Respiratory Disease of Neoborn
Transient Tachypnea of the Newborn (TTN)
TTN is most common after term cesarean delivery. The syndrome is believed to be
secondary to slow absorption of fetal lung fluid, resulting in decreased pulmonary
compliance.
It is a self-limited condition characterized by the early onset of tachypnea, sometimes with
retractions, or expiratory grunting and, occasionally, cyanosis that is relieved by minimal
oxygen supplementation (<40%). The chest generally sounds clear without crackles or
wheeze. Most infants recover rapidly, usually within 3 days.
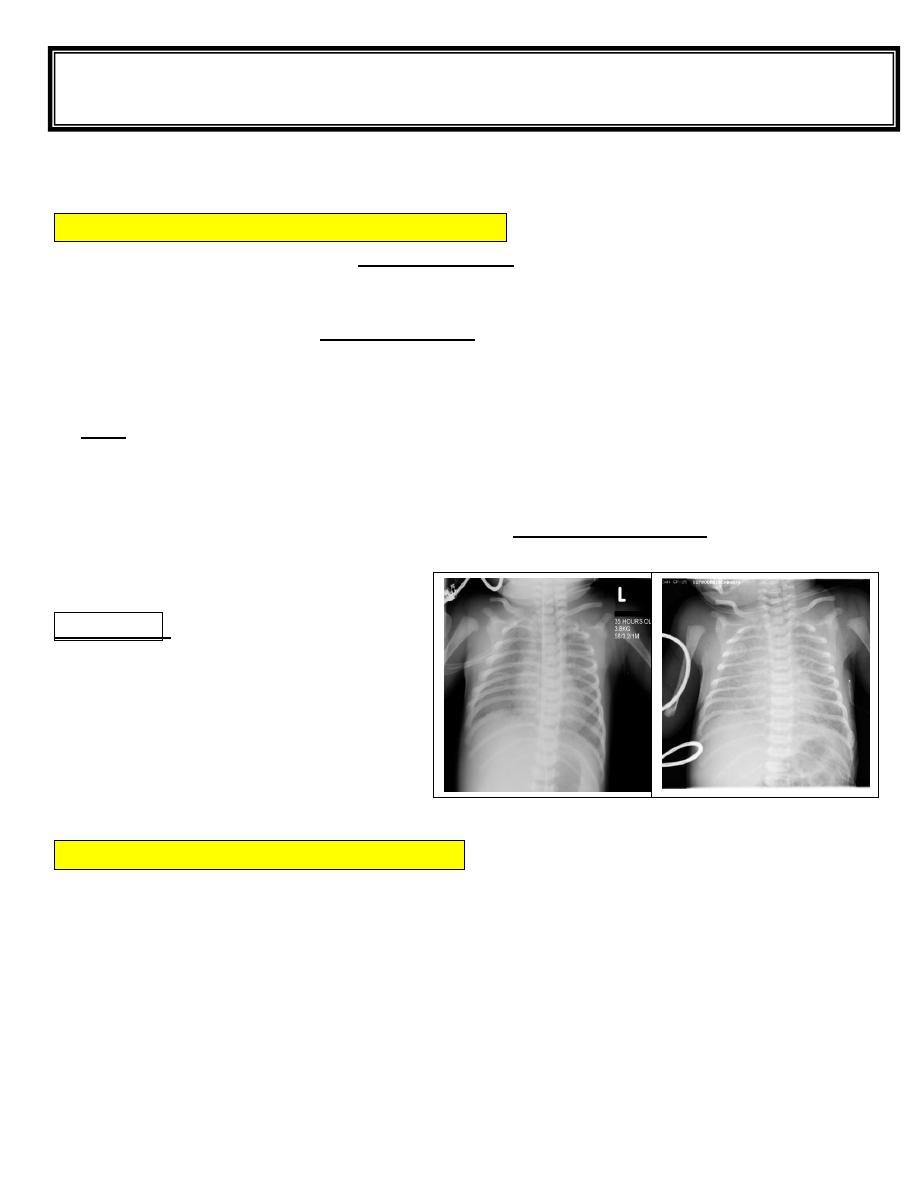
CXR shows prominent pulmonary vascular markings, fluid in the intralobar fissures,
overaeration, flat diaphragms, and, rarely, small pleural effusions.
Hypercapnia and acidosis are uncommon.
