
1
6
th
stage
Pediatrics
lec.3
د.دمحم خضر
Tutorial
2016/8/18
Vaccination
The immune system is a complex network of specialized organs and cells
protects the body from destruction by foreign agents and microbial
pathogens , degrades and removes damaged or dead cells, and exerts a
surveillance function to prevent the development and growth of
malignant cells. The immune system is composed of immune cells and
central and peripheral lymphoid structures. The immune cells move
throughout the body, searching for and destroying foreign substances
but avoiding cells regarded as self.
Natural immunity:
It is not produced by the immune response. This type of immunity is
present at birth and appears to be present in all members of a species.
Acquired immunity:
It develops after birth as a result of exposure to an antigen, thereby
activating the immune response. Acquired immunity can be either active
or passive, depending on whether the immune response took place in
the host or a donor.
Differences of immune system of children and adult
The normal human no fully active immune system at birth because of
immaturity. It relies instead on passively transferred antibodies from the
mother. This maternal antibody slowly decreases in concentration and
for all practical purposes, has waned by 1 year.
The infant own production of antibody begins to be meaningful at 7 or 8
months of age when the total of maternal and infant antibody is low.

2
One has waned and the other is not up to full strength. This is age when
many of the infectious disease processes of infancy begin /e.g. otitis
media, pneumonia.
Vaccination
• Administration of a substance to a person with the purpose of
preventing a disease
• Traditionally composed of a killed or weakened microorganism
• Vaccination works by creating a type of immune response that
enables the memory cells to later respond to a similar organism
before it can cause disease
• Early History of Vaccination
• Pioneered India and China in the 17th century
• The tradition of vaccination may have originated in India in AD
1000
• Powdered scabs from people infected with smallpox was used to
protect against the disease
• Smallpox was responsible for 8 to 20% of all deaths in several
European countries in the 18th century
• In 1721 Lady Mary Wortley Montagu brought the knowledge of
these techniques from Constantinople (now Istanbul) to England
• Two to three percent of the smallpox vaccinees, however, died
from the vaccination itself
• Benjamin Jesty and, later, Edward Jenner could show that
vaccination with the less dangerous cowpox could protect against
infection with smallpox
• The word vaccination, which is derived from vacca, the Latin word
for cow.
Era of Vaccination
3
English physician Edward Jenner
observed that milkmaids stricken with a viral disease called
cowpox were rarely victims of a similar disease, smallpox
Jenner took a few drops of fluid from a pustule of a woman who
had cowpox and injected the fluid into a healthy young boy who
had never had cowpox or smallpox
Six weeks later, Jenner injected the boy with fluid from a smallpox
pustule, but the boy remained free of the dreaded smallpox.
Era of Vacinnation
In those days, a million people died from smallpox each year in
Europe alone, most of them children.
Those who survived were often left with blindness, deep scars,
and deformities
In 1796, Jenner started on a course that would ease the suffering
of people around the world for centuries to come.
By 1980, an updated version of Jenner vaccine lead to the total
eradication of smallpox.
In 1879 Louis Pasteur showed that chicken cholera weakened by
growing it in the laboratory could protect against infection with
more virulent strains
1881 he showed in a public experiment at Pouilly-Le-Fort that his
anthrax vaccine was efficient in protecting sheep, a goat, and
cows.
In 1885 Pasteur developed a vaccine against rabies based on a live
attenuated virus
A year later Edmund Salmon and Theobald Smith developed a
(heat) killed cholera vaccine.
