Fenamates group (Mefenamic acid and Meclofenamic acid).
Diclofenac group.
Ketorolac.
Phenyl-butazone group.
2. COX-2 inhibitors
(Celecobix).
3. Acetaminophen (paraaminophenol group).
B. Opioid:
1. Agonist:
Strong:
1. Morphine.
2. Meperidine.
3. Methadone.
4. Fentanyl.
Moderate:
1. Oxycodone.
2. Codeine.
Weak (Propoxyphene).
2. Mixed agonist-antagonist and partial agonist:
Pentazocine (mixed agonist-antagonist).
Buprenorphine (partial agonist).
3. Antagonists:
Naloxone.
Naltrexone.
Nalmefene.
Other analgesics
(Tramadol).

85
*Local Anesthesia:
Ester L.A
Amide L.A
1. Cocaine.
2. Procaine.
3. Propoxycaine.
4. Tetracaine.
5. Benzocaine.
6. Dyclonine hydrochloride.
7. Chlorobutanol.
1. Lidocaine (Xylocaine).
2. Prolocaine (Citanest).
3. Articaine (Septocaine).
4. Mepivacaine hydrochloride.
5. Bupivicaine (Marcaine).
*General Anesthesia:
Inhaled
Intravenous
1. Volatile liquid:
Halothane.
Enflurane.
Isoflurane.
Desflurane.
Sevoflurane
2. Gas:
Nitrous oxide (N
2
O)
"laughing gas".
1. Barbiturates:
Thiopental.
Methohexital.
2. Dissociative:
Ketamine (Ketalar).
3. Miscellaneous:
Etomidate.
Propofol (Diprovan).
Fospropofol.
4. Opioids:
Fentanyl.
5. Benzodiazepines:
Midazolam (versed).
Lorazepam (ativan).
Diazepam (valium).

86
*Autacoids and Autacoids Antagonists
Histamine:
To minimize histamine reactions:
1. Physiological antagonism → Epinephrine.
2. Inhibit the release of histamine → Cromolyn.
3. Pharmacological antagonism → Anti-Histamine.
Generations of H1-RA:
1st generation (sedating)
2nd generation (non sedating)
3rd generation
Diphen-hydramine.
Doxyl-amine.
Ter-fenadine.
Chloro-phenir-amine.
Meclizine.
Hydroxine.
Cetirizine.
Loratadine.
Fexo-fenadine.
Levo-cetrizine.
Des-loratadine.
H2-RA
(examples)
:
1. Cime-tidine.
2. Rani-tidine.
3. Famo-tidine.
4. Niza-tidine.
Serotonine:
Agonists
Antagonists
4. Sumatriptan
4. Cyproheptadine (periactin)
5. Fluoxetine
5. Ondansetron
6. Buspirone
6. Clozapine
Migraine:
Drugs used to treat Migraine headache:
3. Non-specific treatment (symptomatic)
4. Specific migraine therapy
Analgesics → NSAIDs.
Anti-emetic drugs → Prochlor-perazine.
Opioids.
Triptans → Suma-triptan, Zolmi-triptan
and Almo-triptan.
Dihydro-ergotamine.
*Both are → 5-HT1D-receptor agonists.
Prophylaxis:
1. β-blockers → Propranolol and Nadolol.
2. Tri-cyclic antidepressant → Ami-triptyline.
3. Anticonvulsant → Dival-proex.
4. Calcium-channel blocker → Verapamil.

87
Pharmacology
MSQ
1. Parasympathetic nervous system:
Q1. Which one of the following statements concerning the
parasympathetic nervous system?
A. The parasympathetic system uses nor-epinephrine as a
neurotransmitter.
B. The parasympathetic system often discharges as a single, functional
system.
C. The parasympathetic division is involved in accommodation of near
vision, movement of food, and urination.
D. The postganglionic fibers of the parasympathetic division are long
compared to those of the sympathetic nervous system.
E. The parasympathetic system controls the secretion of the adrenal
medulla.
*Correct answer = C: The parasympathetic system maintains essential
bodily functions, such as vision, movement of food, and urination.
It uses acetylcholine, not nor-epinephrine, as a neurotransmitter, and it
discharges as discrete fibers that are activated separately.
The postganglionic fibers of the parasympathetic system are short
compared to those of the sympathetic division.
The adrenal medulla is under control of the sympathetic system.
Q2. Which one of the following is characteristic of parasympathetic
stimulation?
A. Decrease in intestinal motility.
B. Inhibition of bronchial secretion.
C. Contraction of sphincter muscle in the iris of the eye (miosis).
D. Contraction of sphincter of urinary bladder.
E. Increase in heart rate.
Correct answer = C: The parasympathetic nervous system is essential in
maintenance activities, such as digestion and waste removal.
Therefore, increased intestinal motility to facilitate peristalsis,
relaxation of urinary bladder sphincters to cause urination, and
increased bronchial secretions result. Increase in heart rate is a
function of the sympathetic nervous system.
Inas Waleed
3rd Stage of Dentistry