Distinguishing the disease from RDS and other respiratory disorders (e.g., pneumonia) may
be difficult, and transient tachypnea is frequently a diagnosis of exclusion; the distinctive
features of TTN are rapid recovery of the infant and the absence of radiographic findings for
RDS.
Treatment
:
Supportive.
One study demonstrated efficacy of
inhaled salbutamol in enhancing resolution
of transient tachypnea of the newborn.
Meconium Aspiration Syndrome (MAS)
Meconium-stained amniotic fluid is found in 10-15% of births and usually occurs in term or
postterm infants.
Meconium aspiration syndrome (MAS) develops in 5% of such infants.
Aspiration of amniotic fluid contaminated with particulate meconium may occur in utero in
a distressed, gasping fetus; but more often, meconium is aspirated into the lung immediately
after delivery.
Fifth stage Lec-7
Dr :Athl Humo
pediatric
10/11/2016
Clinical manifestations
MAS is characterized by tachypnea, hypoxia, hypercapnia, and small airway obstruction
causing a ball-valve effect, leading to air trapping, overdistention, and extra-alveolar air
leaks.
Complete small airway obstruction produces atelectasis.
Within 24 to 48 hours, a chemical pneumonitis develops in addition to the mechanical
effects of airway obstruction.
Abnormal pulmonary function may be caused by the meconium, in part, through inactivation
of surfactant.
Primary pulmonary hypertension of the newborn (PPHN) frequently accompanies meconium
aspiration, with right-to-left shunting caused by increased pulmonary vascular resistance.
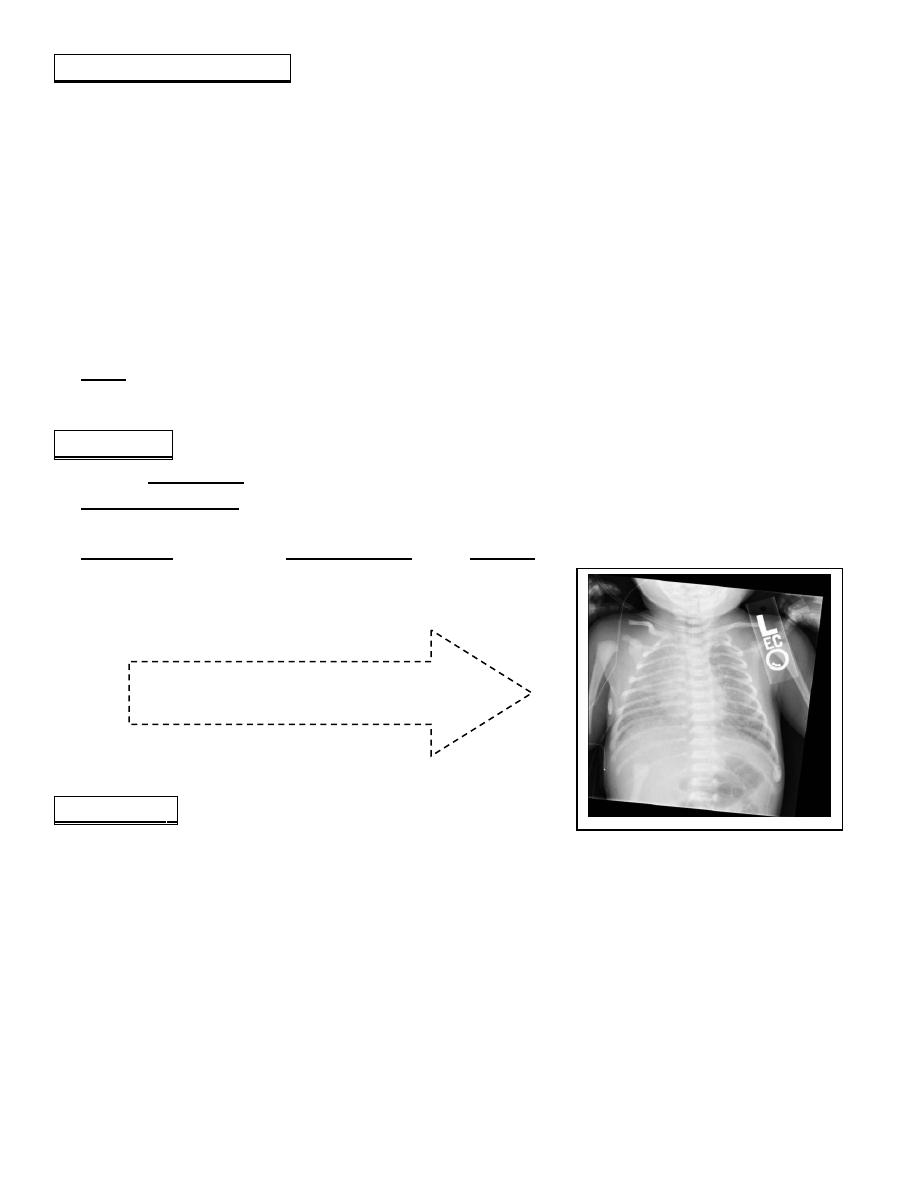
CXR reveals patchy infiltrates, overdistention, flattening of the diaphragm, increased
anteroposterior diameter, and a high incidence of pneumomediastinum and pneumothoraces.
