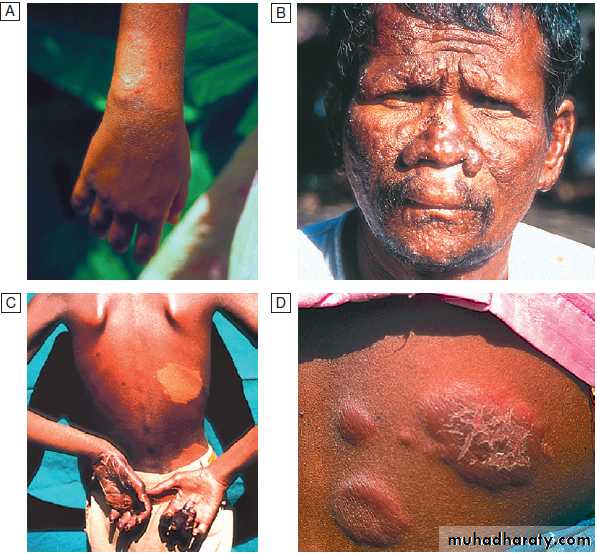
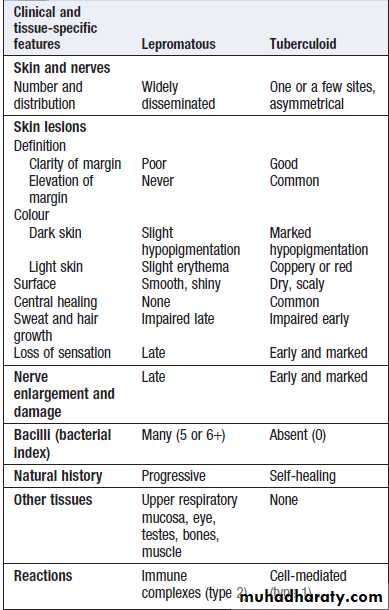
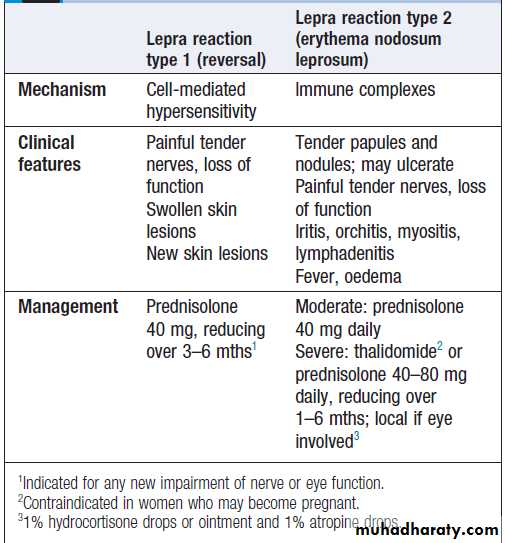
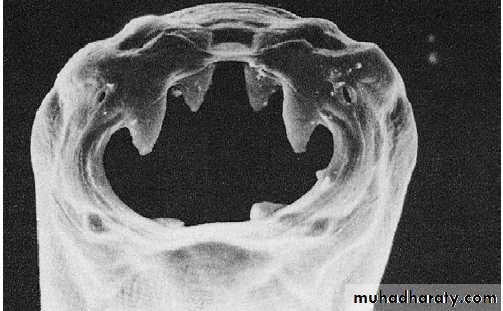
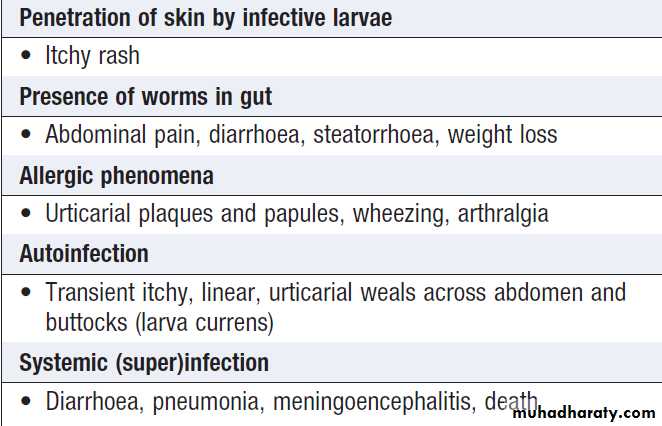
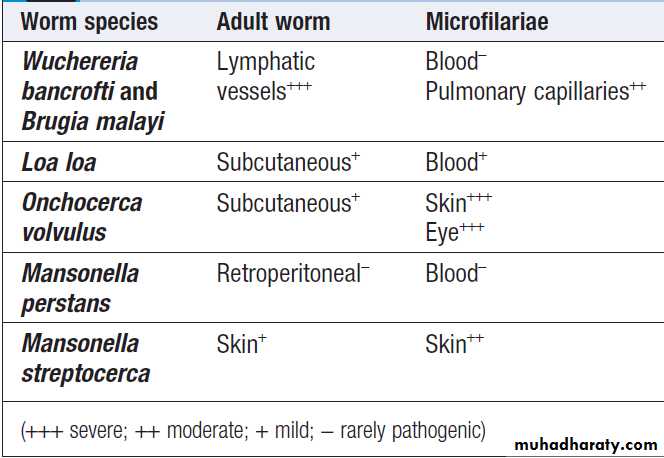
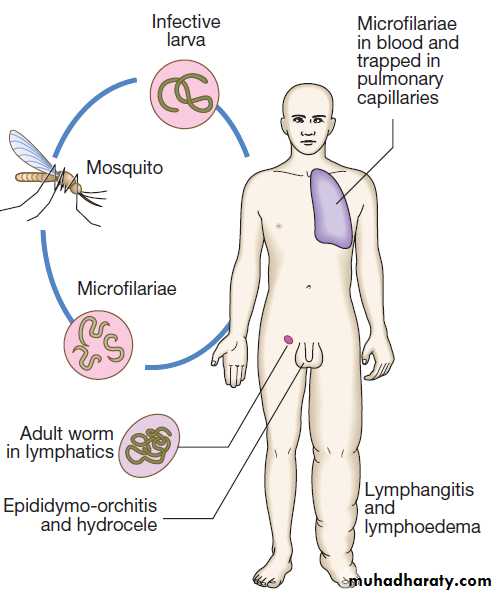
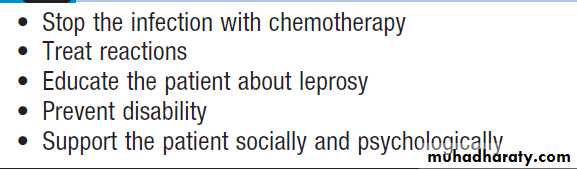Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
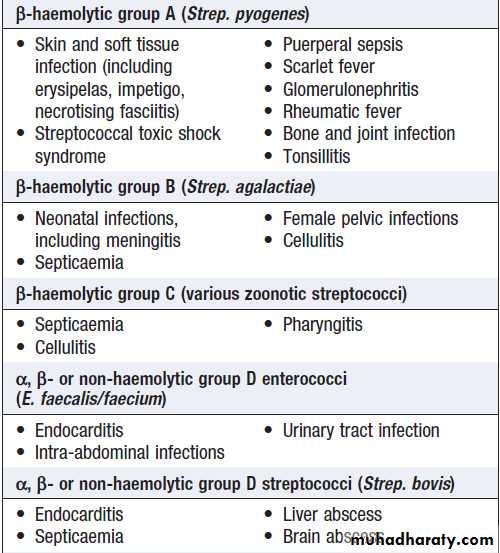
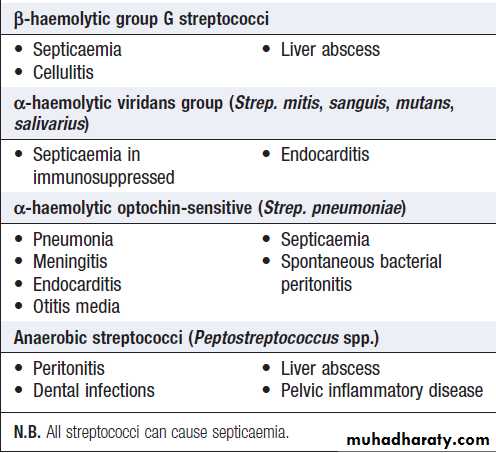
Infectious diseaseFever
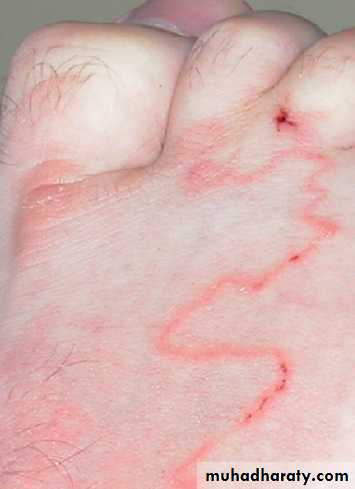
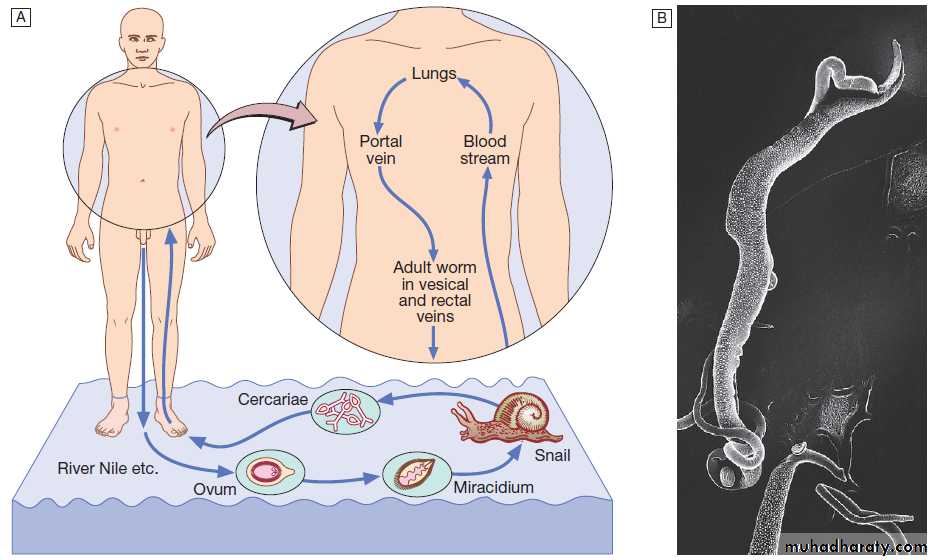
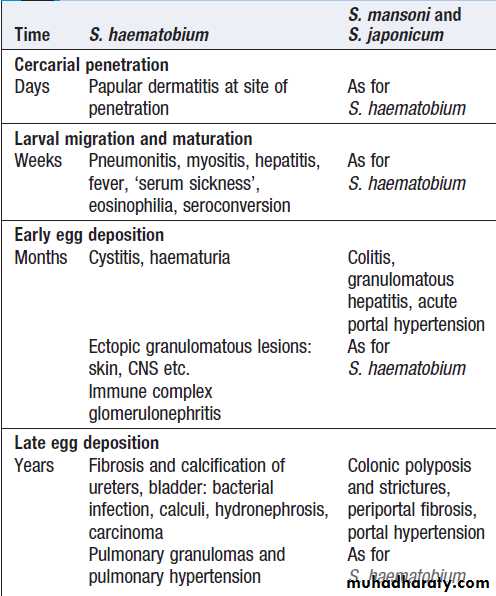
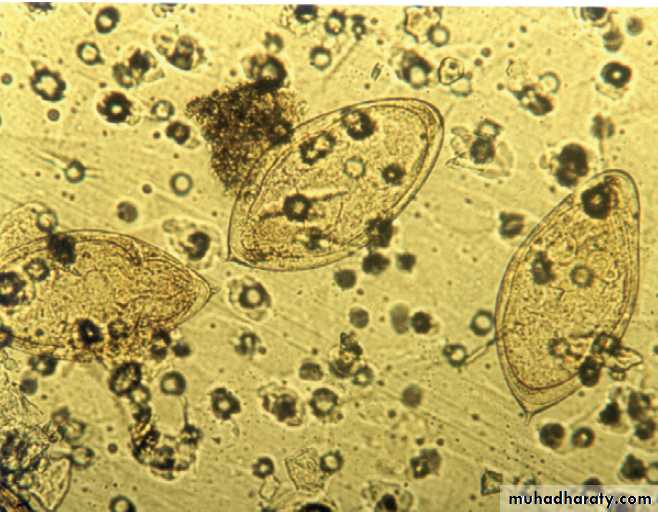
• Documentation of fever.Fever is diagnosed a body temperature >38.0°C has been recorded. Axillary and aural measurement is less accurate than oral or rectal.
