
DIABETES MELLITUS •
11
407
Twice-daily injections: short-acting and intermediate-acting insulin
(usually soluble and isophane insulins), given in combination before break-
fast and the evening meal. Usually two-thirds of the total daily insulin
requirement is given in the morning in a ratio of 1 : 2, short : intermediate-
acting insulins. The remaining third is given in the evening. Pre-mixed
formulations are available containing different proportions of soluble and
isophane insulins (e.g. 30 : 70 and 50 : 50). These are useful in patients who
have diffi culty mixing insulins, but preclude adjustment of the individual
components.
Multiple injections: short-acting insulin taken before each meal, and
intermediate-acting insulin injected at bedtime (basal–bolus regimen). This
allows greater freedom for timing of meals. Fast-acting insulin analogues
may be used before meals, and are particularly useful if the evening meal
is late, as they do not induce nocturnal hyperinsulinaemia. However, a long
interval between meals allows the blood glucose to rise. A common problem
is fasting hyperglycaemia (the ‘dawn phenomenon’) caused by the release
of counter-regulatory hormones during the night, which increases insulin
requirement before wakening.
Complications of insulin therapy
• Hypoglycaemia. • Weight gain. • Peripheral oedema (insulin treatment
causes salt and water retention in the short term). • Insulin antibodies
(animal insulins). • Local allergy (rare). • Lipodystrophy at injection sites.
FUTURE THERAPIES
Transplantation of isolated pancreatic islets (usually into the liver via the
portal vein) has been achieved in a small number of humans. The develop-
ment of methods of inducing tolerance to transplanted islets and the use of
stem cells or transformation of hepatocytes to make insulin by genetic
engineering mean that this may still prove the most promising approach in
the long term.
Whole pancreas transplantation presents particular problems relating to
the exocrine pancreatic secretions, and long-term immunosuppression is
necessary. While results are steadily improving, they remain less favourable
than for renal transplantation. However, it is questionable whether it will
ever be considered justifi able to perform transplantation in young diabetic
patients before vascular disease is clinically apparent.
Alternative methods and routes of insulin delivery other than subcutane-
ous injection are being sought. Inhaled, oral and transcutaneous (patch)
routes of delivery are being explored.
LONG-TERM COMPLICATIONS OF DIABETES
People with diabetes have a mortality rate double that of age- and sex-
matched controls. Large blood vessel disease accounts for 70% of all

408
DIABETES MELLITUS •
11
deaths. Atherosclerosis in diabetic patients occurs earlier and is more exten-
sive and severe. Diabetes enhances the effects of the other major cardio-
vascular risk factors: smoking, hypertension and dyslipidaemia.
Microangiopathy (small blood vessel disease) is a specifi c complication
of diabetes. It contributes to mortality by provoking renal failure (diabetic
nephropathy) and causes substantial morbidity and disability: blindness,
diffi culty in walking, chronic foot ulceration, and bowel and bladder dys-
function. The risk of microangiopathy is related to the duration and degree
of hyperglycaemia. Diabetic retinopathy, nephropathy, neuropathy and
atherosclerosis are thought to result from the local response to the general-
ised vascular injury.
Preventing diabetes complications
No study has produced any evidence of improved glycaemic control revers-
ing either retinopathy or nephropathy, and in some cases retinopathy wors-
ened abruptly soon after control was improved. Despite this, in the long
term the rate of progression of both retinopathy and nephropathy was
reduced by continuing better control. The Diabetes Control and Complica-
tions Trial (DCCT) was a large study in type 1 diabetic patients to answer
the question: are diabetic complications preventable? The trial demon-
strated a 60% overall reduction in the risk of developing diabetic complica-
tions in those on intensive therapy with strict glycaemic control (mean
HbA
1c
7%), compared with those on conventional therapy (HbA
1c
9%). This
trial showed that diabetic complications are preventable and treatment aim
should be ‘near-normal’ glycaemia. Strict glycaemic control was compli-
cated by weight gain and severe hypoglycaemic episodes. This increased
risk of hypoglycaemia may alter the risk : benefi t ratio in patients who have
impaired awareness of hypoglycaemia, have severe macrovascular disease
(previous myocardial infarction/cerebrovascular accident), or are at the
extremes of life.
The UK Prospective Diabetes Study (UKPDS) in type 2 diabetes found
that diabetic complications were reduced and progression was slower with
good glycaemic control (mean HbA
1c
7%) and effective treatment of hyper-
tension, irrespective of the type of therapy used. RCTs have also shown
that aggressive management of lipids and BP limits the complications of
diabetes. ACE inhibitors are valuable in improving outcome in heart disease
and in preventing diabetic nephropathy.
