Carbohydrate
Colour test for carbohydrate1-Molish test
“general test for carbohydrate “
Molish reagent : α -Naphthol in alcohol with conc. H2SO4
Principle of the test :
• It is found that conc. H2SO4 acts as a dehydrating agent . (removing water molecules from carbohydrate and changing carbohydrate to furfural or its derivative depending upon the sugar).
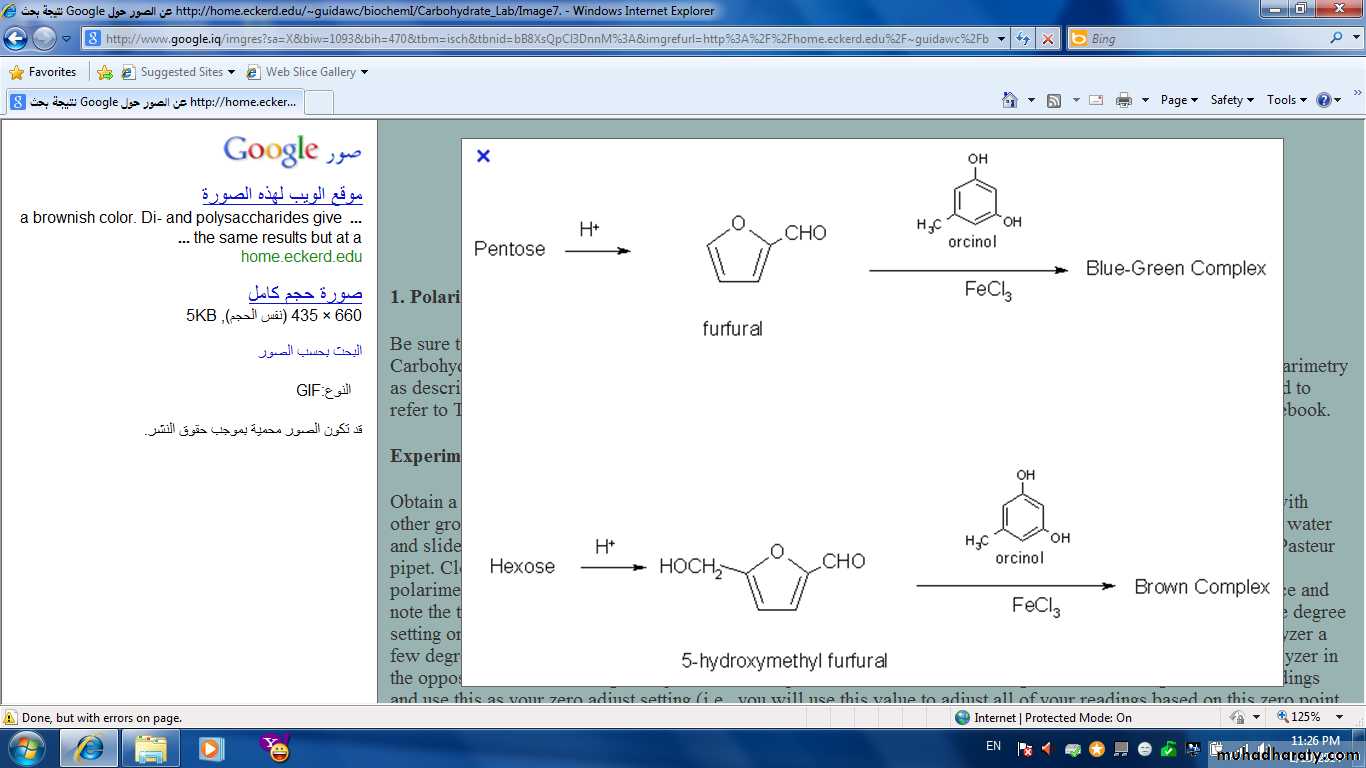
• If the carbohydrate is pentose (5C), It will change to furfural.
• If it is hexose (6C), It will change to hydroxyl methyl furfural.
Hexose + H2SO4 Hydroxy methyl furfural
Pentose + H2SO4 Furfural
-3H2O
-3H2O
Note 1:
• All carbohydrates (poly or di ) converted to mono saccharides by conc.H2SO4 ,then to furfural or its derivative then it condensed with α- Naphthol to form a violet colour complex as a ring.this will indicate the presence of carbohydrate
CHO + H2SO4 MONO + H2SO4 Furfural + α- Naphthol Violate ring
-3H2O
Procedure :
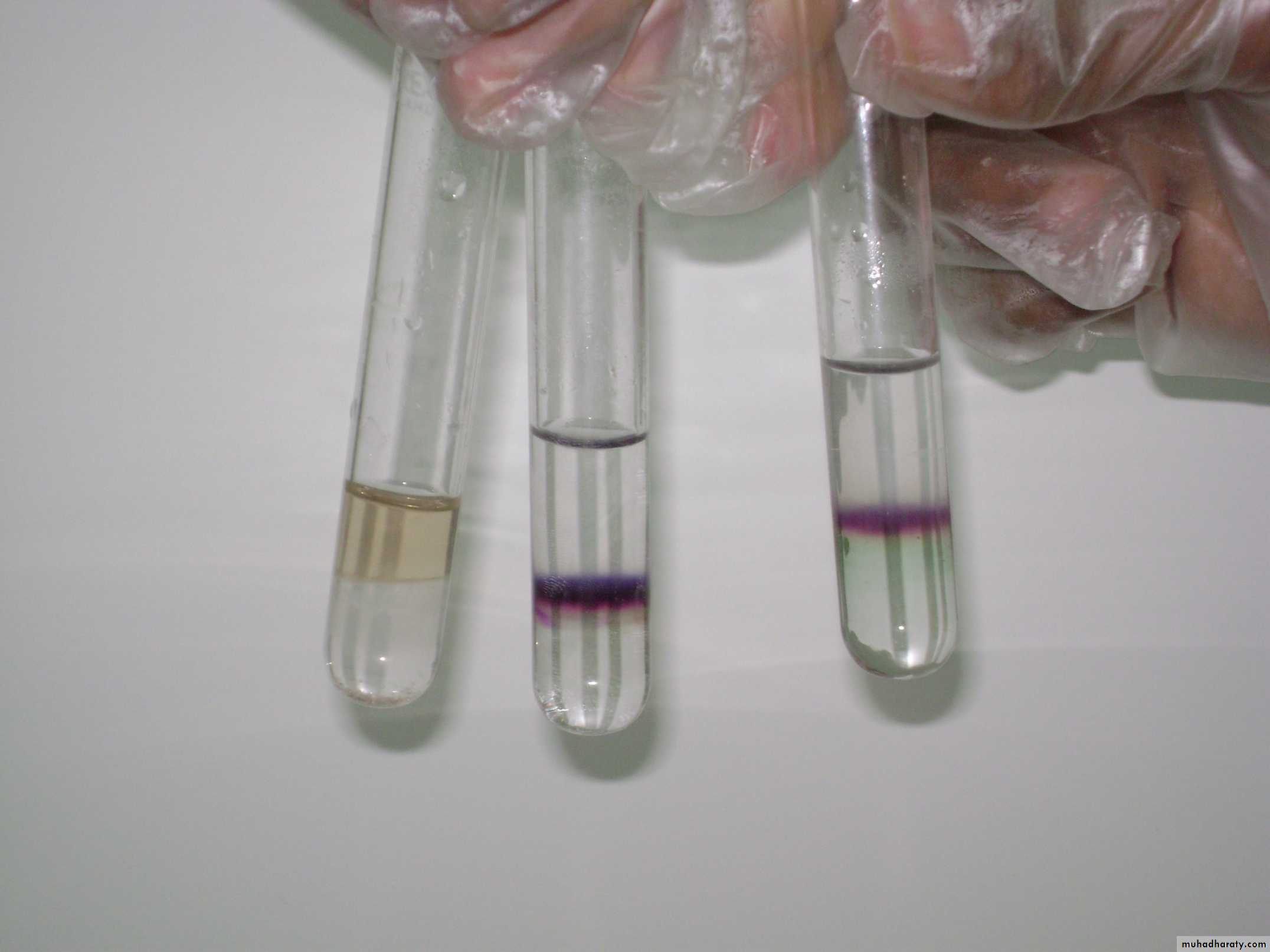
• In clean test tube put (5 drops) of carbohydrate, Then added (2-3) drops of molish reagent , mixed very well , then added about equal volume of conc. H2SO4 on the side of the test tube , a violet ring will appear between two layers .Note 2 :
• In some cases a brown or black colour will form, This is due to the addition of conc. H2SO4 very quickly so the best method for addition of H2SO4 is slowly and on the side of the test tube .
Note 3:
