
Jabir Ibn Hayyan Medical College
Biochemistry
for 1
st
class students
Lecture 1
Introduction to biological molecules
March 9, 2017
DR. MONA ABDEL RIDHA AL-BARQAAWI
References
• Marks’ Basic Medical Biochemistry Chapter 6
• Lippincott’s Illustrated Reviews: Biochemistry Chapter 1
2
Glossary
Acid: A chemical that can dissociate to release
hydrogen ions (H
+
), i.e. it is a proton donor.
Amino acid: The building blocks of proteins. They are composed of a central carbon atom
attached to four other chemical groups: an amino group (-NH
2
), a carboxyl group (COOH), a
hydrogen atom and a variable group (R).
Amphipathic: A molecule that has both a polar (hydrophilic) and non-polar (hydrophobic)
end is said to be amphipathic.
Base: A chemical that can combine with a hydrogen ion (H
+
), i.e. it is a proton acceptor.
Buffer: A solution of a weak acid and its conjugate base that resists a change in pH when a
small amount of acid or alkali is added.
Covalent bond: A bond formed between two atoms by the sharing of electrons.
3

Glossary
Hydrogen bond: A weak electrostatic interaction between a hydrogen atom bound to an
electronegative atom (N, O) and another electronegative atom.
Hydrophilic: A polar molecule that is able to interact with water molecules is said to be
hydrophilic.
Hydrophobic: A non-polar molecule that is unable to interact with water is said to be
hydrophobic.
Ionic bond: A bond formed between two atoms where there is a complete transfer of an
electron resulting in the formation of two ions (one positive and one negative).
Isoelectric point: The pH at which a protein has no overall net charge.
Peptide bond: A type of covalent bond which joins amino acids in proteins. The bond forms
between the carboxyl group of one amino acid and the amino group of the second.
pH: A measurement of the concentration of H
+
ions in solution. pH = -log
10
[H
+
]
Protein: A polymer composed of amino acids joined by peptide bonds.
4
The Cell
Cells are the structural and functional units of all living organisms, they are
capable of carrying out all the activities necessary for life.
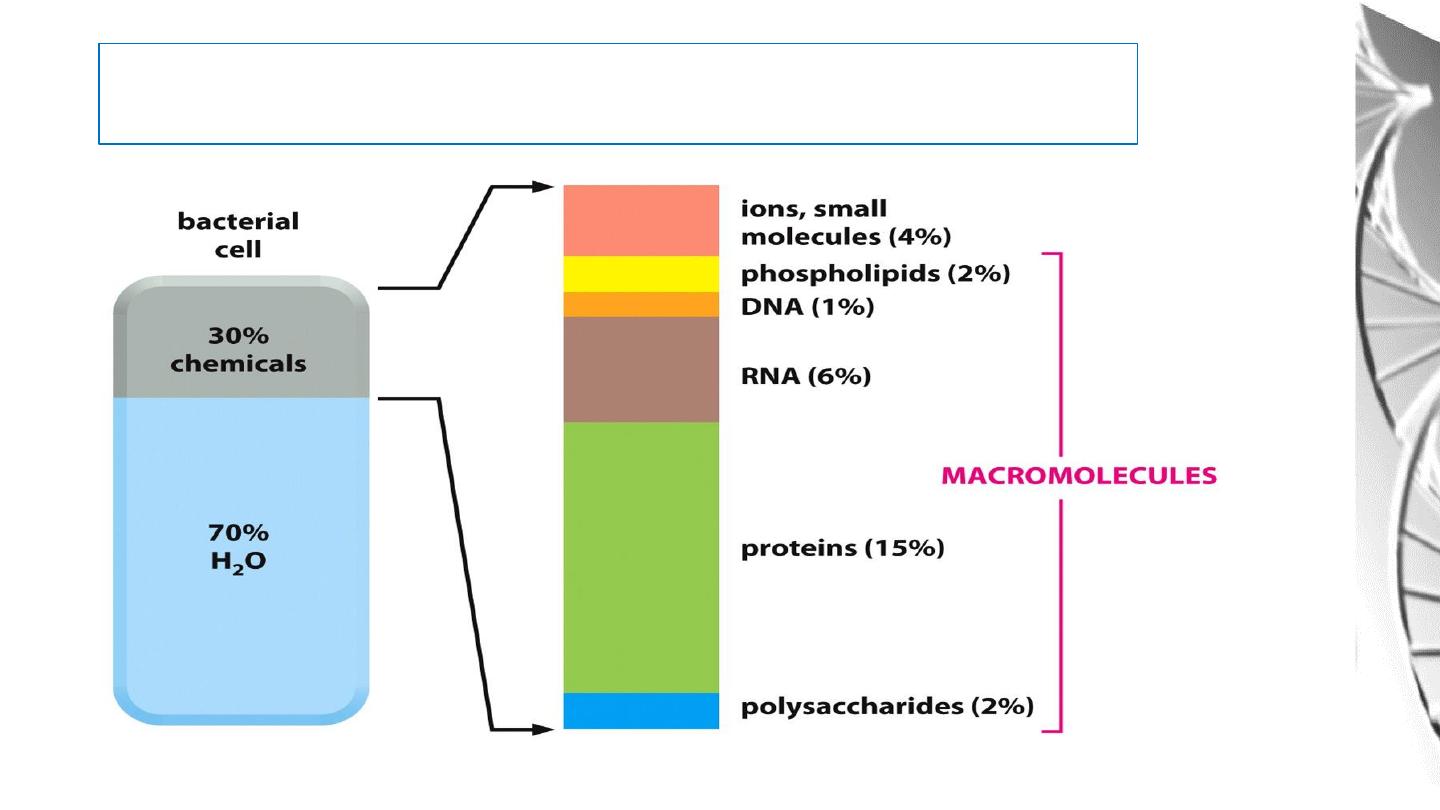
Cells are small, membrane enclosed units filled with a concentrated Aqueous
Solution of Chemicals and provide with the surprising ability to create copies
of themselves by growing and dividing in two.
5
Cell Components
1. Plasma membrane: It is a phospholipid bilayer that responsible for the
cell morphology and movement, and transport of ions and small molecules.
2. Cytosol: Liquid portion of cytoplasm
(cytoplasm composed of all materials contained within cytosol). Metabolism
of carbohydrates, amino acids and nucleotides, and fatty acid synthesis are
carried out in cytoplasm.
3. Organelles: Complex intracellular locations where processes necessary for
eukaryotic cellular life occur. Most organelles are membrane enclosed
structures, each organelle carries out a specific function.
6
Cell Components
7
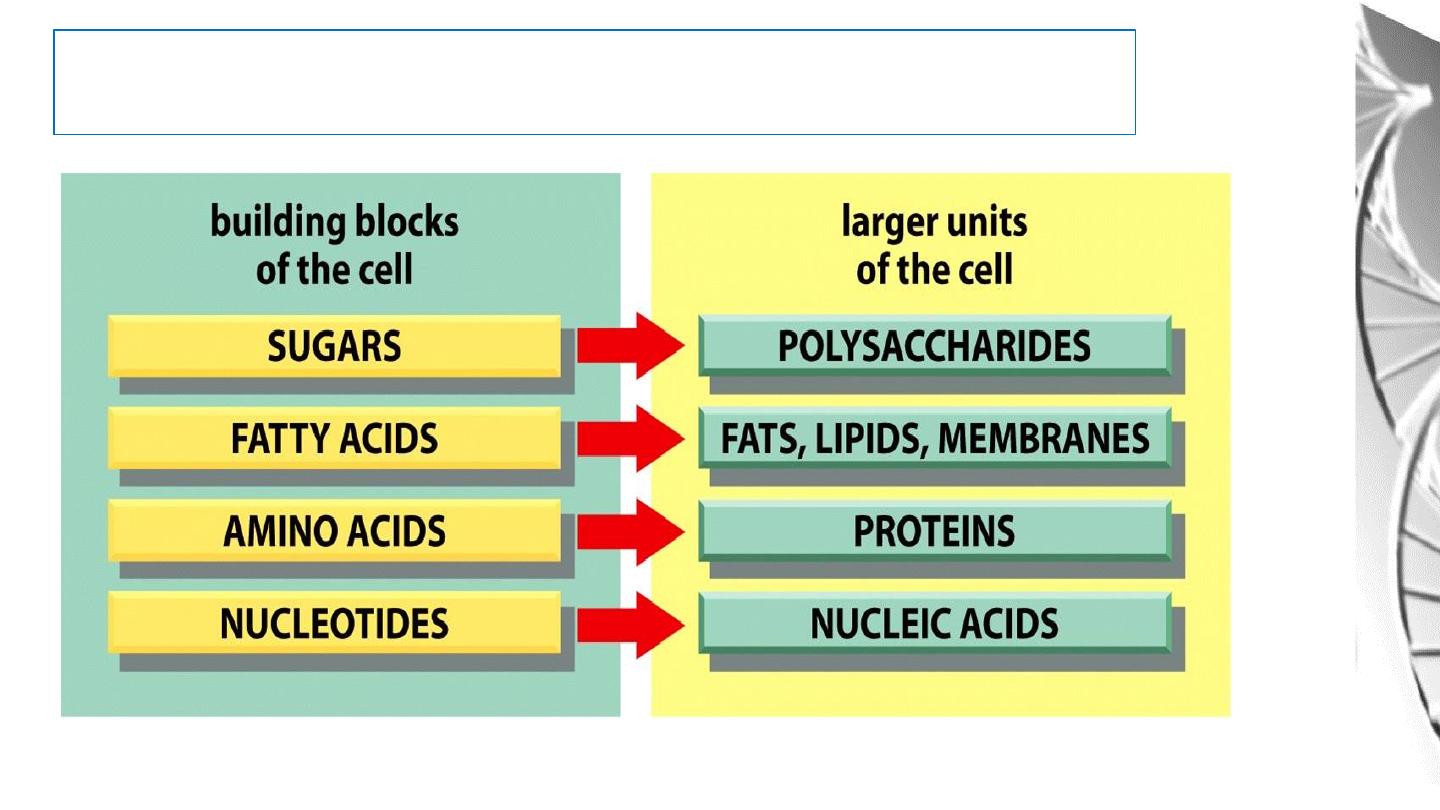
Macromolecules
8

Water, properties & functions
• Weak interactions are the key means by which molecules interact with one
another.
• The strength and specificity of weak interactions between biomolecules
are highly dependent on the medium (aqueous) in which they take place.
• Two properties of water are biologically important:
1.Water is polar molecule.
2.Water is highly cohesive (hydrogen bonds between water molecules).
•Water is the principal fluid medium of the cell, which is present in most cells,
in a concentration of 70 to 85 per cent. Many cellular chemicals are dissolved
in the water.
•Water Helps in regulation of temperature since it is able to absorb large
amounts of heat.
•Helps in regulation of intracellular pH since it is amphoteric solvent.
•Used for transport – delivers nutrients and removes waste from cells
9
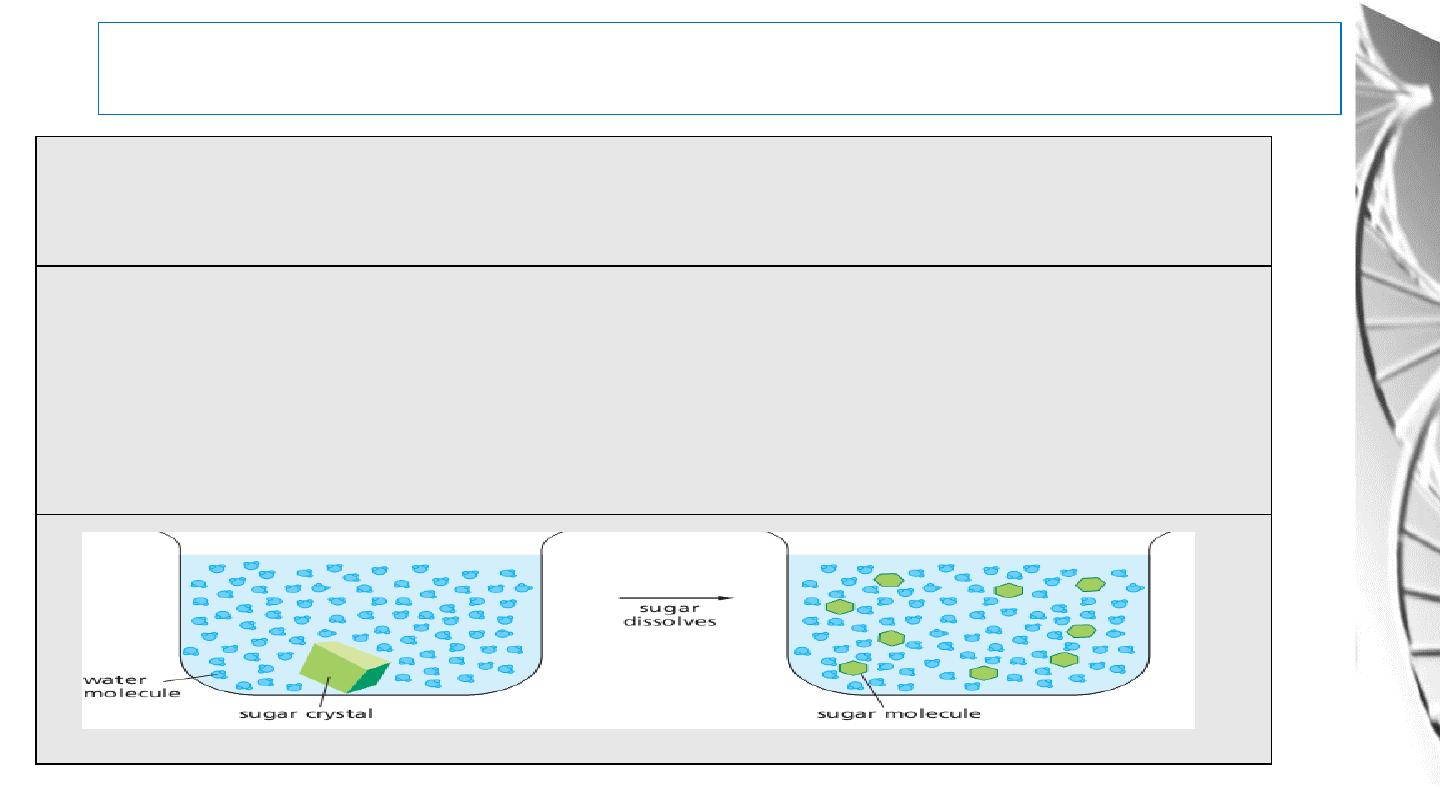
Water As A Solvent
According to the ability of water to dissolve their molecules, substances can be classified
in to Hydrophilic, Hydrophobic, and Amphipathic.
