1
Respiratory distress syndrome (Hyaline membrane disease);
Incidence is inversely related to gestational age and birth weight. It occurs in 60-80% of infants
less than 28 wk of gestational age, in 15-30% of those between 32 and 36 wk, in about 5% beyond
37 wk, and rarely at term.
The risk of developing RDS increases with; maternal diabetes, multiple births, cesarean section
delivery, precipitous delivery, asphyxia, cold stress, and history of previously affected infants.
The risk of RDS is reduced in; pregnancies with chronic or pregnancy-associated hypertension,
maternal heroin use, prolonged rupture of membranes, and antenatal corticosteroid prophylaxis.
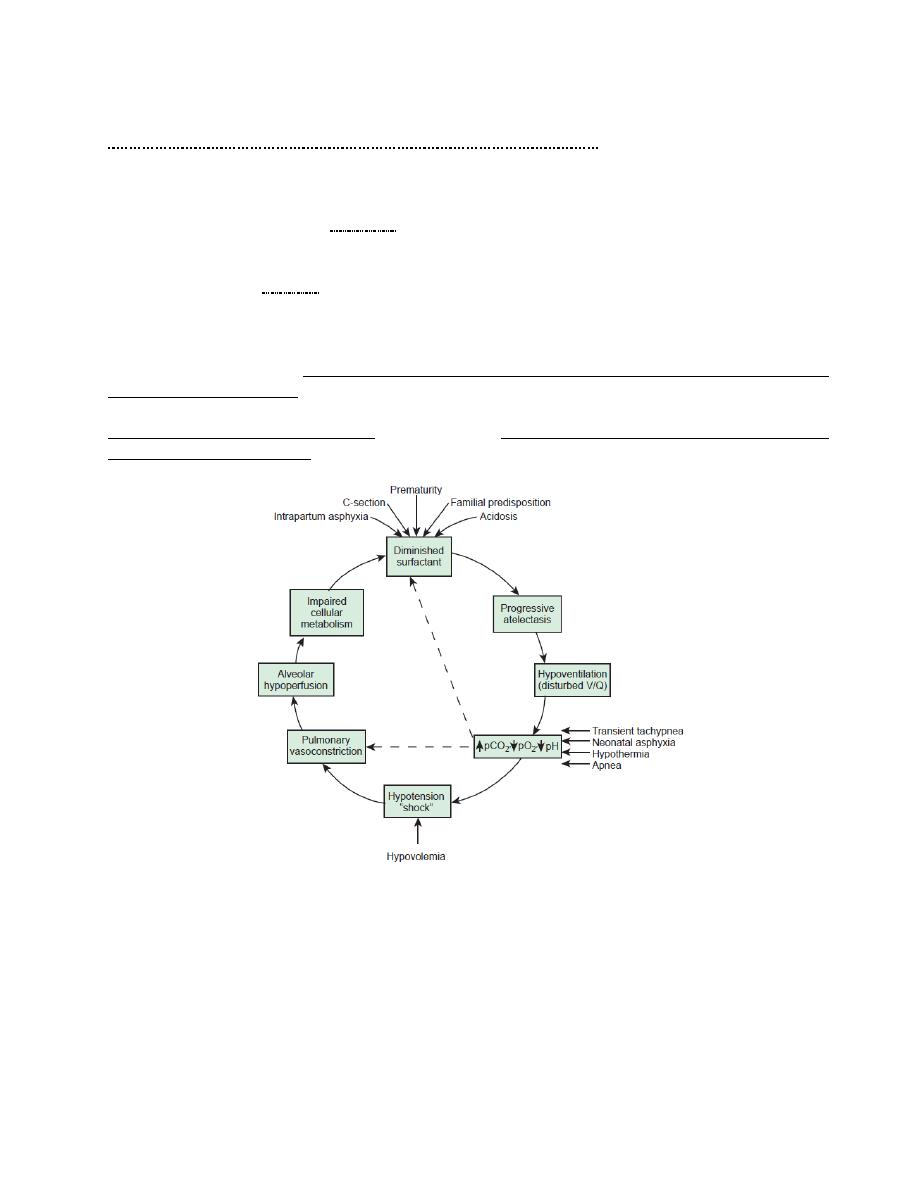
Etiology; decreased production and secretion of surfactant (which is released into the alveoli from
type II alveolar cells to reduce surface tension and maintain alveolar stability by preventing
collapse at end expiration) is the primary cause of RDS, thus the lungs are prone for atelectasis.