Over the next 20 years killed typhoid and plague vaccines were
developed
4
In 1927 the bacille Calmette-Guérin (BCG vaccine) against
tuberculosis vere developed
Since Jenner's time, vaccines have been developed against more
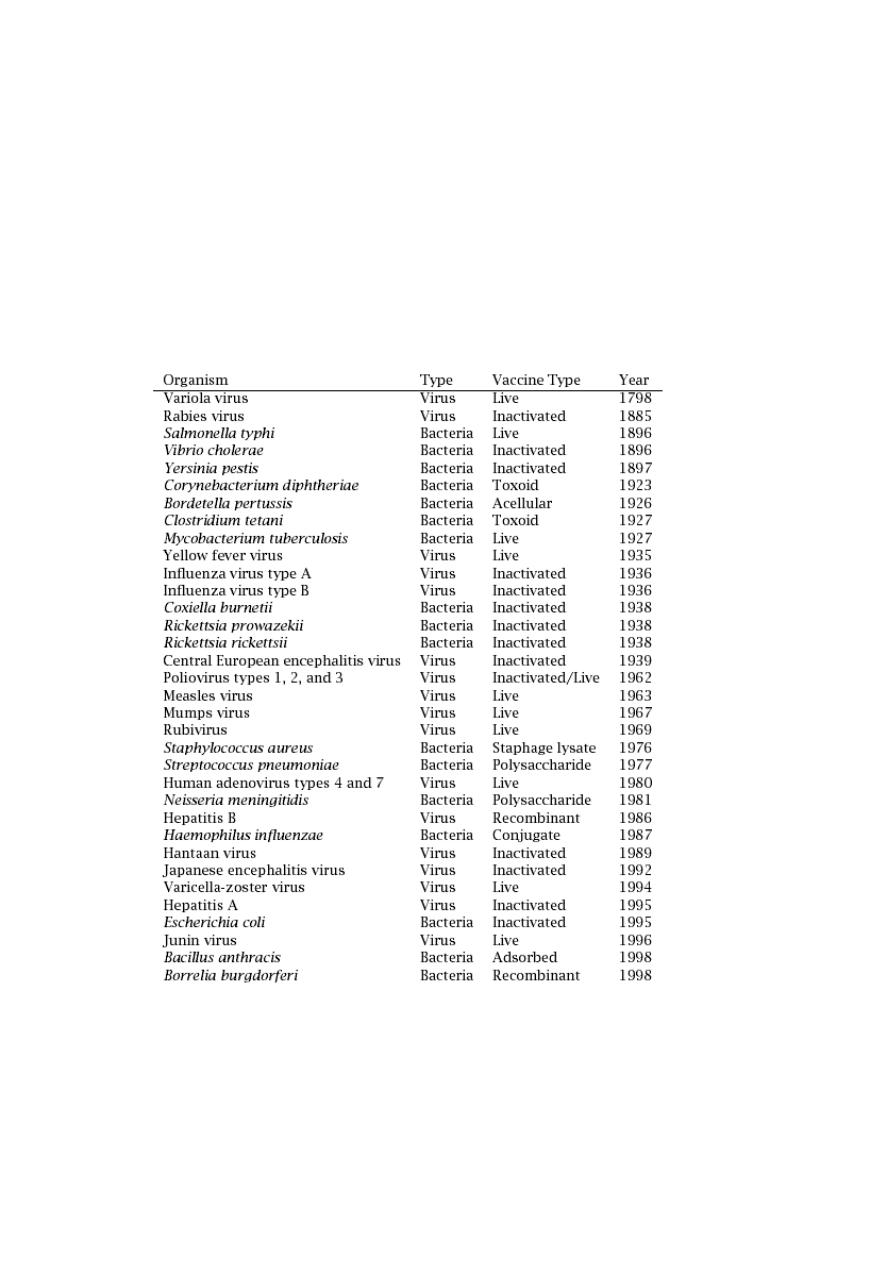
than 20 infectious diseases
• The date of introduction of first generation of vaccines for use
in humans*
o
1798 Smallpox
o
1885 Rabies
o
1897 Plague
o
1923 Diphtheria
o
1926 Pertussis
o
1927 Tuberculosis (BCG)
o
1927 Tetanus
o
1935 Yellow Fever
• After World War II
o
1955 Injectable Polio Vaccine (IPV)
o
1962 Oral Polio Vaccine (OPV)
o
1964 Measles
o
1967 Mumps
o
1970 Rubella
o
1981 Hepatitis B
• Vaccination Today
• Vaccines have been made for only 34 of the more than 400 known
pathogens that are harmful to man.