• Rigors. Shivering (followed by excessive sweating) occurs with a rapid rise in body temperature from any cause.
• Night sweats. Associated with infections (e.g. tuberculosis, infective endocarditis), but sweating from any cause is worse at night.
• Excessive sweating. Alcohol, anxiety, thyrotoxicosis, diabetes, acromegaly, lymphoma and environmental heat all cause sweating without temperature elevation.
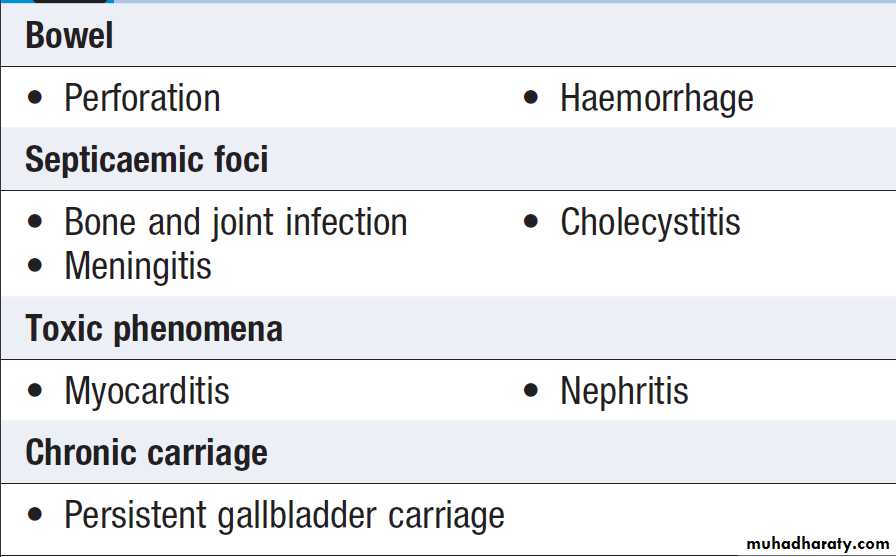
• Recurrent fever.
e.g. Borrelia recurrentis, bacterial abscess.
• Accompanying features.
HEADACHE.
Severe headache and photophobia, although
characteristic of meningitis, may accompany other.
DELIRIUM.
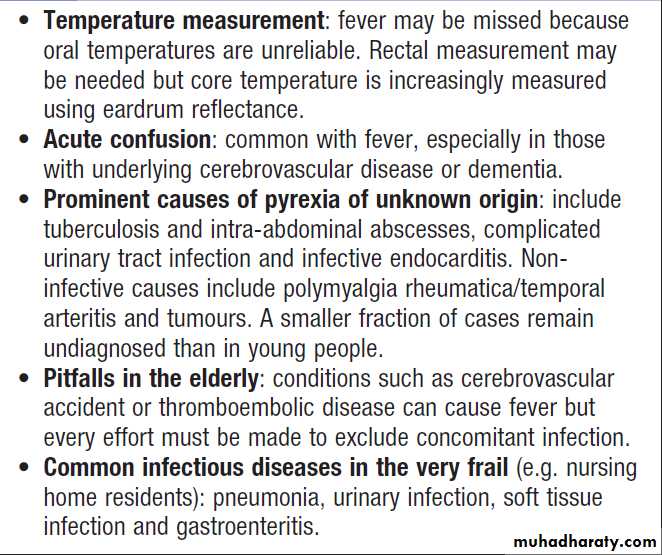
Mental confusion during fever is more common in young children or the elderly.
MUSCLE PAIN.
Myalgia may occur with viral infections, such as influenza, and with septicaemia, including meningococcal sepsis.
SHOCK.
Shock may accompany severe infections and sepsis
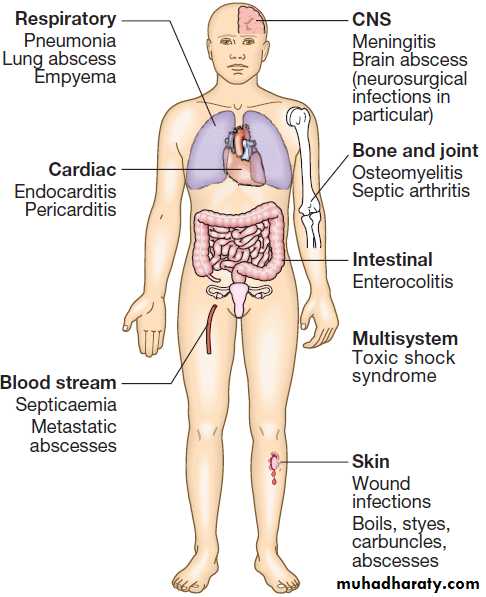
PRESENTING PROBLEMS IN INFECTIOUS DISEASES
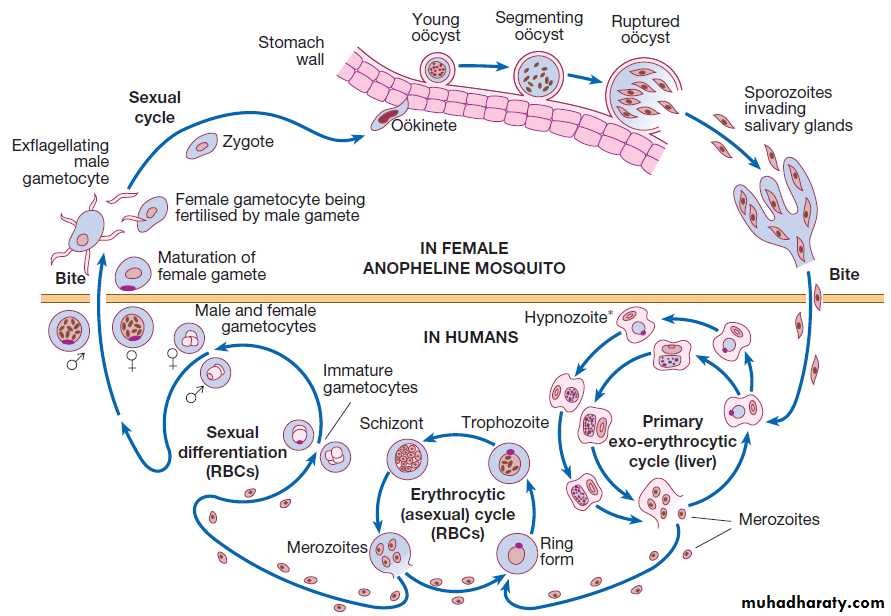
Fever‘Fever’ implies an elevated core body temperature of
more than 38.0°C . Fever is a response to cytokines
and acute phase proteins and occurs in infections and in non-infectious conditions.
Clinical assessment
The differential diagnosis is very broad.
Investigations
If the clinical features do not suggest a specific infection, then initial investigations should include:
• a full blood count (FBC) with differential, including eosinophil count
• urea and electrolytes, liver function tests (LFTs),
blood glucose and muscle enzymes
• inflammatory markers, ESR and CRP
• a test for antibodies to HIV-1
• autoantibodies, including antinuclear antibodies (ANA)
• chest X-ray and ECG
• urinalysis and urine culture
• blood culture
• throat swab for culture
• other specimens, as indicated by history and examination.
Management
Fever and its associated symptoms can be treated with paracetamol, and by tepid sponging to cool the skin. Replacement of salt and water is important . Further management is focused on the underlying cause.