Mature levels of pulmonary surfactant are usually present after 35 wk of gestation.
Synthesis of surfactant is suppressed in cases of; asphyxia, hypoxemia, pulmonary ischemia,
hypotension, and cold stress.
Contributing factors to Hyaline membrane disease
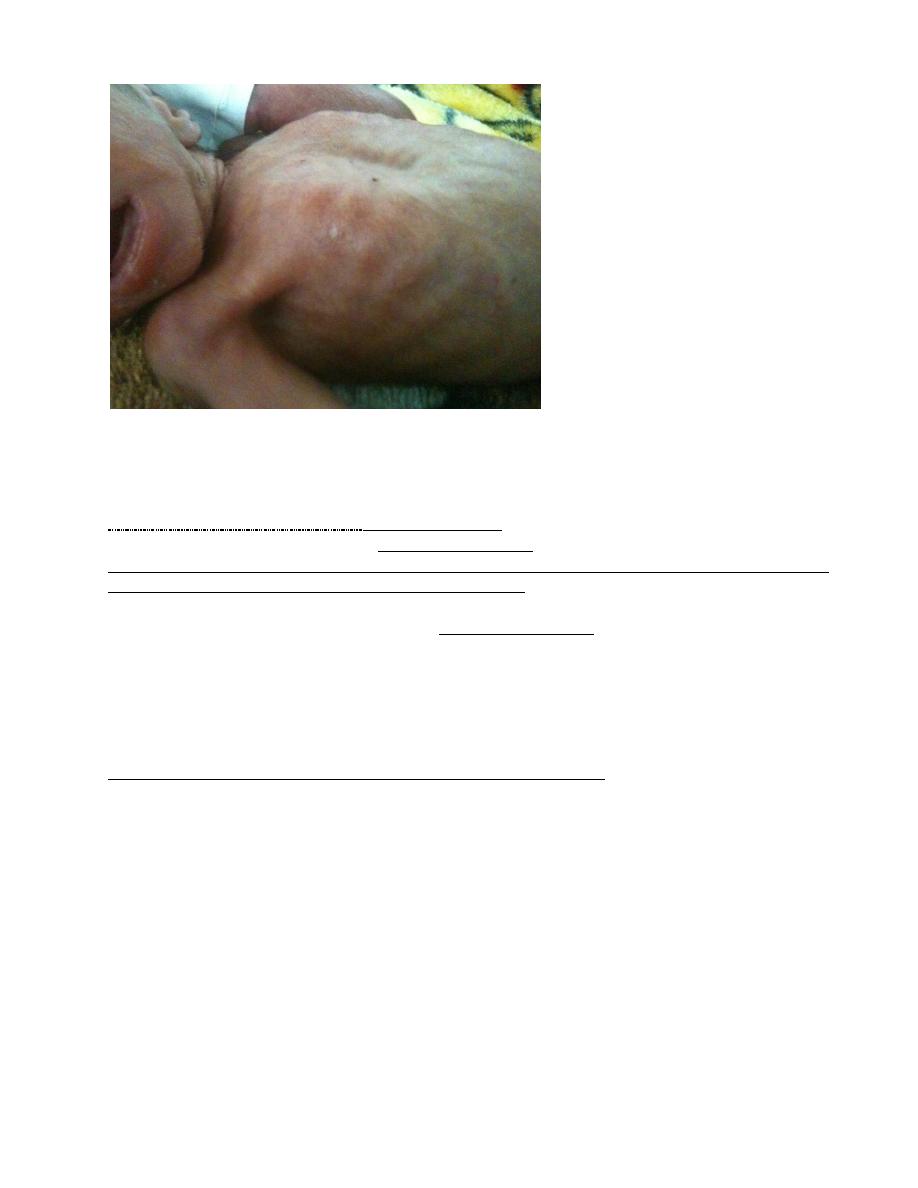
Clinical presentation; tachypnea and SOB usually appear within minutes of birth, yet it could
appear in hours in larger premature infants. Late onset of tachypnea should suggest other
conditions. Some patients require resuscitation at birth because of intrapartum asphyxia.