5
• Immunization saves the lives of 3 million children each year, but
that 2 million more lives could be saved if existing vaccines were
applied on a full-scale worldwide
• Human Vaccines against pathogens
12
Human Vaccines
against

6
Type of Vaccination
Live Vaccines
• Characteristics

7
• Able to replicate in the host
• Attenuated (weakened) so they do not cause disease
• Advantages
• Induce a broad immune response (cellular and humoral)
• Low doses of vaccine are normally sufficient
• Long-lasting protection are often induced
• Disadvantages
• May cause adverse reactions
• May be transmitted from person to person
• Subunit Vaccines
• Relatively easy to produce (not live)
• Classically produced by inactivating a whole virus or bacterium
• Heat
• Chemicals
• The vaccine may be purified
• Selecting one or a few proteins which confer protection
• Bordetella pertussis (whooping cough)
• Create a better-tolerated vaccine that is free from whole
microorganism cells
• Subunit Vaccines: Polysaccharides
• Polysaccharides
• Many bacteria have polysaccharides in their outer
membrane
• Polysaccharide based vaccines

8
• Neisseria meningitidis
• Streptococcus pneumoniae
• Generate a T cell-independent response
• Inefficient in children younger than 2 years old
• Overcome by conjugating the polysaccharides to
peptides
• Used in vaccines against Streptococcus
pneumoniae and Haemophilus influenzae.
• Subunit Vaccines: Toxoids
• Toxins
• Responsible for the pathogenesis of many bacteria
• Toxoids
• Inactivated toxins
• Toxoid based vaccines
• Bordetella pertussis
• Clostridium tetani
• Corynebacterium diphtheriae
• Inactivation
• Traditionally done by chemical means
• Altering the DNA sequences important to toxicity
• Subunit Vaccines: Recombinant
• The hepatitis B virus (HBV) vaccine
• Originally based on the surface antigen purified from the
blood of chronically infected individuals.

9
• Due to safety concerns, the HBV vaccine became the first to
be produced using recombinant DNA technology (1986)
• Produced in bakers’ yeast (Saccharomyces cerevisiae)
• Virus-like particles (VLPs)
• Viral proteins that self-assemble to particles with the same
size as the native virus.
• VLP is the basis of a promising new vaccine against human
papilloma virus (HPV)
• Merck
• In phase III
• Genetic Vaccines
• Introduce DNA or RNA into the host
• Injected (Naked)
• Coated on gold particles
• Carried by viruses
• vaccinia, adenovirus, or alphaviruses
• bacteria such as
• Salmonella typhi, Mycobacterium tuberculosis
• Advantages
• Easy to produce
• Induce cellular response
• Disadvantages
• Low response in 1st generation
Type of Vaccination
11
Live attenuated Vaccine
OPV
Measles
Rubella
Mumps
BCG
Varicella Vaccine
Inactivated organism or their products
Diphtheria
Tetanus
Pertussis( whole cell/acellular)
Hepatits Avaccine
Hepatitis B
Pneumococcal Polysaccharide vaccine
Influenza
IPV
Hib
Passive Immunity
Transfer of antibody produced by one human or other animal to
another
Transplacental most important source in infancy
Temporary protection
Sources of Passive Immunity
Almost all blood or blood products
11
Homologous pooled human antibody (immune globulin)
Homologous human hyperimmune globulin
Heterologous hyperimmune serum (antitoxin)
IMMUNOGLOBULIN PREPARETION
Normal human Ig.
Normal human Ig is an antibody-rich fraction, obtained from a
pool of at least 1000 donors. The preparation should contain at
least 90% intact IgG; it should be as free as possible from IgG
aggregates; all IgG sub-classes should be present; there should be
a low IgA concentration; the level of antibody against at least two
bacterial species and two viruses should be ascertained
Normal human Ig used to prevent measles in highly susceptible
individuals and to provide temporary protection /up to 12 weeks/
against hepatitis A infection.
Live vaccines should not normally be given for 12 weeks after an
injection of normal human Ig.
· Specific human Ig.
These preparations are made from the plasma of patient who
have recently recovered from an infection or are obtained from
individuals who have been immunized against a specific infection.