• Some time a green colour that appear is not due to the presence of CHO but due to interaction between the α- Naphthol and conc. H2SO42- Benedict test :
• Is the test for reducing sugar.Benedict reagent :
Cupper sulphate (CuSO4).
Sodium carbonate (Na2CO3).
Sodium or potassium citrate .
CuSO4 + Na2Co3 CuCO3 Soluble complex
Na or K citrate
Insoluble white ppt.
• Benedict test it the test for reducing sugar.
• Reducing sugar ex. : glucose , fructose , maltose .• It contain aldo or keto group .
• Non reducing sugar ex. sucrose & starch it is not contain aldo or keto sugar.
Principle of Benedict Test :
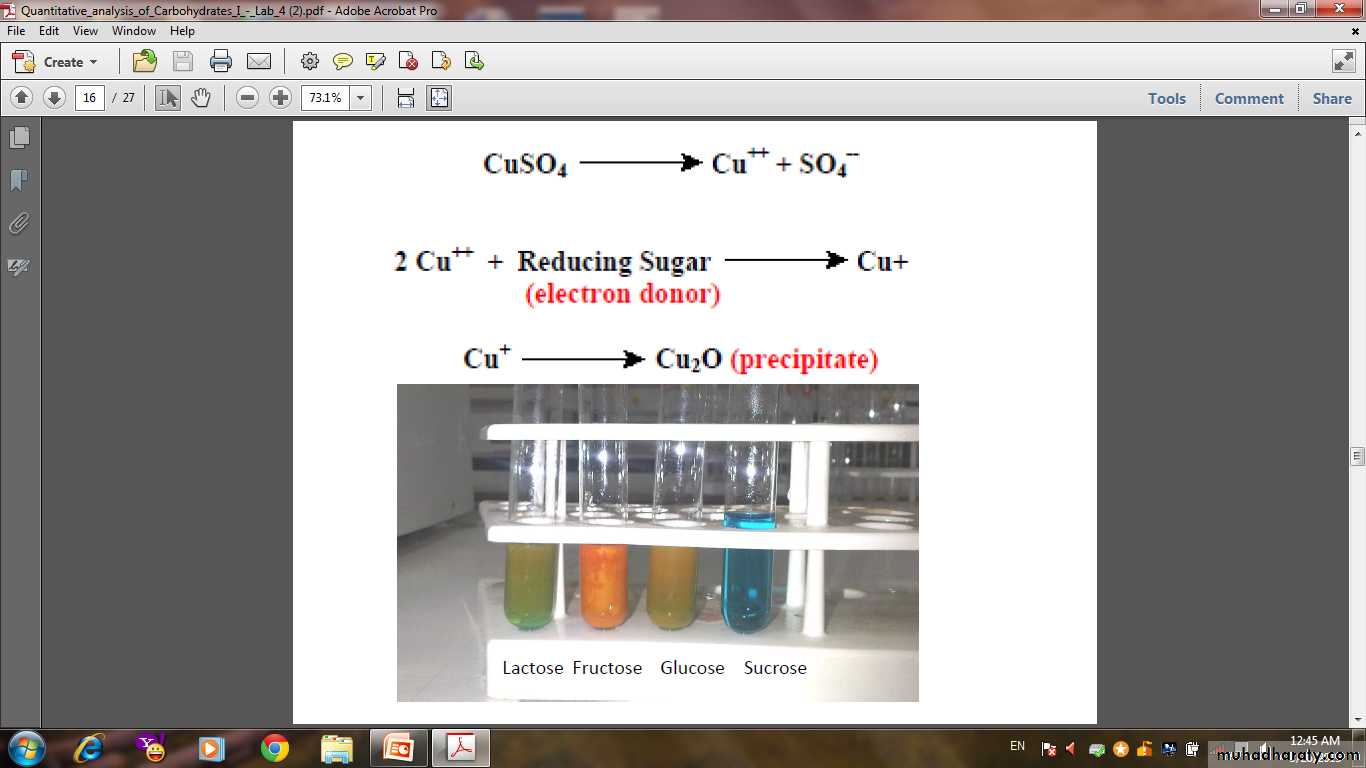
• Sugar with aldo or keto group reduced cupric ion (Cu++) in alkaline medium to cuprous ion (Cu+), Red ppt. of Cu2O will appears.Glucose + Cu ++ Cu2O
Alkaline mediumPrinciple of Benedict :
• All sugar contain free group of aldehyde or ketone undergo enolization in alkaline medium.• Aldo or keto in alkaline medium change to enol form. • It is unstable decompose upon heating and converted to fragment of aldehyde this is reducing a gent changing Cu++ (cupric ) to Cu+ (cuprous)
Aldo or Keto sugar Enol form Fragmental aldehyde
Enolization in
alkaline mediumRed ppt. of Cu2O
Benedict regentsProcedure:
• In clean test tube put (10drops) of benedict reagent then add (5drop) of carbohydrate, mix very well then put the test tube in to a boiling water bath for (5 minutes).• Formation of red ppt. indicate the presence of reducing sugar.