DIABETIC RETINOPATHY
Diabetic retinopathy is a common cause of blindness in adults in developed
countries. Hyperglycaemia increases retinal blood fl ow and metabolism,
and has direct effects on retinal endothelial cells, resulting in impaired
vascular autoregulation. This leads to chronic retinal hypoxia which stimu-
lates production of growth factors causing new vessel formation and
increased vascular permeability.

DIABETES MELLITUS •
11
409
Clinical features
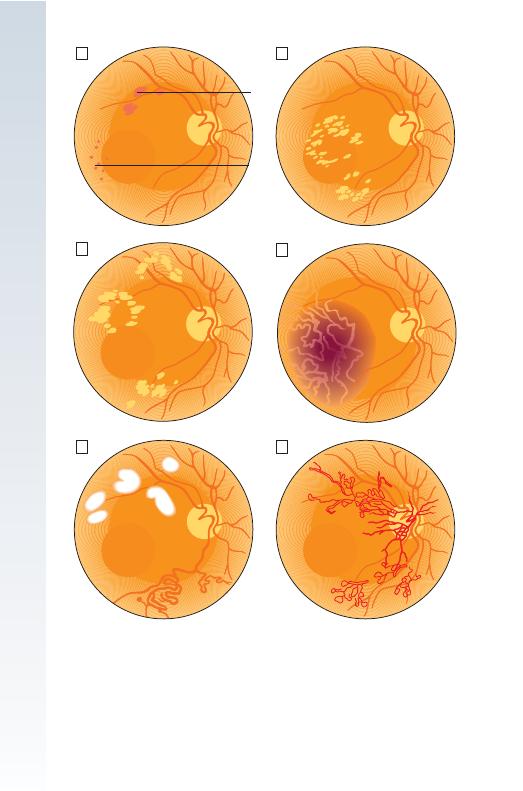
Microaneurysms: Tiny, discrete, circular, dark red spots near to the
retinal vessels (Fig. 11.3A) are the earliest abnormality detected.
Haemorrhages: These are found in the deeper layers of the retina and
are round (‘blot’ haemorrhages, Fig. 11.3A). They are often grouped with
microaneurysms as ‘dots and blots’. Superfi cial fl ame-shaped haemor-
rhages in the nerve fi bre layer also occur, particularly in hypertensive
patients.
Exudates: Characteristic of diabetic retinopathy, these vary in size from
specks to confl uent patches, and tend to occur around the macula (Fig.
11.3B).
Cotton wool spots: Usually occurring within fi ve disc diameters of the
optic disc (Fig. 11.3E), these represent arteriolar occlusions causing retinal
ischaemia and occur in pre-proliferative, often rapidly advancing retinopa-
thy or uncontrolled hypertension.
Intraretinal microvascular abnormalities: These are dilated, tortuous
capillaries representing patent capillaries in an area where most have been
occluded (severe pre-proliferative retinopathy).
Neovascularisation: Vessels arise on the optic disc or retina in response
to an ischaemic retina (Fig. 11.3F). They are fragile and leaky, causing
haemorrhage which may be pre-retinal or vitreous. Leaking serous products
stimulate a connective tissue reaction, called gliosis, by which retinal
detachment can occur due to adhesions between the vitreous and the
retina.
Venous changes: These range from venous dilatation (background)
to venous beading and increased tortuosity (‘oxbow lakes’/loops, pre-
proliferative retinopathy).
Rubeosis iridis: This is the development of new vessels on the anterior
surface of the iris. Vessels may obstruct the drainage angle, causing second-
ary glaucoma.
Classifi cation
A classifi cation based on prognosis is shown in Box 11.11. Background
retinopathy does not threaten vision unless associated with macular disease.
Macular oedema is suspected if there is impairment of visual acuity with
peripheral non-proliferative retinopathy. New vessels may be symptomless
until sudden visual loss occurs from pre-retinal or vitreous haemorrhage.
This resolves, although recurrence is common. Fibrous tissue may interfere
with vision by obscuring the retina or causing detachment.
Prevention
Good glycaemic control reduces the risk of retinopathy. Early diagnosis
and treatment is important for patients with type 2 diabetes; 25% present
with established retinopathy. A rapid reduction in blood glucose may cause
an initial deterioration of retinopathy by causing relative ischaemia.
Improvement in glycaemic control should therefore be effected gradually.
BP lowering is of proven benefi t. Elevated cholesterol is a risk factor, but
410
DIABETES MELLITUS •
11
Blots
Dots
C
E
A
D
F
B
Fig. 11.3 Diagrammatic representation of diabetic eye disease.
䊐
A Background diabetic
retinopathy showing microaneurysms and blot haemorrhages.
䊐
B Background retinopathy
showing exudates.