Characteristically, tachypnea, grunting, intercostal and subcostal retractions, nasal flaring, and
duskiness are noted. Cyanosis increases and is often relatively unresponsive to oxygen
administration.
2
Breath sounds may be normal or diminished with a harsh tubular quality, fine crepitations may be
heard over the lung bases.
If untreated, blood pressure may fall, gets fatigue, cyanosis, increasing pallor, and grunting
decreases.
Apnea and irregular respirations are ominous signs, requiring immediate intervention.
The symptoms and signs reach a peak within 3 days, after which improvement is gradual.
Improvement is often
1
heralded by spontaneous diuresis and the
2
ability to oxygenate the infant at
lower inspired oxygen levels or lower ventilator pressures.
Death; is rare on the first day, usually occurs between days 2 and 7, and is associated with
1
alveolar
air leaks (interstitial emphysema, pneumothorax),
2
pulmonary hemorrhage, or
3
intraventricular
hemorrhage.
Diagnosis;
1.The clinical course
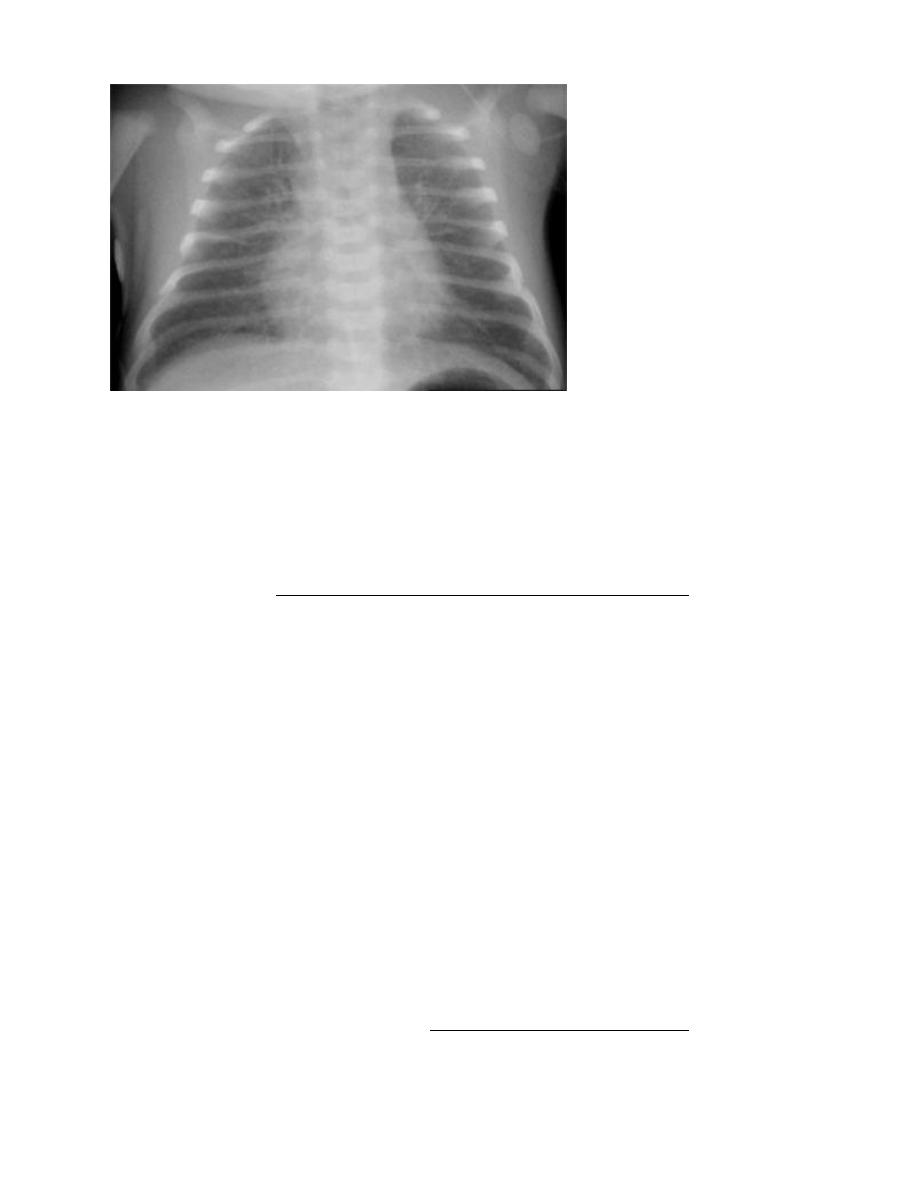
2. X-ray of the chest (the lungs may have a characteristic, but not pathognomonic appearance of
fine reticular granularity of the parenchyma and air bronchograms. The initial roentgenogram is
occasionally normal, with the typical pattern developing at 6-12 hr
3. blood gas and acid-base values (hypoxemia, hypercapnia and metabolic acidosis) help establish
the diagnosis.
3
Differential diagnosis; includes; early onset sepsis, cyanotic congenital heart disease, meconium
aspiration, persistent pulmonary hypertension, diaphragmatic hernia, pulmonary lobar emphysema,
transient tachypnea of the newborn.
Prevention;
1. avoid unnecessary C/S.
2. avoid premature deliveries.
3. administration of betamethasone to women 48 hr before the delivery between 24 and 34 wk of