• Sugars used in this test are : Glucose ,Sucrose, Maltose and Starch.
Note:
• All monosaccarides are reducing sugar , polysaccharides are non-reducing sugar, disaccharides some of them are reducing sugar like maltose and lactose, but others are non reducing like sucrose.
3. Barfoied test: (for mono sugar)
Barfoied reagent: cupper acetate with acetic acid glacial.
Principle:
• It᾽s found that the reducing agent or active group which present in mono is more active than in di or poly (in acid medium).
• Monosaccaride only will reduce the cupper which is present in cuppric acetate to Cu2O (Cuprous oxide)
Mono sugar + Cu- acetate Cu2O + Sugar
Heating3 min.
• In this test, boiling for (3minutes) only because :− disaccharide hydrolysed to mono and this interfere with the result.Procedure:
• In clean test tube put (10drops) of Barfoied reagent, then add (5drops) of sugar, mix it very well, then put the test tube in a boiling water bath for (3 minutes) only. Red ppt. in the bottom of the test tube will appears, this indicate the presence of monosaccharide.• Sugar used in this test are : glucose and maltose.
Conclusion
ObservationTest
Benedict test
Reducing sugar+ ve / Orange colour
Glucose
Reducing sugar
+ ve / Orange colour
Maltose
Non reducing sugar
• ve / No reaction−
Starch
Barfoied test
Mono sugar• + ve / Red ppt.
• Glucose
Not mono sugar
• ve / No reaction−• Maltose
Bials test• To improve the pentose sugar.
Bials reagent :
Orcinol in conc. HCl and traces of ferric ion (Fe+3 ).
Principle of Bials test
The strong HCl act as dehydrating agent removing water molecule from pentose to form furfural, then it reacting with orcinol in the presence of ferric ion Fe+++ , a green colour will be appears which indicate the presence of pentose.
Pentose Furfural
Conc. HClHeat
Furfural + Orcinol Green colourFe+3
Note :
• If the conc. of pentose sugar is too high, a violet colour will be form instead of the green colour.Procedure :
• In clean test tube put (10 drops) of bials reagent , then added (6 drops) of sugar , mix very well , then put the test tube in a bioling water bath for (2 minutes), a green colour will be formed that indicate the presence of pentose sugar (Arabinose).• Sugar used in this test : Arabinose & Glucose
Negative Negative Positive
Seliwanoff′s test
• To improve the keto sugar.
Seliwanoff′s reagent :
Recorcinol in HCl
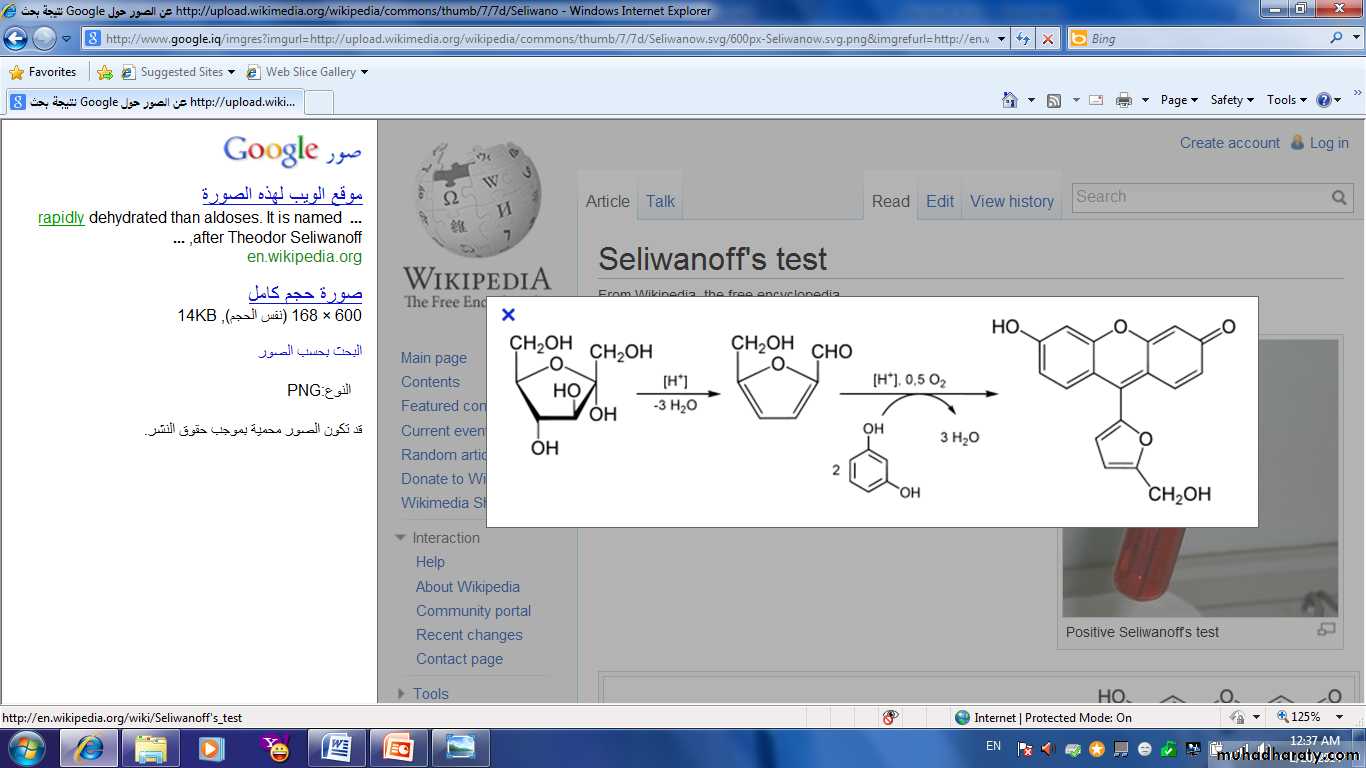
Principle of Seliwanoff′s :
Keto hexoses are dehydrated more rapidly than aldo hexoses to give hydroxy methyl furfural and levuolinic acid (C5H8O3), this form will react with recorcinol to give a pink to orange colour complex.
