Pathogenesis of Diabetes
In both of the common types of diabetes, environmental factors interact with genetic susceptibility to determine which people develop the clinical syndrome, and the timing of its onset.However, the underlying genes, precipitating environmental factors and pathophysiology differ substantially between type 1 and type 2 diabetes.
Type 1 diabetesPathology
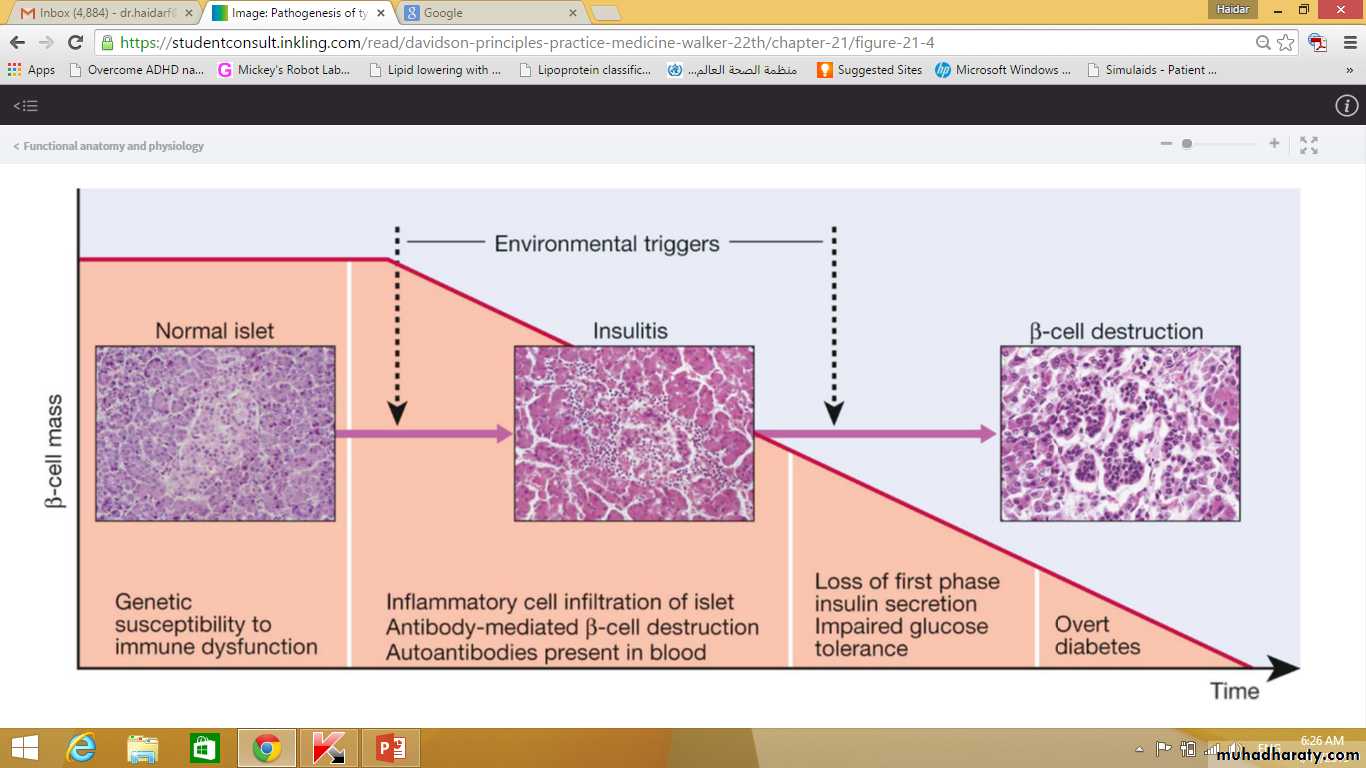
Type 1 diabetes is a T cell-mediated autoimmune disease involving destruction of the insulin-secreting β cells in the pancreatic islets.Progressive loss of β cell function takes place over a prolonged period (months to years), but marked hyperglycaemia, accompanied by the classical symptoms of diabetes, occurs only when 80–90% of the functional capacity of β cells has been lost.
Pathogenesis of type 1 Diabetes
Metabolic disturbances in type 1 diabetes
Patients with type 1 diabetes present when progressive β-cell destruction has crossed a threshold at which adequate insulin secretion and normal blood glucose levels can no longer be sustained.Above a certain level, high glucose levels may be toxic to the remaining β cells, so that profound insulin deficiency rapidly ensues, causing the metabolic sequelae shown in figure in the next slide.
Insufficient insulin
Increase FFA & Glycerol to liver
Increase proteolysis
Increase lipolysis
Increase gluconeogenesis
Increase glycogenolysis
Decreased glucose uptake & Utilization
Increase ketogenesis
Metabolic acidosis
Hyperglycemia
Glycosuria
Osmotic diuresis
Dehydration
Secondary hyperldosteronism
K+ deficiency
Hyperosmolarity
Impaired renal function
Increased lactate
Hyperglycaemia leads to glycosuria and dehydration, causing fatigue, polyuria, nocturia, thirst and polydipsia, susceptibility to urinary and genital tract infections, and later tachycardia and hypotension.
Unrestrained lipolysis and proteolysis result in weight loss.
Ketoacidosis occurs when generation of ketone bodies exceeds the capacity for their metabolism.
Elevated blood H+ ions drive K+out of the intracellular compartment, while secondary hyperaldosteronism encourages urinary loss of K+.
Thus patients usually present with a short history (typically a few weeks) of hyperglycaemic symptoms (thirst, polyuria, nocturia and fatigue), infections and weight loss, and may have developed ketoacidosis.
Type 2 diabetesPathology
Type 2 diabetes is a diagnosis of exclusion, i.e. it is made when type 1 diabetes and other types of diabetes are ruled out, and is highly heterogeneous.