gestation significantly reduces the incidence, severity, mortality and morbidity of RDS. Repeated
weekly doses of betamethasone until 32 wk, may reduce neonatal morbidities and the duration of
mechanical ventilation.
4. continues positive airway pressure ventilation (CPAP) started at birth is as effective as
prophylactic or early surfactant and it is the approach of choice for management of preterm at risk
for RDS.
Treatment;
A. Supportive
1. Admission to neonatal intensive care unit (NICU).
2. Early supportive care of low birth weight (LBW) infants and treatment of acidosis, hypoxia,
hypotension and hypothermia.
3. Careful monitoring of heart rate, respiratory rate, blood gas and acid-base values,
electrolytes, blood sugar, vital signs, HCT.
4. Calories and fluids should initially be provided intravenously. Subsequently, electrolytes
should be added and fluid volume increased gradually.
B. Oxygen
1. Humidified oxygen; should be provided initially at a concentration sufficient to keep arterial
levels at 91-95% saturation and minimizing the risk of oxygen toxicity.
If the PaO
2
cannot be maintained above >90% at inspired oxygen concentrations of 40-70% or
greater then,
2. CPAP at a pressure of 5-10 cm H
2
O (continuous positive airway pressure) by nasal prongs is
indicated and usually produces a sharp rise in PaO
2
. Infants with severe RDS and those with
complications that result in persistent apnea will require assisted mechanical ventilation.
4
C. Surfactant
Rescue treatment is initiated with endotracheal surfactant soon after birth within the 1
st
24hr of
life of 2-4 doses will reduce the severity of RDS.
Multi-dose endotracheal installation of exogenous surfactant to VLBW infants improves
survival and reduces the incidence of pulmonary air leaks.