Keto sugar
hydroxy methyl furfuralPink to orange colour
Recorcinol
HeatHCl
Short time
+ Levalonic acid
Aldo sugar
HClLong time
hydroxy methyl furfural
Note :
• In case of sucrose, it give a pink to orange colour with seliwanoff′s , this is not due to the presence of sucrose but due to hydrolysis of sucrose spontaneously by HCl in seliwanoff′s reagent to form fructose & glucose.Sucrose
HCl inSeliwanoff′s reagent
Fructose + Glucose
Procedure :
In a clean test tube put (5 drops) of seliwanoff′s reagent,then added (3 drops) of sugar, mix it very well, then put it in a boiling water bath for (3 minutes), a pink to orange colour will be appears that indicated the presence of keto sugar.
Sugar used in this test :
Fructose , Glucose & Sucrose
Conclusion
ObservationTest
Bials test
presence of pentose sugar+ ve / green to blue colour
Arabinose
absence of pentose sugar
ve−
Glucose
Seliwanoff′s test
presence of keto sugar+ ve / pink to orange
Fructose
absence of keto sugar
ve−
• Glucose
du to hydrolysis to frctose & glucose
+ ve / Pink to orange
• Sucrose
PROTEINS
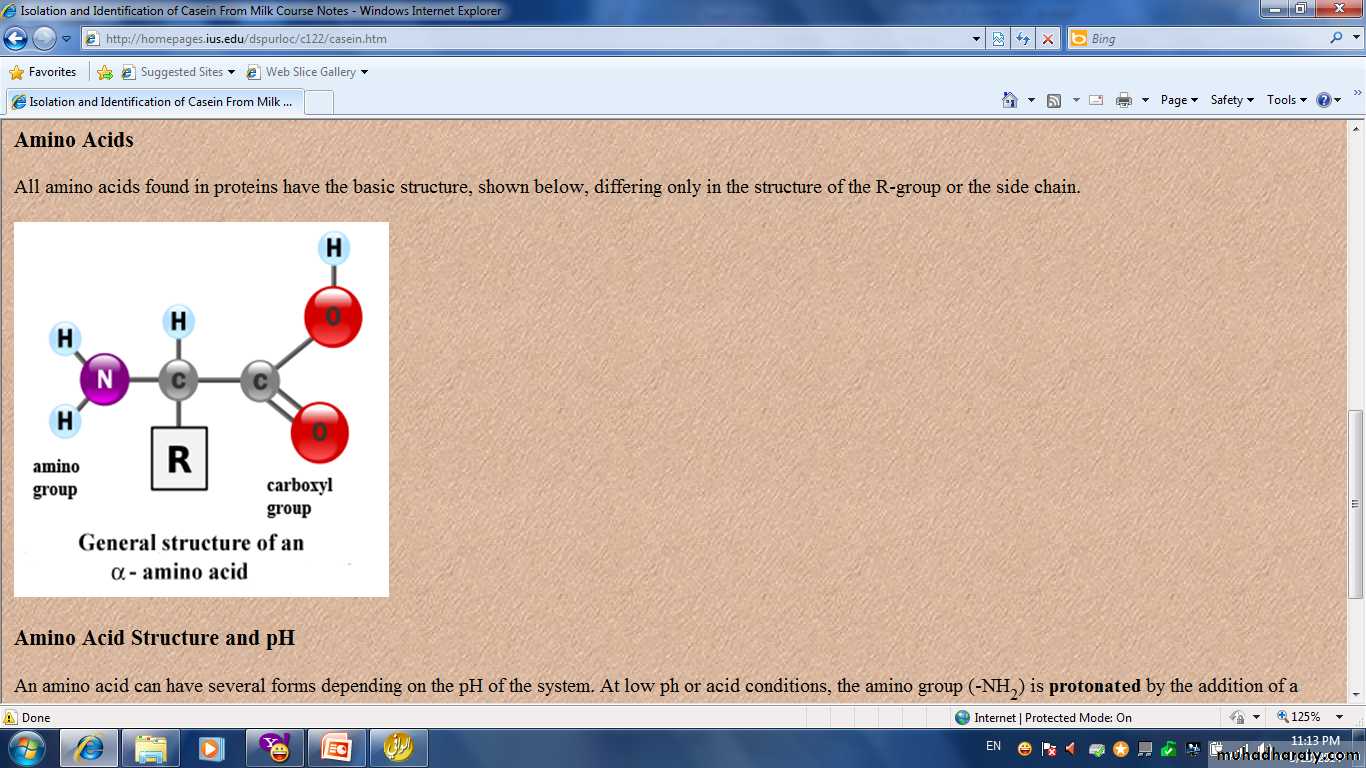
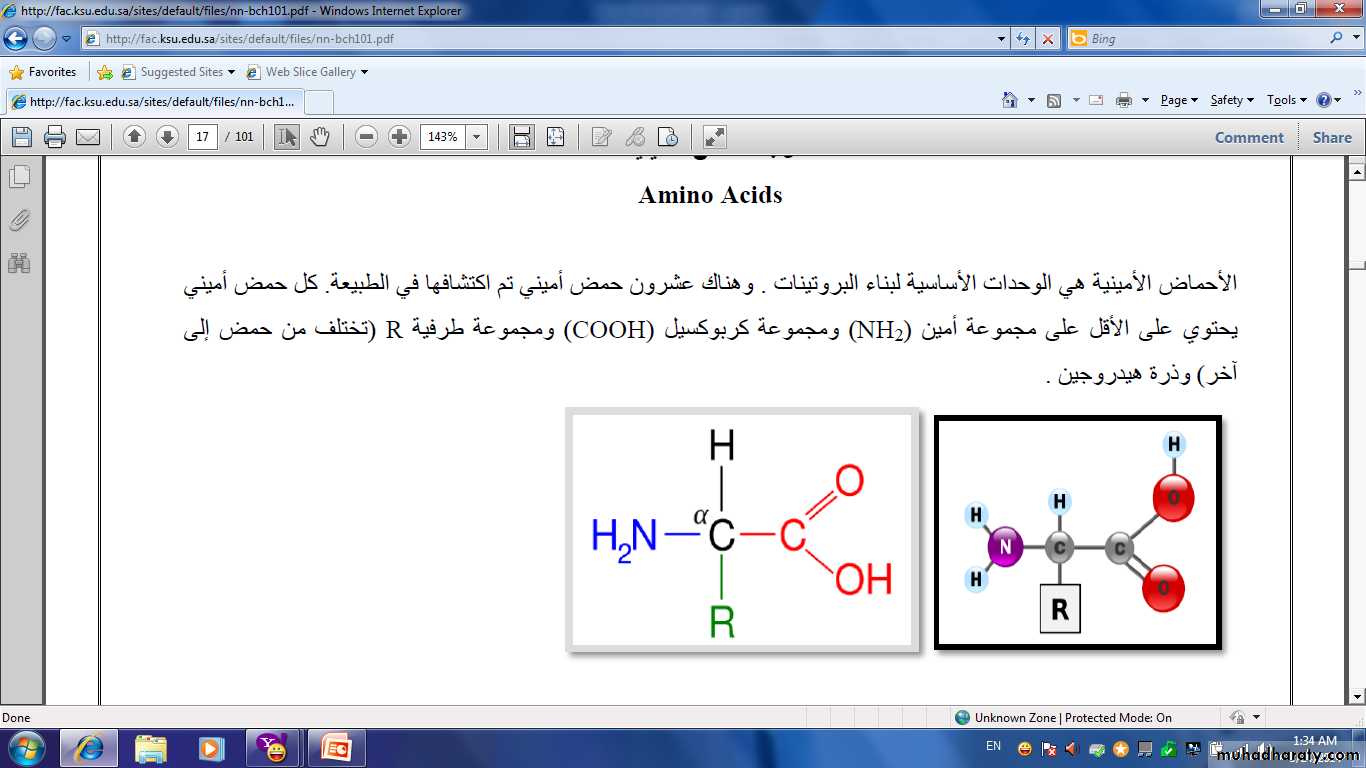
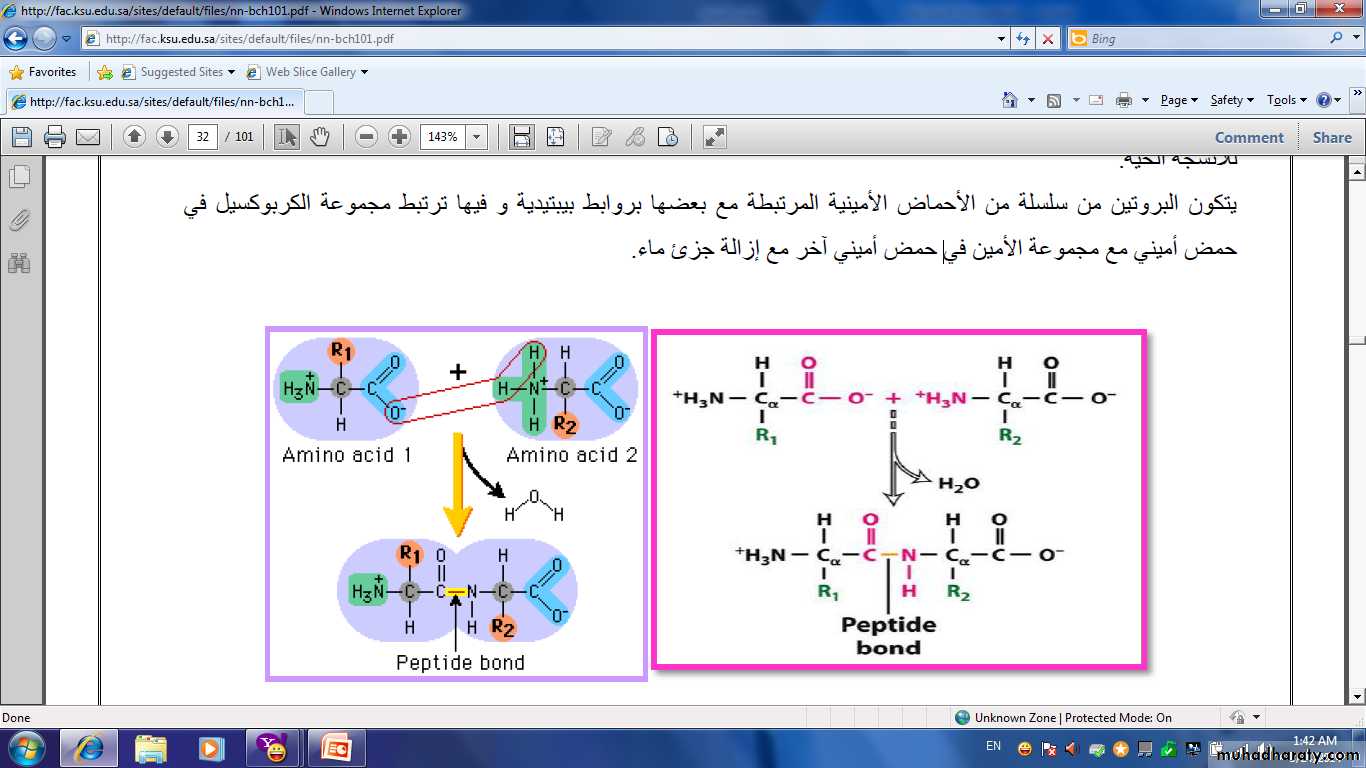
• Proteins are a complex nitrogenous group with high molecular weight .It consist of a large number of amino acid connected together with a special bond known as peptied bond.− C− NH−
=O
• The difference between protein and carbohydrate is that protein contain nitrogen in addition of C , H and O.
Characters of proteins:
• All proteins give a number of a colour reaction due to the existence of certain amino acids in the protein molecule or to special chemical group associated with molecular structure of protein.• All proteins from colloidal solutions which are usually opalescent and can be precipitated by variety of reagents.
Elements present in proteins
• All proteins contain N,C,H,O and small quantity of : sulfur, iodine and phosphate.
Procedure:
In to a dry clean test tube place a small quantity powder of albumin. Place a piece of moistened red litmus paper on the mouth of the test tube and heat until we get a smell of burning hair.1. The red litmus paper will change to blue colour due to evolution of ammonia from heating protein indicating the presence of N .
2. Moisture on the part of the inside of the test tube due to the condensation of water and indicate the presence of H and O.