Hydrophilic molecules: substances that dissolve readily in water. They are composed of
ions or polar molecules that attract water molecules through electrical charge effects.
Water molecules surround each ion or polar molecule on the surface of such a solid and
carry it into solution.
10
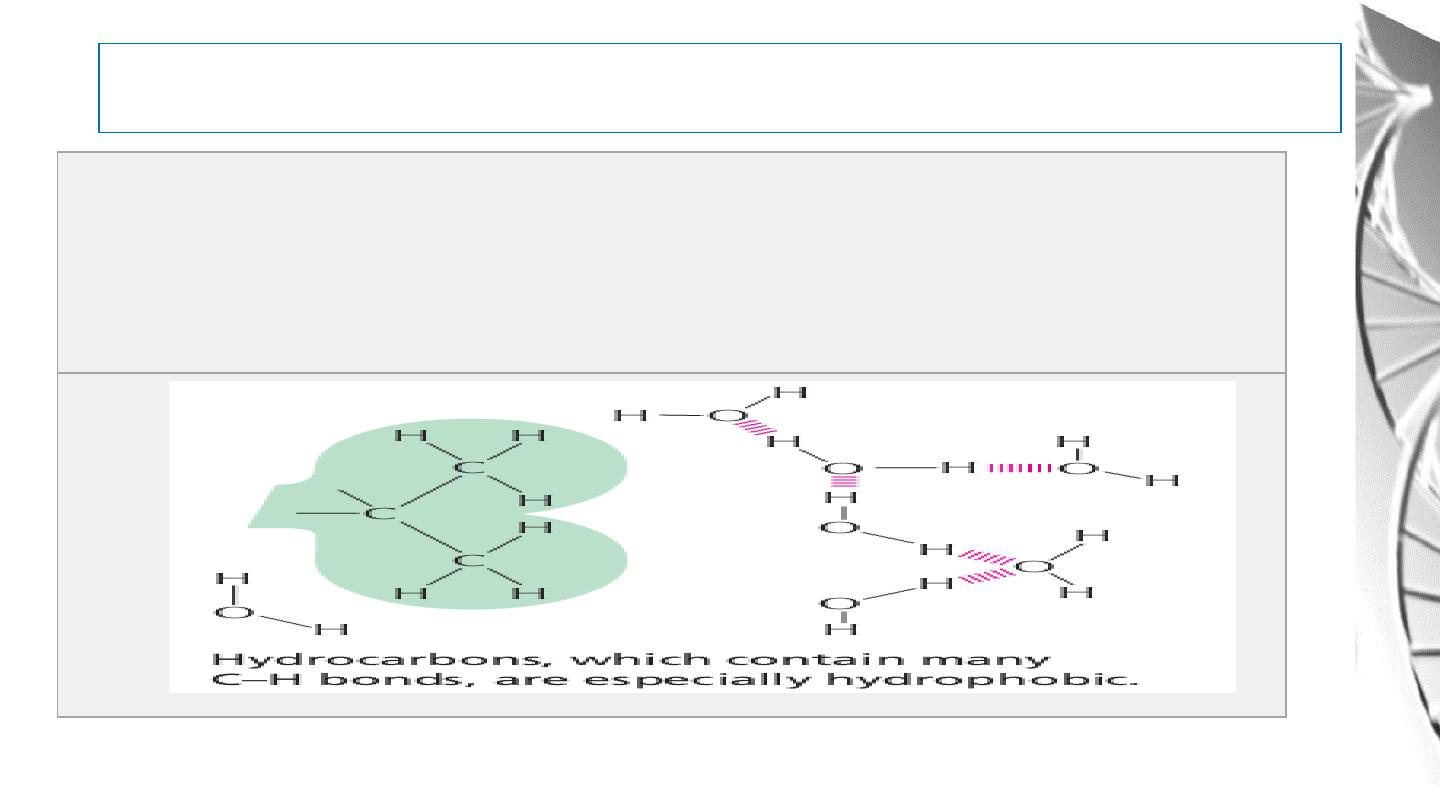
Water As A Solvent
Hydrophobic Molecules: Substances that contain nonpolar bonds and
insoluble in water. Water molecules are not attracted to their molecules and
so have little tendency to surround them and carry them into solution.
11
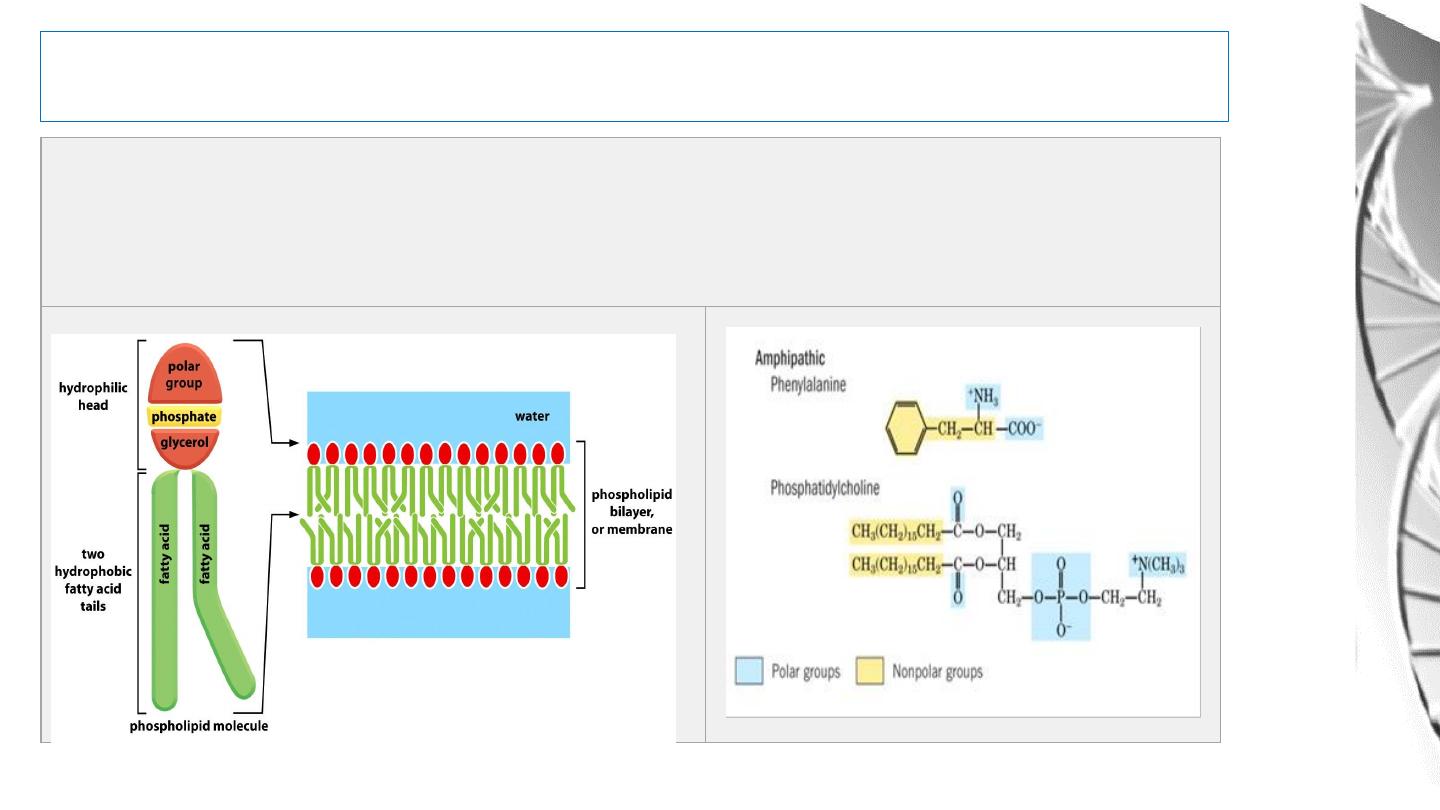
Water As A Solvent
Amphipathic Molecules:
Molecules that have both hydrophilic and
hydrophobic properties are said to be amphipathic
12
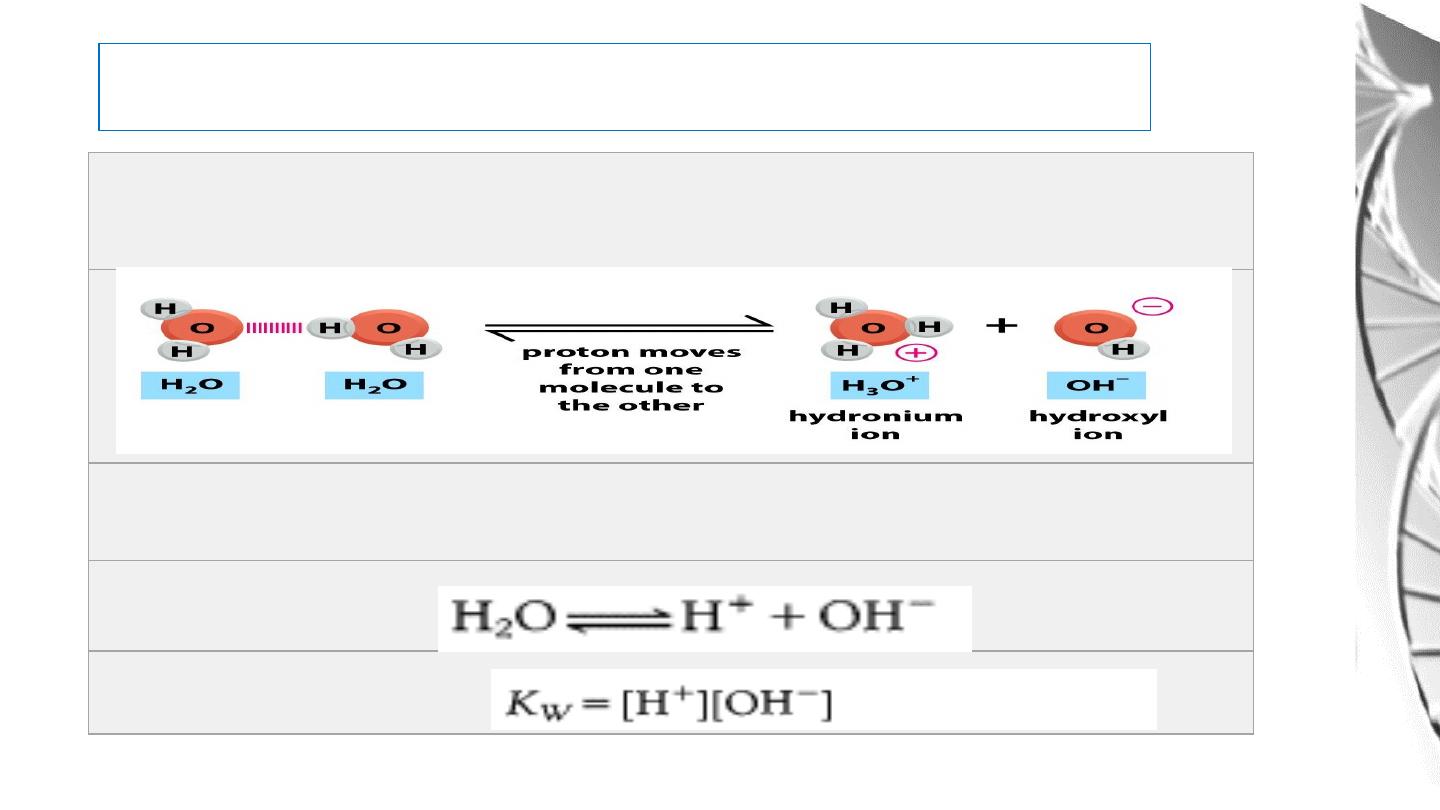
Water dissociates into hydronium (H3O+) and hydroxyl (OH-) ions
For simplicity, we refer to the hydronium ion as a hydrogen ion (H+)
and write the equilibrium as
Ionization of Water
13
Ionization of Water
K
w is the ion product of water.
At 25
°C,
K
w is
1.0
× 10
-14
.
Note that the con. of H
+
and
OH
-
are reciprocally related.
If the concentration of H+ is
high, then the concentration of
OH- must be low, and vice
versa.
For example, if [H+] = 10
-2
M,
then [OH-] = 10
-12
M.