88
Q3. Which of the following is characteristic of the sympathetic nervous
system?
A Discrete response to activation.
B. Actions mediated by muscarinic and nicotinic receptors.
C. Effects only mediated by nor-epinephrine.
D. Responses predominate during physical activity or when
experiencing fright.
E. Subjected to voluntary control.
Correct answer = D: The sympathetic nervous system is activated by
“fight or flight” stimuli. To achieve rapid activation of this system, the
sympathetic nervous system often discharges as a unit. The receptors
that mediate sympathetic nervous system effects on neuro-effector
organs are α and β receptors. Because the sympathetic nervous system
is a division of the autonomic nervous system, it is not subject to
voluntary control and functions below conscious thought.
Q4. Patient presents with salivation, lacrimation, urination and
defecation as side effects of a medication. Which one of the following
receptors mediates the actions of this drug?
A. Nicotinic receptors.
B. α Receptors.
C. Muscarinic receptors.
D. β Receptors.
Correct answer = C: The muscarinic receptors of the parasympathetic
nervous system maintain essential body functions such as digestion and
waste elimination. The nicotinic receptors are a receptor for
acetylcholine. It plays a major role in skeletal muscles, ganglia and
synthesis of catecholamines in the adrenal medulla. α and β receptors
are receptors for nor-epinephrine and epinephrine and activation of
these receptors does not produce these effects.

89
2. Cholinergic agonist:
Q1. A patient with an acute attack of glaucoma is treated with
pilocarpine. The primary reason for its effectiveness in this condition is
its:
A. Action to terminate acetylcholinesterase.
B. Selectivity for nicotinic receptors.
C. Ability to inhibit secretions, such as tears, saliva, and sweat.
D. Ability to lower intraocular pressure.
E. Inability to enter the brain.
Correct answer = D: Pilocarpine can abort an acute attack of glaucoma,
because it causes pupillary constriction to lower intraocular pressure. It
binds mainly to muscarinic receptors and can enter the brain. It is not
effective in inhibiting secretions.
Q2. A soldier's unit has come under attack with a nerve agent. The
symptoms exhibited are skeletal muscle paralysis, profuse bronchial
secretions, miosis, bradycardia, and convulsions. The alarm indicates
exposure to an organophosphate. What is the correct treatment?
A. Do nothing until you can confirm the nature of the nerve agent.
B. Administer atropine, and attempt to confirm the nature of the nerve
agent.
C. Administer atropine and 2-PAM (pralidoxime).
D. Administer pralidoxime.
Correct answer = C: Organophosphates exert their effect by irreversibly
binding to acetylcholinesterase (AChE) and, thus, can cause a
cholinergic crisis. Administration of atropine will block the muscarinic
sites, but it will not reactivate the enzyme, which will remain blocked
for a long period of time. Therefore, it is essential to also administer
pralidoxime as soon as possible to reactivate the enzyme before aging
occurs. Administering pralidoxime alone will not protect the patient
against the effects of acetylcholine resulting from AChE inhibition

90
Q3. A patient on a diagnostic test for myasthenia gravis would be
expected to have improved neuromuscular function after being treated
with:
A. Donepezil.
B. Edrophonium.
C. Atropine.
D. Echothiophate.
E. Neostigmine.
Correct answer = B: Edrophonium is a short-acting inhibitor of
acetylcholinesterase (AChE) that is used to diagnose myasthenia gravis.
It is a quaternary compound and does not enter the central nervous
system. Donepezil, isoflurophate, and neostigmine are also AChEs but
with longer actions. Donepezil is used in the treatment of Alzheimer
disease. Echothiophate has some activity in treating open-angle
glaucoma. Neostigmine is used in the treatment of myasthenia gravis
but is not used in its diagnosis. Atropine is a cholinergic antagonist.
Q4. The drug of choice for treating decreased salivation accompanying
head and neck irradiation is:
A. Physostigmine.
B. Scopolamine.
C. Carbachol.
D. Acetylcholine.
E. Pilocarpine.
Correct answer = E: Pilocarpine, taken orally, has proven to be
beneficial in this situation. All the others choices except scopolamine
are cholinergic agonists. However, their ability to stimulate salivation is
less than that of pilocarpine, and their other effects are more
troublesome.