Fever in old age
Fever with localising symptoms or signsIn most patients, the site of infection is apparent ,and the likelihood of infection is reinforced by investigation results (e.g. neutrophilia with raised ESR and CRP in bacterial infections). Not all apparently localising symptoms are reliable, however; headache, breathlessness and diarrhoea can occur in sepsis without localised infection in the CNS, respiratory or GIT . Careful interpretation of the clinical features is vital (severe headache , photophobia, rash and neck stiffness suggests meningitis, whereas moderate headache with cough and rhinorrhoea is consistent with a viral upper respiratory tract infection). Further investigation and management are specific to the cause, but may include empirical antimicrobial therapy pending confirmation of the microbiological diagnosis.
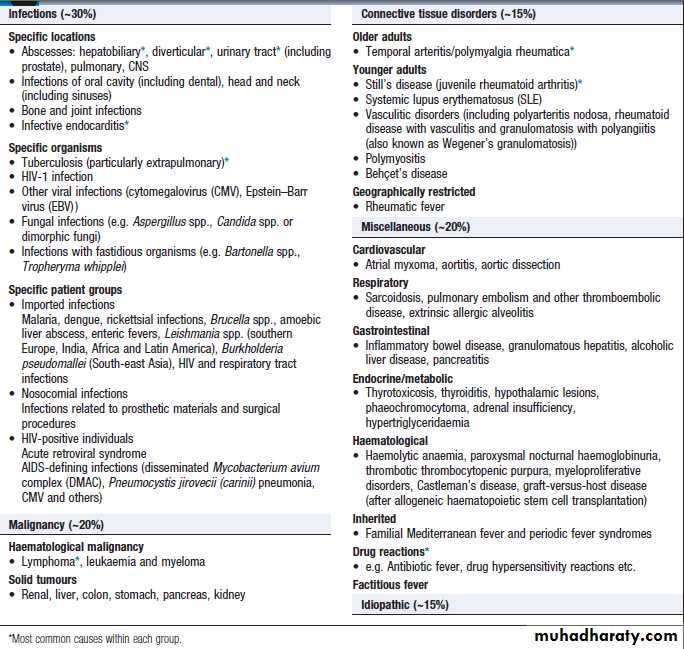
Pyrexia of unknown origin (PUO)
Defined as a temperature persistently above 38.0°C for more than 3 weeks, without diagnosis, despite initial investigation during 3 days of inpatient care or after more than two outpatient visits.Subsets of PUO are described by medical setting:
HIV-1 related, immune-deficient or nosocomial.
Up to one-third of cases of PUO remain undiagnosed.
Clinical assessment
Major causes of PUO are outlined in Box.
Rare causes, such as periodic fever syndromes , should be considered in those with a positive family history. Children and younger adults are more likely to have infectious causes – in particular, viral infections.
Older adults are more likely to have certain infectious and non-infectious causes .
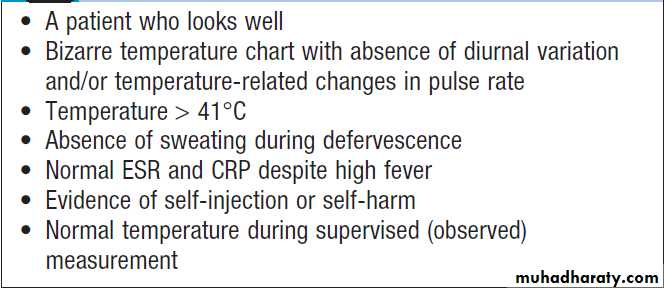
Detailed history and examination should be repeated at regular intervals to detect emerging features (e.g. rashes, signs of infective endocarditis or features of vasculitis). In men, the prostate should be considered as a potential source of infection. Clinicians should be alert to the possibility of factitious fever.
Investigations
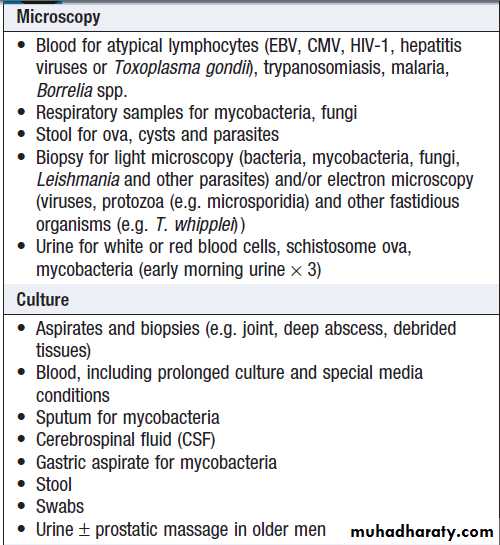
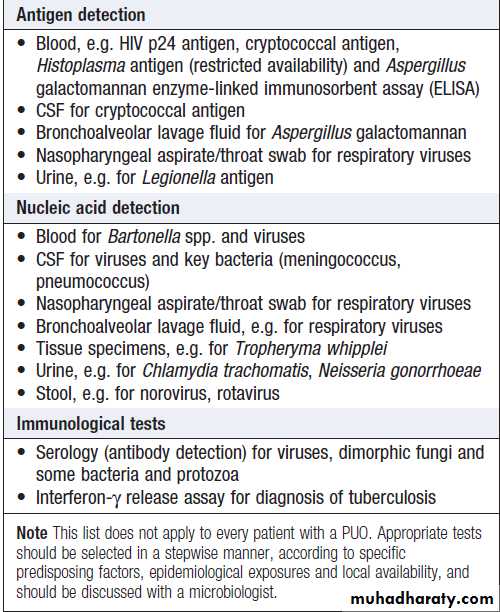
If initial investigation is negative, a series of further microbiological and non-microbiological investigations should be considered. These will usually include:
• induced sputum or other specimens for mycobacterial stains and culture
• serological tests
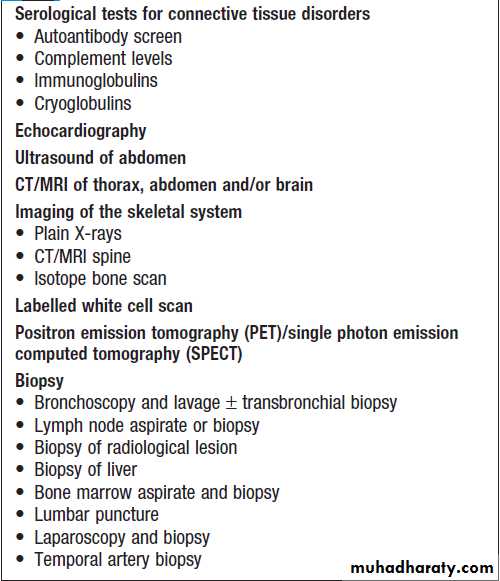
• imaging of the abdomen by ultrasonography or CT• echocardiography.
Lesions identified on imaging should usually be
biopsied in order to seek evidence of relevant pathogens
by culture, histopathology or nucleic acid detection.
Liver biopsy may be justified, e.g. to identify idiopathic
granulomatous hepatitis, if there are biochemical or
radiological abnormalities.
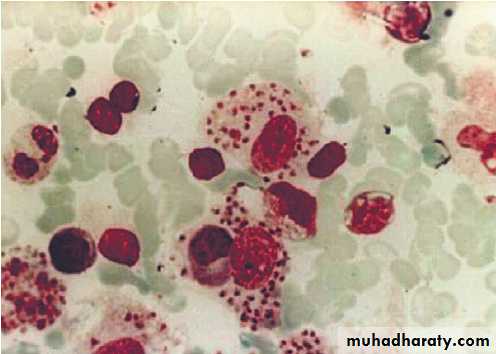
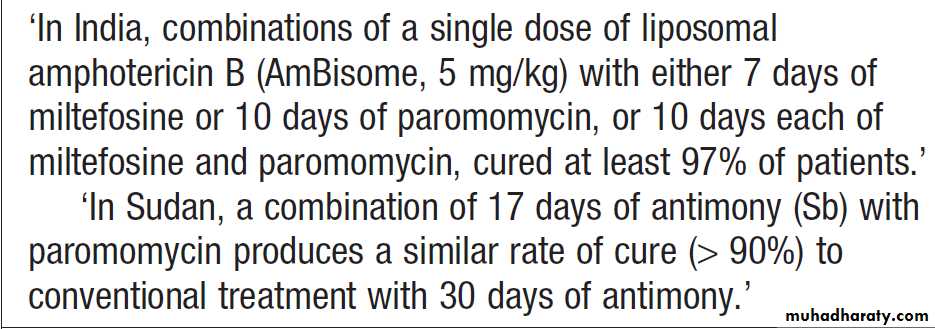
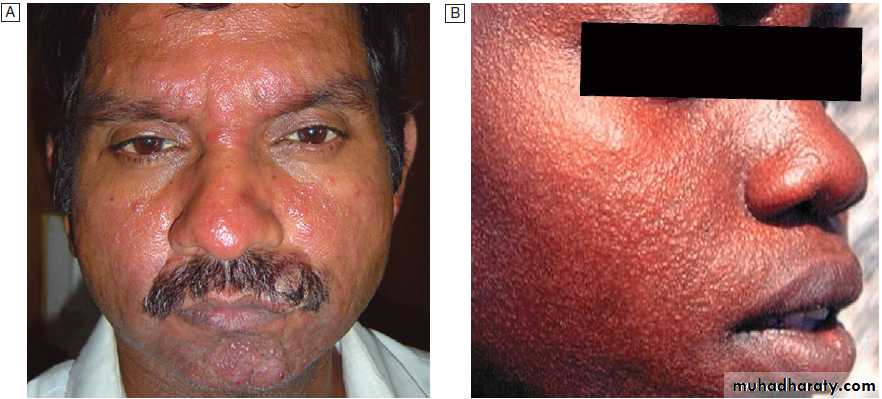
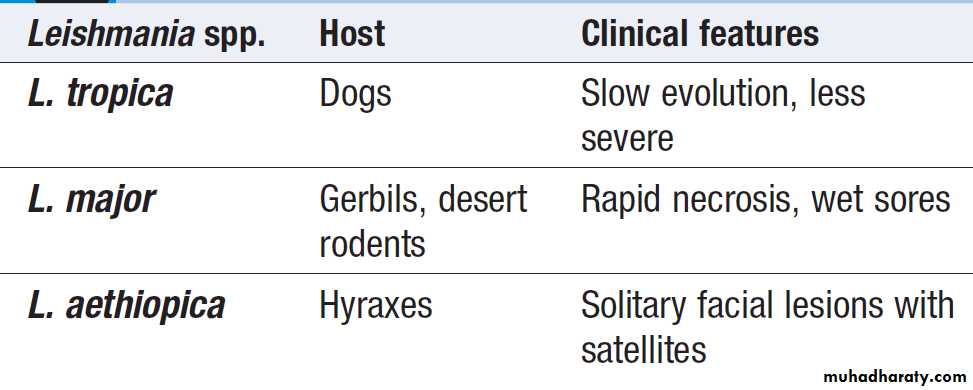
Bone marrow biopsies have a diagnostic yield of up to 15%, most often revealing haematological malignancy, myelodysplasia or tuberculosis, and also identifying brucellosis, typhoid fever or visceral leishmaniasis.