14
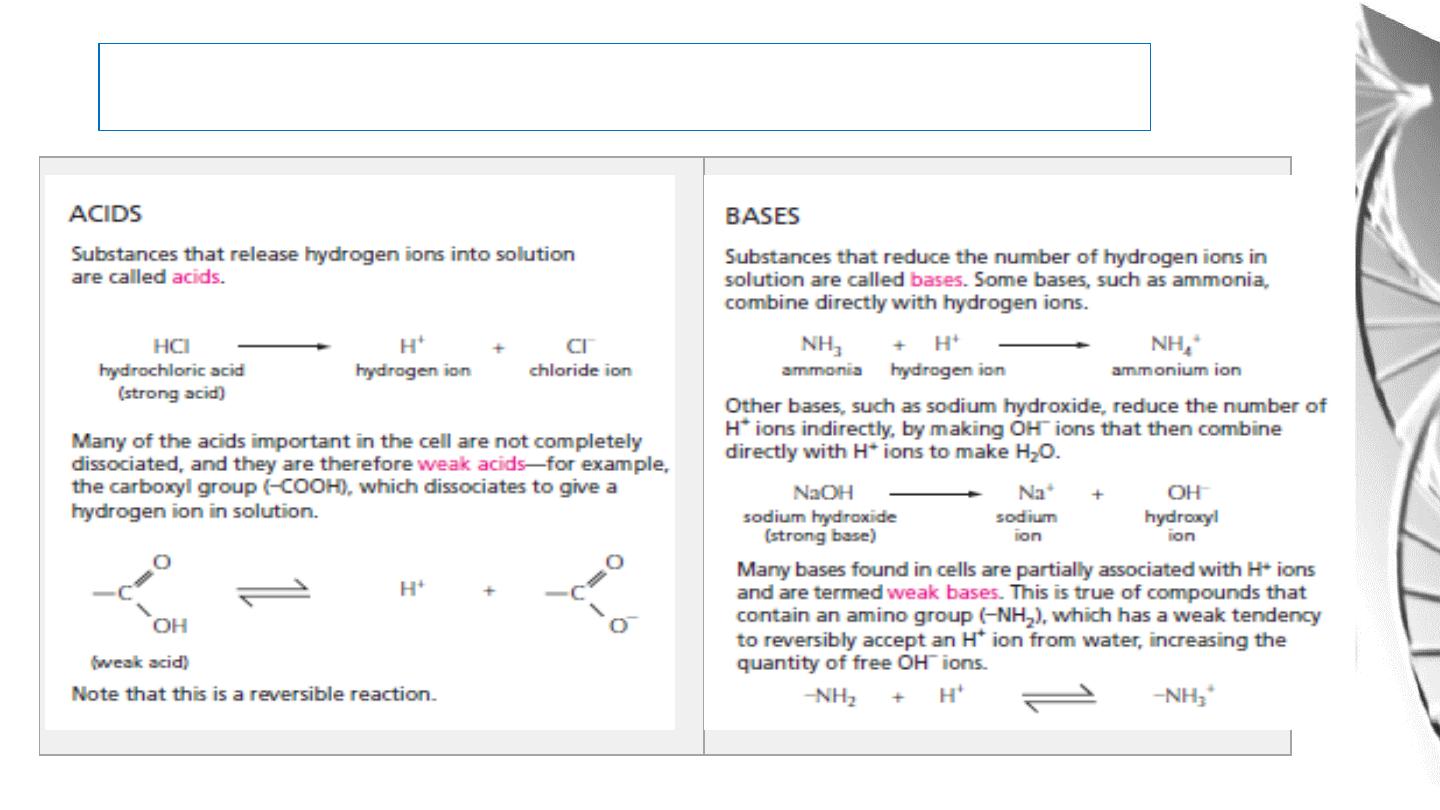
Acids and bases
15

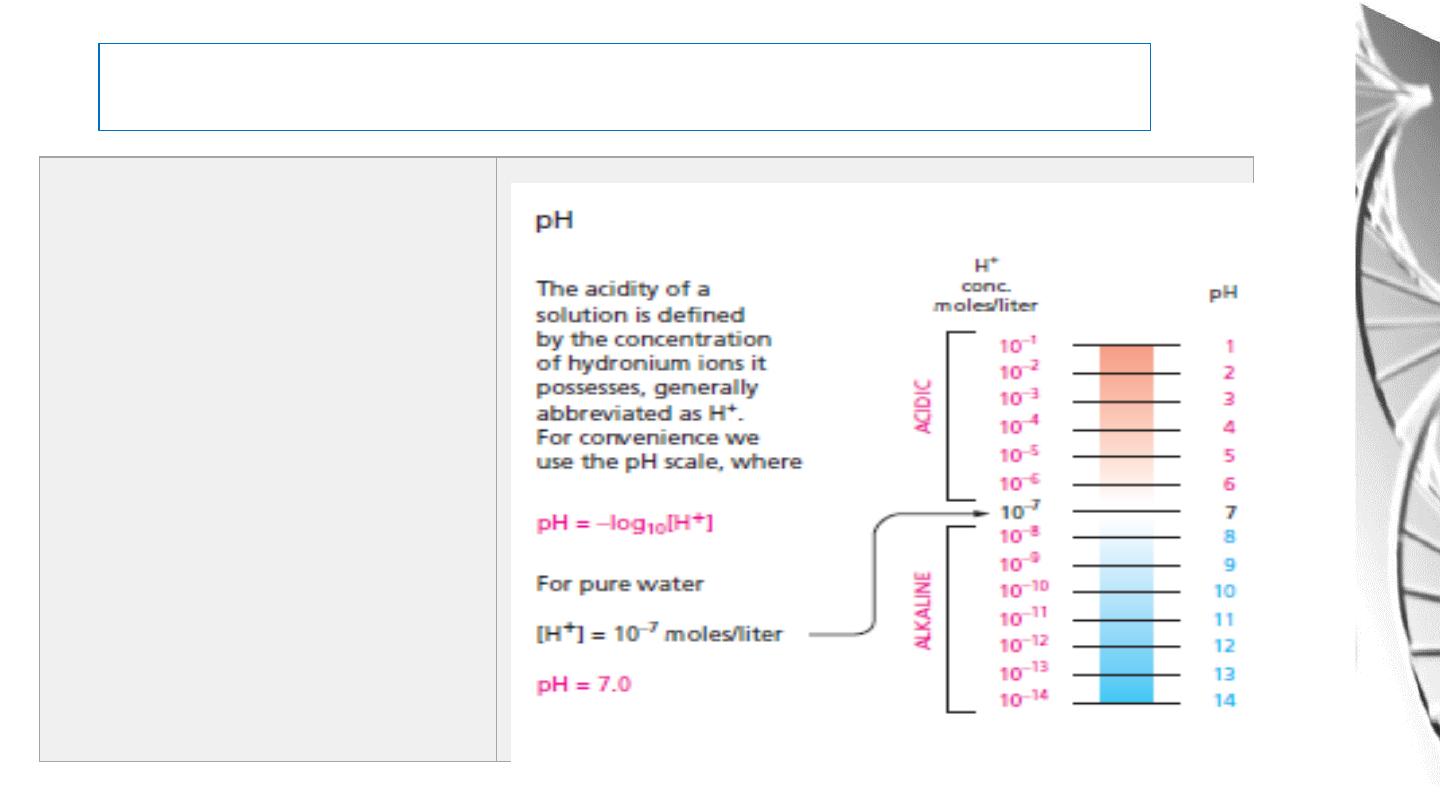
Definition of pH and pK
The pH of a solution is a measure of its concentration of H+. The pH is defined as:
The ionization equilibrium of a weak acid is given by:
The apparent equilibrium constant Ka for this ionization is:
The pKa of an acid is defined as:
The pKa of an acid is the pH at which it is half dissociated, when [A-]=[HA]
16
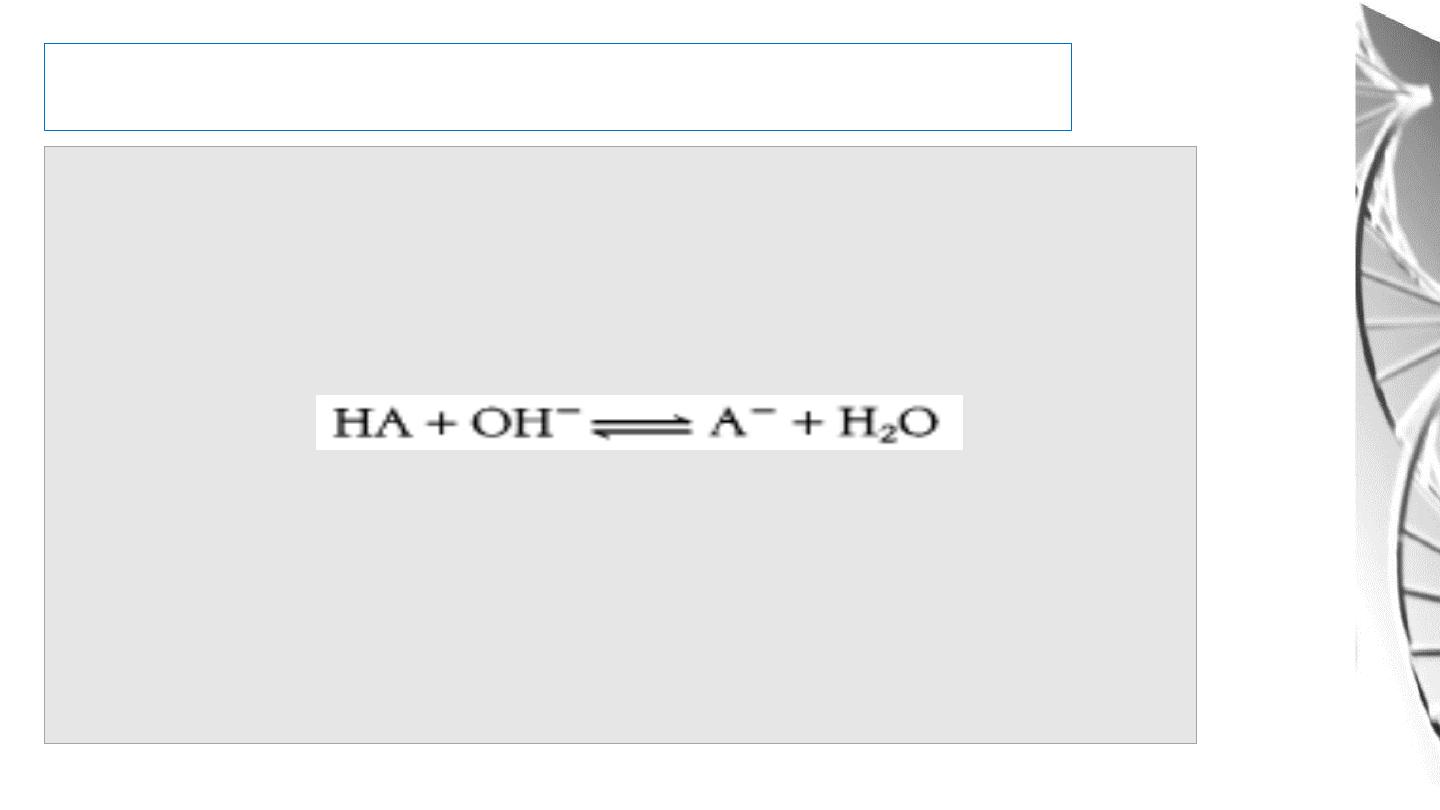
Buffers
An acid-base conjugate pair (such as acetic acid and acetate ion) has an
important property: it resists changes in the pH of a solution. In other words,
it acts as a
buffer
. Consider the addition of OH- to a solution of acetic acid
(HA):
A plot of the dependence of the pH of this solution on the amount of OH-
added is called a
titration curve.
there is an inflection point in the curve at pH 4.8, which is the p
K
a of acetic
acid.
17
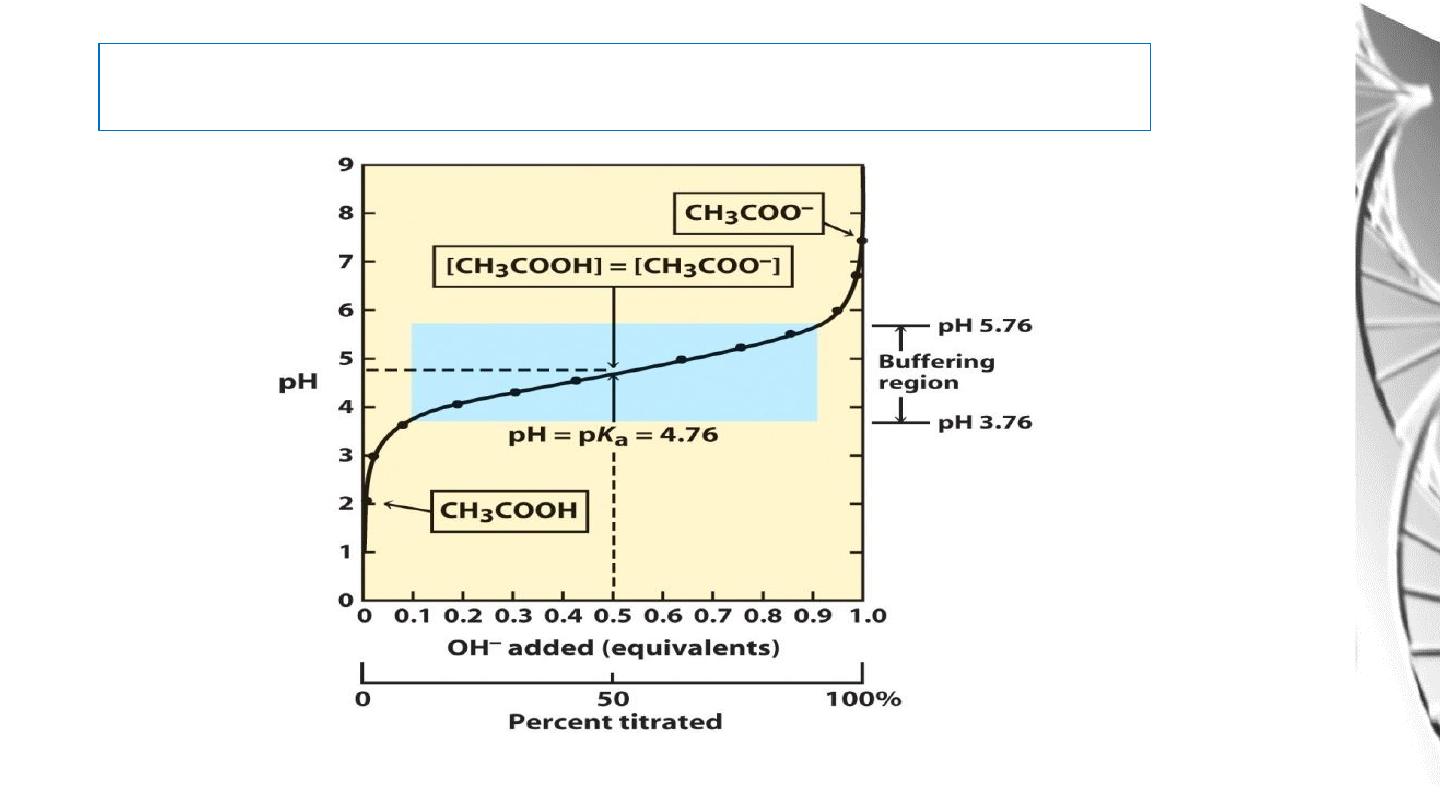
Titration curve of acetic acid
18

What is the relation between pH and the ratio of acid to base?
We know:
Therefore
And
This equation is commonly known as the Henderson-Hasselbalch
equation.