3. Charring of the heated protein indicate the presence of C.
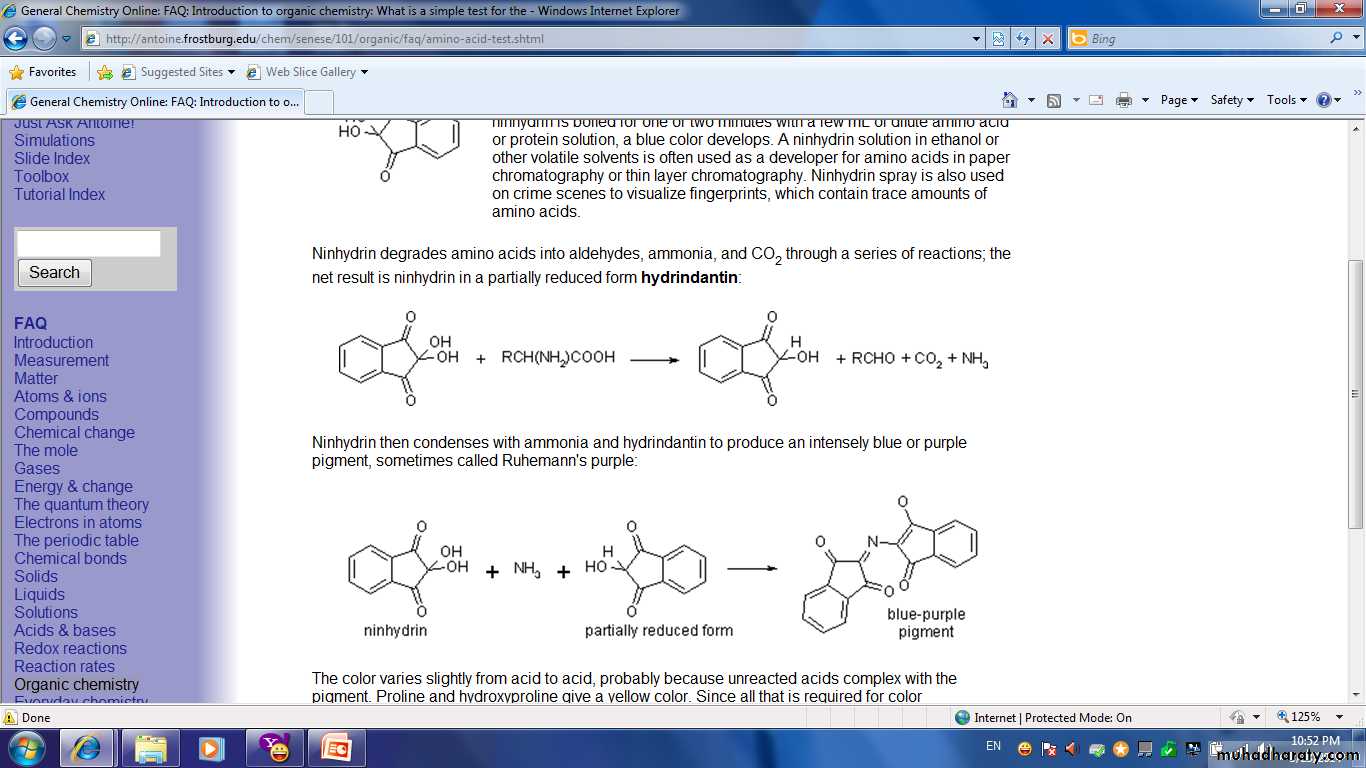
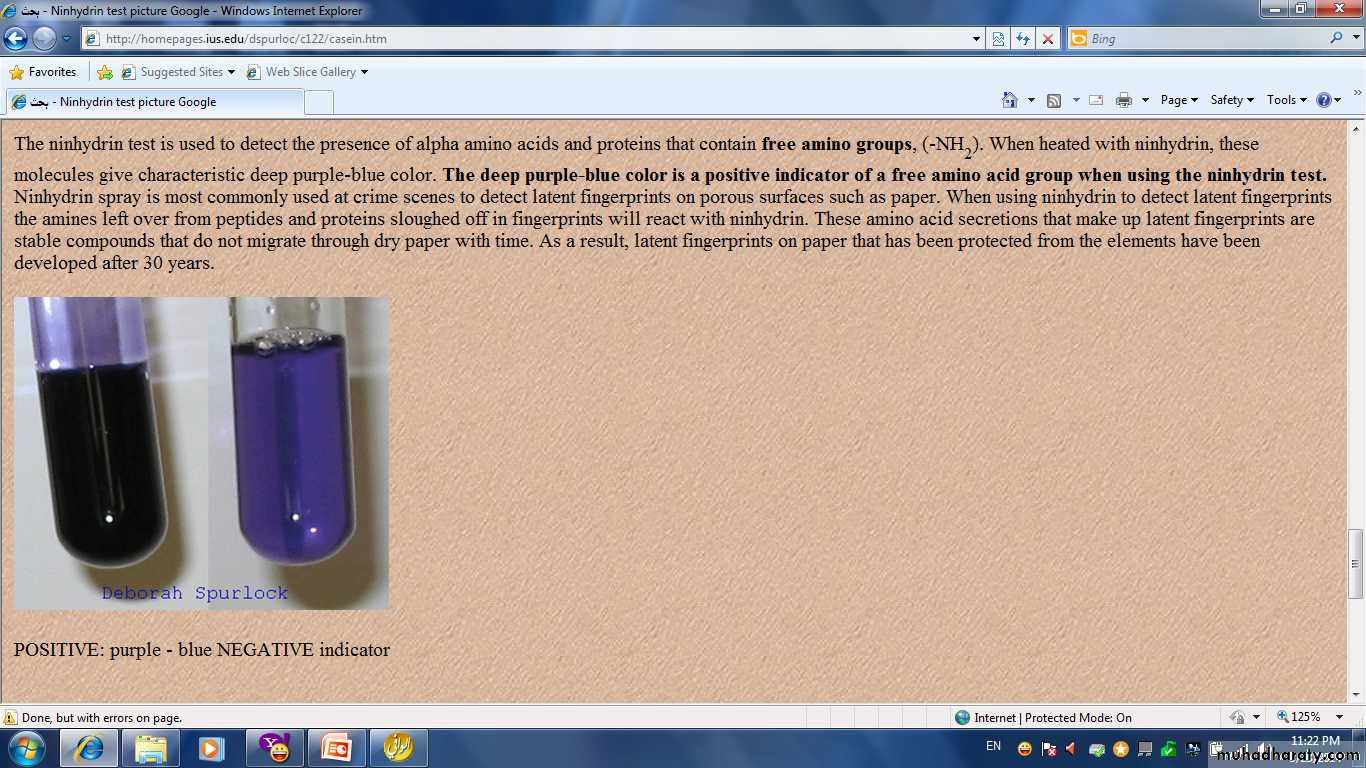
Ninhydrin Test:
• It is the general test for protein and for all products of protein hydrolysis including amino acid.• The Ninhydrin test is used to detect the presence of alpha amino acids and proteins that contain free amino groups, (-NH2)
• Ninhydrine is powerful oxidizing agent which oxidize amino acid according to the following general equation.