Treatment
general supportive care and assisted ventilation.
treated for PPHN.
If severe hypoxia does not subside with conventional or high-frequency ventilation,
surfactant therapy, and inhaled nitric oxide, ECMO may be beneficial.
Prevention
careful in utero monitoring to prevent asphyxia.
When meconium-stained fluid is observed, the obstetrician should suction the infant’s
oropharynx before delivering the rest of the infant’s body.
If the infant is depressed with poor tone, minimal respiratory effort, and cyanosis, the
infant’s oropharynx should be suctioned, the vocal cords visualized, and the area below the
vocal cords suctioned to remove any meconium from the trachea.
Saline intrauterine amnioinfusion during labor may reduce the incidence of aspiration and
pneumonia.
Meconium aspiration:
white spread coarse opacity

Respiratory Distress Syndrome(RDS)
RDS (HYALINE MEMBRANE DISEASE) occurs after the onset of breathing and is
associated with an insufficiency of pulmonary surfactant.
This lipoprotein surfactant is 90% lipid and is composed predominantly of saturated
phosphatidylcholine (lecithin), but also contains phosphatidylglycerol, other phospholipids,
and neutral lipids. The surfactant proteins, SP-A, SP-B, SP-C, and SP-D, are packaged into
the lamellar body and contribute to surface-active properties and recycling of surfactant.
These surface-active agents are released into the alveoli, where they reduce surface tension
and help maintain alveolar stability by preventing the collapse of small air spaces at
end-expiration. Without surfactant, surface tension forces are not reduced, and atelectasis
develops during end expiration as the alveolus collapses.
By 34 to 36 weeks, sufficient surface-active material is produced by the type II cells in the
lung, is secreted into the alveolar lumen, and is excreted into the amniotic fluid.
The concentration of lecithin in amniotic fluid indicates fetal pulmonary maturity. Because
the amount of lecithin is difficult to quantify, the ratio of lecithin (which increases with
maturity) to sphingomyelin (which remains constant during gestation) (L/S ratio) is
determined. An L/S ratio of 2:1 usually indicates pulmonarymaturity.
Risk
1. Premature and have an immature L/S ratio.
2. Delivery of a previous preterm infant with RDS.
3. Delivery by cesarean section without labor.
4. Being the second-born of twins.
5. Maternal diabetes.
6. Fetal distress
7. Asphyxia.
8. Male sex.
9. White race.
Clinical manifestations
Signs of RDS usually appear within minutes of birth, although they may not be recognized
for several hours in larger premature infants. A later onset of tachypnea should suggest other
conditions.
NB Characteristically develop tachypnea, grunting, intercostal and subcostal retractions,
nasal flaring, and cyanosis.
Breath sounds may be normal or diminished with a harsh tubular quality, and on deep
inspiration, fine crackles may be heard.
The natural course of untreated RDS is characterized by progressive worsening of cyanosis
and dyspnea. If the condition is inadequately treated, blood pressure may fall; cyanosis and
pallor increase, and grunting decreases or disappears, as the condition worsens.
Apnea and irregular respirations are ominous signs requiring immediate intervention.
Respiratory failure may occur in infants with rapid progression of the disease.
In most cases, the signs reach a peak within 3 days, after which improvement is gradual.
Improvement is often heralded by spontaneous diuresis and improved blood gas values at
lower inspired oxygen levels and/or lower ventilator support.