19
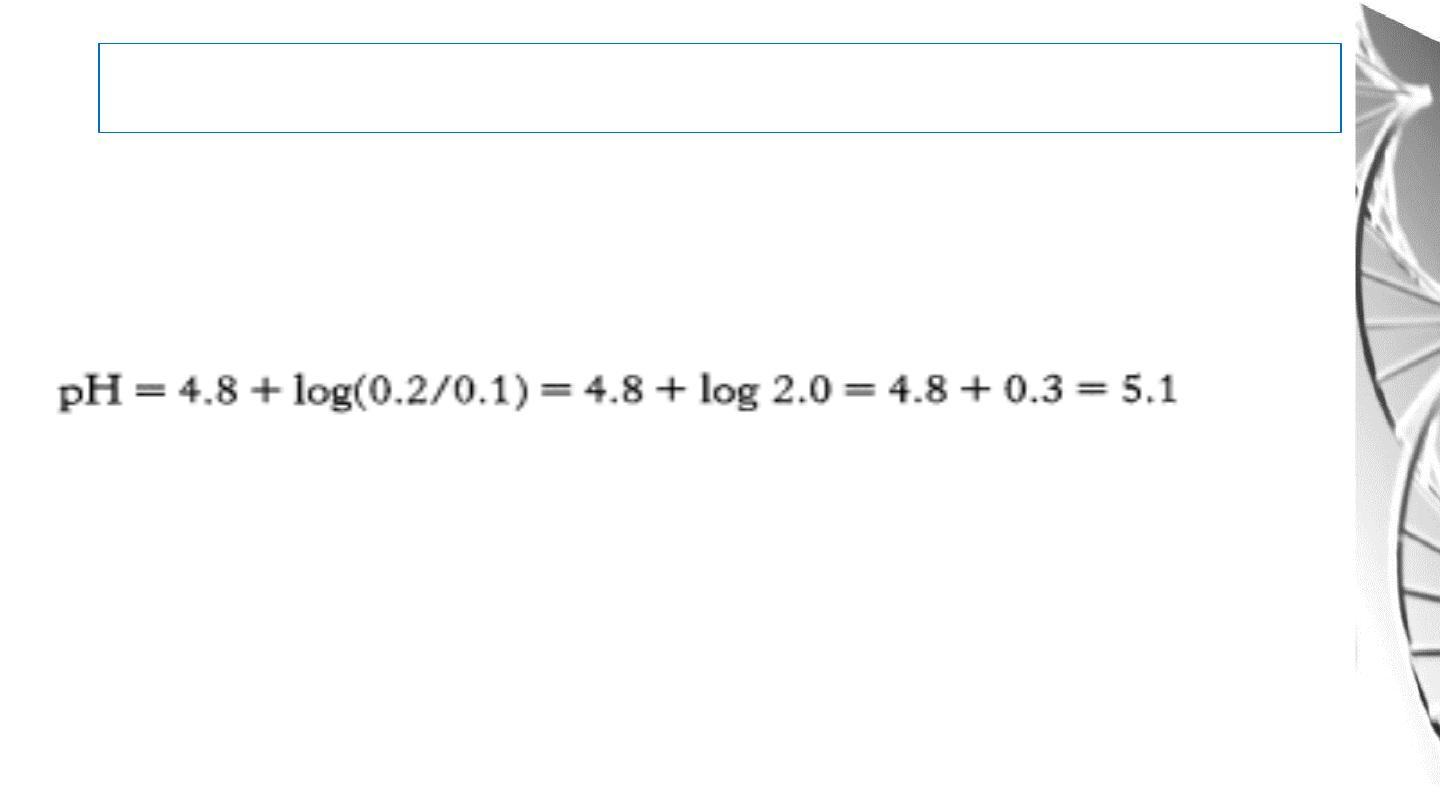
Application of Henderson equation
• The pH of a solution can be calculated from this equation if the molar
proportion of A- to HA and the p
K
a of HA are known.
Example:
• Conversely, the p
K
a of an acid can be calculated if the molar proportion of A-
to HA and the pH of the solution are known.
• And, the molar proportion of A- to HA can be calculated if the pH and p
K
a
values are known.
20
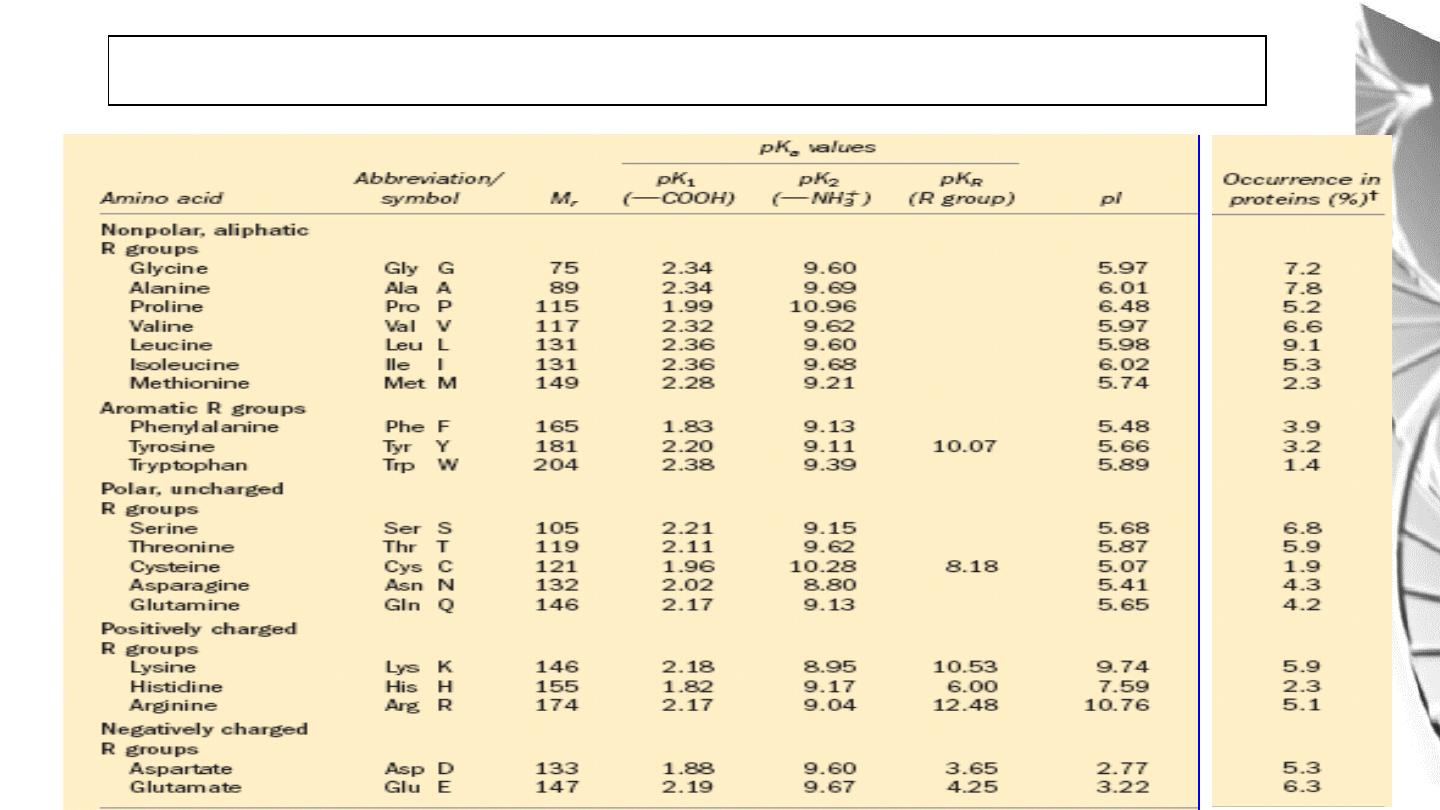
Amino Acids
• Amino acids are the building blocks of proteins.
• There are 20 different amino acids that are commonly found in proteins. All
are α-amino acids.
• They all have a similar structure: a carboxyl group (-COO
-
) and an
amino group (-NH
3
+
) are covalently bound to a central carbon atom. In
addition, the side chain or R group, which differs between amino acids, is
also bonded to the central carbon.
21
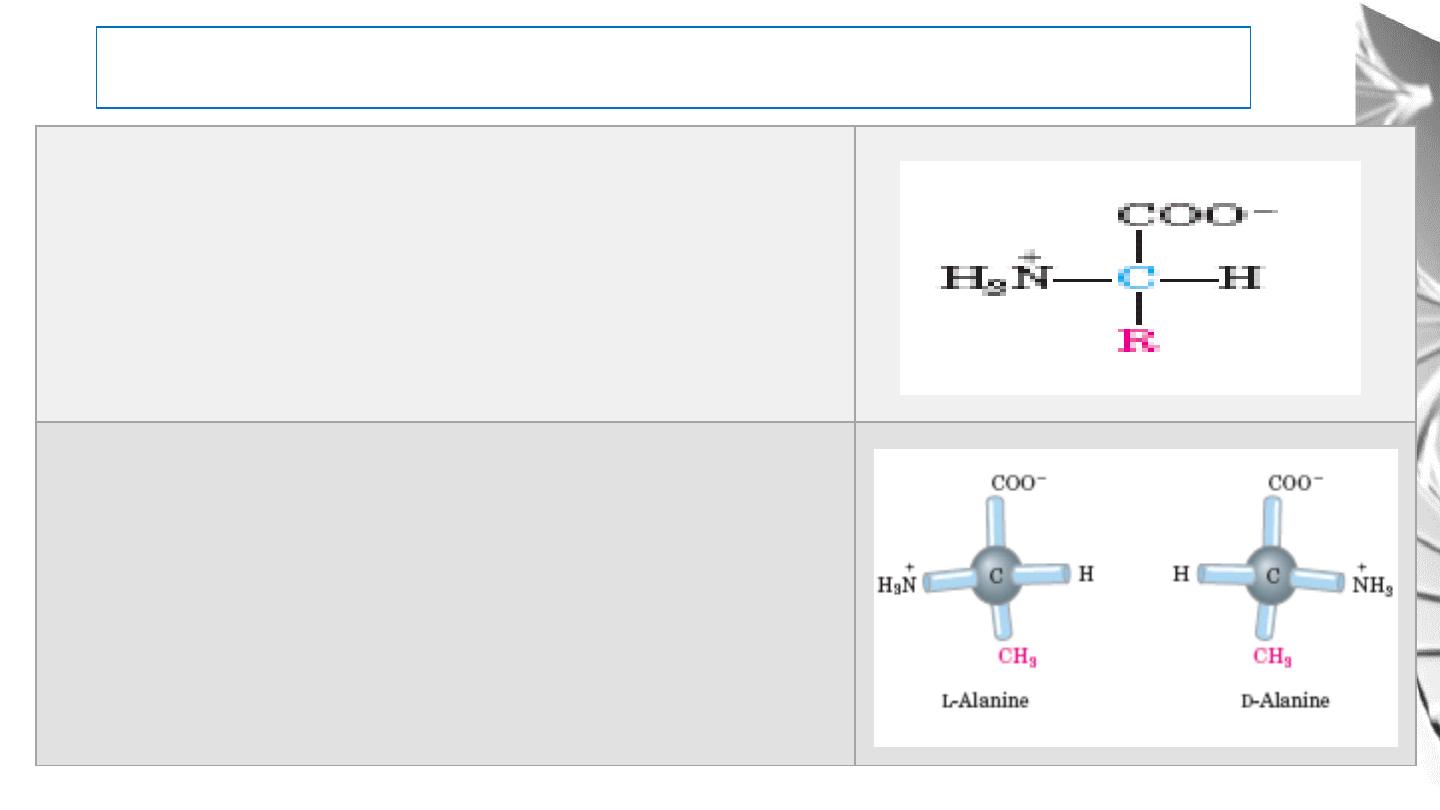
Amino Acids
R groups are vary in structure, size, and electric charge. It
influences the solubility of the amino acids in water.
The α-carbon atom is a chiral center; so, amino acids have
two possible stereoisomers, L or D
Cells are able to specifically synthesize the L isomers of
amino acids because the active sites of enzymes are
asymmetric, causing the reactions they catalyze to be
stereospecific.