91
3. Cholinergic antagonists:
Q1. A 75-year-old man who was a smoker is diagnosed with chronic
obstructive pulmonary disease and suffers from occasional
bronchospasm. Which of the following would be effective in treating
him?
A. Ipratropium aerosol.
B. Scopolamine patches.
C. Mecamylamine.
D. Oxygen.
E. Nicotine.
Correct answer = A: This is a drug of choice, especially in a patient who
cannot tolerate an adrenergic agonist, which would dilate the
bronchioles. Scopolamine's main effect is atropinic, and it is the most
effective anti–motion sickness drug. Mecamylamine is a ganglionic
blocker and completely inappropriate in this situation. Oxygen would
improve aeration but would not dilate the bronchial musculature.
Nicotine would exacerbate his condition.
Q2. Which of the following may precipitate an attack of open-angle
glaucoma if instilled into the eye?
A. Physostigmine.
B. Atropine.
C. Pilocarpine.
D. Echothiophate.
E. Tropicamide.
Correct answer = B: The mydriatic effect of atropine can result in the
narrowing of the canal of Schlemm leading to an increase in intraocular
pressure. Physostigmine, pilocarpine, and echothiophate would cause
miosis. Tropicamide produces mydriasis without increasing intraocular
pressure because of its shorter duration of action.

92
Q3. The prolonged apnea sometimes seen in patients who have
undergone an operation in which succinylcholine was used as a muscle
relaxant has been shown to be due to:
A. Urinary atony.
B. Depressed levels of plasma cholinesterase.
C. A mutation in acetylcholinesterase.
D. A mutation in the nicotinic receptor at the neuromuscular junction
E. Weak histamine-releasing action.
Correct answer = B: These patients have a genetic deficiency of the
nonspecific plasma cholinesterase that is required for the termination
of succinylcholine's action. The other choices would not produce apnea.
Q4. A 50-year-old male farm worker is brought to the emergency room.
He was found confused in the orchard and since then has lost
consciousness. His heart rate is 45, and his blood pressure is 80/40 mm
Hg. He is sweating and salivating profusely. Which of the following
treatments is indicated?
A. Physostigmine.
B. Norepinephrine.
C. Trimethaphan.
D. Atropine.
E. Edrophonium.
Correct answer = D: The patient is exhibiting signs of cholinergic
stimulation. Because he is a farmer, insecticide poisoning is a likely
diagnosis. Thus, either intravenous or intramuscular doses of atropine
are indicated to antagonize the muscarinic symptoms. Physostigmine
and edrophonium are cholinesterase inhibitors and would exacerbate
the problem. Norepinephrine would not be effective in combating the
cholinergic stimulation. Trimethaphan, being a ganglionic blocker,
would also worsen the condition.

93
Q5. Nondepolarizing neuromuscular blockers are associated with all of
the following except:
A. Initial activation of ACh receptor and depolarization of the motor end
plate.
B. Effects are reversed by acetylcholinesterase inhibitors.
C. Intermediate to long duration of action.
D. Bind but do not activate ACh receptor.
E. Most of these agents have minimal cardiovascular effects.
Correct answer = A. Activation of the ACh receptor is attributed to
depolarizing agents (succinylcholine). B, C, and D are true for
nondepolarizing agents. Pancuronium may cause tachycardia and
hypertension; rocuronium and vecuronium have favorable
cardiovascular safety profiles.