Death can result from :
1. severe impairment of gas exchange
2. alveolar air leaks (interstitial emphysema, pneumothorax)
3. pulmonary hemorrhage
4. IVH.
5. BPD is a form of chronic lung disease that often develops in infants with severe RDS.
Diagnosis
Clinical course.
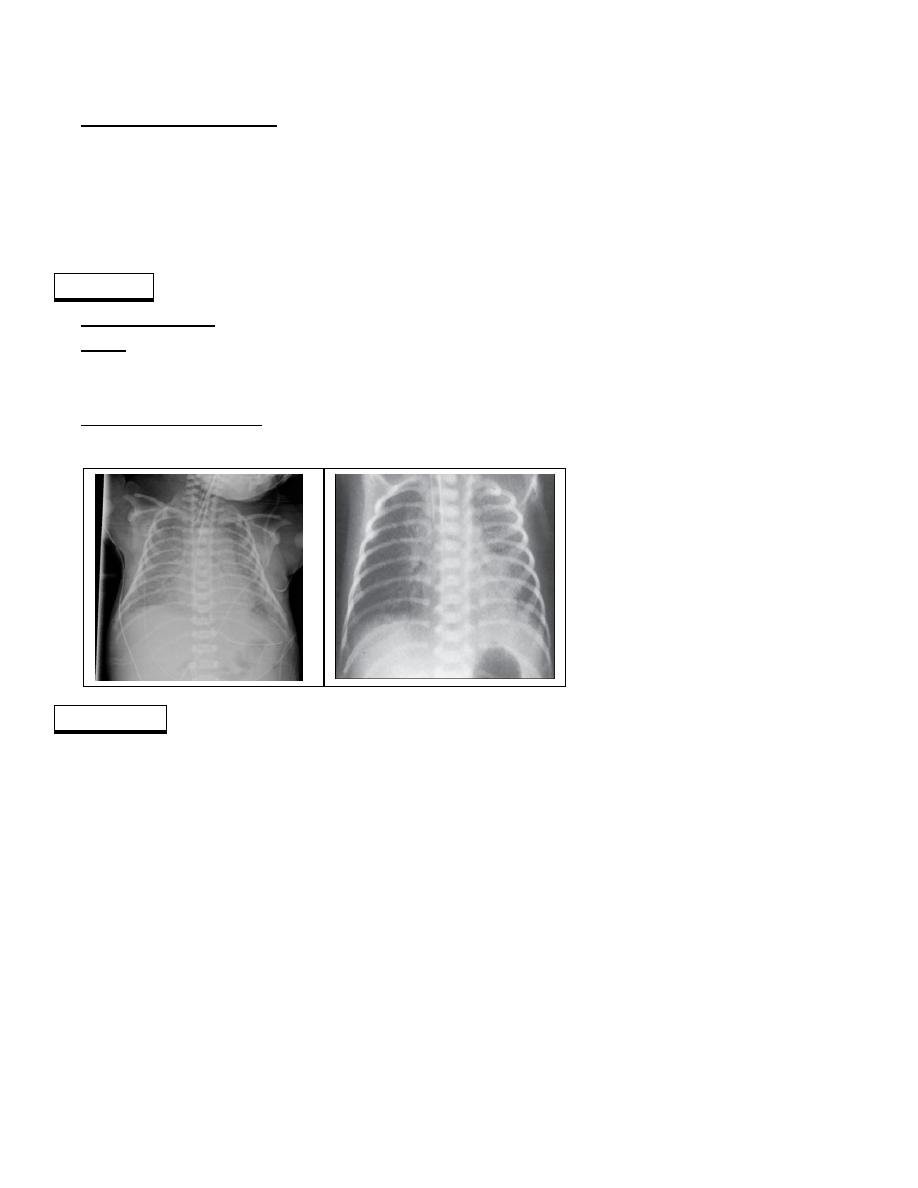
CXR: atelectasis, ground-glass haze in the lung surrounding air-filled bronchi (the air
bronchogram). In severe RDS, CXR may show an airless lung field (whiteout), even
obliterating the distinction between the atelectatic lungs and the heart.
Laboratory findings: are characterized initially by hypoxemia and later by progressive
hypoxemia, hypercapnia, and variable metabolic acidosis.
Prevention
Avoidance of unnecessary or poorly timed early cesarean section (<39 wk) or induction of
labor.