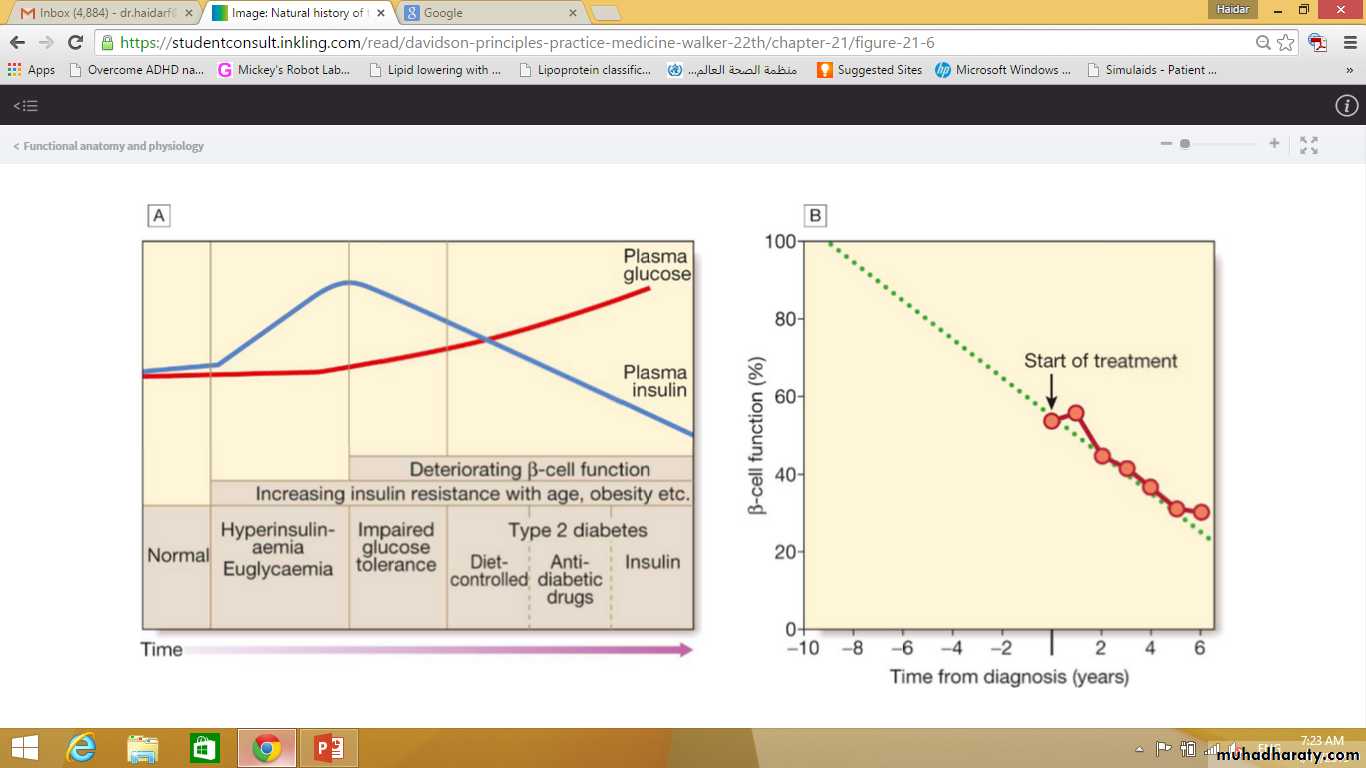
The natural history of typical type 2 diabetes is shown in following figure.
Initially, insulin resistance leads to elevated insulin secretion in order to maintain normal blood glucose levels.
However, in susceptible individuals, the pancreatic β cells are unable to sustain the increased demand for insulin and a slowly progressive insulin deficiency develops.
Some patients develop diabetes at a young age, usually driven by insulin resistance due to obesity and ethnicity; others, particularly the elderly, develop diabetes despite being non-obese and may have more pronounced β-cell failure.
The key feature is a ‘relative’ insulin deficiency, such that there is insufficient insulin production to overcome the resistance to insulin action.
This contrasts with type 1 diabetes, in which there is rapid loss of insulin production and an absolute deficiency, resulting in ketoacidosis and death if the insulin is not replaced.
Basic pathophysiology in Type 2 DM
Insulin resistanceObesity
Genetic predisposition
Environmental factors
Aetiological classification of diabetes mellitus
Type 1 diabetesImmune-mediated
Idiopathic
Type 2 diabetes
Gestational diabetes
Other specific types
Aetiological classification of diabetes mellitus
Other specific types
Genetic defects of β-cell function
Genetic defects of insulin action (e.g. leprechaunism, lipodystrophies)
Pancreatic disease (e.g. pancreatitis, pancreatectomy, neoplastic disease, cystic fibrosis, haemochromatosis, fibrocalculous pancreatopathy)
Aetiological classification of diabetes mellitus
Other specific typesExcess endogenous production of hormonal antagonists to insulin, e.g.
Growth hormone – acromegaly
Glucocorticoids – Cushing’s syndrome
Glucagon – glucagonoma
Catecholamines – phaeochromocytoma
Thyroid hormones – thyrotoxicosis
Aetiological classification of diabetes mellitus
Other specific typesDrug-induced (e.g. corticosteroids, thiazide diuretics, phenytoin)
Uncommon forms of immune-mediated diabetes (e.g. IPEX (immunodysregulation polyendocrinopathy X) syndrome)
Associated with genetic syndromes (e.g. Down’s syndrome; Klinefelter’s syndrome; Turner’s syndrome; DIDMOAD (Wolfram’s syndrome) – diabetes insipidus, diabetes mellitus, optic atrophy, nerve deafness; Friedreich’s ataxia; myotonic dystrophy)
Monogenic diabetes mellitusMaturity-onset diabetes of the young (MODY)
Functional defect
Main type*
Gene mutated*
β-cell glucose sensing
MODY2
GCK
The set point for basal insulin release is altered, causing a high fasting glucose, but sufficient insulin is released after meals. As a result, the HbA1c is often normal and microvascular complications are rare. Treatment is rarely required
β-cell transcriptional regulation
MODY3HNF-1α
MODY5
HNF-1βMODY1
HNF-4αDiabetes develops during adolescence/early adulthood and can be managed with diet and tablets for many years, but ultimately, insulin treatment is required. The HNF-1α and 4α forms respond particularly well to sulphonylurea drugs. All types are associated with microvascular complications. HNF-1β mutations also cause renal cysts and renal failure
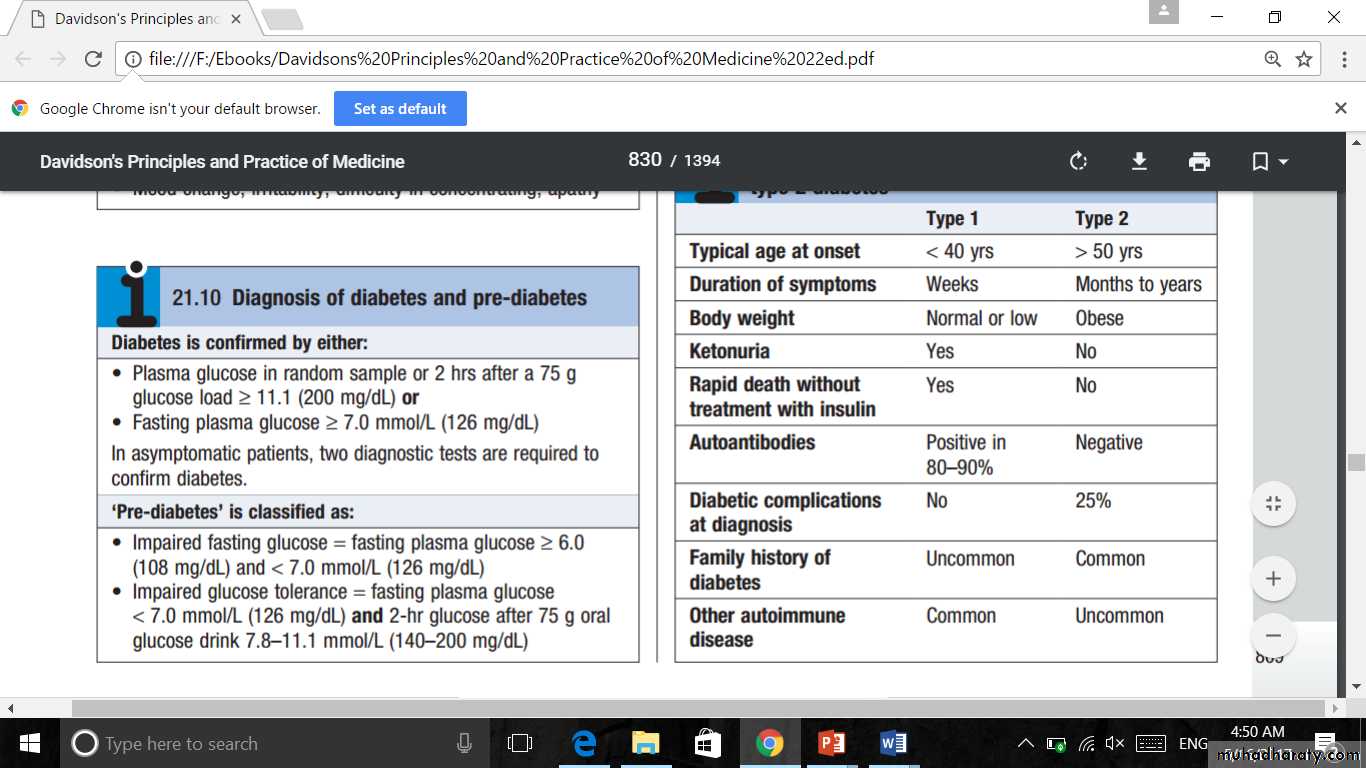
Classical features of type 1 and type 2 diabetes
InvestigationsUrine testingGlucose
Testing the urine for glucose with dipsticks is a common screening procedure for detecting diabetes.If possible, testing should be performed on urine passed 1–2 hours after a meal to maximise sensitivity.
Glycosuria always warrants further assessment by blood testing
The greatest disadvantage of urinary glucose measurement is the individual variation in renal threshold for glucose.