Bone marrow should be sent for culture, as well as microscopy. Laparoscopy is occasionally undertaken with biopsy of abnormal tissues. Splenic aspiration in specialist centres is the diagnostic test of choice for suspected visceral leishmaniasis. Temporal artery biopsy should be considered in patients over the age of 50 years, even in the absence of physical signs or a raised ESR. ‘Blind’ biopsy of other structures in the absence of localising signs, or laboratory or radiology results is unhelpful.
Prognosis
The overall mortality of PUO is 30–40%, mainly attributable to malignancy in older patients. If no cause is found, the long-term mortality is low and fever often settles
spontaneously.
Aetiology of pyrexia of unknown origin (PUO)
Clues to the diagnosis of factitious fever
Microbiological investigation of PUO
Microbiological investigation of PUO – cont’d
Additional investigations in PUO
Fever in the injection drug-userIV injection of recreational drugs is widespread .
Infective organisms are introduced by non-sterile injection and infection is facilitated by immunodeficiency. The risks increase with prolonged drug use and injection into large veins of the groin and neck because of progressive thrombosis of superficial peripheral veins. The most common causes are soft tissue or respiratory infections.
Clinical assessment
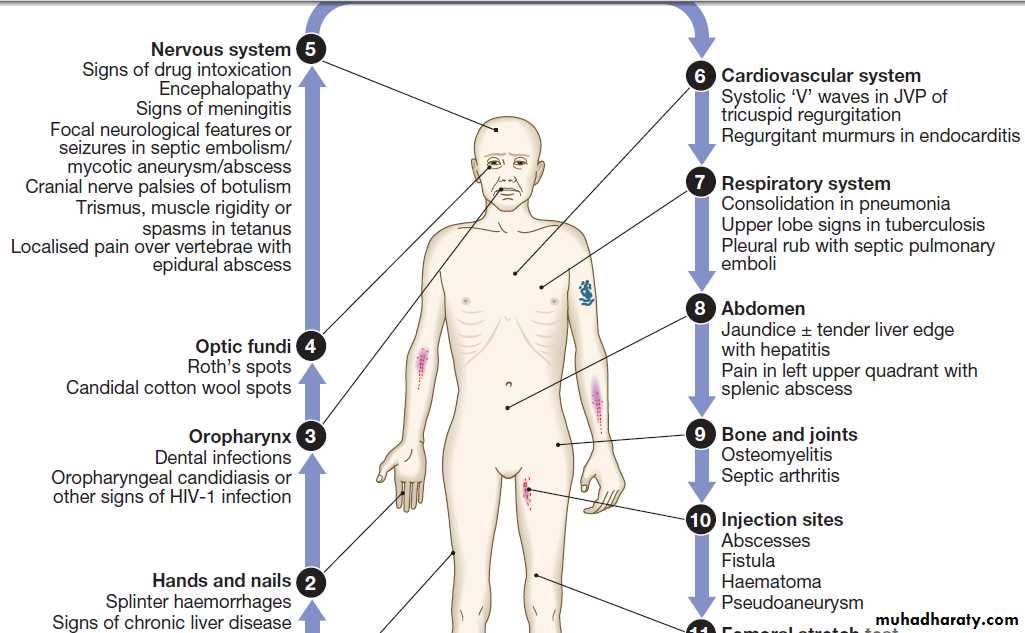
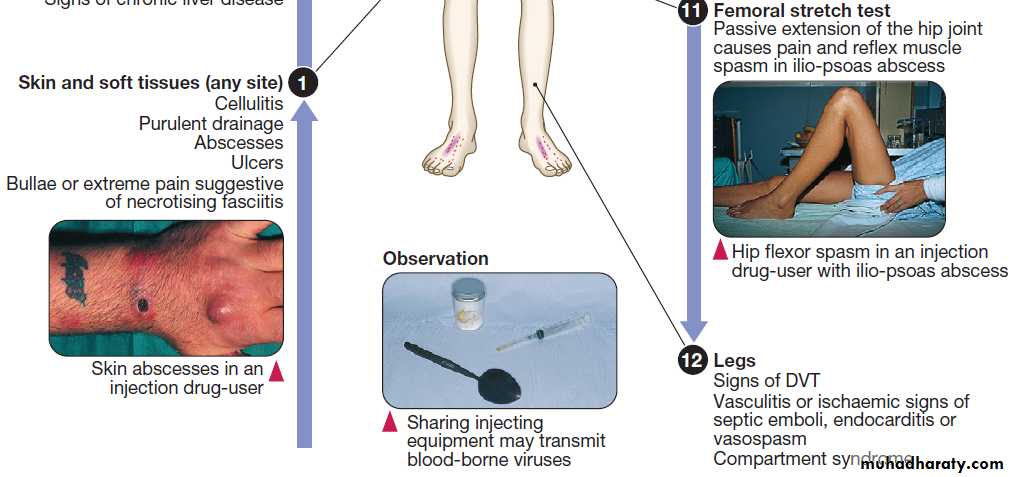
The history should address the following risk factors:
• Site of injection. Femoral vein injection is associated
with vascular complications such as DVT (50% of which are septic) and accidental arterial injection with false aneurysm formation or a compartment syndrome.
Local complications include iliopsoas abscess, and septic arthritis of the hip joint or sacroiliac joint. Injection of the jugular vein can be associated with cerebrovascular complications. Subcutaneous and intramuscular injection has been related to infection by clostridial species, the spores of which contaminate heroin. Tetanus, wound botulism and gas gangrene also occur.
• Technical details of injection. Sharing of needles and
other injecting paraphernalia (including spoons and
filters) greatly increases the risk of blood-borne virus infection (e.g. HIV-1, hepatitis B or C virus). Some users lubricate their needles by licking them prior to injection, thus introducing mouth organisms (e.g. anaerobic streptococci, Fusobacterium spp. and Prevotella spp).
• Substances injected. Injection of cocaine is associated
with a variety of vascular complications.• Blood-borne virus status. Results of previous HIV-1
and hepatitis virus tests or vaccinations for hepatitis
viruses should be recorded.
• Surreptitious use of antimicrobials. Addicts may use
antimicrobials to self-treat infections, masking initial
blood culture results.
It can be difficult to distinguish the effects of infection from the effects of drugs or drug withdrawal (excitement, tachycardia, sweating, marked myalgia, confusion). Stupor and delirium may result from drug administration but may also indicate meningitis or encephalitis.
Investigations
The initial investigations are as for any fever ,a chest X-ray and blood cultures. Echo to detect infective endocarditis
should be performed in all injection drug-users.
Endovascular infection should also be suspected if lung abscesses or pneumatocoeles are detected radiologically. Additional imaging should be focused on sites of injection or of localising symptoms and signs .
Any pathological fluid collections should be sampled.
Urinary toxicology tests may suggest a non-infectious cause, testing for hepatitis B and C virus and HIV-1.
Management
Empirical therapy of fever in the injection drug-userincludes an antistaph-penicillin (e.g. flucloxacillin) or, if meticillin-resistant Staph. aureus (MRSA) is prevalent, a glycopeptide (e.g. vancomycin), with modification when antimicrobial susceptibility is available. Right-sided endocarditis due to Staph. aureus treated with high-dose IV flucloxacillin. In left-sided Staph. aureus endocarditis, aminoglycoside therapy may be added. Right-sided endocarditis caused by MRSA is usually treated with 4 weeks of vancomycin plus gentamicin. For localised infections of the skin and soft tissues, oral therapy with agents active against staphylococci, streptococci and anaerobes is appropriate (e.g. flucloxacillin plus co-amoxiclav or clindamycin).
Fever in the injection drug-user: key features of clinical examination.
Fever in the immunocompromised host
Include those with congenital immunodeficiency , HIV infection and iatrogenic immunosuppression induced by chemotherapy , transplantation or immunosuppressant medicines, including high-dose corticosteroids. Metabolic abnormalities, such as under-nutrition or hyperglycaemia, may also contribute.Multiple elements of the immune system are potentially compromised, impaired neutrophil function from
chemotherapy, impaired T-cell and/or B-cell responses
due to underlying malignancy, T-cell and phagocytosis
defects due to corticosteroids, mucositis from chemotherapy
and an impaired skin barrier due to insertion of
a central venous catheter.
Fever may result from infectious or from noninfectious
causes, including lymphoproliferative disease, graft-versus-host disease ,drugs, vasculitis, neoplasm, or Sweet’s syndrome (reddish nodules or plaques with fever and leucocytosis, in association with haematological malignancy).
Clinical assessment
• Identification of the immunosuppressant factors.
• Any past infections and their treatment.
• Exposure to infections, including opportunistic infections.
• Prophylactic medicines and vaccinations administered. Examination should include inspection of the normal
physical barriers , skin and mucosal surfaces and, in particular, central venous catheters, mouth, sinuses, ears and perianal area (digital rectal examination should be avoided). The areas around finger and toenails should also be inspected.