The advantages of Ig-s are:
1. freedom from hepatitis B
2. concentration of the antibodies into a small volume for
intramuscular use.
3. stable antibody content, if properly stored.
Vaccine Preventable Diseases
An infectious disease for which an effective preventive vaccine
exists.

12
If a person dies from it, the death is considered a vaccine-
preventable death.
FULLY IMMUNIZED CHILD
A child who received
One dose of BCG,
Three doses of DPT and OPV,
One dose of measles
before one year of age.
This gives a child the best chance for survival.
Control
Reduction of prevalence or incidence of disease to lower
acceptable level.
Elimination
Eradication of disease from a large geographic region or political
jurisdiction
Either reduction of infectious disease’s prevalence in regional
population to zero or reduction of global prevalence to a
negligible amount.
Eradication
Termination of all transmissions of infection by extermination of
infectious agent through surveillance and containment.
Reduction of infectious disease’s prevalence in global host
population to zero.
EXPANDED PROGRAMME ON IMMUNISATION (EPI)
EPI launched in 1974
Build on smallpox infrastructure
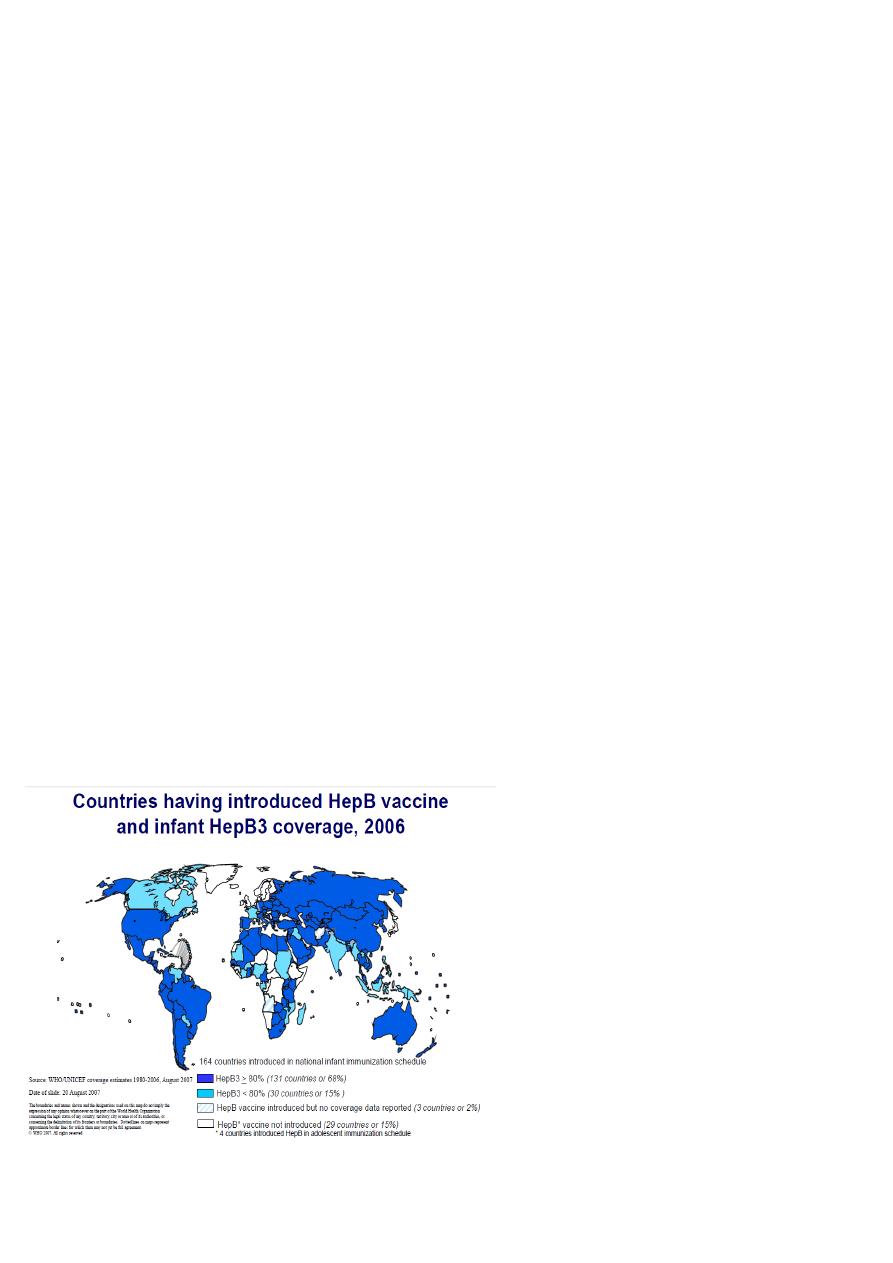
13
Targeted 6 diseases
EPI progressively adopted by all countries
Universal by early 1098s
Addition to EPI
Yellow fever in 1988
• For endemic countries only : 33 in Africa, 11 in S. America.