The most frequent cause of glycosuria is a low renal threshold, which is common during pregnancy and in young people; the resulting ‘renal glycosuria’ is a benign condition unrelated to diabetes.
Another disadvantage is that some drugs (such as β-lactam antibiotics, levodopa and salicylates) may interfere with urine glucose tests.
Urine testingKetones
Ketone bodies can be identified by the nitroprusside reaction, which measures acetoacetate, using either tablets or dipsticks.Ketonuria may be found in normal people who have been fasting or exercising strenuously for long periods, who have been vomiting repeatedly, or who have been eating a diet high in fat and low in carbohydrate.
Ketonuria is therefore not pathognomonic of diabetes but, if associated with glycosuria, the diagnosis of diabetes is highly likely. In diabetic ketoacidosis, ketones can also be detected in plasma using test sticks
Urine testingProtein
Standard dipstick testing for albumin detects urinary albumin at concentrations above 300 mg/L, but smaller amounts (microalbuminuria,) can only be measured using specific albumin dipsticks or by quantitative biochemical laboratory measurement.Microalbuminuria or proteinuria, in the absence of urinary tract infection, is an important indicator of diabetic nephropathy and/or increased risk of macrovascular disease.
Blood testingGlucose
Laboratory glucose testing in blood relies upon an enzymatic reaction (glucose oxidase) and is cheap, usually automated and highly reliable. However, blood glucose levels depend on whether the patient has eaten recently, so it is important to consider the circumstances in which the blood sample was taken.
Blood glucose can also be measured with colorimetric or other testing sticks, which are often read with a portable electronic meter. These are used for capillary (fingerprick) testing to monitor diabetes treatment
There is some debate as to whether self-monitoring in people with type 2 diabetes improves glycaemic control. Many countries now only offer self-monitoring to people with type 2 diabetes taking insulin therapy. To make the diagnosis of diabetes, the blood glucose concentration should be estimated using an accurate laboratory method rather than a portable technique.
Glucose concentrations are lower in venous than arterial or capillary (fingerprick) blood. Whole blood glucose concentrations are lower than plasma concentrations because red blood cells contain relatively little glucose. Venous plasma values are usually the most reliable for diagnostic purposes.
Glycated haemoglobin
Glycated haemoglobin provides an accurate and objective measure of glycaemic control integrated over a period of weeks to months.In diabetes, the slow non-enzymatic covalent attachment of glucose to haemoglobin (glycation) increases the amount in the HbA1 (HbA1c) fraction relative to non-glycated adult haemoglobin (HbA0).
These fractions can be separated by chromatography; laboratories may report glycated haemoglobin as total glycated haemoglobin (GHb), HbA1or HbA1c. In most countries, HbA1c is the preferred measurement. The rate of formation of HbA1c is directly proportional to the ambient blood glucose concentration; a rise of 1% in HbA1c corresponds to an approximate average increase of 2 mmol/L (36 mg/dL) in blood glucose.
Although HbA1c concentration reflects the integrated blood glucose control over the lifespan of erythrocytes (120 days), HbA1c is most sensitive to changes in glycaemic control occurring in the month before measurement.
Various assay methods are used to measure HbA1c, but most laboratories have been reporting HbA1c values (as %) aligned with the reference range that was used in the Diabetes Control and Complications Trial (DCCT).