The Amino Acid Residues in Proteins Are L -
Stereoisomers
22
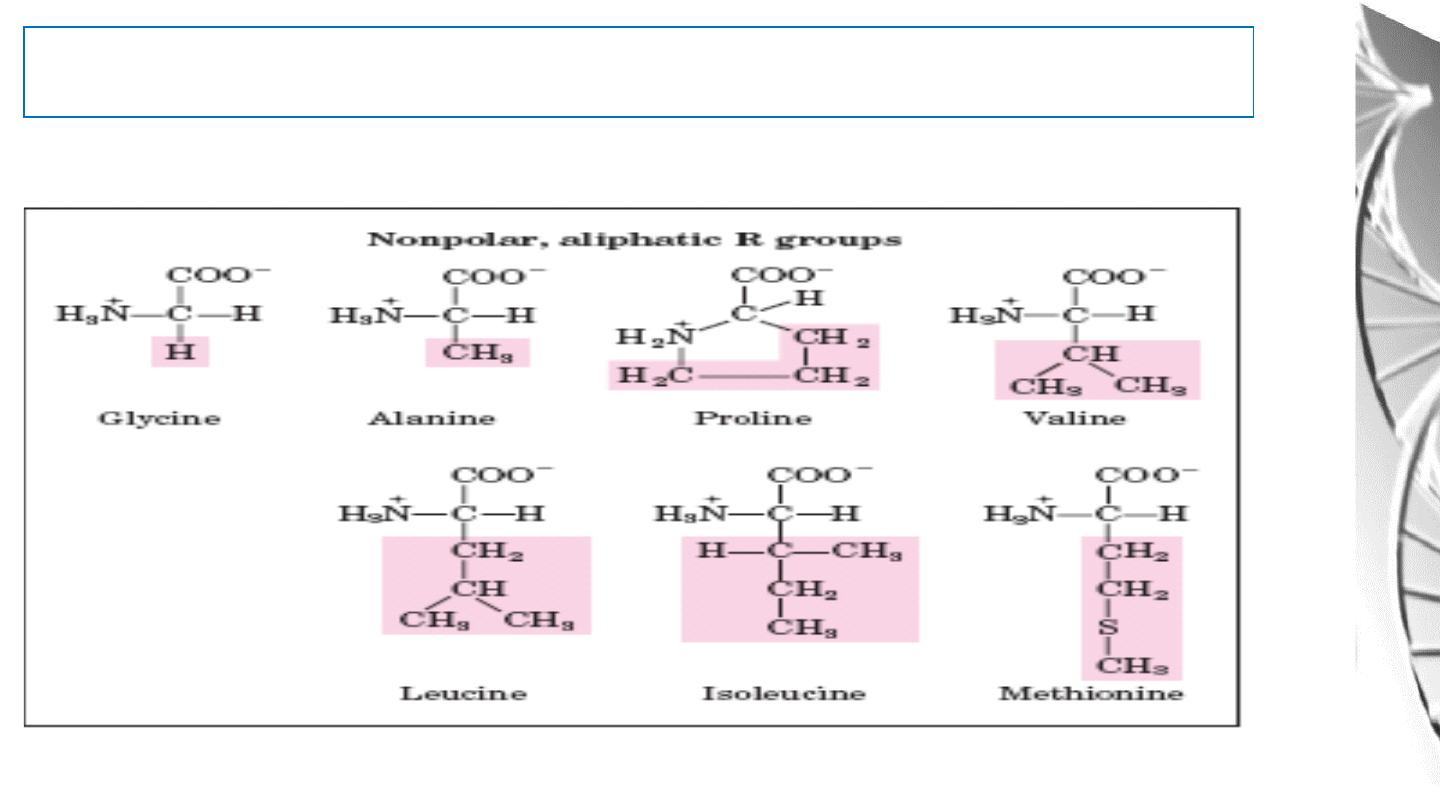
Classification of Amino Acids by R Group
1. Non polar, aliphatic R groups
23
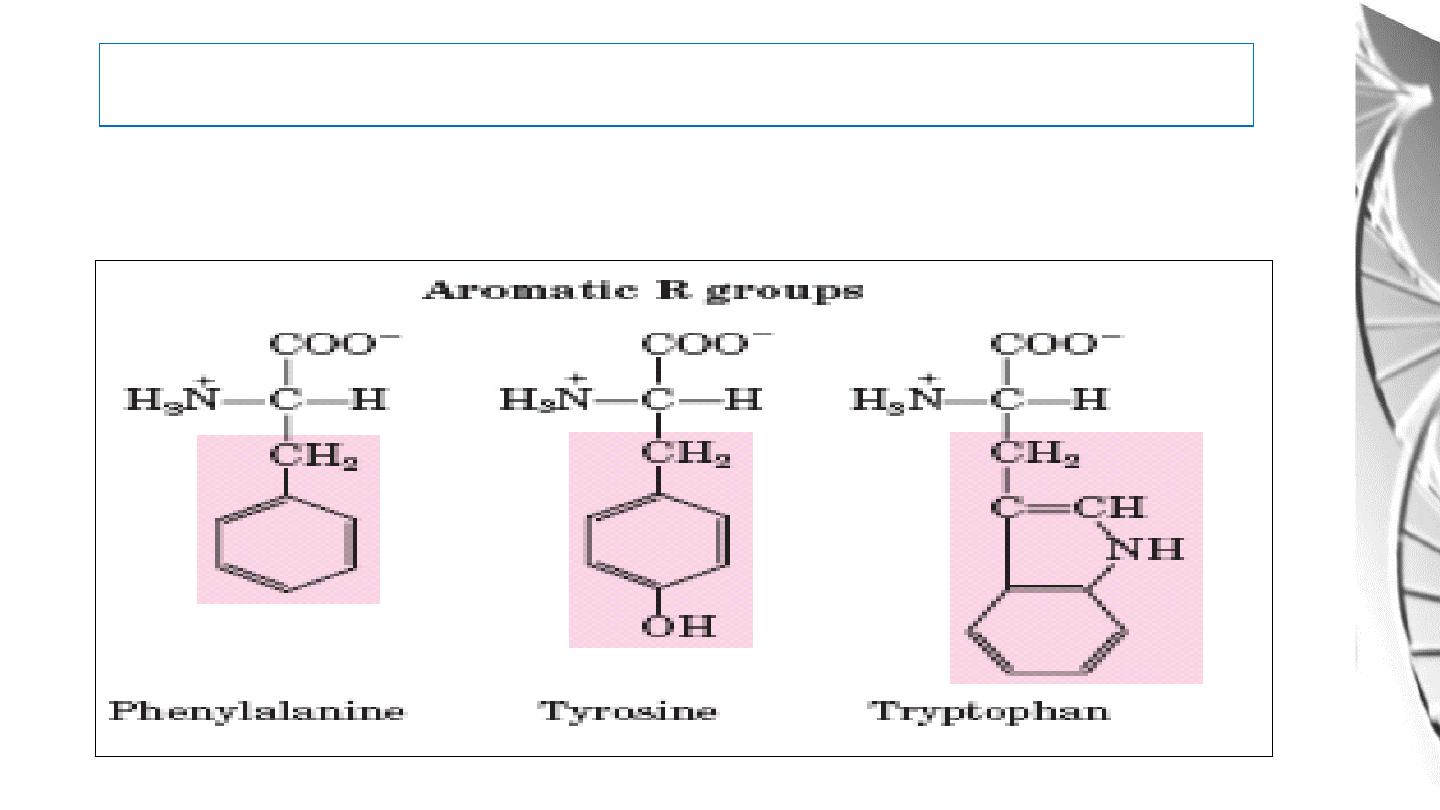
2. Aromatic R Groups
Classification of Amino Acids by R Group
24
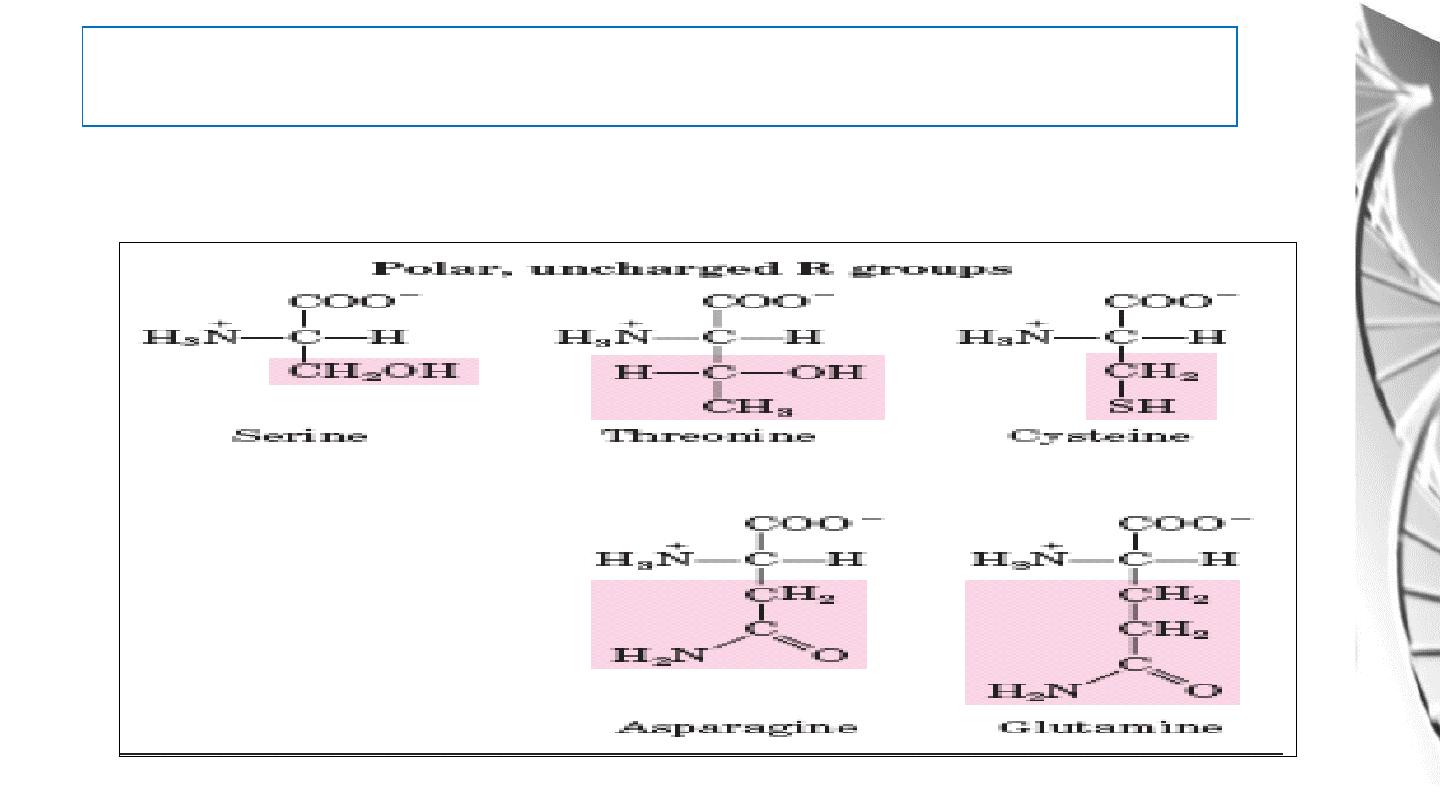
Classification of Amino Acids by R Group
3. Polar, uncharged R groups
25
MGD 2016/ DR. Al-BARQAAWI
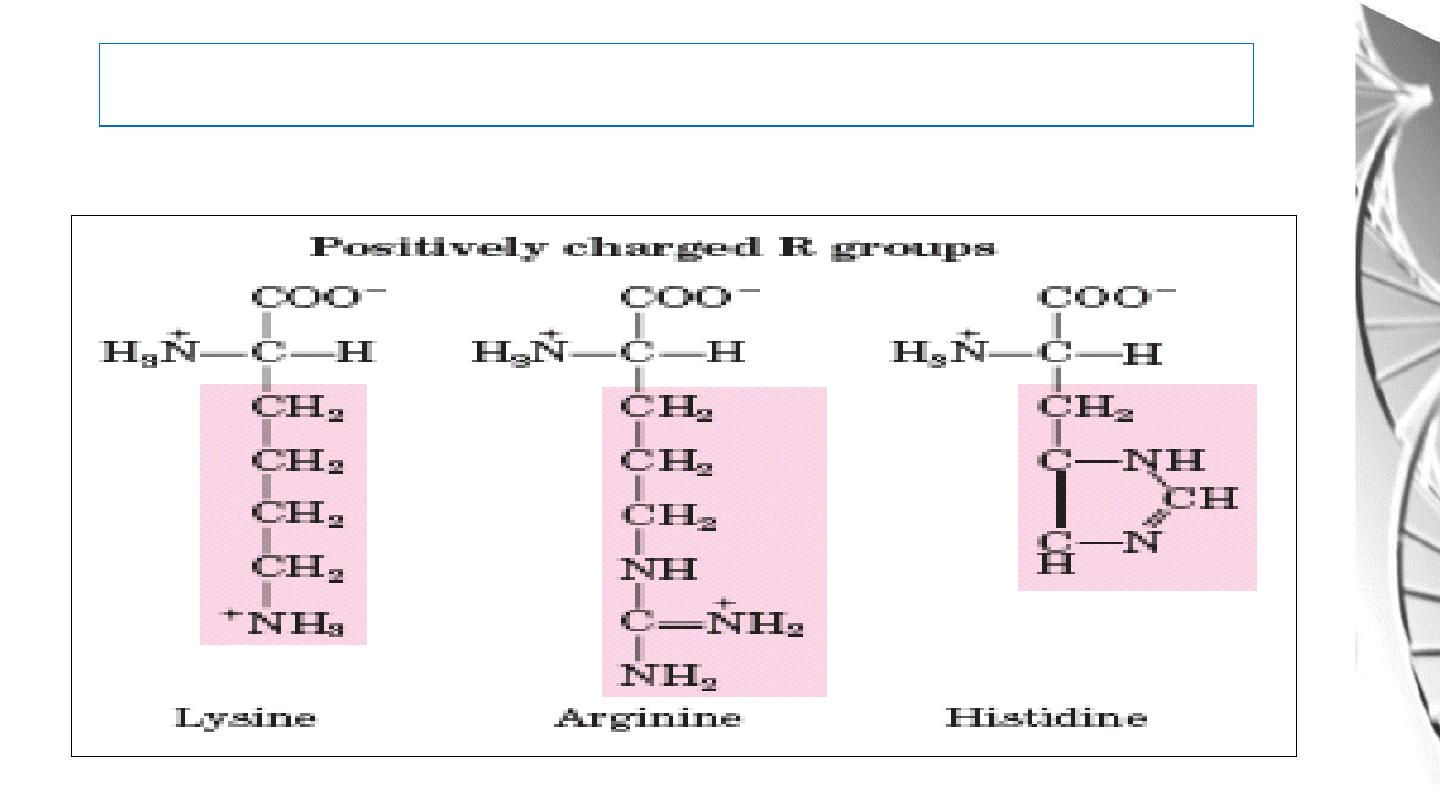
Classification of Amino Acids by R Group
4. Positively Charged (Basic) R Group:
26
MGD 2016/ DR. Al-BARQAAWI
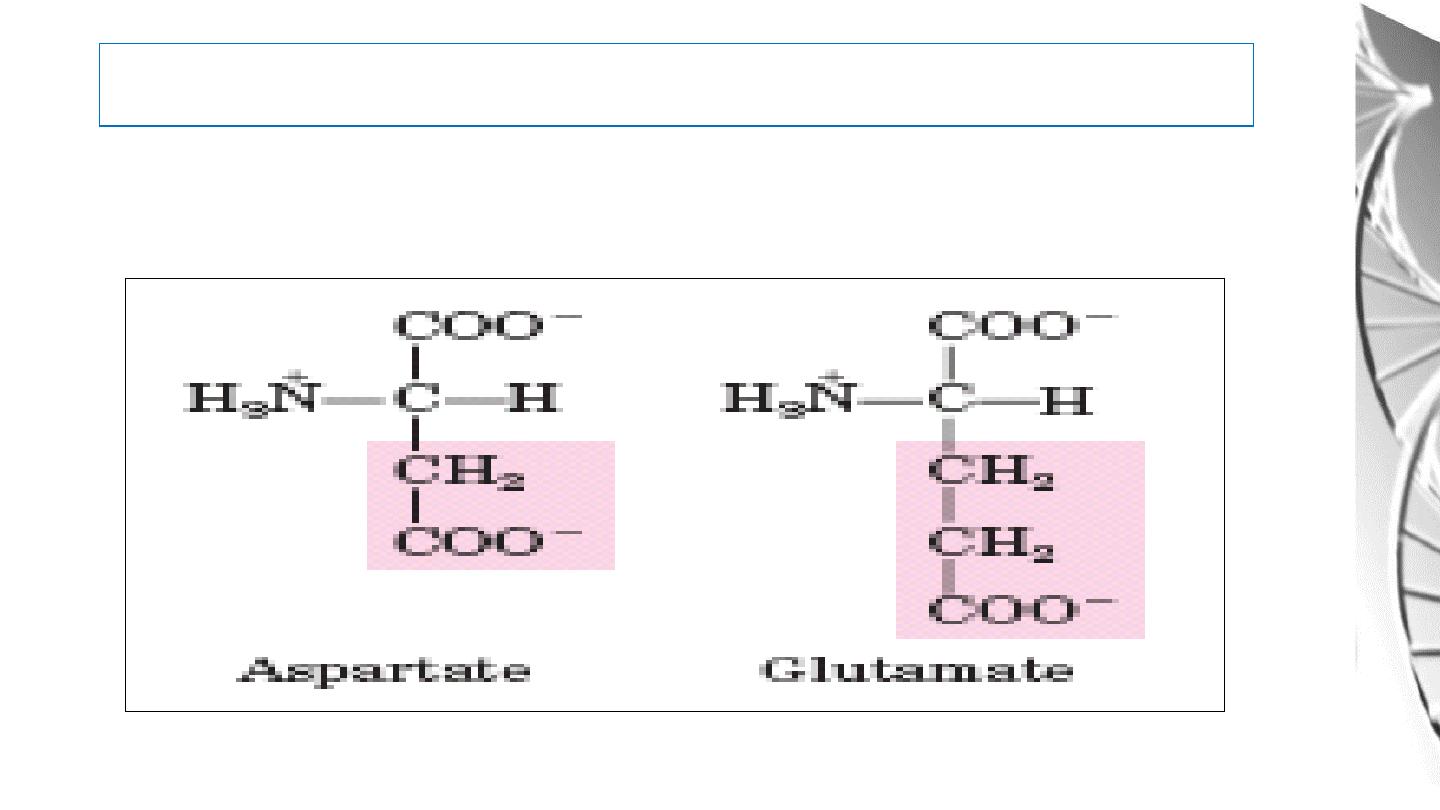
Classification of Amino Acids by R Group
5. Negatively Charged (Acidic) R Groups
27
Classification of Amino Acids by R Group
28
MGD 2016/ DR. Al-BARQAAWI
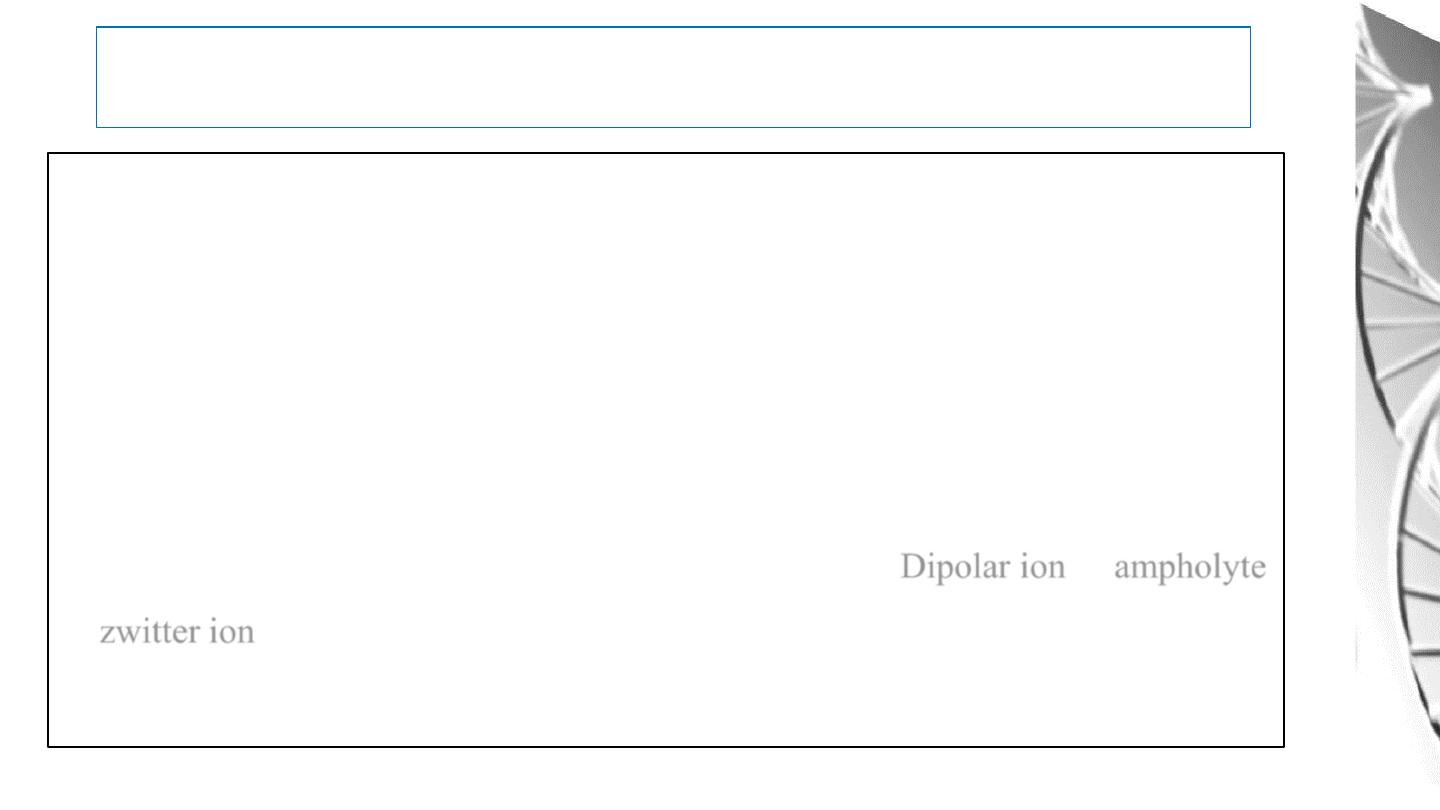
Acid-Base Behavior of Amino acids
The acid base properties of amino acids depends on the:
• amino and carboxyl groups attached to the α- carbon
• on the basic, acidic, or other functional groups represented by R, & The pH of the
meduim.