• Given with measles vaccine
Hepatitis B in 1992
• In high seroprevalence countries by 1995
• In all countries by 1997
Haemophilus influenzae type b (Hib)
• 1998 : based on disease burden and capacity
• 2006 : all countries. ( lack of data should not be obstacle)
3
slides of coverage fm unicef
14
COMPONENTS of program
1. Immunization of pregnant women against tetanus.
2. Immunization of children in their first year of life against 6 VPDs.
15
3) Immunisation in preterm infants
All vaccines except Hepatitis B
If BW < 2Kg & mother HBsAg negative :- postpone till baby
attaines 2kg wt or 2 mths of age.
If BW < 2Kg & mother HBsAg positive :- give vaccine +
immunoglobulin.
4) Children receiving corticosteroids
Children receiving corticosteroids at the dose of 2 mg/kg/day for
more than 14 days should not receive live virus vaccines until
steroid has been discontinued for at least 1 month.
Tetanus toxoid
Intramuscular – upper arm – 0.5 ml

16
Pregnancy – 2 doses - 1
st
dose as early as possible and second
dose after 4 weeks of first dose and before 36 weeks of pregnancy
Pregnancy – booster dose (before 36 weeks of pregnancy) – If
received 2 TT doses in a pregnancy within last three years. Give TT
to woman in labour, if she has not received TT previously
TT booster for both boys and girls at 10 years and 16 years
No TT required between two doses in case of injury
BCG
At birth or as early as possible till one year of age
0.1 ml (0.05ml until one month of age)
Intra-dermal
Left upper arm
Hepatitis B
Birth dose – within 24 hours of birth
0.5 ml
Intramuscular
Antero-lateral side of mid-thigh
Rest three doses at 6 weeks, 10 weeks and 14 weeks
OPV
Zero dose – within first 15 days of birth
2 drops
Oral
First, second and third doses at 6, 10 and 14 weeks with DPT-1, 2