Investigations
Immunocompromised often have decreased inflammatory responses leading to attenuation of physical signs, such as neck stiffness with meningitis, radiological and laboratory findings, such as leucocytosis. Chest CT scan should be considered in addition to chest X-ray when respiratory symptoms occur. Abdominal imaging . Blood cultures from a central venous catheter, urine cultures, and stool cultures. Nasopharyngeal aspirates, skin lesions should be biopsied if nodules are present. PCR for CMV and Aspergillus spp. Antibody detection is rarely useful. Patients with respiratory signs or symptoms should be considered for bronchoscopy to obtain BAL fluid to detect Pneumocystis jirovecii (carinii), bacteria, fungi and viruses.Neutropenic fever
Neutropenic fever defined as a neutrophil count of < 0.5 × 109/L and a single axillary temperature above 38.5°C or three recordings above 38.0°C over a 12-hour period. Patients are particularly prone to bacterial or fungal infection. Gram-positive are the most common. Empirical broad-spectrum therapy is commenced as soon as neutropenic fever occurs and cultures have been obtained. The most common regimens , broad-spectrum penicillins, such as piperacillin–tazobactam IV. If fever has not resolved after 3–5 days, empirical antifungal therapy (e.g. caspofungin) is added .Post-transplantation fever
Fever in transplant recipients may be due to infection,episodes of graft rejection in solid organ transplant
recipients, or graft-versus-host disease following haematopoietic stem cell transplantation (HSCT).
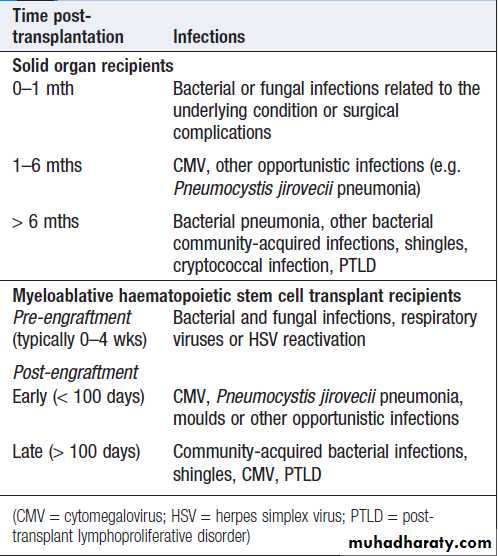
Infections in solid transplant recipients are grouped
according to the time of onset . Those in the
first month are related to the underlying condition or
surgical complications.
Those occurring 1–6 months after transplantation are characteristic of impaired T-cell function.
Infections in transplant recipients
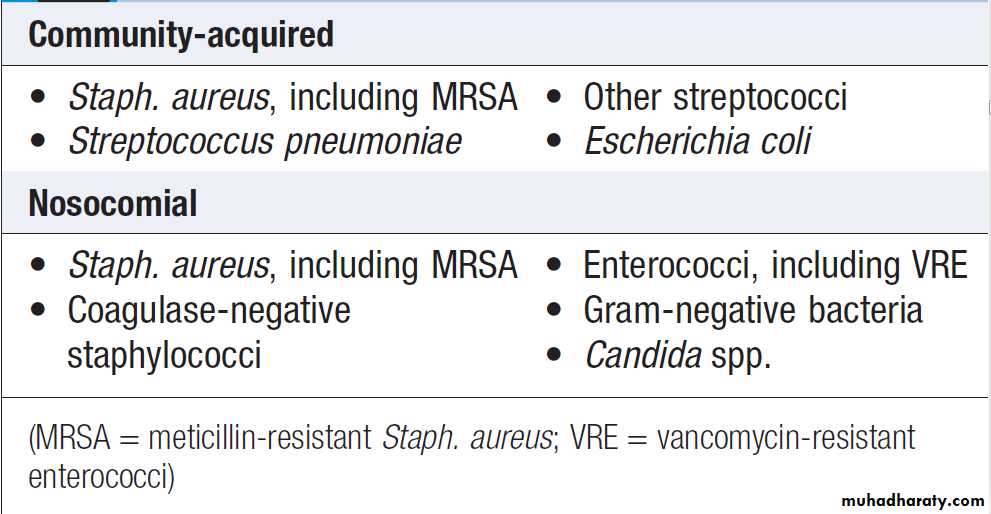
Common causes of blood-stream infection
Positive blood culturePositive blood cultures may be caused by contaminants.
When isolated from only one bottle, or from all bottlesfrom one venesection, coagulase-negative staphylococci
often represent contamination. Repeated isolation of this
organism, however, should raise suspicion of infective
endocarditis or, in a patient with any form of prosthetic
material, prosthesis infection. Viridans streptococci
occasionally cause transient non-significant bacteraemia
or blood culture contamination but, in view of their association with infective endocarditis, significant infection
must always be sought clinically.
Further investigations are influenced by the causative
organism and setting.
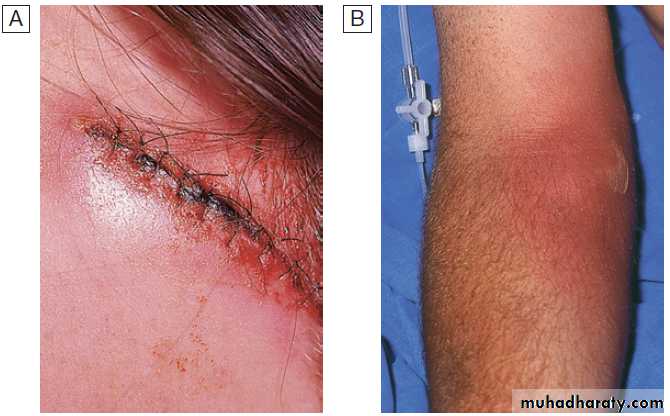
Central venous catheter infections
Typically involve the catheter lumen and are associated with fever, positive blood cultures and, in some cases, signs of purulence or exudate at the site of insertion. Infection is
more common in temporary catheters inserted into the
groin or jugular vein than those in the subclavian vein.
Staphylococci account for 70–90%, with coagulase-negative staphylococci more common than Staph. Aureus. Other causes include enterococci and Gram-negative bacilli. Candida spp. are a common cause of line infections,
particularly in association with total parenteral
nutrition.
Non-tuberculous mycobacteria may cause tunnel infections.
Infection prevention is a key component of the management of vascular catheters. Measures include strict
attention to hand hygiene, optimal siting, full aseptic
technique on insertion and subsequent interventions,
skin antisepsis with chlorhexidine and isopropyl
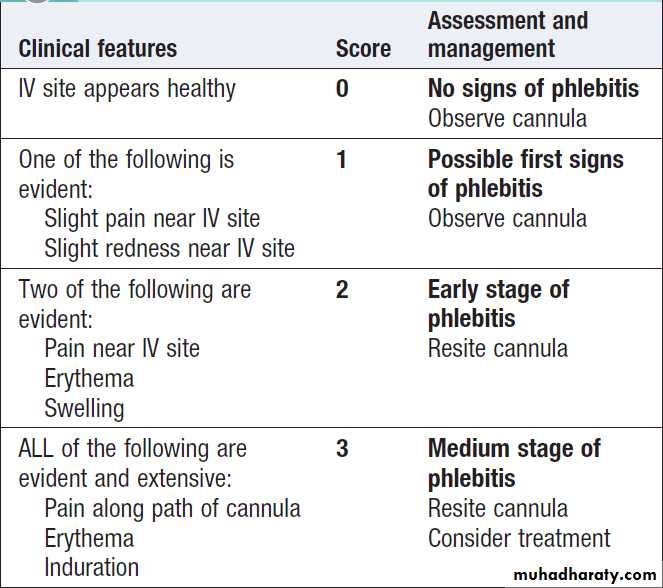
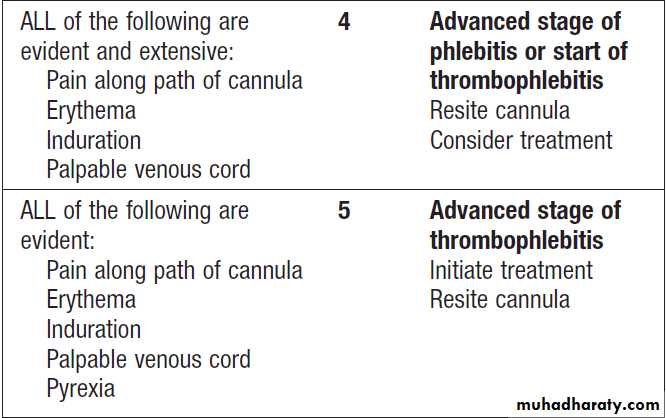
alcohol, daily assessment of catheter sites (e.g. with
visual infusion phlebitis (VIP) score , and daily
consideration of the continuing requirement for catheterisation.
The use of catheters impregnated with antimicrobials
such as chlorhexidine or silver is advocated in some settings.
Severe necrotising soft tissue infections
Necrotising fasciitis
In necrotising fascitis, cutaneous erythema and oedemaprogress to bullae or areas of necrosis. Unlike in cellulitis, pain may be disproportionately intense in relation to the visible cutaneous features. The infection spreads quickly along the fascial plane.
Type 1 necrotising fasciitis is a mixed infection with Gram-negative bacteria and anaerobes, often seen post-operatively in diabetic or immunocompromised hosts. Subcutaneous gas may be present.
Type 2 necrotising fasciitis is caused by group
A or other streptococci. Approximately 60% of cases are
associated with streptococcal toxic shock syndrome .
Necrotising fasciitis is a medical emergency, requiring
immediate surgical débridement with inspection ofthe involved muscle groups, in addition to antimicrobial
therapy . Empiric treatment is with broadspectrum
agents (e.g. piperacillin–tazobactam plus clindamycin
and ciprofloxacin; meropenem monotherapy;
or third-generation cephalosporin plus metronidazole).
Hyperbaric oxygen therapy may be considered for polymicrobial infection.
Group A streptococcal infection is treated with benzylpenicillin plus clindamycin, and often immunoglobulin.
Gas gangrene
Although Clostridium spp. may colonise or contaminatewounds, no action is required unless there is evidence
of spreading infection. Infection may be limited to tissue
that is already damaged (anaerobic cellulitis) or involve
healthy muscle (gas gangrene).