To allow worldwide comparisons of HbA1c values, the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has developed a standard method; IFCC-standardised HbA1c values are reported in mmol/mol. In 2011, many countries adopted the IFCC reference method.
HbA1c estimates may be erroneously diminished in anaemia or during pregnancy, and may be difficult to interpret with some assay methods in patients who have uraemia or a haemoglobinopathy.
Management
The aims of management are:to improve symptoms of hyperglycaemia
to minimise the risks of long-term microvascular and macrovascular complications.
Treatment methods for diabetes include:
dietary/lifestyle modification,
oral anti-diabetic drugs
injected therapies.
In patients with suspected type 1 diabetes, urgent treatment with insulin is required and prompt referral to a specialist is usually needed.
In patients with suspected type 2 diabetes, first-line therapy involves advice about dietary and lifestyle modification. Oral anti-diabetic drugs are usually added in those who do not achieve glycaemic targets as a result, or who have severe symptomatic hyperglycaemia at diagnosis and a high HbA1c. However, the guidelines in some countries are to introduce medication immediately upon diagnosis of diabetes without waiting to assess the impact of diet and lifestyle changes.
In parallel with treatment of hyperglycaemia, other risk factors for complications of diabetes need to be addressed, including treatment of hypertension and dyslipidaemia, and advice on smoking cessation.
Educating patients
It is essential that people with diabetes understand their disorder and learn to handle all aspects of their management as comprehensively and quickly as possible.
Ideally, this can be achieved by a multidisciplinary team (doctor, dietitian, specialist nurse and podiatrist) in the outpatient setting. For those with newly diagnosed type 2 diabetes, structured education can be given in groups by trained educators.
Those requiring insulin need to learn how to measure doses of insulin accurately with an insulin syringe or pen device, how to inject, and how to adjust the dose on the basis of blood glucose values and in relation to factors such as exercise, illness and episodic hypoglycaemia.
They must therefore acquire a working knowledge of diabetes, be familiar with the symptoms of hypoglycaemia, and have ready access to medical advice when the need arises. Information should be provided about driving (national statutory regulations and practical safety measures.
Providing this education is time-consuming but essential if patients are to undertake normal activities safely while maintaining good control.
Glucose monitoring—HbA1c1,2
HbA1c should be performedTwice yearly in those who have achieved the target
Four times in a year in those who have not achieved the target or whose treatment has been changed
HbA1c strongly predicts diabetic complications
An HbA1c goal of 6.5% to 7% is recommended for optimal control of diabetes based on individual needs
International Diabetes Federation, 2005. Global guidelines for type 2 diabetes. www.idf.org/webdata/docs/IDF%20GGT2D.pdf.
American Diabetes Association. Standards of Medical Care in Diabetes—2011. Diabetes Care. 2010;34(Suppl 1):S11-S61.
The ADA recommendations
Detailed instructions on SMBG techniques and use of the data for treatment modifications should be providedSMBG should be used to:
Assess the individual response to therapy
Assess glycaemic targets and
Adjust medications (by the physician)
Continuous glucose monitoring (CGM) can be an additional tool to SMBG:
In patients with hypoglycaemia unawareness
In patients with frequent hypoglycaemic episodes
In patients with frequent glucose fluctuations (hypo-hyper)
American Diabetes Association. Standards of Medical Care in Diabetes—2011. Diabetes Care. 2010;34(suppl ):S11-S61.
Nutritional therapy1,2
A vital part of initial and continuing diabetes education programmes
Objectives:
To attain and preserve optimal body weight or BMI
To prevent hypoglycaemia
To provide optimal nutritional requirement
To attain optimal blood lipid status
Maintain desired blood pressure levels
Global guidelines for type 2 diabetes. www.idf.org/webdata/docs/IDF%20GGT2D.pdf.
Medical Guidelines for the Management of Diabetes. WHO website. http://www.emro.who.int/lebanon/ncd/diabetes%20book%20part%202.pdf.
Lifestyle interventions—Weight management
Benefits of moderate weight loss (5% of body weight)Klien S, Sheard N, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes. Diabetes Care. 2004;27(8) :2067-2073.
Lifestyle interventions—Weight management
Weight loss recommendationsKlien S, Sheard N, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes. Diabetes Care. 2004;27(8):2067-2073.
Recommended for everyone with BMI >25 kg/m2
Diet and physical activity are the primary approach
Calorie intake should be reduced to ~500–1000 kcal/d
Diet should contain at least 1000–1200 kcal/d for women and 1200–1600 kcal/d for men
Regular moderate-intensity physical activity is recommended
30- to 45-min aerobics/30-min jogging/1-hr of walking per day is recommended
Lifestyle interventions—Total calorie distribution
Clinical management of type 2 diabetes mellitus. WHO website. http://www.emro.who.int/lebanon/2006%20Guideline%20diab2.pdf.
Breads,
grains andother starches
Lifestyle interventions—Diabetes food pyramid
Fats, oils
and sweetsMeat, meat substitutes and other proteins
Fruits
Milk
Vegetables
Dietary recommendations in special situations1,2
• Nephropathy patients• Dietary protein restricted to 0.6 g/kg
• Restrict excessive salt intake (>7 g/d)
Overweight patients
Moderate calorie restriction (500 or 1000 kcal less than previous intake)
Hypertensive patients
Restrict salt intake to <3 g/d
Dyslipidaemia patients
<200 mg cholesterol/day
<10% of energy intake from saturated fat
Alcohol—Moderate consumption can be permitted
Smoking to be avoided
Medical Guidelines for the Management of Diabetes. WHO website. http://www.emro.who.int/lebanon/ncd/diabetes%20book%20part%202.pdf. Accessed January 3, 2012.
Clinical management of type 2 diabetes mellitus. WHO website http://www.emro.who.int/lebanon/2006%20Guideline%20diab2.pdf. Accessed January 3, 2012.
Lifestyle interventions—Physical activity
Regular physical activity is an essential component of T2DM management1
Benefits of physical exercise:
Increased cardiorespiratory fitness
Improved glycaemic control
Decreased insulin resistance
Improved lipid profile
Maintenance of weight loss
International Diabetes Federation. http://www.idf.org/international-curriculum-diabetes-health-professional-education.
Drugs to reduce hyperglycaemia
For many years, there were only a few choices of drugs available for type 2 diabetes – the biguanide metformin, the sulphonylureas and insulin.Insulin is the only treatment for type 1 diabetes. Acarbose was also available but little used in most countries.