Complications; include;
1
complications of tracheal intubation (asphyxia from obstruction of the tube, cardiac arrest during
intubation or suctioning, development of subglottic stenosis), bleeding from trauma during
intubation, persistent hoarseness, stridor or edema of the larynx
2
risks associated with umbilical arterial catheterization (vascular embolization, thrombosis and
vascular perforation), risks associated with umbilical vein catheterization
3
bronchopulmonary dysplasia.
Neonatal birth injures
Erythema, abrasions, ecchymoses, and subcutaneous fat necrosis of facial or scalp soft tissues may
be noted after forceps or vacuum-assisted deliveries.
Subconjunctival and retinal hemorrhages are frequent.
Fractures of the skull; may occur as a result of pressure from forceps or from the maternal
symphysis pubis, sacral promontory. Linear fractures, the most common, cause no symptoms and
require no treatment. Depressed fractures are generally a complication of forceps delivery. Affected
infants may be asymptomatic unless they have associated intracranial injury.
Cranium injuries
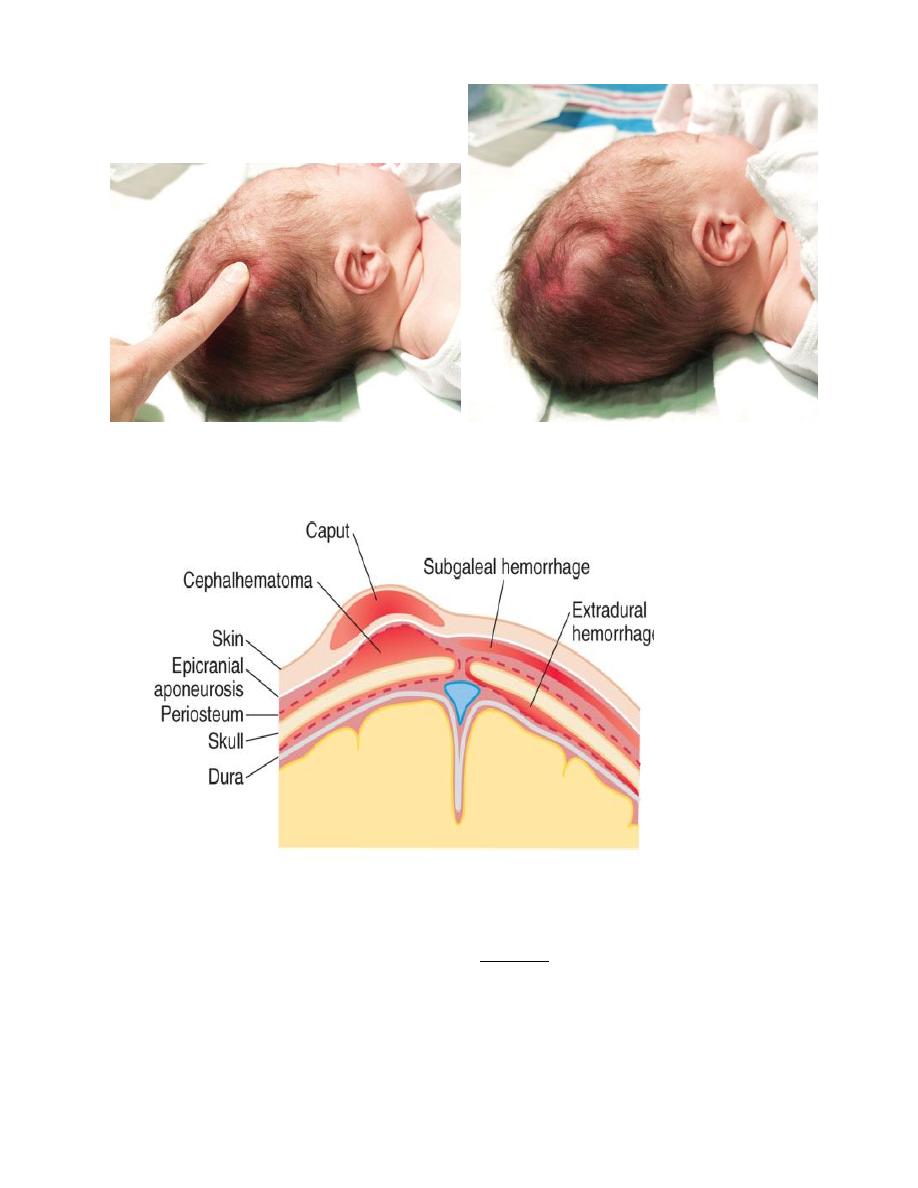
Caput succedaneum; is a diffuse, sometimes ecchymotic, edematous swelling of the soft tissues of
the scalp involving the area presenting during vertex delivery. It may extend across the midline and
across suture lines.