In anaerobic cellulitis, usually that due to C. perfringens
or to other strains infecting devitalised tissue following
a wound, gas forms locally and extends along
tissue planes but bacteraemia does not occur. Prompt
surgical débridement of devitalised tissue and therapy
with penicillin or clindamycin is usually effective.
Gas gangrene (clostridial myonecrosis) is defined as
acute invasion of healthy living muscle undamaged by
previous trauma, and is most commonly caused by C.
perfringens. In at least 70% of cases, it follows deep penetrating injury sufficient to create an anaerobic (ischaemic) environment and allow clostridial introduction
and proliferation. Severe pain at the site of the injury
progresses rapidly over 18–24 hours. Skin colour changes
from pallor to bronze/purple discoloration and the skin
is tense, swollen, oedematous and exquisitely tender.
Gas in tissues may be obvious, with crepitus on clinical
examination, or visible on X-ray, CT or ultrasound.
Signs of systemic toxicity develop rapidly, with high leucocytosis, multi-organ dysfunction, raised creatine kinase
and evidence of disseminated intravascular coagulation
and haemolysis. Antibiotic therapy with high-dose
intravenous penicillin and clindamycin is recommended,
coupled with aggressive surgical débridement of the
affected tissues. Alternative agents include cephalosporins
and metronidazole. Hyperbaric oxygen has a
putative but controversial role.
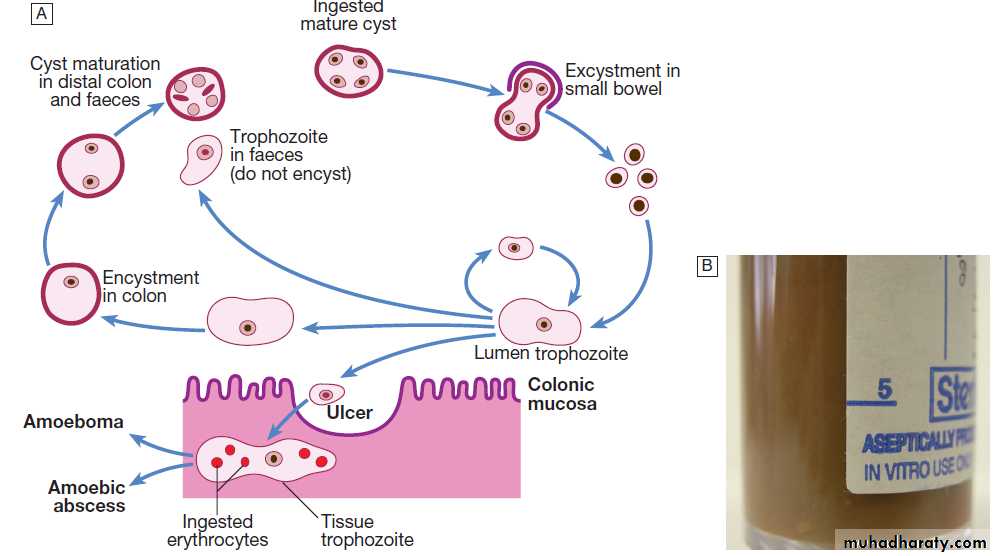
Acute diarrhoea and vomiting
Acute diarrhoea , sometimes with vomiting, is the predominant symptom in infective gastroenteritis . Acute diarrhoea may also be a symptom of other infectious and non-infectious diseases . Stress, whether psychological or physical, can also produce loose stools. The majority of episodes are due to infections spread by the faecal–oral route and transmitted either on fomites, on contaminated hands, or in food or water. Measures such as the provision of clean drinking water, appropriate disposal of human and animal sewage, and the application of simple principles of food hygiene can all limit gastroenteritis.Some organisms (Bacillus cereus, Staph. aureus and
Vibrio cholerae) elute exotoxins which cause vomiting
and/or so-called ‘secretory’ diarrhoea (watery diarrhoea
without blood or faecal leucocytes, reflecting small
bowel dysfunction). In general, the time from ingestion
to the onset of symptoms is short and, other than dehydration, little systemic upset occurs.
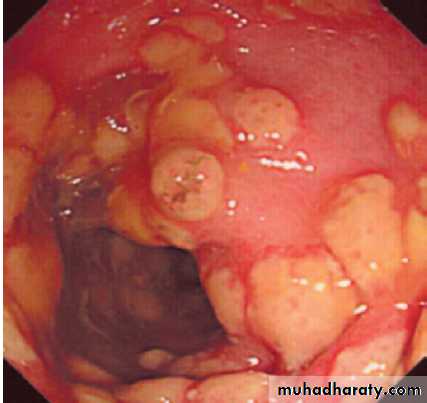
Other organisms, such as Shigella spp., Campylobacter spp. and enterohaemorrhagic E. coli (EHEC), may directly invade the mucosa of the small bowel or produce cytotoxins that cause mucosal ulceration, typically affecting the terminal small bowel and colon. The incubation period is longer and more systemic upset occurs, with prolonged bloody diarrhoea.
Salmonella spp. are capable of invading enterocytes, and of causing both a secretory response and invasive disease with systemic features.
This is seen with Salmonella typhi and S. paratyphi (enteric fever), and, in the immunocompromised host, with non-typhoidal Salmonella spp.
Clinical assessment
The history should address foods ingested , duration and frequency of diarrhoea, presence of blood or steatorrhoea, abdominal pain and tenesmus, and
whether other people have been affected.
Fever and bloody diarrhoea suggest an invasive, colitic, dysenteric process.
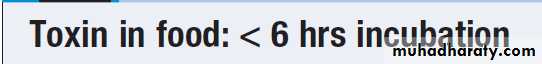
An incubation period of <18 hours suggests toxin-mediated food poisoning, and >5 days suggests diarrhoea caused by protozoa or helminths. Person-to-person spread suggests certain infections, such as shigellosis or cholera.
Examination includes assessment of the degree of
dehydration by skin turgor, pulse and blood pressure
measurement. The urine output and ongoing stool losses
should be monitored.
Investigations
These include stool inspection for blood and microscopy
for leucocytes, and also an examination for ova, cysts
and parasites. Stool culture should be performed and C. difficile toxin sought. FBC and serum electrolytes indicate the degree of inflammation and dehydration.
In a malarious area, a blood film for malaria parasites should be obtained.
Blood and urine cultures and a chest X-ray may identify alternative sites of infection, particularly if the clinical features suggest a syndrome other than gastroenteritis.
Management
All patients with acute, potentially infective diarrhoea
should be appropriately isolated to minimise personto-
person spread of infection. If the history suggests a
food-borne source, public health measures must be
implemented to identify the source and to establish
whether other linked cases exist .
Fluid replacement
Replacement of fluid losses is crucial and may be life-saving. Although normal daily fluid intake in an adult is only 1–2 L, there is considerable additional fluid movement in and out of the gut in secretions . Altered gut resorption with diarrhoea can result in substantial fluid loss, e.g. 10–20 L of fluid may be lost in 24 hours in cholera. The fluid lost in diarrhoea is isotonic, so both water and electrolytes need to be replaced. Absorption of electrolytes from the gut is an active process requiring energy. Infected mucosa is capable of very rapid fluid and electrolyte transport if carbohydrate is available as an energy source. Oral rehydration solutions (ORS) therefore contain sugars, as well as water and electrolytes .ORS can be just as effective as intravenous replacement fluid, even in the management of cholera. In mild to moderate gastroenteritis, adults should be encouraged to drink fluids and, if possible, continue normal dietary food intake. If this is impossible, e.g. due to vomiting, IV fluid administration will be required. In very sick patients, or
those with cardiac or renal disease, monitoring of urine
output and central venous pressure may be necessary. The volume of fluid replacement required should be
estimated based on the following considerations.
• Replacement of established deficit. After 48 hours of
moderate diarrhoea (6–10 stools per 24 hours), the
average adult will be 2–4 L depleted from diarrhoea
alone. Associated vomiting will compound this.
Adults should therefore be given rapid replacement of 1–1.5 L, either orally (ORS) or by IV infusion (normal saline), within the first 2–4 hours of presentation. Longer symptomatology or more persistent/severe diarrhoea rapidly produces fluid losses comparable to diabetic ketoacidosis and is a metabolic emergency.
• Replacement of ongoing losses. The average adult’s
diarrhoeal stool accounts for a loss of 200 mL of
isotonic fluid. Stool losses should be carefully charted and an estimate of ongoing replacement fluid calculated. Commercially available rehydration sachets are conveniently produced to provide 200 mL of ORS; one sachet per diarrhea stool is an appropriate estimate of supplementary replacement requirements.
• Replacement of normal daily requirement. The average
adult has a daily requirement of 1–1.5 L of fluid inaddition to the calculations above. This will be
increased substantially in fever or a hot environment. Antimicrobial agents
In non-specific gastroenteritis, antibiotics have been
shown to shorten symptoms by only 1 day in an illness
usually lasting 1–3 days. This benefit, when related to
the potential for the development of antimicrobial resistance or side-effects, does not justify treatment, except if there is systemic involvement, immunocompromised or significant comorbidity. Evidence suggests that, in EHEC infections, the use of antibiotics may make haemolytic uraemic syndrome (HUS) more likely due to increased toxin release. Antibiotics should therefore not be used in this condition.
Conversely, antibiotics are indicated in Sh. dysenteriae
infection and in invasive salmonellosis – in particular,typhoid fever. Antibiotics may also be advantageous in
cholera epidemics, reducing infectivity and controlling
the spread of infection.
Antidiarrhoeal, antimotility and antisecretory agents
These agents are not usually recommended in acute
infective diarrhoea. Loperamide, diphenoxylate and
opiates are potentially dangerous in dysentery in childhood, causing intussusception. Antisecretory agents,
such as bismuth and chlorpromazine, may be effective but can cause significant sedation. They do not reduce stool fluid losses, although the stools may appear more bulky. Adsorbents, such as kaolin or charcoal, have little effect.
• Bacillus cereus • Staph. Aureus • Clostridium spp.
Causes of infectious gastroenteritis
• Enterotoxigenic E. coli (ETEC,) • Shiga toxin-producing E. coli (EHEC)*• Enteroinvasive E. coli (EIEC,)* • Vibrio cholerae • Salmonella • Shigella* • Campylobacter* • C. difficile*
• Rotavirus • Norovirus
• Giardiasis • Cryptosporidium • Microsporidiosis • Amoebic dysentery * • Isosporiasis)*Associated with bloody diarrhoea.