In the physiological pH range of 7.35- 7.45, the carboxyl group of an amino acid is
dissociated and the amino group is protonated
,
it is called Dipolar ion or ampholyte,
or zwitter ion.
29
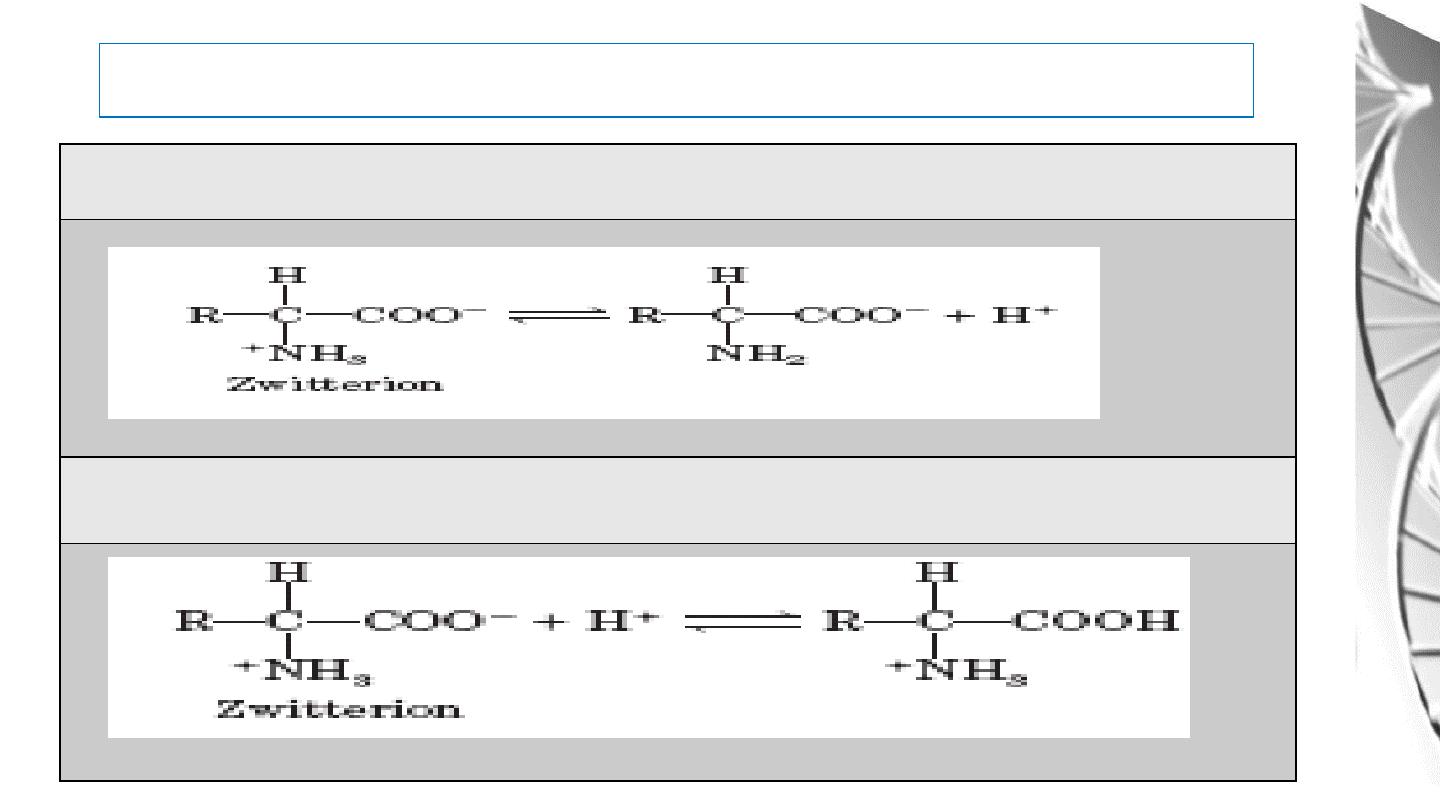
Acid-Base Behavior of Amino acids
The Zwitterion can donate proton (H
+
)
And can accept proton, too.
30
Acid-Base Behavior of Amino acids
At low pH an amino acid is in its cationic form with both its amino and carboxyl groups are
protonated (NH3+ and COOH).
As the pH rises, the carboxyl group loses its proton and the ampholyte form appear at about
pH 6.
With a further increase in pH the amino group (NH3+) is deprotonated, resulting in the
anionic form of the molecule.
For example:
31
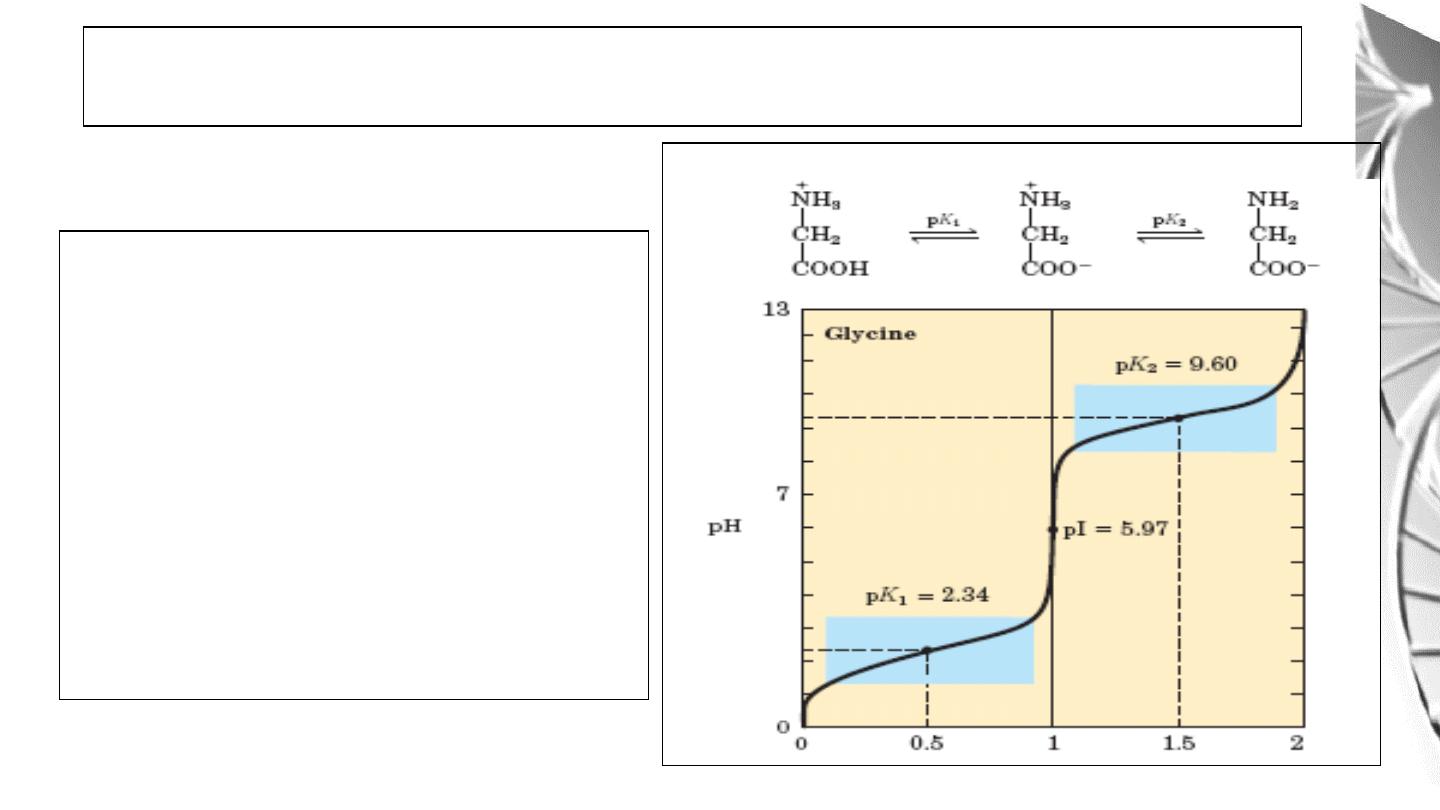
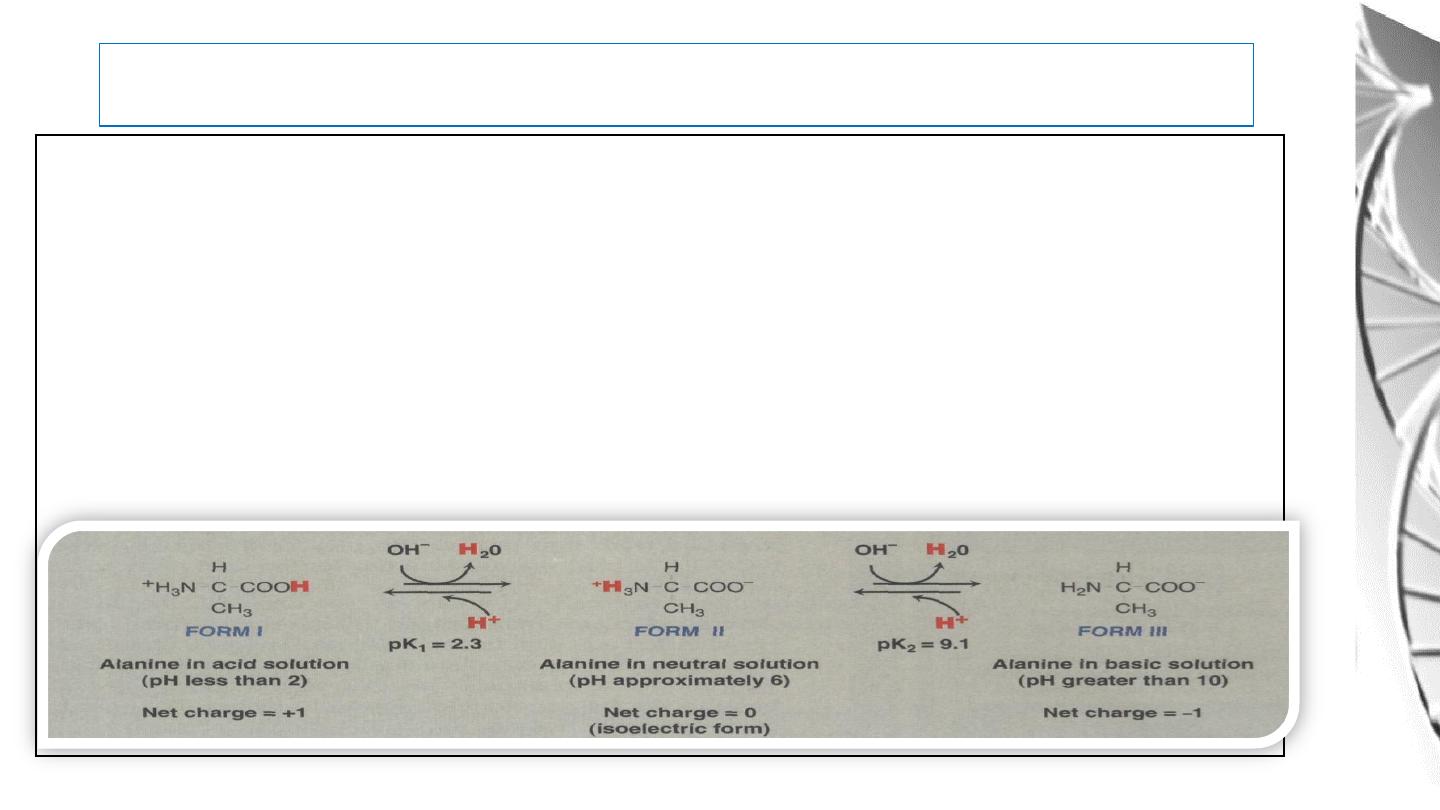
The isoelectric point is the pH at which an
amino acid is electrically neutral that is, in
which the sum of the positive charges equals
the sum of the negative charges. For alanine,
that has only two dissociable hydrogens
(one from the α-carboxyl and one from the
α-amino group), the pI is the average of pK1
and pK2
Titration curve of amino acids with
not ionizable R groups.