94
4. Adrenergic drugs:
Q1. A 68-year-old man presents to the emergency department
with acute heart failure. The patient requires immediate drug therapy to
improve his cardiac function. Which one of the following drugs would be
most beneficial?
A. Albuterol.
B. Dobutamine.
C. Epinephrine.
D. Norepinephrine.
E. Phenylephrine.
Correct answer = B: Dobutamine increases cardiac output without
significantly increasing heart rate, a complicating condition in heart
failure. Because epinephrine can significantly increase heart rate, it is
not typically used for acute heart failure. Both nor-epinephrine and
phenylephrine have significant α1-receptor–stimulating properties. The
subsequent increase in blood pressure would worsen the heart failure.
Albuterol, a β2-selective–receptor agonist, would not significantly
improve contractility of the heart.
Q2. Remedies for nasal stuffiness often contain which one of the
following drugs?
A. Albuterol.
B. Atropine.
C. Epinephrine.
D. Norepinephrine.
E. Phenylephrine.
Correct answer = E. Phenylephrine is an a1 agonist that constricts the
nasal mucosa, thereby decreasing airway resistance. Norepinephrine
and epinephrine also constrict the mucosa but have much too short a
duration of action. Albuterol is a β2 agonist and has no effect on
mucosal volume. Atropine, a muscarinic antagonist, only dries the
mucosa but does not decrease its volume

95
Q3. Which one of the following drugs, when administered intravenously,
can decrease blood flow to the skin, increase blood flow to skeletal
muscle, and increase the force and rate of cardiac contraction?
A. Epinephrine.
B. Isoproterenol.
C. Norepinephrine.
D. Phenylephrine.
E. Terbutaline.
Q4. Which of the following is most likely to be Drug X?
A. Physostigmine.
B. Acetylcholine.
C. Terbutaline.
D. Phenylephrine.
E. Isoproteren.
Correct answer = D: Phenylephrine is the only drug in the list that
causes mydriasis, because it stimulates α receptors. Both
physostigmine and acetylcholine cause pupillary constriction. The β-
blockers terbutaline and isoproterenol do not influence pupillary
diameter.

96
5. Analgesic drugs:
Q1. In which one of the following conditions would aspirin be
contraindicated?
A. Myalgia.
B. Fever.
C. Peptic ulcer.
D. Rheumatoid arthritis.
E. Unstable angina.
Correct answer = C: Among the non-steroidal anti-inflammatory drugs,
aspirin is one of the worst for causing gastric irritation. Aspirin is an
effective analgesic and is used to reduce muscle pain. It also has
antipyretic actions, so it can be used to treat fever. Because of its anti-
inflammatory properties, aspirin is used to treat pain related to the
inflammatory process (for example, in the treatment of rheumatoid
arthritis). Low doses of aspirin also decrease the incidence of transient
ischemic attacks.
Q2. Which one of the following statements concerning COX-2 inhibitors
is correct?
A. The COX-2 inhibitors show greater analgesic
activity than traditional NSAIDs.
B. The COX-2 inhibitors decrease platelet function.
C. The COX-2 inhibitors do not affect the kidney.
D. The COX-2 inhibitors show anti-inflammatory activity similar to that
of the traditional NSAIDs.
E. The COX-2 inhibitors are cardioprotective.
Correct answer = D: The COX-2 inhibitors show similar analgesic and
anti-inflammatory activity compared to traditional NSAIDs. They do not
affect platelets. Like NSAIDs, COX-2 inhibitors may cause the
development of acute renal failure due to renal vasoconstriction. COX-2
inhibitors have the potential for increasing the risk of myocardial
infarction.

97
Q3. An 8-year-old girl has a fever and muscle aches from a presumptive
viral infection. Which one of the following drugs would be most
appropriate to treat her symptoms?
A. Acetaminophen.
B. Aspirin.
C. Celecoxib.
D. Codeine.
E. Indomethacin.
Correct answer = A: Aspirin should be avoided in children because of an
association with Reye’s syndrome. Indomethacin has antipyretic
activity but is too toxic for use in these circumstances. Celecoxib is
indicated for alleviation of pain, and codeine has no antipyretic effects.
Q4. A 70-year-old man has a history of ulcer disease. He has recently
experienced swelling and pain in the joints of his hands. His physician
wants to begin therapy with an NSAID. Which one of the following drugs
might also be prescribed along with the NSAID to reduce the risk of
activating this patient’s ulcer disease
A. Allopurinol.
B. Colchicine.
C. Misoprostol.
D. Probenecid.
E. Sulindac.
Correct answer = C: Misoprostol is a prostaglandin analog that can
reduce gastric acid and pepsin secretion and promote the formation of
mucus in the stomach. It is indicated for the purpose of decreasing the
risk of ulcer activation in patients taking NSAIDs. The other choices are
not appropriate for alleviating the gastric irritation caused by NSAIDs.
Q5. Which of the following is a potent analgesic but a weak anesthetic?
A. Etomidate.
B. Halothane.
C. Midazolam.
D. Nitrous oxide.
E. Thiopental
Correct answer = D: Etomidate is a hypnotic agent but lacks analgesic
activity. Midazolam is a common sedative / amnestic. Halothane and
thiopental are potent anesthetics with weak analgesic properties
Nitrous oxide provides good analgesia but is a weak anesthetic that
must be combined with other agents to provide complete anesthesia
.