Appropriate management of high-risk pregnancy and labor.
Antenatal and intrapartum fetal monitoring may decrease the risk of fetal asphyxia; asphyxia
is associated with an increased incidence and severity of RDS.
Administration of antenatal corticosteroids to women before 34 wk of gestation significantly
reduces the incidence and mortality of RDS. (e.g.betamethasone) stimulates fetal lung
production of surfactant; this approach requires multiple doses for at least 48 hours.
After birth, RDS may be prevented or its severity reduced by intratracheal administration of
exogenous surfactant immediately after birth in the delivery room or within a few hours of
birth.

Treatment
Early supportive care of premature infants, especially in the treatment of acidosis,
hypoxia, hypotension, and hypothermia, may lessen the severity of RDS.
Therapy requires careful and frequent monitoring of vital sign, oxygen saturation, arterial
blood gas & PH, serum bicarbonate, electrolytes, glucose & hematocrit.
Arterial catheterization is frequently necessary.
Warm humidified oxygen should be provided at a concentration initially sufficient to keep
arterial oxygen pressure between 50 and 70 mm Hg (91-95% saturation) in order to maintain
normal tissue oxygenation while minimizing the risk of oxygen toxicity. If oxygen saturation
cannot be kept >90%, applying CPAP at a pressure of 5-10 cm H2O via nasal prongs is
indicated and usually produces a rapid improvement in oxygenation.
If an infant with RDS undergoing CPAP cannot keep oxygen saturation >90% while
breathing 40-70% oxygen, assisted ventilation and surfactant are indicated.
Systemic CS have arole in treating RDS but associated with side effect. Administration of
inhaled steroids to ventilated preterm infants during the 1st 2 wk after birth reduced the need
for systemic steroids and tended to decrease rates of death and/or BPD at 36 wk without an
increase in adverse effects.
Inhaled nitric oxide (iNO) decreases the need for extracorporeal membrane oxygenation
(ECMO) in term and near-term infants with hypoxic respiratory failure or persistent
pulmonary hypertension of the neonate.
Sodium bicarbonate, 1-2 mEq/kg for treatment of metabolic acidosis.
If hypotension, administration of volume (crystalloid) and early use of vasopressors.
Dopamine is more effective in raising blood pressure than dobutamine. Hypotension may be
refractory to pressors, but responsive to glucocorticoids, especially in neonates <1,000 g.
Because of the difficulty of distinguishing group B streptococcal or other bacterial
infections from RDS, empirical antibiotic therapy is indicated until the results of blood
cultures are available.
Complication
1. PDA
2. BPD (Bronchopulmonary Dysplasia)
BronchoPoulmonary Dysplasia (BPD)
BPD (Chronic Lung Disease) is a clinical diagnosis defined by oxygen dependence at 36
weeks postconceptual age and accompanied by characteristic clinical and radiographic
findings that correspond.
Oxygen concentrations greater than 40% are toxic to the neonatal lung.
Assisted ventilation with high peak pressures produces barotrauma.
Inflammation from prolonged assisted ventilation and repeated systemic and pulmonary
infections may play a major role.
Failure of RDS to improve after 2 weeks, the need for prolonged mechanical ventilation, and
oxygen therapy required at 36 weeks’ postconceptual age are characteristic of patients with
RDS in whom BPD develops.
Clinical manifestations
Oxygen dependence, hypercapnia with a compensatory metabolic alkalosis.
Pulmonary hypertension, poor growth, and development of right sided heart failure.
Increased airway resistance with reactive airway bronchoconstriction also is noted and is
treated with bronchodilating agents.
Fluid retention into the interstitial space , necessitating fluid restriction and the
administration of diuretics.