Since the late 1990s, however, several new classes of agents have been approved for use in type 2 diabetes, with more in development. Newer drugs include thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, and sodium and glucose transporter 2 (SGLT2) inhibitors.
The effects of these drugs are compared in the next slide
Insulin
SU/Meglitinides
Metformin
Acarbose
Thiazolidinediones (glitazones)
DPP-4 inhibitors (gliptins)
GLP-1 receptor agonists
SGLT2 inhibitors
Fasting blood glucose
↓
↓
↓
↘
↓
↓
↓
↓
Post-prandial blood glucose
↓
↓
↓
↓
↓
↓
↓
↓
Plasma insulin
↑
↑
↓
↓
↓
↑
↑
↓
Body weight
↑
↑
→
→
↑
→
↓
↓
Risk of hypoglycaemia
++
+
–
–
–
–
–
–
Tolerability
Good
Good
Moderate
Moderate
Moderate
Good
Moderate
Limited experience
This makes for an exciting time in diabetes pharmacotherapy, but exactly how, when and in what order these agents should be used remains uncertain.
The older drugs are cheaper and have established benefits for reducing microvascular disease; they are therefore usually recommended as first-line therapy.
Use of the newer drugs is not supported by evidence for reduction in microvascular disease (because the trials have not yet been done) and they are much more expensive, so are often reserved for later therapy after failure of metformin and sulphonylureas.
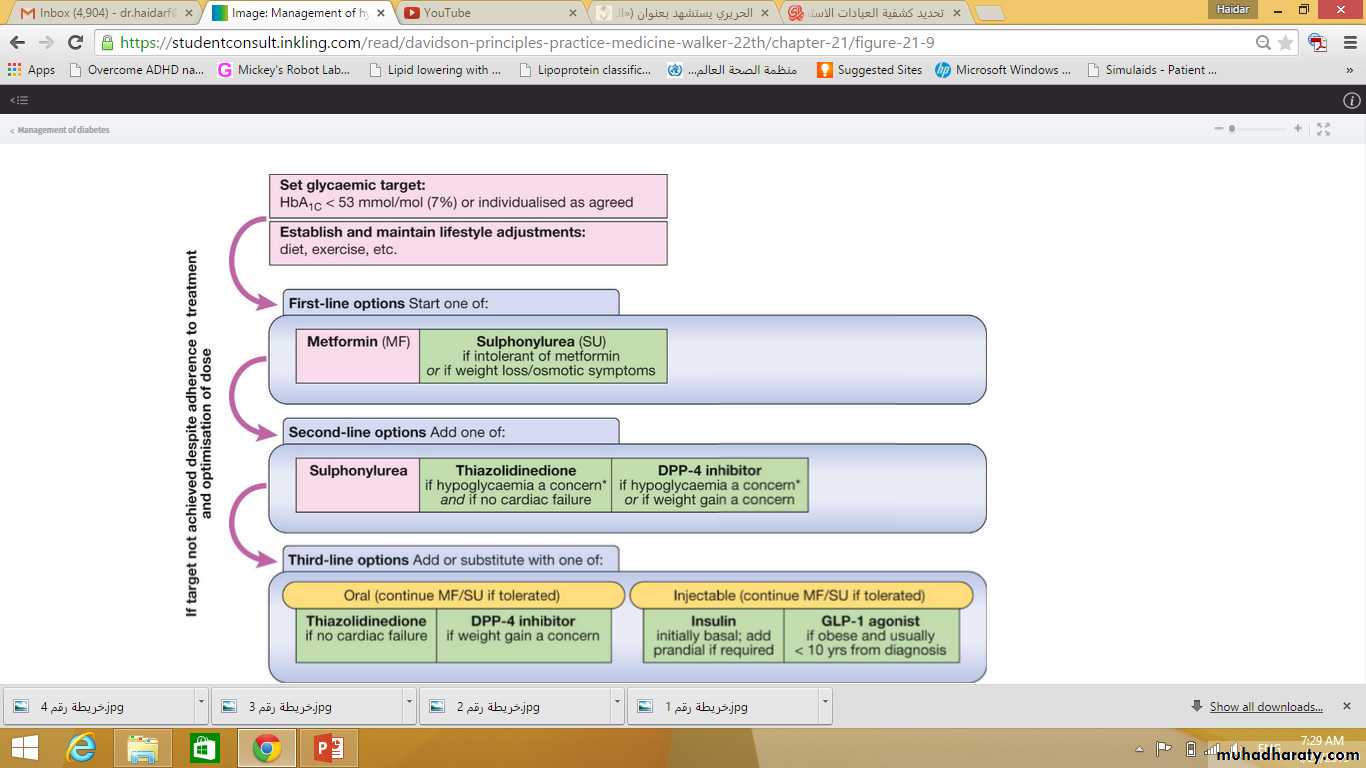
One guideline which follows these principles is shown in the next slide.
However, this does not necessarily reflect the optimum positioning of these newer drugs. As large trials, which aim to establish their cardiovascular benefit, report in the next few years, these recommendations may change dramatically.
3. ANTI-HYPERGLYCEMIC THERAPY
• Glycemic targets• HbA1c < 7.0% (mean PG 150-160 mg/dl [8.3-8.9 mmol/l])
• Pre-prandial PG <130 mg/dl (7.2 mmol/l)
• Post-prandial PG <180 mg/dl (10.0 mmol/l)
• Individualization is key:
• Tighter targets (6.0 - 6.5%) - younger, healthier
• Looser targets (7.5 - 8.0%+) - older, comorbidities, hypoglycemia prone, etc.
• Avoidance of hypoglycemia
PG = plasma glucose
ADA-EASD Position Statement Update:Management of Hyperglycemia in T2DM, 2015
Diabetes Care 2012;35:1364–1379; Diabetologia 2012;55:1577–1596
Biguanides
Metformin is the only biguanide now available. Its long-term benefits were shown in the UK Prospective Diabetes Study (UKPDS) (p. 827), and it is now widely used as first-line therapy for type 2 diabetes, irrespective of body weight.
Metformin is also given increasingly as an adjunct to insulin therapy in obese patients with type 1 diabetes.
Approximately 25% of patients develop mild gastrointestinal side-effects with metformin, but only 5% are unable to tolerate it even at low dose. The main side-effects are diarrhoea, abdominal cramps, bloating and nausea.