䊐
C Maculopathy with exudates.
䊐
D Maculopathy showing oedema.
䊐
E Pre-proliferative retinopathy showing venous changes and cotton wool spots.
䊐
F Proliferative
retinopathy showing neovascularisation.

DIABETES MELLITUS •
11
411
evidence for statin use is awaited. Regular screening for retinopathy is
essential in all diabetic patients, especially those with risk factors such as
long duration of diabetes, hypertension, poor control, pregnancy, smoking,
excess alcohol and evidence of microangiopathy. The preferred screening
option is a digital imaging photographic system, with referral to an oph-
thalmologist when needed.
Management
Severe non-proliferative and proliferative retinopathy is treated with retinal
photocoagulation, which has been shown to reduce severe visual loss by
85% (50% in maculopathy). Argon laser photocoagulation is used to:
• Destroy areas of retinal ischaemia. • Seal leaking microaneurysms. •
Reduce macular oedema. • Gliose new vessels on the retinal surface.
11.11 CLASSIFICATION OF DIABETIC RETINOPATHY BY
PROGNOSIS FOR VISION
Type of retinopathy
Prognosis/Action required
Background retinopathy
Venous dilatation
Peripheral microaneurysms, blot haemorrhages,
exudates
No immediate threat to vision
Control blood glucose, lipids
and BP, advise to stop
smoking
Fundoscopy every 6–12 mths,
refer if progression
Maculopathy
Macular exudation, haemorrhage, ischaemia
Macular oedema
Sight-threatening—refer for
specialist opinion
Review of risk factors,
glycaemic control, BP and lipid
levels
Pre-proliferative retinopathy
Venous loops and beading
Clusters of microaneurysms, blot
haemorrhages, large retinal haemorrhages
Intraretinal microvascular abnormalities, cotton
wool spots
Macular oedema
Perimacular exudates
± haemorrhages
Sight-threatening—refer for
specialist opinion
Rapid lowering of blood
glucose may worsen
retinopathy (see text)
Proliferative retinopathy
Pre-retinal haemorrhage
Neovascularisation
Fibrosis, exudative maculopathy
Sight-threatening—urgent
review and treatment by
specialist mandatory

412
DIABETES MELLITUS •
11
Patients must be reviewed regularly to check for recurrence. Extensive
bilateral photocoagulation can cause visual fi eld loss, interfering with
driving and night vision. Vitrectomy may be used where visual loss is due
to recurrent vitreous haemorrhage which has failed to clear, or retinal
detachment resulting from retinitis proliferans. Rubeosis iridis is managed
by early pan-retinal photocoagulation.
OTHER CAUSES OF VISUAL LOSS IN DIABETES
Around 50% of visual loss in people with type 2 diabetes is due to causes
other than diabetic retinopathy. These include:
• Cataract. • Macular degeneration. • Retinal vein occlusion. • Retinal
arterial occlusion. • Ischaemic optic neuropathy. • Glaucoma.
Cataract occurs prematurely in people with diabetes due to the metabolic
insult to the lens.
DIABETIC NEPHROPATHY
Diabetic nephropathy is among the most common causes of end-stage renal
failure (ESRF) in developed countries. About 30% of patients with type 1
diabetes have developed diabetic nephropathy after 20 yrs, but the risk
after this time falls to less than 1% per year. Risk factors for developing
nephropathy include:
• Poor glycaemic control. • Duration of diabetes. • Asian ethnicity. • Hyper-
tension. • Other microvascular complications. • A family history of
nephropathy or hypertension.
Pathologically, thickening of the glomerular basement membrane is fol-
lowed by nodular deposits (Kimmelstiel–Wilson nodules). As glomerulo-
sclerosis worsens, heavy proteinuria develops, sometimes in the nephrotic
range, and renal function progressively deteriorates.
Diagnosis and screening
Microalbuminuria (30–300 mg/24 hrs) is a risk factor for developing overt
diabetic nephropathy, although it is also found in other conditions. Progres-
sively increasing albuminuria, or albuminuria accompanied by hyperten-
sion, is more likely to be due to early diabetic nephropathy. Patients with
type 1 diabetes should be screened annually from 5 yrs after diagnosis;
those with type 2 diabetes should be screened annually from the time of
diagnosis.
Management
If nephropathy is identifi ed, progression can be reduced by improved blood
glucose control, and aggressive reduction of BP and other cardiovascular
risk factors.
• ACE inhibitors and angiotensin receptor blockers have been shown to
provide greater benefi t than equal BP reduction achieved with other drugs.
• Diabetic control becomes diffi cult in renal impairment. Metformin should
be stopped when creatinine is
>150 μmol/l (1.7 mg/dl; risk of lactic acido-

DIABETES MELLITUS •
11
413
sis). Long-acting sulphonylureas should be replaced by short-acting agents.