Ninhydrin + RCH(NH2) COOH
Triketohydrindene hydrate (oxidizing agent)RCHO + NH3 + CO2 + reduced Ninhydrin
General amino acidReduced Ninhydrin + NH3 + Ninhydrin
blue-violet complex• The librated NH3 reacts with the hydrindantin (reduced ninhydrin) with a second molecule of Ninhydrin to produce a blue ̶ violet colour complex.
( hydrindantin)
ninhydrin amino acid hydrindantin
− 2 H2OProcedure:
In a clean two test tube put in the first one 5 drops of diluted albumin and in the second 5 drops of a.a. Then add to each test tube 2 drops of ninhydrin solution, mixed very well then put the two test tube in to a boiling water bath for 3 min. a blue-violet colour complex will form indicating the presence of amino acid.Note:
• Proline and hydroxy proline which lack an amino group, yield a yellow colour with Ninhydrin test, which contains a secondary amino acid.• Some colour test are specific for certain amino acids.
General colour test of protein
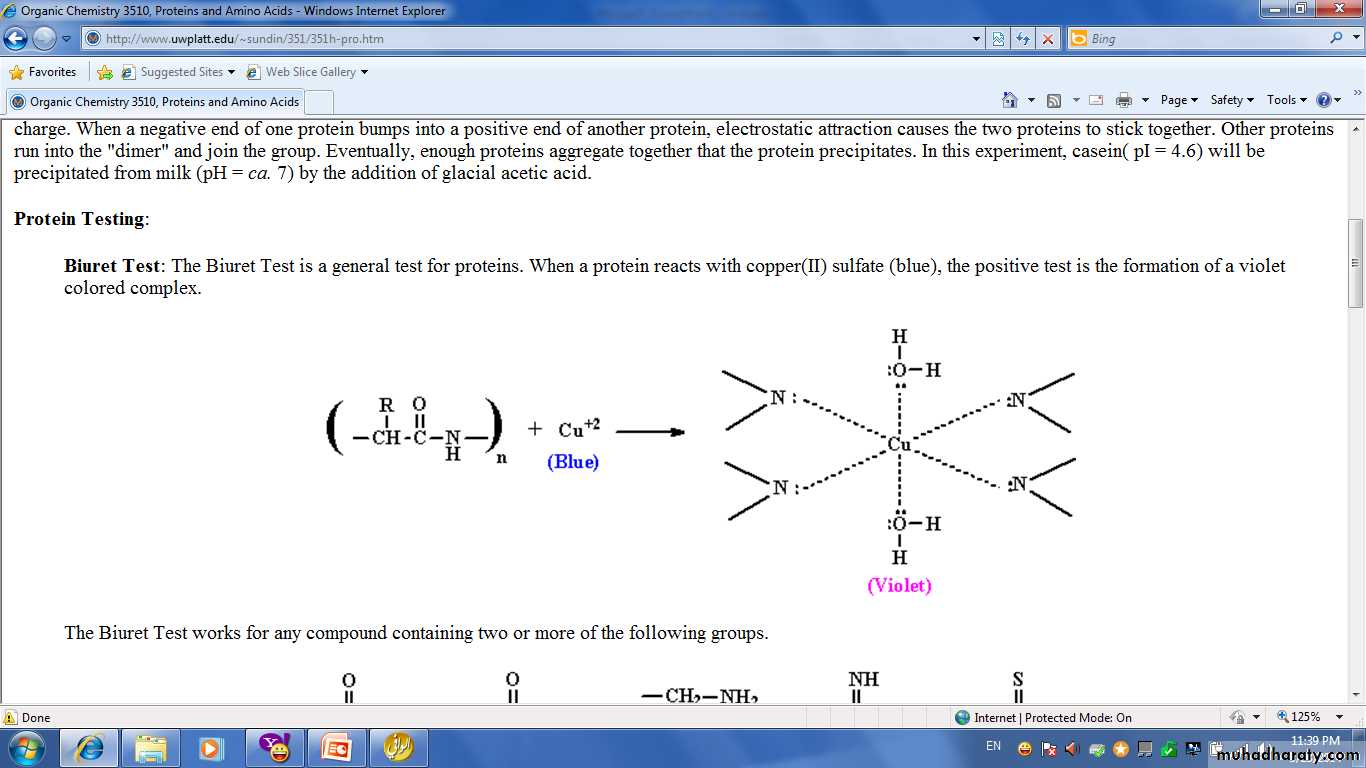
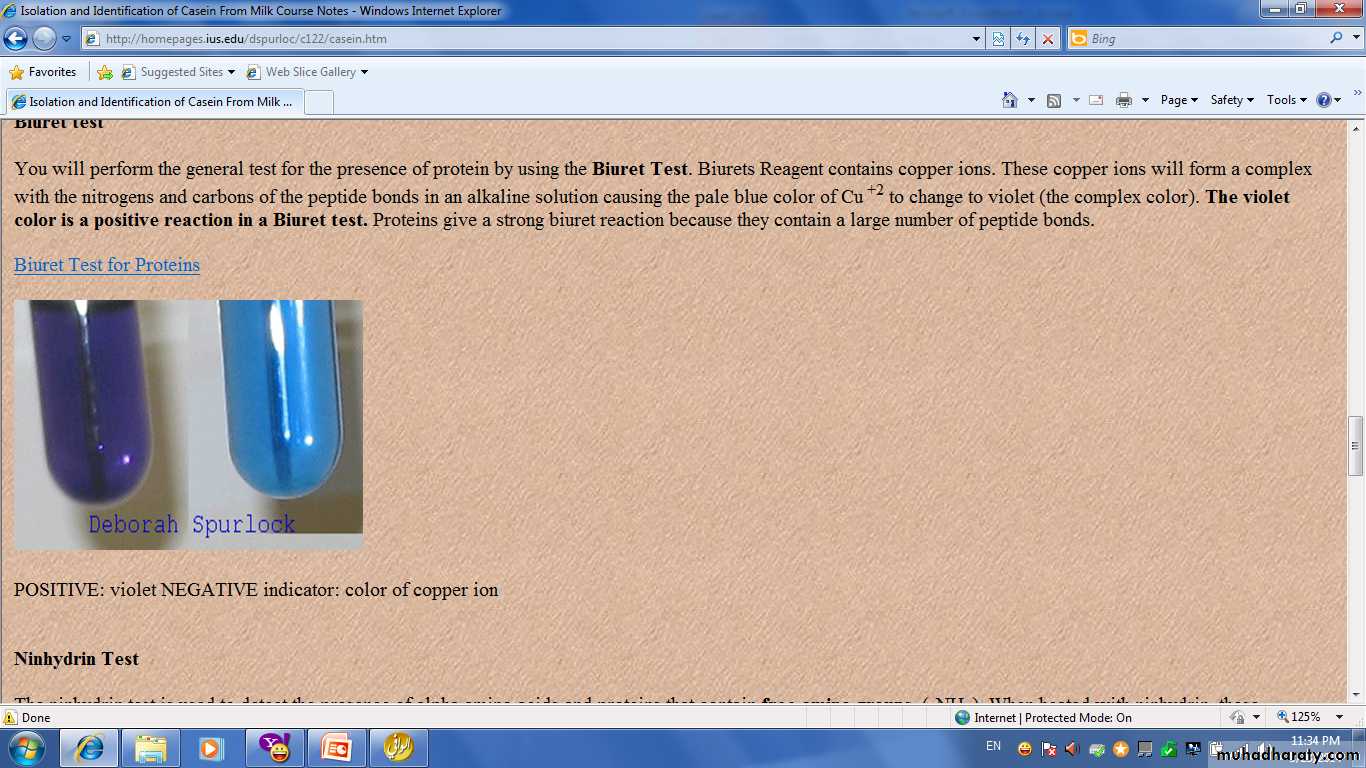
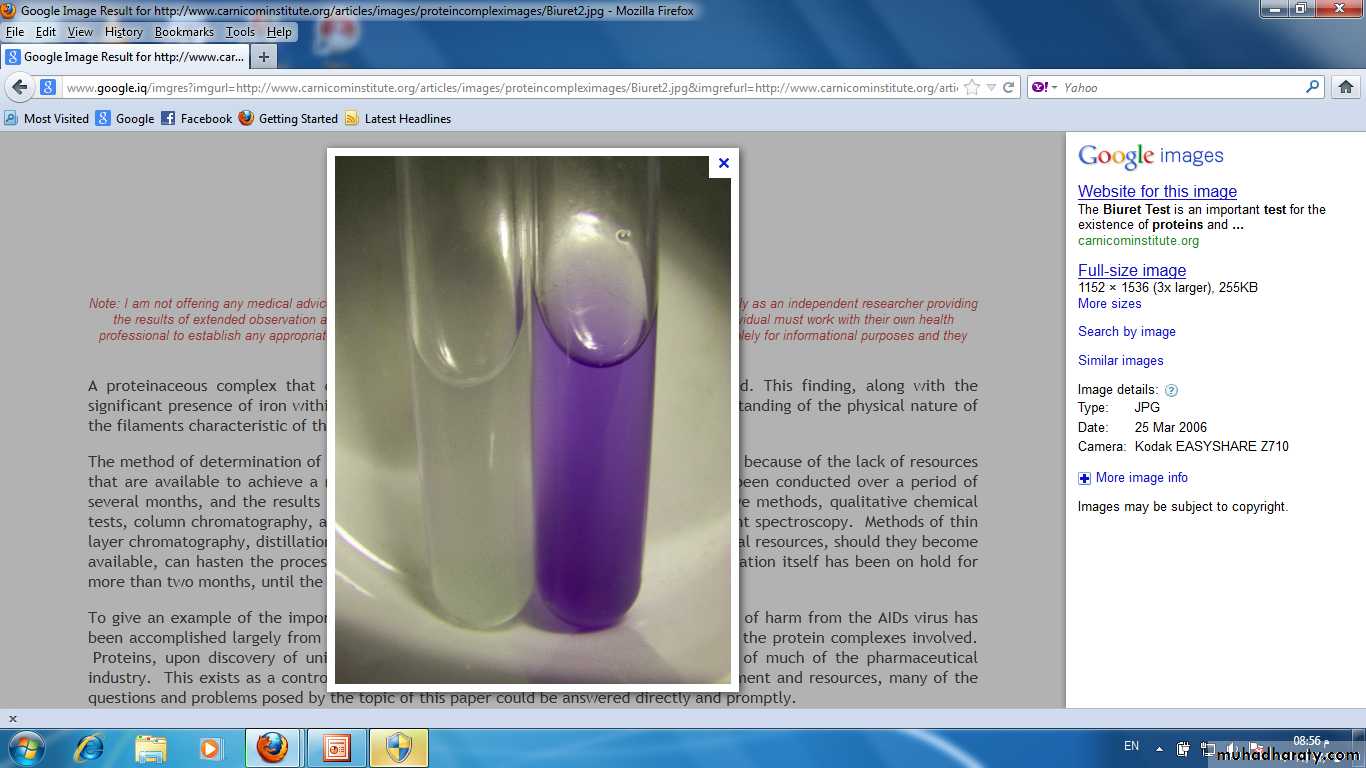
The most useful general test of protein are Biuret and Ninhydrin test.Biuret Test:
Is commonly used to detect the presence of proteins and peptides by treating a sample with an alkaline solution of dilute copper sulfate to yield a pink-violet to purple-violet colour. At least two peptide bonds (tripeptide) are required for positive test Biuret. The intensity of the colour complex produced is directly proportional to the number peptide bond present.
• Biuret reagent is CuSO4 in alkaline NaOH.
Procedure:
Add 5 drops of dilute protein solution add, 5 drops of 10% of NaOH, mixed and then add 2 drops of 0.5% CuSO4 solution. Compare with a blank tube containing 5 drops of water and the same amount of NaOH and CuSO4. Describe any colour change that occurred.Note:
• Excess copper sulphate should be avoided because copper hydroxide will be form which has a white ppt. interfering with the violet or blue colour of Biuret reaction.CuSO4 + NaOH
White ppt.Cu(OH)2
ExcessConclusion
ObservationTest
• presence of α- amino acids
+ ve / blue - violet colourAlbumin
presence of α- amino acids
+ ve / blue - violet colour
Amino acids
Biuret test
presence of peptide bond+ ve / violet colour
Albumin
presence of peptide bond
ve / blue colour−
• Amino acids
blank
− ve
• D.W