32
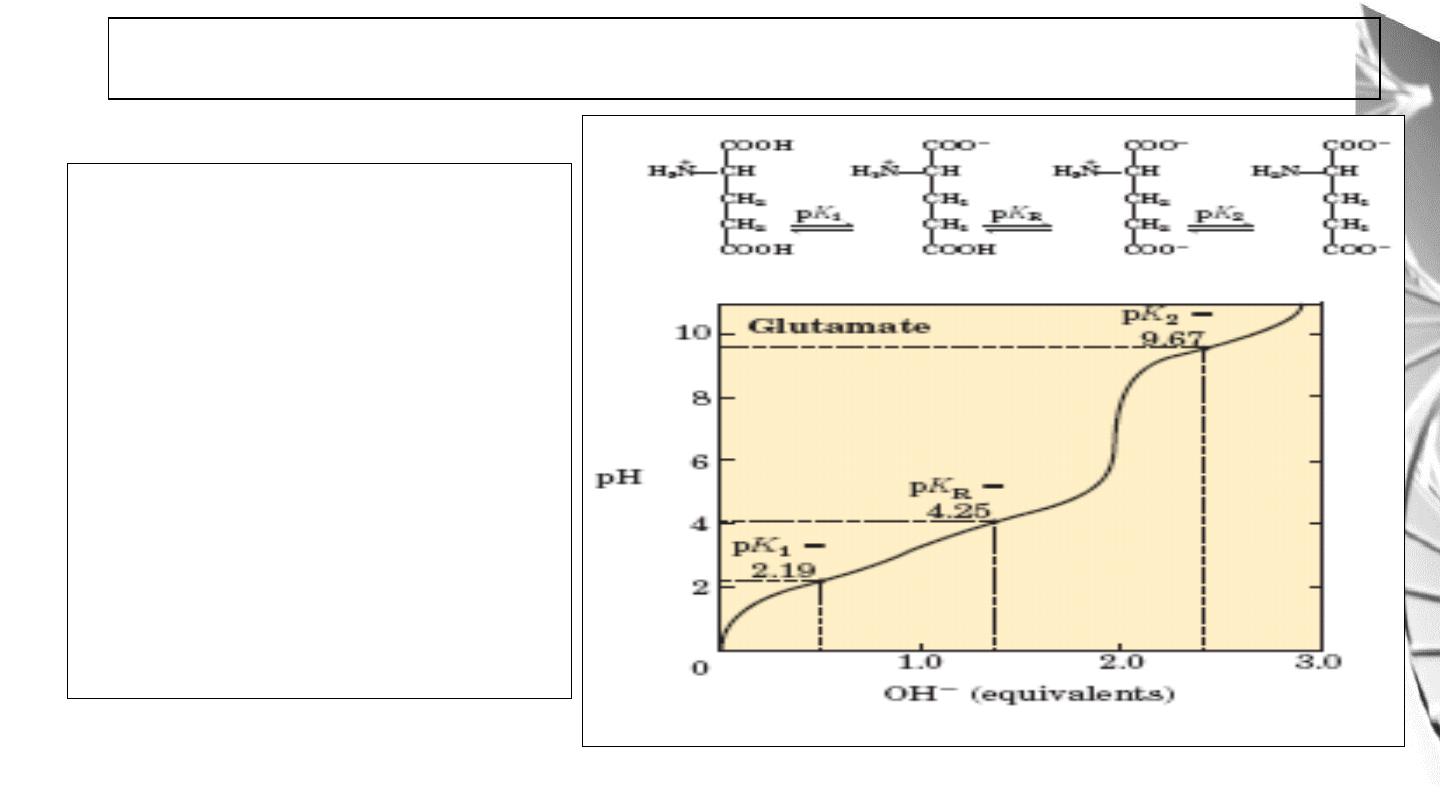
Amino acids with ionizable R
groups have additional ionic
species, depending on the pH of
the medium and the pKa of the R
group.
For glutamic acid:
pI is the average of pK1 and
pK
R.
Titration curve of Amino acids with ionizable R groups
33
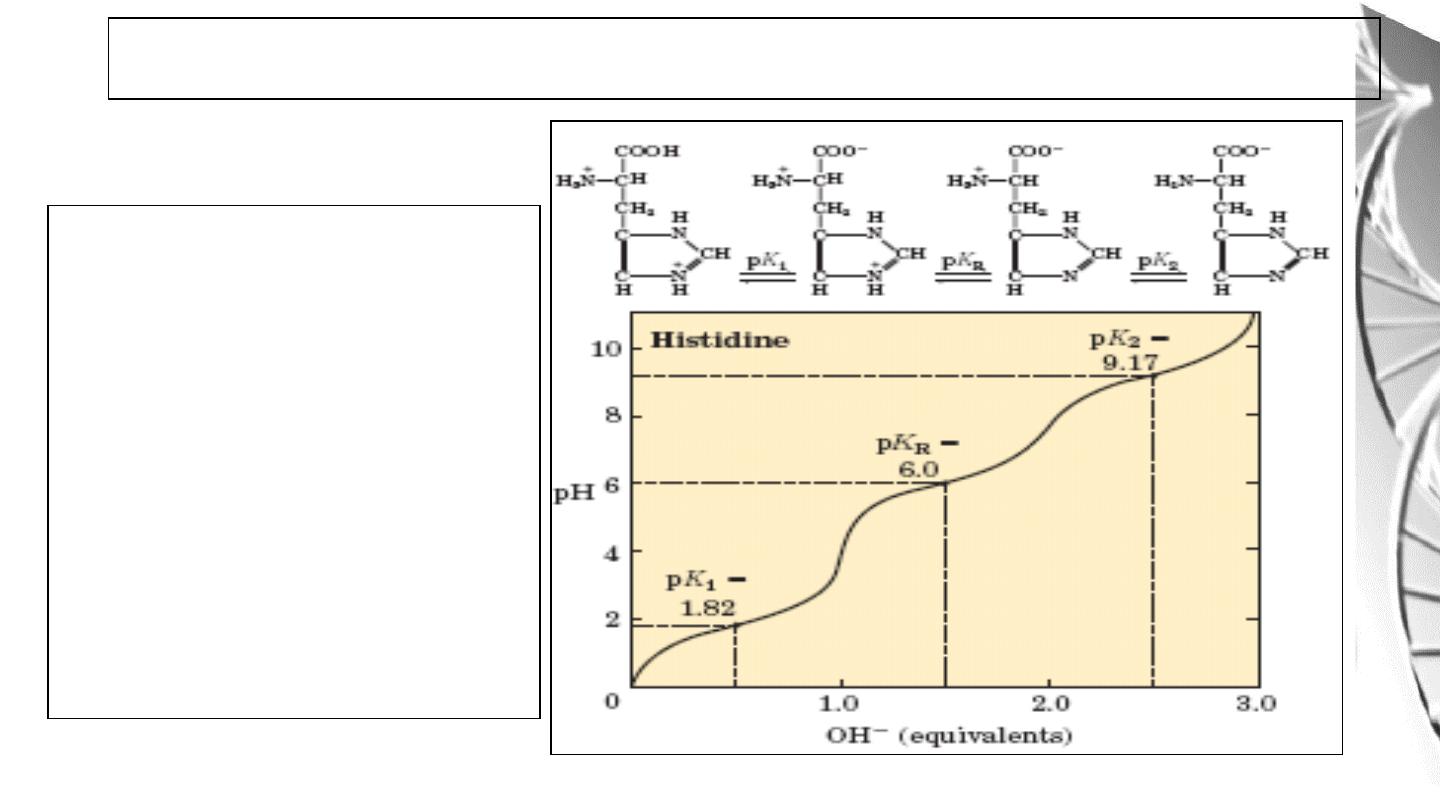
For histidine and other basic
amino acids:
pI is the average of pK
2
and
pK
R.
At pH = pI, amino acid bears no
net charge and therefore does
not move in an electric field.
Titration curve of Amino acids with ionizable R groups
34
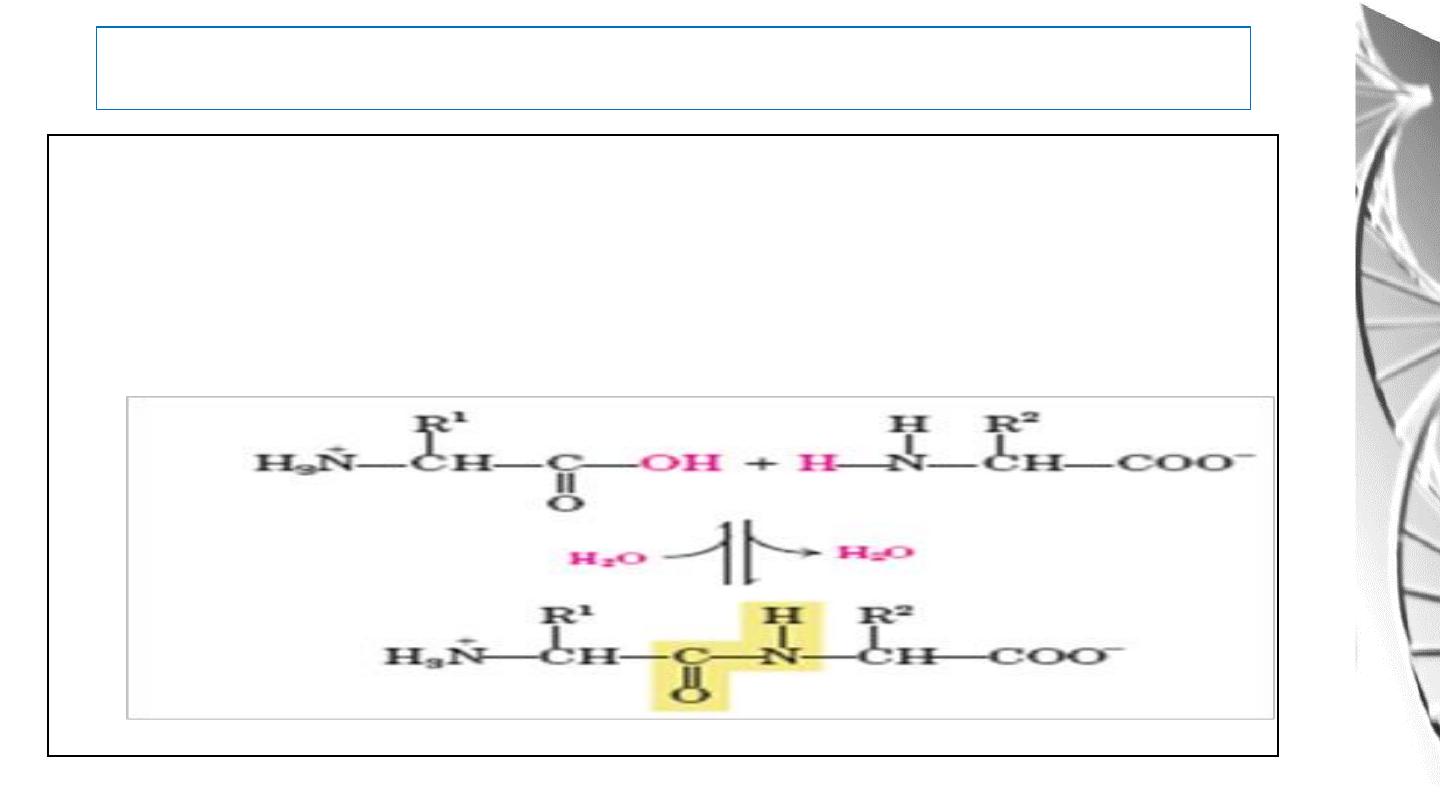
Peptide Bond
One of the most Important Reactions of Amino Acids is the formation
of peptide bond by the condensation of two amino acids.