The edema disappears within the 1
st
few days of life. Molding of the head and overriding of the
parietal bones are frequently associated, and become more evident after the caput has receded; they
disappear during the 1st weeks of life.
5
Cephalohematoma; is a subperiosteal hemorrhage, so always limited to the surface of one cranial
bone.
Cephalohematomas occur in 1-2% of live births. No discoloration of overlying scalp occurs, and
swelling is not usually visible for several hours after birth because subperiosteal bleeding is a slow
process. The lesion becomes a firm tense mass with a palpable rim localized over 1 area of the
skull. Most cephalohematomas are resorbed within 2wk-3mo, depending on their size.

6
They begin to calcify by the end of the 2nd week. A few remain for years as bony protuberances
and are detectable by x-ray as widening of the diploic space. An underlying skull fracture, usually
linear and not depressed, may be associated with 10-25% of cases. A sensation of central
depression suggesting but not indicative of an underlying fracture or bony defect is usually
encountered on palpation of the organized rim of a cephalohematoma.
Cephalohematomas require no treatment, although phototherapy may be necessary to treat
hyperbilirubinemia.
Nerve injuries
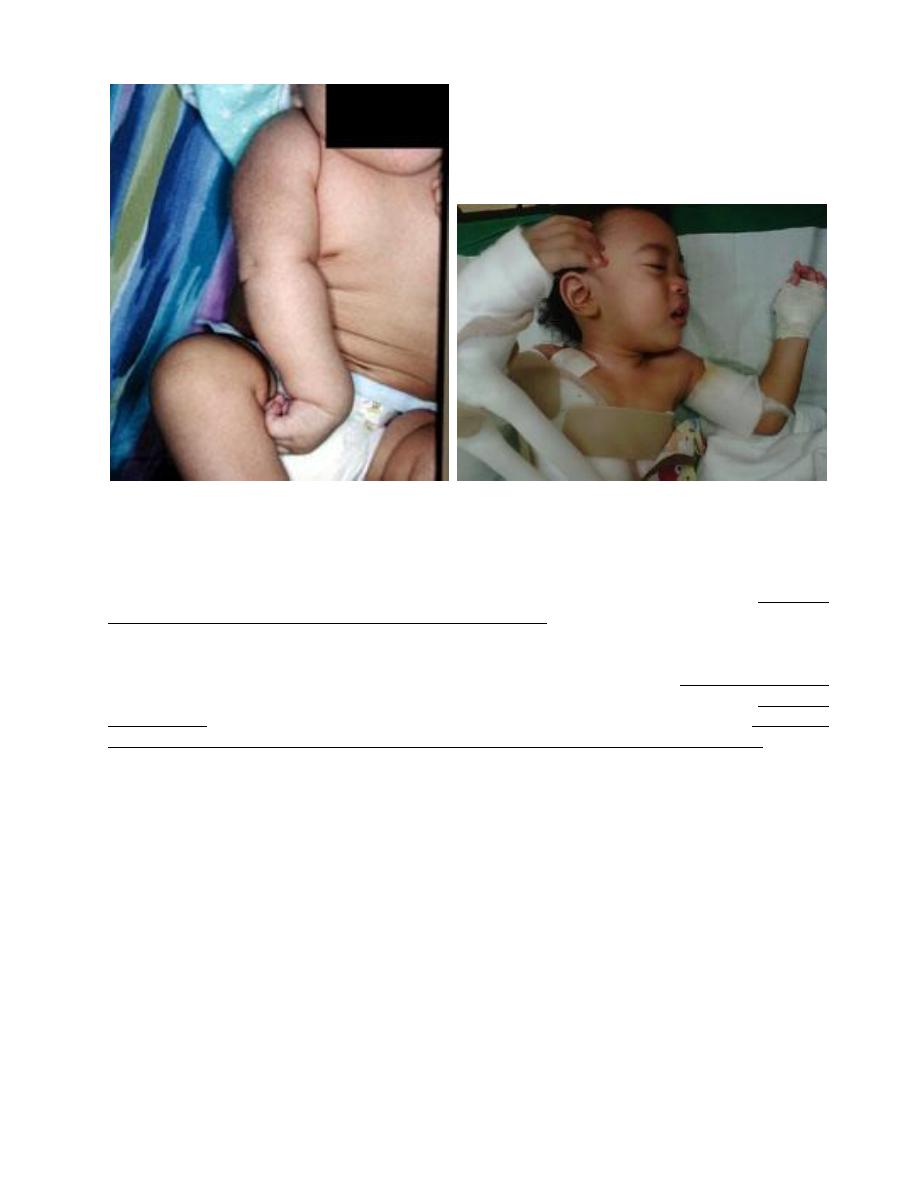
Brachial palsy; injury most common in macrosomic babies, lateral traction of head & neck during
delivery of head in vertex presentation, and when the arms are extended over the head in a breech
presentation with extreme shoulder traction.
Erb-Duchenne paralysis; the injury is limited to C
5
& C
6
. The infant loses the power to
1
abduct the
arm from the shoulder,
2
rotate the arm externally, and
3
supinate the forearm. The characteristic
position consists of adduction and internal rotation of the arm with pronation of the forearm.