• Gastroenteritis• C. difficile infection• Acute diverticulitis• Pelvic inflammatory disease• Sepsis• Meningococcaemia• Pneumonia especially ‘atypical disease• MalariaDifferential diagnosis of acute diarrhoea
and vomitingGastrointestinal• Inflammatory bowel • Bowel malignancy• Overflow from constipation• Enteral tube feedingMetabolic• Diabetic ketoacidosis • Thyrotoxicosis• Uraemia• Neuroendocrine tumoursreleasing (e.g.) VIP or 5-HT
(5-HT = 5-hydroxytryptamine, serotonin; VIP = vasoactive intestinal peptide)
Differential diagnosis of acute diarrhea and vomiting'cont'dDrugs and toxins• NSAIDs• Cytotoxic agents• Antibiotics• Proton pump inhibitors• Dinoflagellates• Heavy metals • Ciguatera fish poisoning• Scombrotoxic fish poisoning• Plant toxins
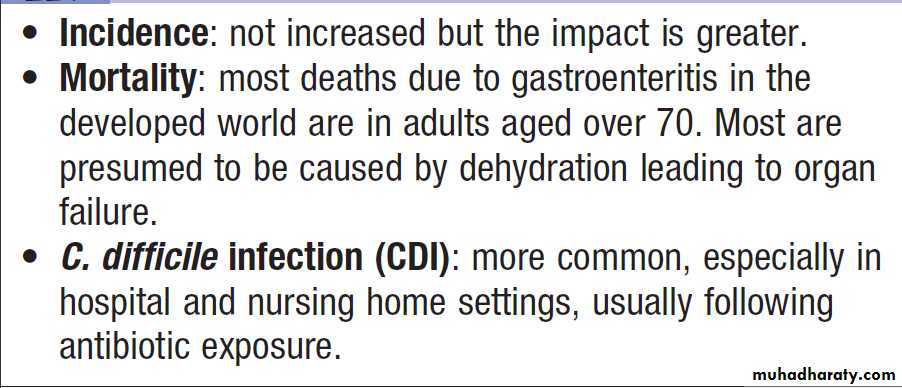
Infectious diarrhoea in old age
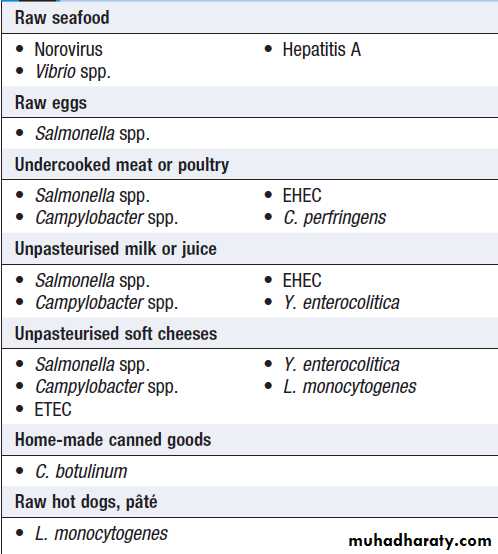
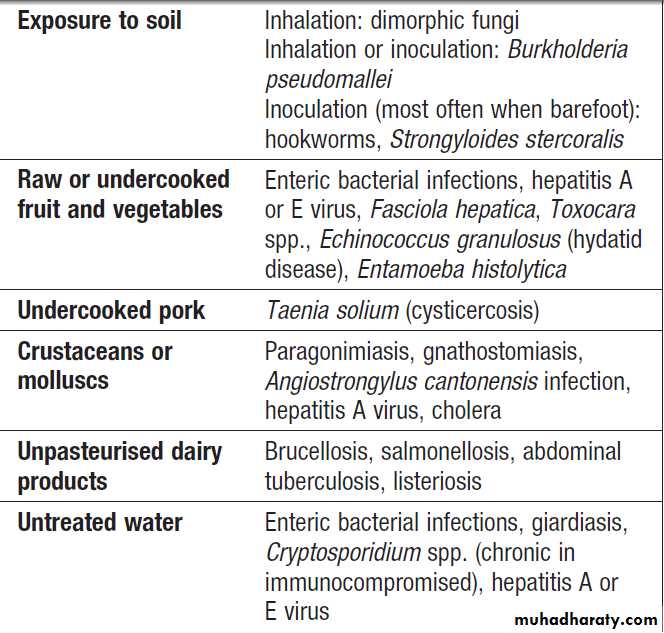
Foods associated with infectious illness,
including gastroenteritisNon-infectious causes of food poisoning
Plant toxinsLegumes and beans produce oxidants which are toxic to people with G6PD deficiency . Consumption produces
headache, nausea and fever, severe haemolysis, haemoglobinuria and jaundice (favism). Red kidney beans, if incompletely cooked, cause acute abdominal pain and diarrhoea from their lectin content. Adequate cooking abolishes this. Alkaloids develop in potato tubers exposed to light, causing green discoloration. Ingestion induces acute vomiting and anticholinesterase-like activity.
Fungi and mushrooms of the Psilocybe spp. produce
hallucinogens.
Many fungal species induce a combination
of gastroenteritis and cholinergic symptoms of
blurred vision, salivation, sweating and diarrhoea.
Amanita phalloides (‘death cap’) causes acute abdominal
cramps and diarrhoea, followed by inexorable hepatorenal failure, often fatal.
Chemical toxins Paralytic shellfish toxin
Paralytic shellfish toxin
Consumption produces gastrointestinal symptoms within 30 minutes, followed by perioral paraesthesia and even respiratory paralysis.
Heavy metals