98
Q6. Which one of the following is a potent intravenous anesthetic but a
weak analgesic?
A. Propofol.
B. Benzodiazepines.
C. Ketamine.
D Etomidate.
E. Isoflurane.
Correct answer = A: Propofol is a potent anesthetic but a weak
analgesic. It is the most widely used intravenously administered
general anesthetic. It has a high lipid solubility. The other choices do
not fit this profile.

99
6. Local Anesthetics:
Q1. Local anesthetics:
A. Affect only small, unmyelinated nerve fibers.
B. Have either a lipophilic or a hydrophilic group.
C. Have either an amide or an ester linkage.
D. Are unaffected by pH of the tissue and pKa of the drug.
E. In their ionized form interact with the protein receptor of calcium
channels.
Correct answer = C: The small, unmyelinated nerve fibers that conduct
impulses for pain, temperature, and autonomic activity are most
sensitive to the action of local anesthetics, but other nerve fibers are
affected also. Local anesthetics have a lipophilic group, joined by either
an amide or ester linkage to a carbon chain which, in turn, is joined to a
hydrophilic group. Onset and duration of action of local anesthetics are
influenced by both pH of the tissue and pKa of the drug. Local
anesthetics work by blocking sodium ion channels.
Q2. A young man is brought into the emergency room. He is
unconscious, and he has pupillary constriction and depressed
respiration. You note needle marks on his legs. You administer
naltrexone, and he awakens. This agent was effective because:
A. The patient was suffering from an overdose of a benzodiazepine.
B. Naltrexone antagonizes opiates at the receptor site.
C. Naltrexone is a stimulant of the central nervous system.
D. Naltrexone binds to the opioid and inactivates it.
E. The patient was was suffering from an overdose of meperidine.
Correct answer = B: The indications are that the patient is suffering
from an overdose of an opioid such as heroin. Naltrexone antagonizes
the opioid by displacing it from the receptor. It is used in preference to
naloxone, because it is longer acting and, thus, can act as long as the
opiate is in the body. Meperidine causes the pupils to dilate.

100
Q3. A heroin addict has entered a rehabilitation program that requires
that she take methadone. Methadone is effective in this situation
because it:
A. Is an antagonist at the morphine receptors.
B. Is a non-narcotic.
C. Is longer acting than heroin, conferring milder
withdrawal than with the latter drug.
D. Does not cause constipation.
E. Is non-addictive.
Correct answer = C: Methadone is used in rehabilitation programs as a
substitute for heroin. It has similar euphorigenic and analgesic activity,
is orally active, and can be easily controlled. Most important, it is long
acting, and the withdrawal the patient undergoes as she is being
weaned off the drug is much milder than would be the case with
heroin. Methadone is a synthetic, orally effective opioid that acts at the
μ receptors. It does cause constipation and can be addictive.
Q4. Which of the following statements about morphine is correct?
A. It is used therapeutically to relieve pain caused by severe head injury.
B. Its withdrawal symptoms can be relieved by naloxone.
C. It causes diarrhea.
D. It is most effective by oral administration.
E. It rapidly enters all body tissues, including the fetus.
Correct answer = E: Morphine causes increased cerebrospinal fluid
pressure secondary to dilation of cerebral vasculature and is
contraindicated in severe head injury. Naloxone is an opioid antagonist
and can precipitate withdrawal symptoms in morphineaddicted
individuals. Morphine is administered parenterally, because absorption
from the gastrointestinal tract is unreliable. It causes constipation.

101
Q5. The pain of a patient with bone cancer has been managed with a
morphine pump. However, he has become tolerant to morphine. Which
of the following might be indicated to ameliorate his pain?
A. Meperidine.
B. Codeine.
C. Fentanyl.
D. Methadone.
E. Buprenorphine.
Correct answer= C: Fentanyl is used in anesthesia. It produces analgesia
and is usually injected epidurally. However, its analgesic action is also
beneficial in cancer patients. It is available as a transdermal patch and an
oral transmucosal preparation. Meperidine and codeine show cross-
tolerance with morphine and, thus, would not be effective.
Buprenorphine, like methadone, is used in opiate detoxification and
could precipitate withdrawal.