• Renal replacement therapy may benefi t diabetic patients at an earlier stage
than other ESRF patients. • Renal transplantation can improve quality of
life, although macrovascular disease and neuropathy/retinopathy show con-
tinued progression. • Pancreatic transplantation can produce insulin inde-
pendence and can reverse microvascular disease, but the supply of organs
is limited.
DIABETIC NEUROPATHY
This complication affects approximately 30% of patients. It is symptomless
in the majority and can occur in motor, sensory and autonomic nerves.
Prevalence is related to the duration of diabetes and the degree of metabolic
control.
Clinical features
Symmetrical sensory polyneuropathy: There is diminished perception
of vibration sensation distally, ‘glove-and-stocking’ impairment of all other
modalities of sensation, and loss of tendon refl exes in the lower limbs.
Although frequently asymptomatic, it can cause paraesthesia in the feet or
hands, pain on the anterior aspect of the legs (worse at night), burning
sensations in the soles of the feet, hyperaesthesia and a wide-based gait.
Toes may be clawed with wasting of the interosseous muscles. A diffuse
small-fi bre neuropathy causes altered pain and temperature sensation and
is associated with symptomatic autonomic neuropathy; characteristic fea-
tures include foot ulcers and Charcot neuroarthropathy. Management
includes strict glycaemic control, tricyclic antidepressants (amitriptyline,
imipramine), anticonvulsants (carbamazepine, gabapentin) and opioids
(tramadol, oxycodone).
Asymmetrical motor diabetic neuropathy (diabetic amyotrophy): This
presents as severe and progressive weakness and wasting of the proximal
muscles of the lower (occasionally upper) limbs, accompanied by severe
pain, hyperaesthesia and paraesthesia. There may also be marked loss of
weight (‘neuropathic cachexia’), tendon refl exes may be absent, and the
CSF protein is often raised. This condition is thought to involve acute inf-
arction of the lumbosacral plexus. Although recovery usually occurs within
12 mths, some defi cits become permanent. Management is mainly
supportive.
Mononeuropathy: Either motor or sensory function can be affected
within a single peripheral or cranial nerve. Unlike other neuropathies,
mononeuropathies are severe and of rapid onset; the patient usually recov-
ers. Most commonly affected nerves are 3rd and 6th cranial nerves, femoral
and sciatic nerves. Multiple nerves are affected in mononeuritis multiplex.
Nerve compression palsies commonly affect the median nerve and lateral
popliteal nerve (foot drop).
Autonomic neuropathy: This is less clearly related to poor metabolic
control, and improved control rarely improves symptoms. Clinical features

414
DIABETES MELLITUS •
11
and management are shown in Box 11.12. Within 10 yrs of developing
autonomic neuropathy, 30–50% of patients are dead. Those with postural
hypotension have the highest subsequent mortality.
THE DIABETIC FOOT
Tissue necrosis in the feet is a common reason for hospital admission in
diabetic patients. Foot ulceration occurs as a result of often trivial trauma
in the presence of neuropathy (peripheral and autonomic) and/or peripheral
vascular disease; infection occurs as a secondary phenomenon. In most
cases all three components are involved in producing tissue necrosis. Most
ulcers are neuropathic or neuro-ischaemic in type. The most common cause
of ulceration is a plaque of callus skin beneath which tissue necrosis occurs,
eventually breaking through to the surface.
Management
Preventative management is the most effective method of dealing with the
diabetic foot. Regular podiatry, patient education and orthotic footwear
(preventing recurrence and protecting deformed feet) are required. Medical
management includes:
• Removal of callus skin (podiatrist only). • Treatment of infection (often
protracted courses, especially in the presence of osteomyelitis). • Avoidance
of weight-bearing. • Good glycaemic control. • Control of oedema. • Angi-
ography if the foot is ischaemic: to assess feasibility of vascular reconstruc-
tion. • Amputation: if there is extensive tissue/bony destruction or intractable
ischaemic pain when vascular reconstruction is not possible or has failed.
11.12 CLINICAL FEATURES AND MANAGEMENT OF
AUTONOMIC NEUROPATHY
Feature
Management
Postural hypotension (drop in systolic
pressure of
≥20 mmHg)
Fludrocortisone, support stockings
Gastroparesis
Metoclopramide, erythromycin
Diarrhoea/constipation
Loperamide, stimulant laxatives
Atonic bladder
Intermittent self-catheterisation
Hyperhydrosis
Topical/oral anti-muscarinics
Erectile dysfunction (30% of males)
Phosphodiesterase inhibitors,
prostaglandin injections