7
Power to extend the forearm is retained, but the biceps reflex is absent; the Moro reflex is absent on
the affected side. Power in the forearm and hand grasp is preserved unless the lower part of the
plexus is also injured; the presence of hand grasp is a favorable prognostic sign.
Klumpke paralysis; is a rare form of brachial palsy; injury to the C
7
, C
8
& T
1
produces a paralyzed
hand and ipsilateral ptosis and miosis (Horner syndrome) if the sympathetic fibers of the 1st
thoracic root are also injured.
MRI demonstrates nerve root rupture or avulsion. If the paralysis was due to edema (neurapraxia)
about the nerve fibers, function should return within a few weeks-months; if due to laceration
(neurotmesis), permanent damage may result and microsurgical repair may be indicated. In general,
paralysis of the upper part of the arm has a better prognosis than paralysis of the lower part.
Treatment: consists of partial immobilization to prevent the development of contracture; the arm
should be abducted 90 degrees with external rotation at the shoulder, full supination of the forearm,
and slight extension at the wrist with the palm turned toward the face. This position may be
achieved with a brace or splint during the 1st 1-2 wk. In lower arm or hand paralysis, the wrist
should be splinted in a neutral position and padding placed in the fist. Gentle massage and range-of-
motion exercises may be started by 7-10 days of age. If persist by 3-6 mo, neuroplasty is done.
Phrenic nerve injury; (3
rd
, 4
th
, 5
th
cervical nerves) with diaphragmatic paralysis must be considered
when cyanosis and irregular and labored respirations develop.
Facial palsy; is usually a peripheral paralysis that results from pressure over the facial nerve in
utero, or from forceps use during delivery.