and 3

17
OPV booster with DPT booster at 16-24 months
DPT
Three primary doses at 6, 10 and 14 weeks with OPV-1, 2 and 3
0.5 ml
Intra-muscular
Antero-lateral side of mid-thigh
One booster at 16-24 m with OPV booster (antero-lateral side of
mid-thigh) and second booster at 5-6 years (upper arm)
Measles
At 9 completed months to 12 months
Give up to 5 years if not received at 9-12 months age
Second dose at 16-24 months (select states after catch-up
campaign) – Measles Containing Vaccine
0.5 ml
Sub-cutaneous
Right upper arm
Along with Vitamin A (1
st
dose) – 1ml (1 lakh IU) - oral
Constraints
Illiteracy
Non uniform coverage
Poor implementation
Poor monitoring
High drop outs
18
Declining coverage in some major states
Over reporting
Poor injection safety
Reorientation of staff being not carried out
Vacany of staff at field level not filled
Poor surveillance of vaccine preventable diseases
Poor vaccine logistics
Poor maintainance of equipments
Extra ordinary emphasis on polio vaccine
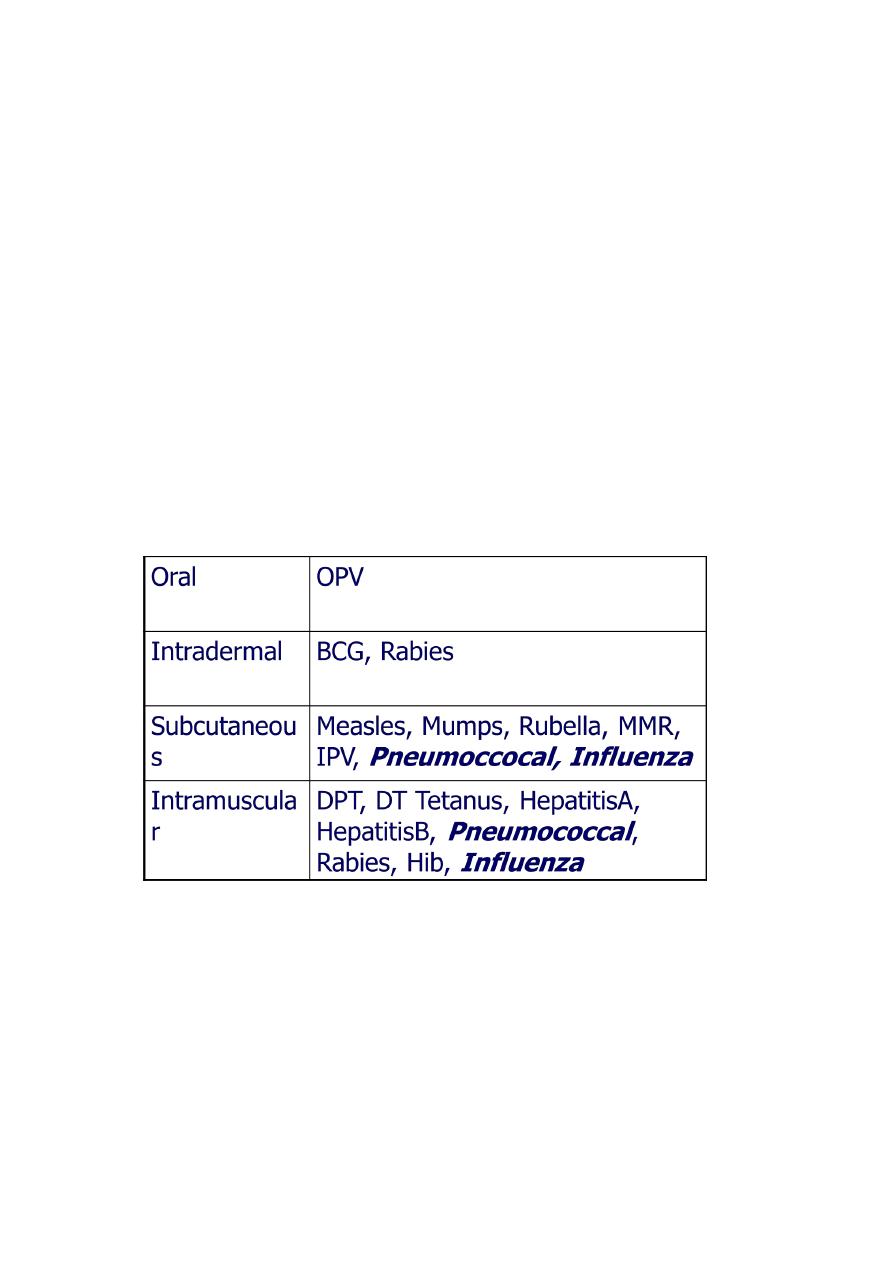
Route of Administration

19
Site of Administration
Who should not be vaccinated?
Allergy
Fever
HIV infection
Immunodeficiency
IG administration
Neurological disorder
Prematurity
Reactions to Previous vaccine
Simultaneous administration of Vaccines
Thrombocytopenia
Neurological disorder
Prematurity
Reactions to Previous vaccine
Simultaneous administration of Vaccines
Thrombocytopenia
21
Allergy
A. Allergic Reactions to Egg-related antigens
1. Yellow fever and influenza vaccines do contain egg proteins
and rarely induce immediate allergic reactions. Skin testing
is recommended before administration with an history of
allergic to egg
2. MMR- Even those with severe hypersensitivity are at low
risk of anaphylaxis.