Mechanism of action
The mechanism of action of metformin has not been precisely defined.Whilst classically considered an ‘insulin sensitiser’ because it lowers insulin levels, its main effects are on fasting glucose and are insulin-independent.
Metformin reduces hepatic glucose production, may also increase insulin-mediated glucose uptake, and has effects on gut glucose uptake and utilisation.
At the molecular level, metformin acts as a weak inhibitor of mitochondrial respiration, which increases intracellular adenosine monophosphate (AMP) and reduces adenosine triphosphate (ATP).
This has direct effects on the flux through gluconeogenesis, and activates the intracellular energy sensor AMP-activated protein kinase (AMPK), leading to multiple beneficial metabolic effects.
Clinical use
Metformin is a potent blood glucose-lowering treatment that is weight-neutral, does not cause hypoglycaemia and has established benefits in microvascular disease. It is employed as first-line therapy in all patients who tolerate it, and its use is maintained when additional agents are added as glycaemia deterioratesMetformin is usually introduced at low dose (500 mg twice daily) to minimise the risk of gastrointestinal side-effects.
The usual maintenance dose is 1 g twice daily.
There is a modified-release formulation of metformin which may be better tolerated by patients with gastrointestinal side-effects.
Metformin can increase susceptibility to lactic acidosis, although this is much less common than was previously thought. As metformin is cleared by the kidneys, it can accumulate in renal impairment, so the dose should be halved when estimated glomerular filtration rate (eGFR) is 30–45 mL/min, and it should not be used below an eGFR of 30 mL/min. Its use is also contraindicated in patients with impaired hepatic function and in those who drink alcohol in excess, in whom the risk of lactic acidosis is significantly increased. It should be discontinued, at least temporarily, if any other serious medical condition develops, especially one causing severe shock or hypoxia. In such circumstances, treatment with insulin should be substituted if required.
Sulphonylureas
Sulphonylureas are ‘insulin secretagogues’, i.e. they promote pancreatic β-cell insulin secretion. Similar to metformin, the long-term benefits of sulphonylureas in lowering microvascular complications of diabetes were established in the UK Prospective Diabetes StudyMechanism of action
Sulphonylureas act by closing the pancreatic β-cell ATP-sensitive potassium (KATP) channel, decreasing K+ efflux, which ultimately triggers insulin secretion. Meglitinides (e.g. repaglinide and nateglinide) also work in this way and, although short-acting, are essentially sulphonylurea-like drugs.
Clinical use
Sulphonylureas are an effective therapy for lowering blood glucose and are often used as an add-on to metformin, if glycaemia is inadequately controlled on metformin aloneThe main adverse effects of sulphonylureas are weight gain and hypoglycaemia.
The weight gain is not ideal in patients with diabetes who are already overweight or obese, although sulphonylureas are effective treatments in this group.
Hypoglycaemia occurs because the closure of KATP channels brings about unregulated insulin secretion, even with normal or low blood glucose levels.
There are a number of sulphonylureas. In the UK, gliclazide is the most commonly used; in contrast, in the USA, glibenclamide (also known as glyburide) is widely used.
Glibenclamide, however, is long-acting and prone to induce hypoglycaemia, so should be avoided in the elderly.
Other sulphonylureas include glimepiride and glipizide. The dose–response of all sulphonylureas is steepest at low doses; little additional benefit is obtained when the dose is increased to maximal levels.
Alpha-glucosidase inhibitors
The α-glucosidase inhibitors delay carbohydrate absorption in the gut by inhibiting disaccharidases.Acarbose and miglitol are available and are taken with each meal. Both lower post-prandial blood glucose and modestly improve overall glycaemic control.
They can be combined with a sulphonylurea. The main side-effects are flatulence, abdominal bloating and diarrhoea. They are used widely in the Far East but infrequently in the UK.
ThiazolidinedionesMechanism of action
These drugs (also called TZDs, ‘glitazones’ or PPARγ agonists) bind and activate peroxisome proliferator-activated receptor-γ, a nuclear receptor present mainly in adipose tissue that regulates the expression of several genes involved in metabolism.TZDs enhance the actions of endogenous insulin, in part directly (in the adipose cells) and in part indirectly (by altering release of ‘adipokines’, such as adiponectin, which alter insulin sensitivity in the liver). Plasma insulin concentrations are not increased and hypoglycaemia does not occur.
TZDs increase pre-adipocyte differentiation, resulting in an increase in fat mass and in body weight.
Clinical use
TZDs have been prescribed widely since the late 1990s, but recently a number of adverse effects have become apparent and their use has declined. One popular TZD, rosiglitazone, was reported to increase the risk of myocardial infarction and was withdrawn in 2010. The other TZD in common use, pioglitazone, does not appear to increase the risk of myocardial infarction but it does exacerbate cardiac failure by causing fluid retention, and recent data show that it increases the risk of bone fracture, and possibly bladder cancer. These observations have reduced the use of pioglitazone dramatically.
Pioglitazone can be very effective at lowering blood glucose in some patients and appears more effective in insulin-resistant patients. In addition, it has a beneficial effect in reducing fatty liver and NASH (p. 805). Pioglitazone is usually added to metformin with or without sulphonylurea therapy (see Fig. 21.9). It may be given with insulin therapy, when it can be very effective, but the combination of insulin and TZDs markedly increases fluid retention and risk of cardiac failure, so should be used with caution.
Incretin-based therapies: DPP-4 inhibitors and GLP-1 analogues
The incretin effect is the augmentation of insulin secretion seen when a glucose stimulus is given orally rather than intravenously, and reflects the release of incretin peptides from the gutThe incretin hormones are primarily glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). These are rapidly broken down by the peptidase DPP-4 (dipeptidyl peptidase 4).
The incretin effect is diminished in type 2 diabetes, and this has stimulated the development of two incretin-based therapeutic approaches.
The ‘gliptins’, or DPP-4 inhibitors, prevent breakdown and therefore enhance concentrations of endogenous GLP-1 and GIP. The first DPP-4 inhibitor to market was sitagliptin; others now available include vildagliptin, saxagliptin and linagliptin. These drugs are very well tolerated and are weight-neutral (see Box 21.27).
The GLP-1 receptor agonists have a similar structure to GLP-1 but have been modified to resist breakdown by DPP-4. These agents are not orally active and have to be given by subcutaneous injection. However, they have a key advantage over the DPP-4 inhibitors: because the GLP-1 activity achieved is supra-physiological, it delays gastric emptying and, at the level of the hypothalamus, decreases appetite. Thus, injectable GLP-1 analogues lower blood glucose and result in weight loss – an appealing therapy, as the majority of patients with type 2 diabetes are obese. Currently available GLP-1 receptor agonists include exenatide (twice daily), exenatide MR (once weekly) and liraglutide (once daily).