Diagnosis
Clinical course
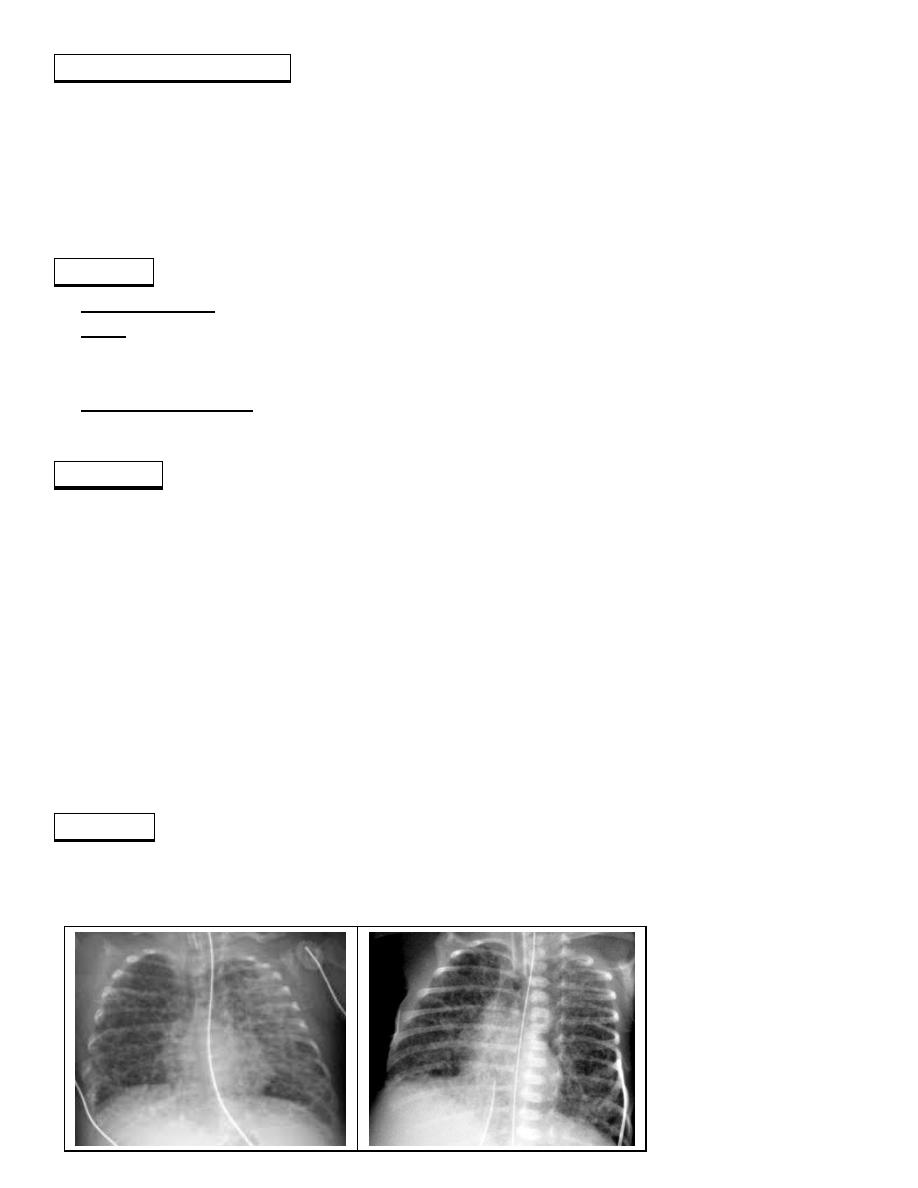
CXR: characterized initially by lung opacification and subsequently by development of
cysts accompanied by areas of overdistention and atelectasis, giving the lung a spongelike
appearance.
The histopathology: reveals interstitial edema, atelectasis, mucosal metaplasia, interstitial
fibrosis, necrotizing obliterative bronchiolitis, and overdistended alveoli.
Treatment
Patients with severe BPD may need treatment with mechanical ventilation for many
months. To reduce the risk of subglottic stenosis, a tracheotomy may be indicated.
To reduce oxygen toxicity and barotrauma, ventilator settings are reduced to maintain blood
gases with slightly lower Pao2 (50 mm Hg) and higher Paco2 (50 to 75 mm Hg) levels than
for infants during the acute phase of RDS.
Dexamethasone therapy may reduce inflammation, improve pulmonary function, and
enhance weaning of patients from assisted ventilation. However, dexamethasone may
increase the risk of cerebral palsy or abnormal neuromotor developmental outcome.
Inhaled bronchodilators improve lung mechanics by decreasing airway resistance.
Methylxanthines are used to increase respiratory drive, decrease apnea, and improve
diaphragmatic contractility.
Diuretic
Prognosis
Older survivors of BPD have hyperinflation, reactive airways, and developmental delay.
They are at risk for severe respiratory syncytial virus pneumonia and as infants should
receive prophylaxis against respiratory syncytial virus.