Allergy
B. Antibiotic-induced allergic reaction
Delayed type local reaction 48-96 hours afterwards and is
usually minor
IPV and OPV – streptomycin, neomycin and polymyxin B
MMR and varicella vaccine-neomycin
Allergy
C. Gelatin- MMR, Varicella vaccine
Fever
Low-grade fever or mild illness is not a contraindication for
vaccination
Children with moderate or severe febrile illnesses can be
vaccinated as soon as they are recovering and no longer acutely ill
Vaccination in Pregnancy
Risk to a developing fetus from vaccination of the mother during
pregnancy is mostly theoretical
Only smallpox vaccine has ever been shown to injure a fetus
The benefits of vaccinating usually outweigh potential risks
21
Vaccination in Pregnancy
Inactivated vaccines
Routine (influenza)
Vaccinate if indicated (hep B, Td, mening, rabies)
Vaccinate if benefit outweighs risk (all other)
Live vaccine – do not administer
Exception is yellow fever vaccine
HIV Infection
No BCG
OPV is Contraindicated
in household contact, in recipient ( asymptomatic or
symptomatic)
IPV for these children and household contacts
MMR vaccination should be considered for all asymptomatic and
to all symptomatic HIV-infected persons who do not have
evidence of severe immunosuprresion or measles immunity
Pneumococcal vaccine, Hib, DTP (or DTaP), Hepatitis B vaccine,
Influenza vaccines are all indicated
Immunosupression
No live viral vaccines and BCG. IPV for these patients, their siblings
and their household contacts
No live vaccine (except varicella) until six months after
immunosuppressive therapy
Neurological disorder
22
Progressive developmental delay or changing neurological findings
(e.g. infantile spasm) - defer pertussis immunization
Personal history of convulsions
Recent seizures - defer pertussis immunization
Conditions predisposing to seizures or neurological deterioration