Unlike sulphonylureas, both incretin-based therapies only promote insulin secretion when there is a glucose ‘trigger’ for insulin secretion. Thus, when the blood glucose is normal, the insulin secretion is not augmented and so these agents do not cause hypoglycaemia.
SGLT2 inhibitors
The sodium and glucose transporter 2 (SGLT2) inhibitor, dapagliflozin, was licensed for use in 2012.Glucose is filtered freely in the renal glomeruli and reabsorbed in the proximal tubules. SGLT2 is involved in reabsorption of glucose. Inhibition results in approximately 25% of the filtered glucose not being reabsorbed, with consequent glycosuria.
Although this helps to lower blood glucose and results in calorie loss and subsequent weight loss, the glycosuria does result in increased urinary tract and genital fungal infections.
With limited experience and evidence to date, the most appropriate position for SGLT2 inhibitors in the therapy of type 2 diabetes has yet to be established.
Insulin therapyManufacture and formulation
Insulin was discovered in 1921 and transformed the management of type 1 diabetes, until then a fatal disorder. Until the 1980s, insulin was obtained by extraction and purification from pancreata of cows and pigs (bovine and porcine insulins), and some patients still prefer to use animal insulins.Recombinant DNA technology enabled large-scale production of human insulin. More recently, the amino acid sequence of insulin has been altered to produce analogues of insulin, which differ in their rate of absorption from the site of injection.
The duration of action of short-acting, unmodified insulin (‘soluble’ or ‘regular’ insulin), which is a clear solution, can be extended by the addition of protamine and zinc at neutral pH (isophane or NPH insulin) or excess zinc ions (lente insulins). These modified ‘depot’ insulins are cloudy preparations. Pre-mixed formulations containing short-acting and isophane insulins in various proportions are available.
ADA-EASD Position Statement Update:
Management of Hyperglycemia in T2DM, 2015
Long (Detemir)
Rapid (Lispro, Aspart, Glulisine)
HoursLong (Glargine)
0 2 4 6 8 10 12 14 16 18 20 22 24
Short (Regular)Hours after injection
Insulin level
(Degludec)
3. ANTI-HYPERGLYCEMIC THERAPY
• Therapeutic options: Insulins
Duration of action (in hours) of insulin preparations
InsulinOnset
Peak
Duration
Rapid-acting(insulin analogues: lispro, aspart, glulisine)
< 0.5
0.5–2.5
3–4.5
Short-acting(soluble (regular))
0.5–1
1–4
4–8
Intermediate-acting(isophane (NPH), lente)
1–3
3–8
7–14
Long-acting(bovine ultralente)
2–4
6–12
12–30
Long-acting(insulin analogues: glargine, detemir)
1–2
None
18–24
Subcutaneous multiple dose insulin therapy
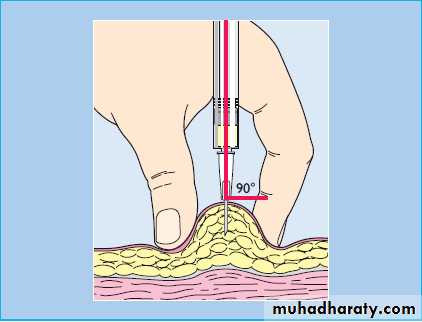
In most patients, insulin is injected subcutaneously several times a day into the anterior abdominal wall, upper arms, outer thighs and buttocks
Accidental intramuscular injection often occurs in children and thin adults.
The rate of absorption of insulin may be influenced by many factors other than the insulin formulation, including the site, depth and volume of injection, skin temperature (warming), local massage and exercise.
Absorption is delayed from areas of lipohypertrophy at injection sites, which results from the local trophic action of insulin, so repeated injection at the same site should be avoided.
Other routes of administration (intravenous and intraperitoneal) are reserved for specific circumstances.
Once absorbed into the blood, insulin has a half-life of just a few minutes.
It is removed mainly by the liver and also the kidneys, so plasma insulin concentrations are elevated in patients with liver disease or renal failure.Rarely, the rate of clearance can be affected by binding to insulin antibodies (induced by use of animal insulins).
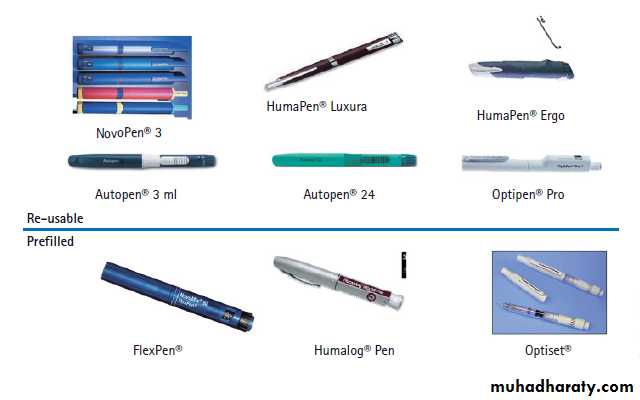
Insulin can be administered using a disposable plastic syringe with a fine needle (which can be re-used several times), but this has largely been replaced by pen injectors containing insulin in cartridges sufficient for multiple dosing. These are also available as pre-loaded disposable pens.
`
solostar
For the most part, insulin analogues have replaced soluble and isophane insulins, especially for people with type 1 diabetes, because they allow more flexibility and convenience
Unlike soluble insulin, which should be injected 30 minutes before eating, rapid-acting insulin analogues can be administered immediately before, during or even after meals.
Long-acting insulin analogues are better able than isophane insulin to maintain ‘basal’ insulin levels for up to 24 hours, so need only be injected once daily.
Despite these pharmacokinetic benefits, the impact of insulin analogues on glycaemic control and adverse events appears to be minor.
The complications of insulin therapy are listed in the next slide ; the most important of these is hypoglycaemia
A common problem is fasting hyperglycaemia (‘the dawn phenomenon’), which arises through a combination of the normal circadian rhythm and release of counter-regulatory hormones (growth hormone and cortisol) during the later part of the night, as well as diminishing levels of overnight isophane insulin.