35
MGD 2016/ DR. Al-BARQAAWI
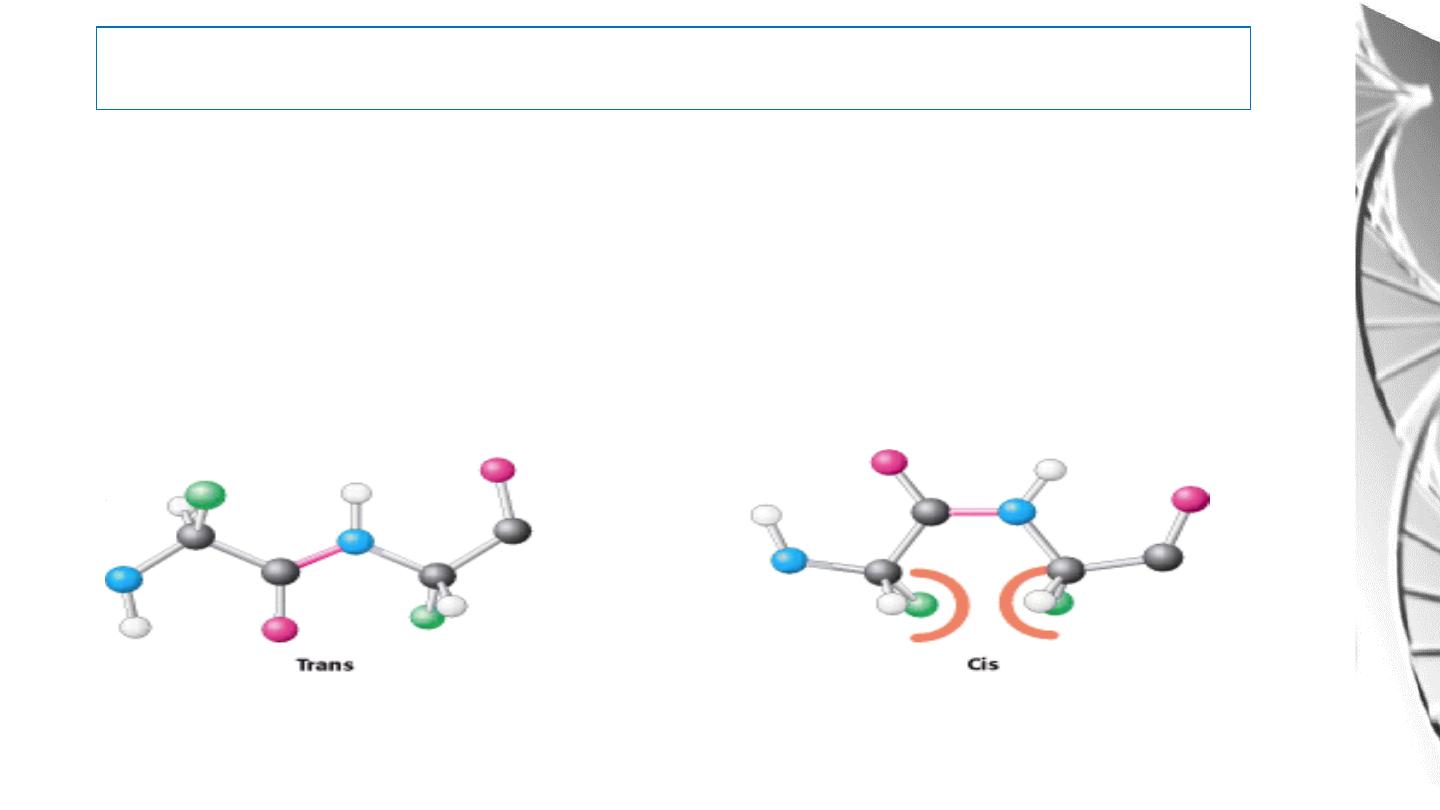
Key feature of the peptide bond
• All the atoms of the bond are in the same plane.
• No rotation about the peptide bonds due to double bond characteristics.
• Carbonyl oxygen and Amide hydrogen are in the
trans
orientation,
because of steric clashes that occur in the cis form.
36
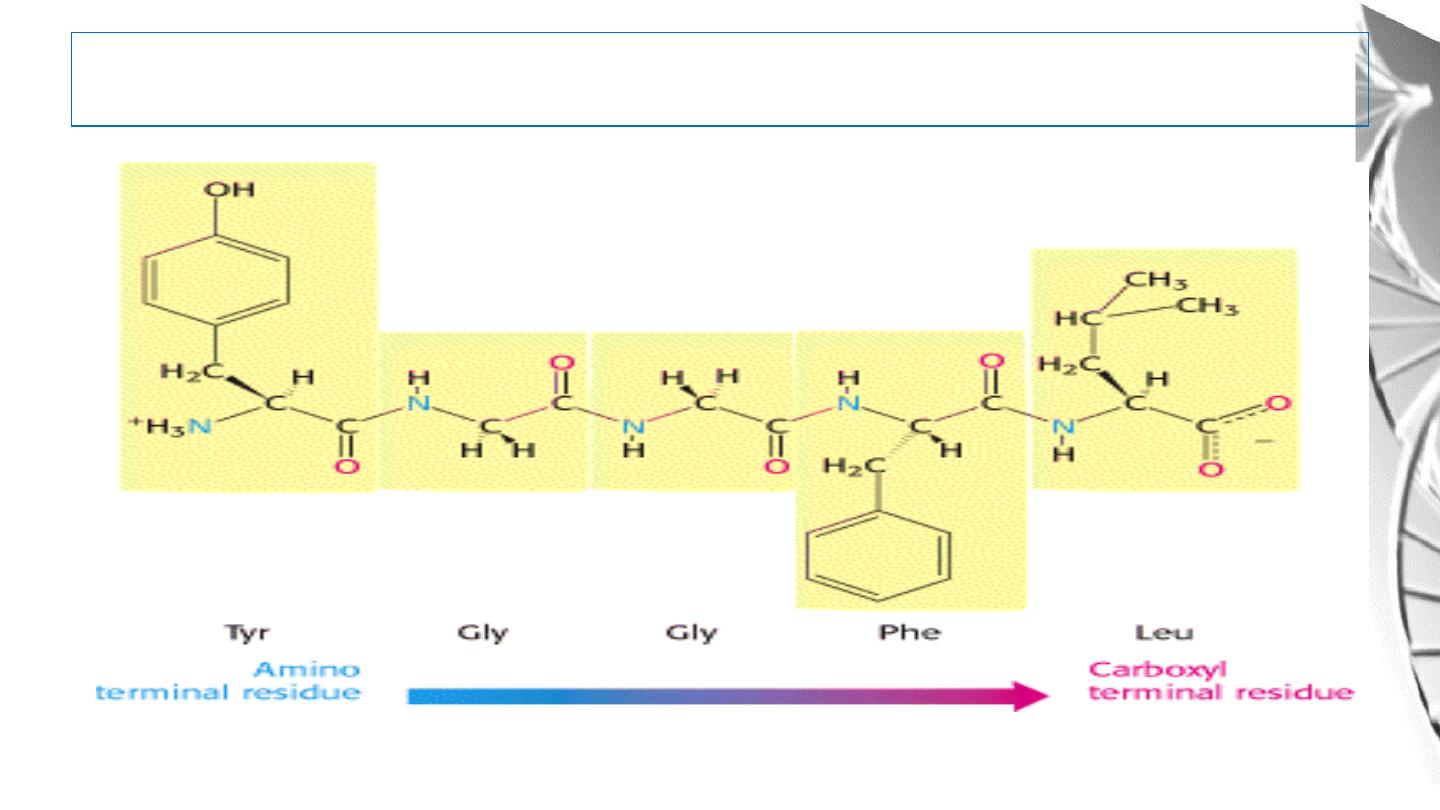
The peptide has a direction
37
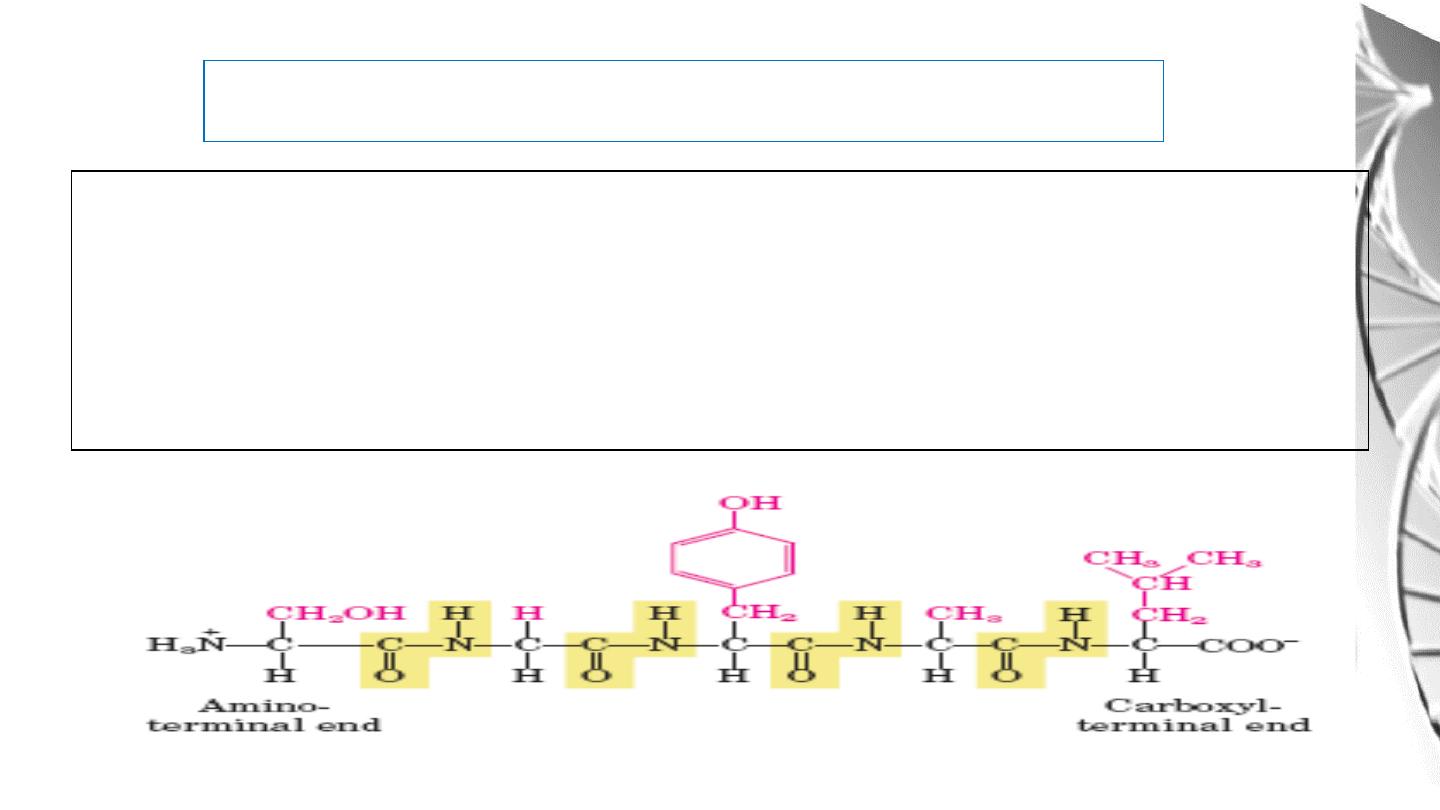
The Peptides are named beginning with the aminoterminal residue, which by convention is
placed
at
the
left.
For
example,
the
below
pentapeptide
named.
serylglycyltyrosylalanylleucine
38
Peptide Nomenclature
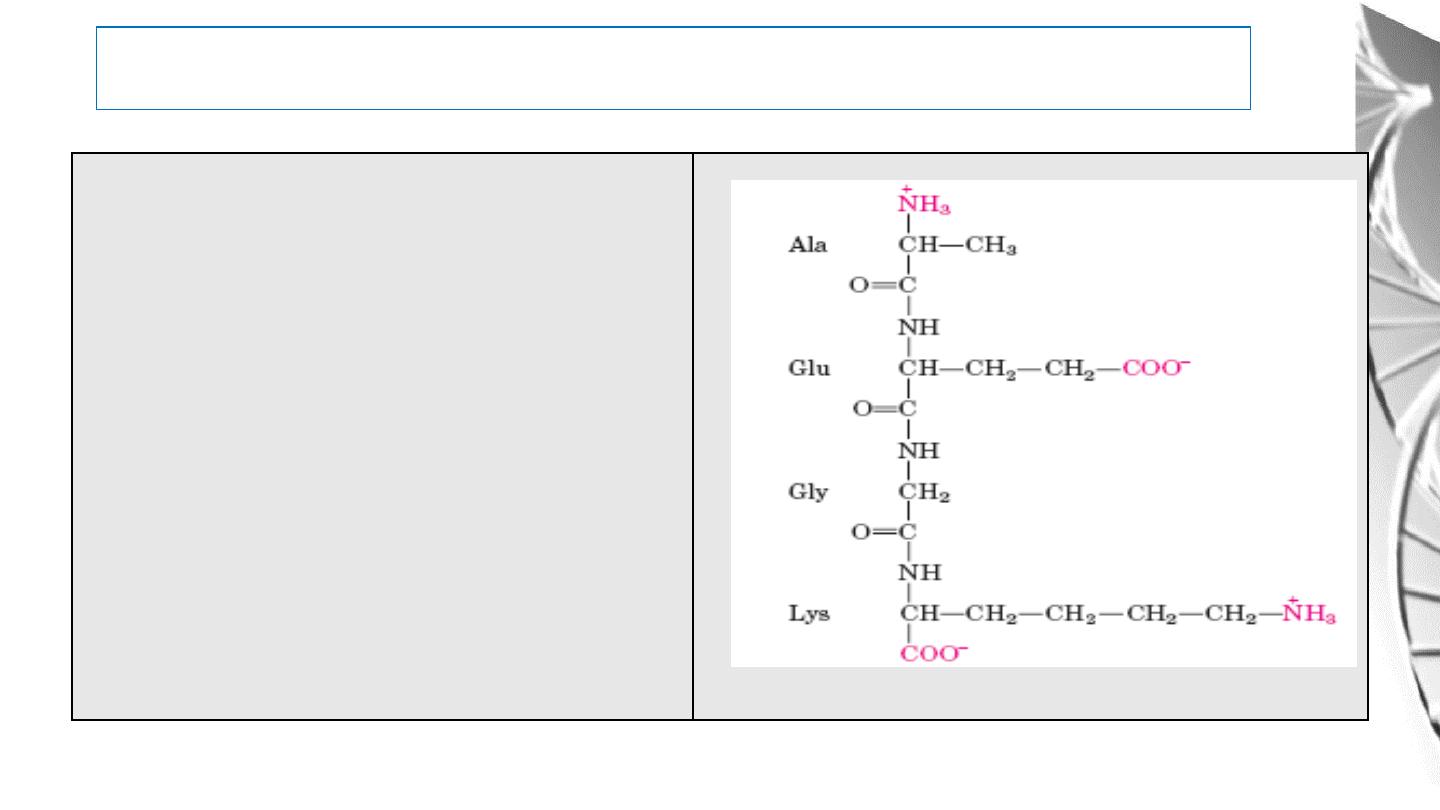
Ionization of peptide
Alanylglutamylglycyllysine.
This
tetrapeptide has one free -amino group,
one free -carboxyl group, and two
ionizable R groups. The groups ionized
at pH 7.0 are in red
39
Proteins
•
Proteins are polypeptides of 20 different amino acids, in a sequence encoded by
the gene
•
The polypeptide chain folds into a complex and highly specific three-
dimensional structure, determined by the sequence of amino acids
•
The folding of proteins depends on the chemical and physical properties of the
amino acids
•
The amino acids that make up a protein contribute to the folding and function of
that protein. The side chains those of the amino acids are more important in a
polypeptide as they contribute to the charge seen on the protein.
40
Isoelectric Point (pI) Of Protein
• The isoelectric point (pI) is the pH at which a protein has no overall net
charge, so can not move in electrical feild.
• Acidic proteins contain many negatively charged amino acids and have a low
pI.
• Basic proteins contain many positively charged amino acids and have a high
pI.
41
42
S
u
m
m
a
r
y

Thank you
for attention
43