(e.g. tuberous sclerosis) - defer pertussis immunization
Reactions
Severe Reactions to DTP
Insonable cry lasting more than 3 hrs with 48 hrs of dose
Seizure with 3 days
Severe local reactions
Family hx of adverse event
Not a contraindication, but consider carefully the benefits and
risks, if need to vaccinate can use acellular DTP for less reactions
Reactions
GBS with 6 weeks after a dose of DTP
Again based on risks and benefits for further dose of DTP
and risk of GBS recurrence.
Contraindication for further dose of DTP
encephalopathy within 7 days of a dose of DTP
VACCINE REACTIONS
Common, minor reactions
vaccine stimulates immune system
settle on their own
warn parents and advise how to manage

23
Rare, more serious reactions
anaphylaxis (serious allergic reaction)
vaccine specific reaction

24
Simutaneous administration of Vaccine
A theoretical risk that administration of multiple live virus vaccine:
OPV, MMR, and varicella ) within 28 days of one another if not
given on the same day will result in a sub optimal immune
response
No data to substantiate this
Recommended Storage Temperatures
Table 5: Vaccination Schedule for Infants and Children 2012
Age Type of vaccine
0-1 Week OPV0 dose , HepB1 , BCG
25
2 Months OPV1 , PENTA1,ROTA1
4 Months OPV2 , TETRA1,ROTA2
6 Months OPV3 , PENTA2,ROTA3
9 Months Measles + VIT A
15 Months MMR (Measles , Mumps , Rubella)
18 Months TETRA2, OPV First Booster dose + VIT A
4-6 Years DPT , OPV Second Booster dose + MMR2
Table 6: National Immunization Schedule for Infants and
Children 2015
Age Type of vaccine
0-1 Week HepB1 , BCG + OPV0dose
2 Months HEXA 1,ROTA1 ,PREV13-1+OPV1
4 Months HEXA2,ROTA2,PREV13-2 + OPV2
6 Months HEXA3,ROTA3,PREV13-3 + OPV3
9 Months Measles + VIT A
15 Months MMR(Measles , Mumps , Rubella)
18 Months PENTA (DTP+IPV+Hib ) OPV + VIT A
4-6 Years TETRA (DTaP +IVP ) + OPV + MMR
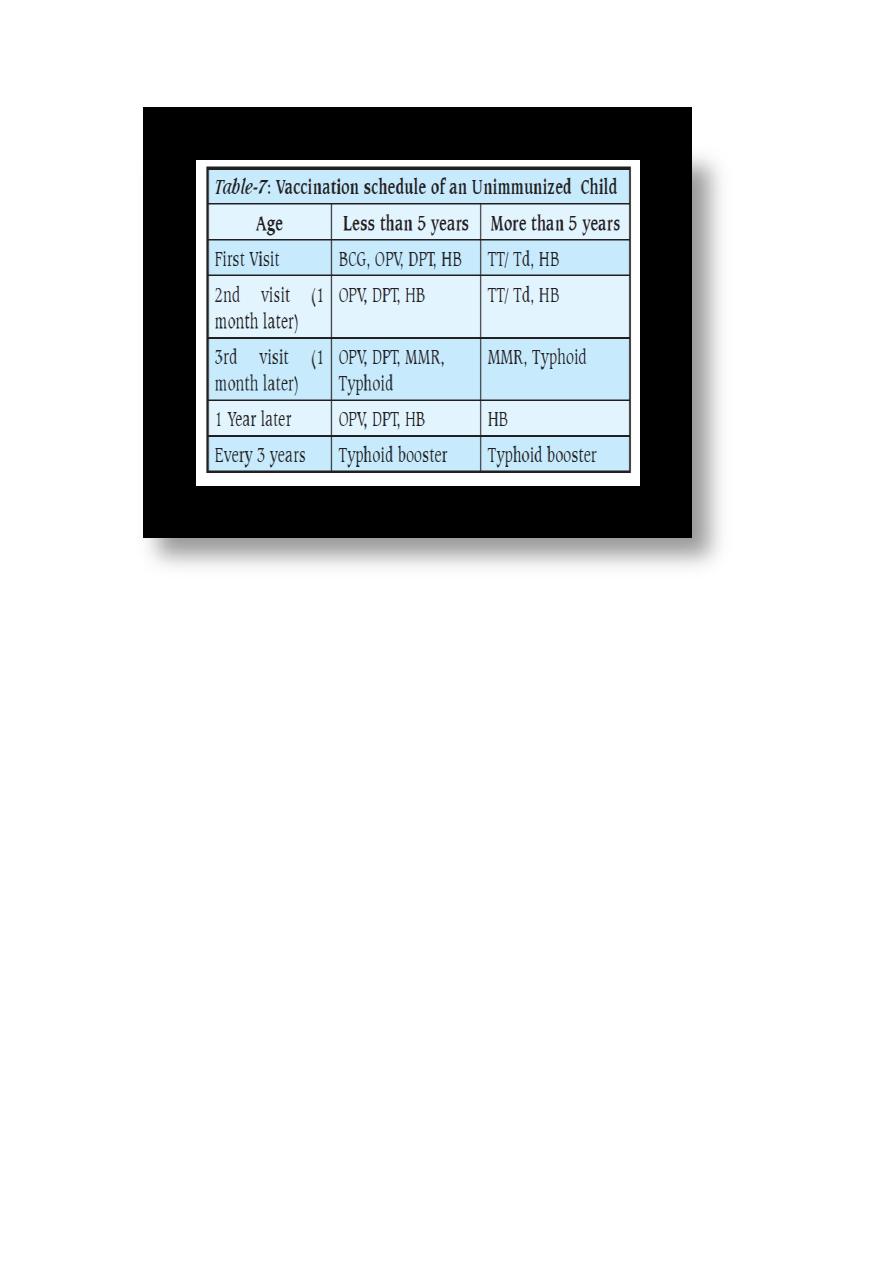
26