Time
• Blood glucose persistently too high• Blood glucose persistently too low
Before breakfast
Increase evening long-acting insulin
Reduce evening long-acting insulin
Before lunch
Increase morning short-acting insulin
Reduce morning short-acting insulin or increase mid-morning snack
Before evening meal
Increase morning long-acting insulin or lunch short-acting insulin
Reduce morning long-acting insulin or lunch short-acting insulin or increase mid-afternoon snack
Before bed
Increase evening short-acting insulin
Reduce evening short-acting insulin
Is insulin dose really empirical?
Calculating insulin doses is still somewhat empirical but may be improved through carbohydrate counting, calculation of insulin-to-carbohydrate ratios, and insulin sensitivity.
Starting doses of insulin vary from 0.4 to 1.0 μg/kg per day (T1DM) and from 0.2 to 1.5 μg/kg per day (T2DM), depending on patient size, degree of glycemia, and severity of insulin resistance.
40-50% TDD basal, 50-60% divided into 3 meals
We are in need to know Carbohydrate counting,
Insulin/carbohydrate ratio:
the approximate insulin requirement to maintain euglycemia following mealtime carbohydrate consumption
Injection therapies for type 2 diabetesGLP-1 agonists
Exenatide, liraglutide and lixisenatide are injectable, long-acting analogues of glucagon-like peptide-1 (GLP-1), a gut hormone involved in the incretin effectThey increase insulin secretion, inhibit glucagon secretion, delay gastric emptying and have central effects on appetite, thus blunting the postprandial rise in plasma glucose and promoting weight loss.
Their main clinical disadvantage is the need for subcutaneous injection, and their major advantage is improving glucose control whilst inducing useful weight reduction.
Unwanted effects include nausea, acute pancreatitis and acute kidney injury. At present, they are used as an alternative to insulin, particularly in the overweight. A once-weekly version of exenatide has been developed
GLP-1 promotes β-cell replication in immature rodents, but there is no evidence to suggest that it can do so in adult humans. GLP-1 receptors are also present in the exocrine pancreas and have potential growth-promoting effects, but the long-term clinical implications of this observation remain unclear.
Insulin treatment
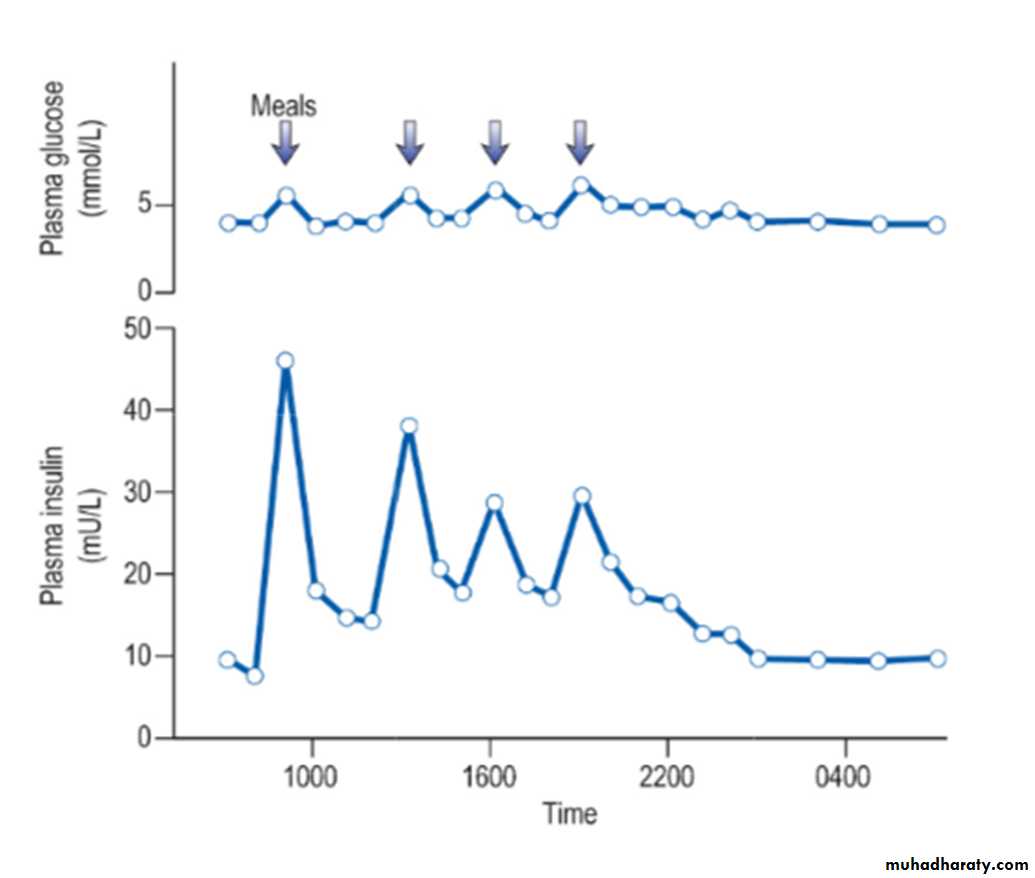
Insulin is found in every vertebrate and the key parts of the molecule show few species differences. Small differences in the amino acid sequence may alter the antigenicity of the molecule. The glucose and insulin profiles in normal subjects are shown in the figure:Short-acting insulins
Insulins were historically derived from beef or pig pancreas but these have now been almost entirely replaced by biosynthetic human insulin. This is produced by adding a DNA sequence coding for insulin or proinsulin into cultured yeast or bacterial cells. Short-acting insulins are used for pre-meal injection in multiple-dose regimens, for continuous intravenous infusion in labour or during medical emergencies, and for use in patients using insulin pumps.
Human insulin is absorbed slowly, reaching a peak 60–90 minutes after subcutaneous injection, and its action tends to persist after meals, predisposing to hypoglycaemia. Absorption is delayed because soluble insulin is in the form of stable hexamers (six insulin molecules around a zinc core) and needs to dissociate to monomers or dimers before it can enter the circulation. Short-acting insulin analogues have been engineered to dissociate more rapidly following injection without altering the biological effect.
Insulin analogues such as the rapid-acting insulins(insulin lispro, insulin aspart and insulin glulisine) enter the circulation more rapidly than human soluble insulin, and also disappear more rapidly. Although widely used, the short-acting analogues have little effect on overall glucose control in most patients, mainly because improved postprandial glucose is balanced by higher levels before the next meal. A Cochrane review has concluded that there is little evidence of their benefit in type 2 diabetes.