Fractures
The clavicle is fractured during labor and delivery more frequently than any other bone; it is
particularly vulnerable with difficult delivery of the shoulder in vertex presentations and the

8
extended arms in breech deliveries. The infant characteristically does not move the arm freely
(pseudoparalysis) on the affected side; crepitus and bony irregularity may be palpated, and
discoloration. The Moro reflex is absent on the affected side. The prognosis is excellent. Treatment,
if any, consists of immobilization of the arm and shoulder on the affected side. A palpable callus
develops at the site within a week and may be the initial evidence of the fracture. Fracture of the
humerus or brachial palsy may also be responsible for limitation of movement of an arm and
absence of a Moro reflex on the affected side. The Moro reflex is often absent from the involved
extremity. Femur fracture is associated with shortening, limitation of active motion, painful passive
motion, and external rotation.
Hypoxia-ischemia
Anoxia; is the consequences of complete lack of oxygen.
Hypoxia; decreased arterial concentration of oxygen.
Ischemia; insufficient blood flow to cells or organs that to maintain their normal function.
Asphyxia; inadequate tissue perfusion, which fails to meet the metabolic demands of the tissues for
oxygen and waste removal.
Hypoxic-ischemic encephalopathy (HIE);
Is an important cause of permanent damage to CNS tissues that may result in neonatal death or manifest
later as cerebral palsy or developmental delay.
Fifteen to 20% of infants with hypoxic-ischemic encephalopathy die in the neonatal period, and 25-30%
of survivors are left with permanent neurodevelopmental abnormalities (cerebral palsy, mental
retardation).
Etiology;
Fetal hypoxia may be caused by various disorders in the mother, including;
(1) inadequate oxygenation of maternal blood from hypoventilation during anesthesia, cyanotic heart
disease, respiratory failure (2) low maternal blood pressure from acute blood loss (3) Inadequate
relaxation of the uterus to permit placental filling as in excessive oxytocin; (4) premature separation of
the placenta (5) defected umbilical cord circulation as a result of compression or knotting of the cord (6)
placental insufficiency from toxemia or postmaturity.
After birth hypoxia may be caused by (1) failure of oxygenation as a result of severe forms of cyanotic
congenital heart disease or severe pulmonary disease (2) anemia severe enough to lower the oxygen
content of the blood (severe hemorrhage, hemolytic disease); (3) shock severe enough to interfere with
the transport of oxygen to vital organs from overwhelming sepsis, massive blood loss, and intracranial
or adrenal hemorrhage.
Pathophysiology; the injury typically correlates to areas of decreased cerebral blood flow. After
hypoxia and ischemia, anaerobic metabolism occurs, which generates lactate and inorganic phosphates.
Increased amounts of intracellular sodium and calcium may result in tissue swelling and cerebral
edema. There is also increased production of free radicals and nitric oxide in these tissues.
The initial circulatory response of the fetus is increased shunting through the ductus arteriosus, and
foramen ovale, with transient maintenance of perfusion of the brain, heart, and adrenals in preference to
the lungs, liver, kidneys, and intestine. Congestion and petechiae in tissues develop.
Prolonged intrauterine hypoxia may result in periventricular leukomalacia(PVL). Pulmonary arterioles
smooth muscle hyperplasia may develop, predisposes the infant to pulmonary hypertension. If fetal

9
distress produces gasping, the amniotic fluid contents (meconium, squamous, lanugo) are aspirated into
the trachea or lungs.
Term infants demonstrate neuronal necrosis of the cortex (later cortical atrophy) and parasagittal
ischemic injury.
Preterm infants demonstrate periventricular leukomalacia (PVL) (later spastic diplegia), status
marmoratus of the basal ganglia, and intraventricular hemorrhage (IVH).
So terms more often than preterm infants have focal or multifocal cortical infarcts that clinically
manifest as focal seizures and hemiplegia.