Thallium and cadmium can cause acute vomiting and diarrhoea resembling staphylococcal enterotoxin poisoning.
Antimicrobial-associated diarrhoea
Antimicrobial-associated diarrhoea (AAD) is a commoncomplication of antimicrobial therapy, especially with
broad-spectrum agents. It is most common in the elderly
but can occur at all ages. Although the specific mechanism
is unknown in most AAD, C. difficile is implicated
in 20–25% of cases and is the most common cause
amongst patients with evidence of colitis. Infection is
diagnosed by detection of C. difficile toxins and is usually
treated with metronidazole or vancomycin .
C. perfringens is a rarer cause which usually remains undiagnosed, and Klebsiella oxytoca is an occasional cause of antibiotic-associated haemorrhagic colitis.
Most common causes of travellers’ diarrhoea
Antimicrobials in travellers’ diarrhoea
Causes of chronic diarrhoea acquired in the tropics
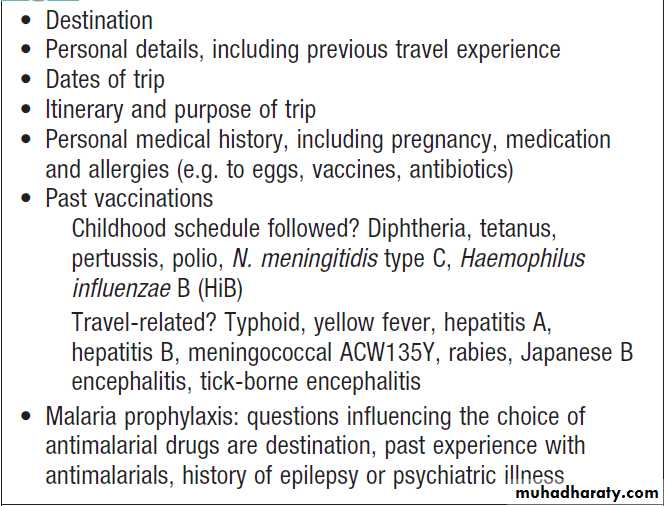
How to assess health needs in travelers before departure
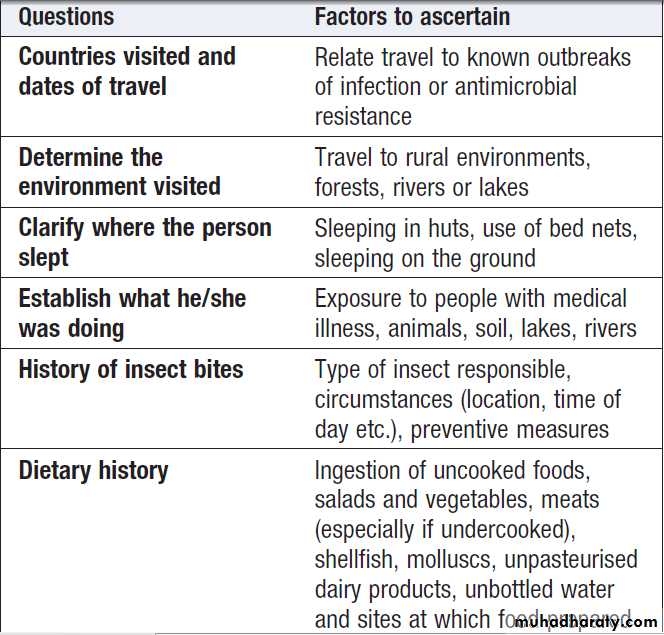
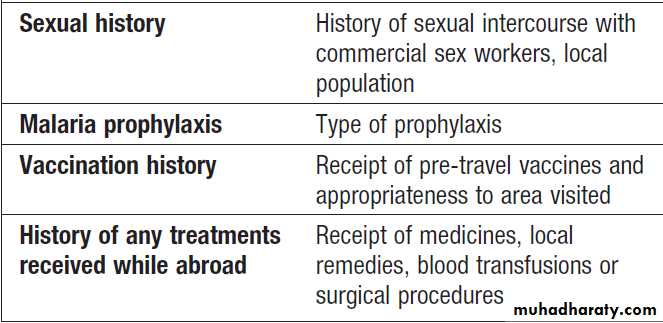
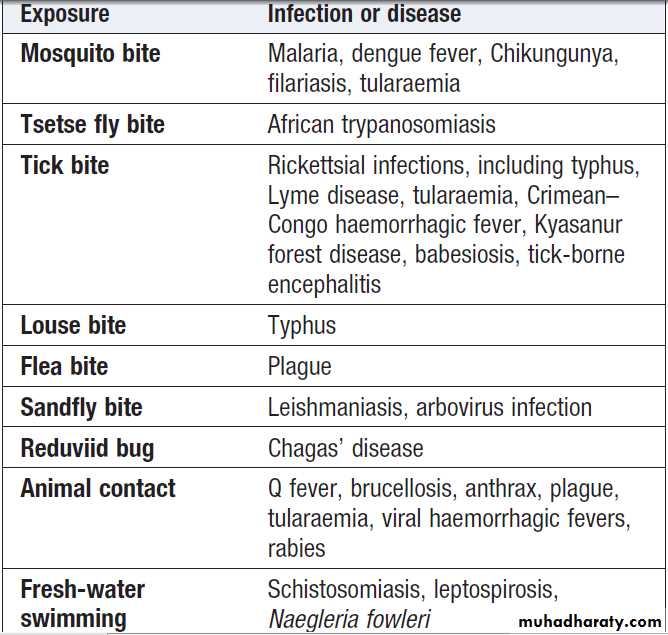
Specific exposures and causes of fever in the tropics
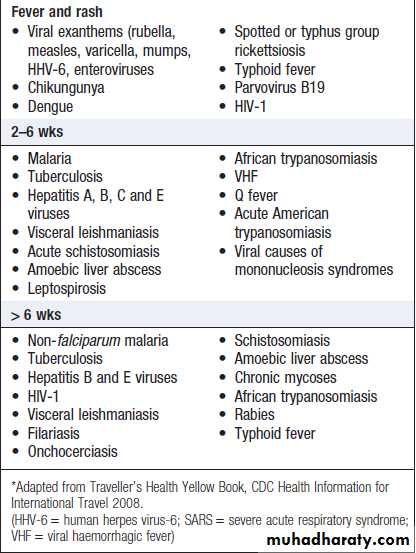
Incubation times and illnesses in travellers*
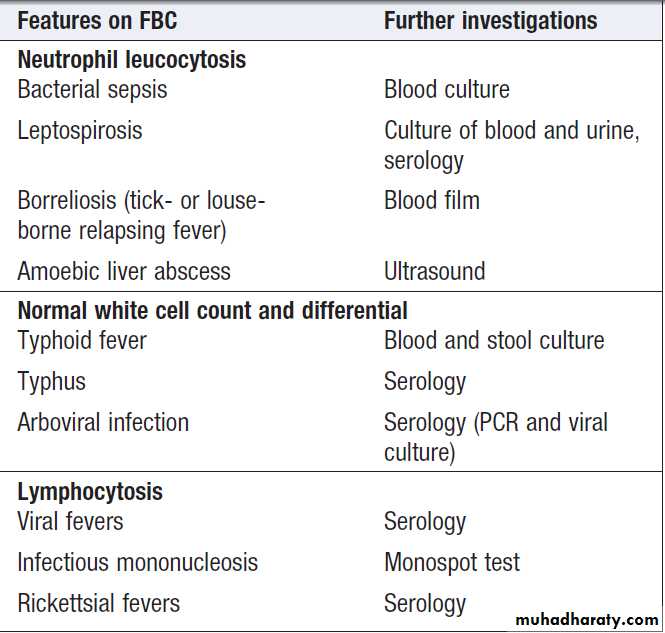
Investigation of tropically acquired
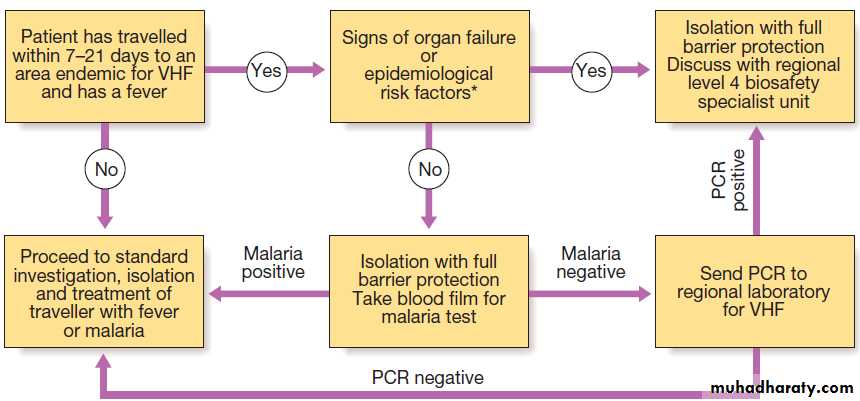
acute fever without localising signsApproach to the patient with suspected viral haemorrhagic fever (VHF). *Epidemiological risk factors: staying with a
febrile individual, caring for a sick individual, or contact with body fluids from a suspected human or animal case of VHF. (PCR = polymerase chain reaction)
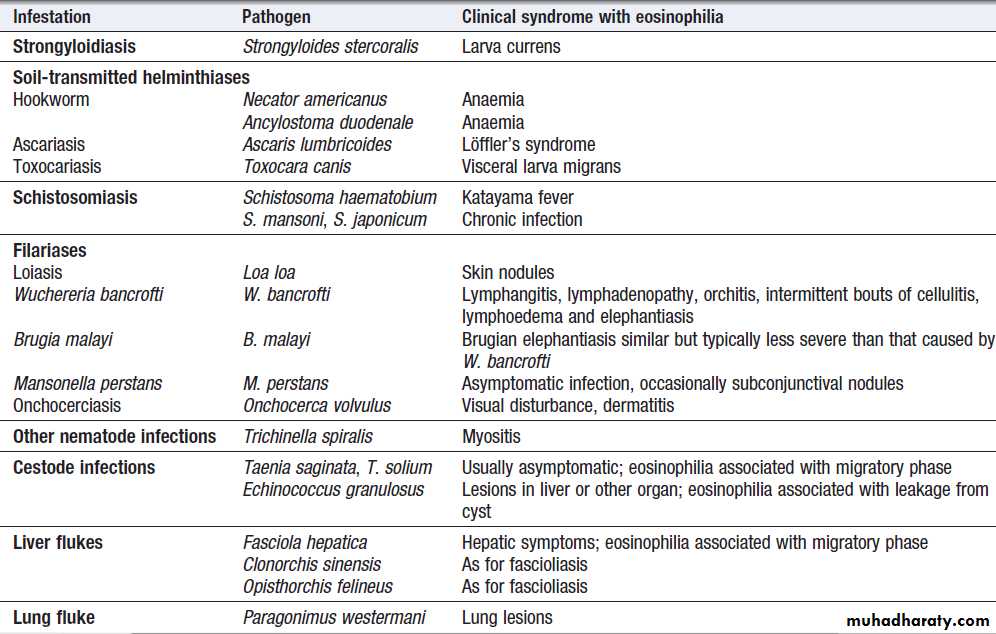
Parasite infections that cause eosinophilia
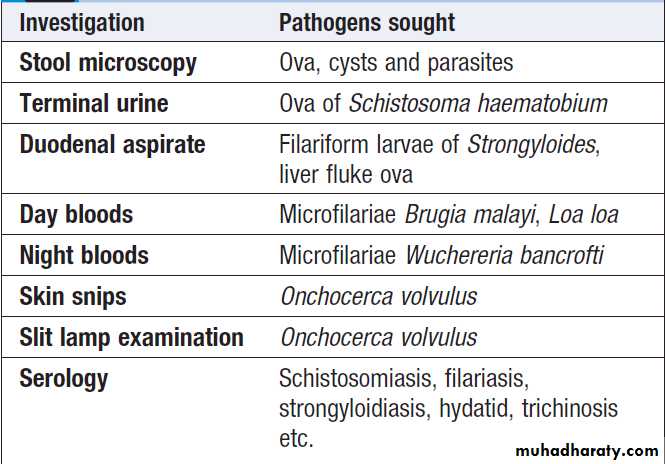
Initial investigation of eosinophilia
Key issues in infectious diseases in
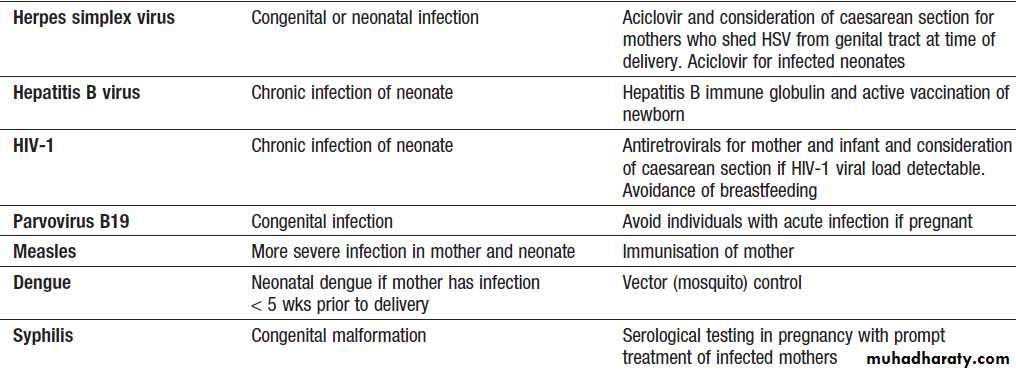
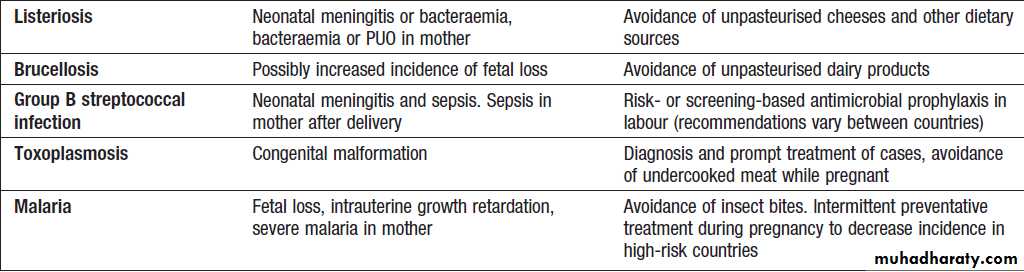
adolescenceInfections during pregnancy
Infections during pregnancy'cont'd
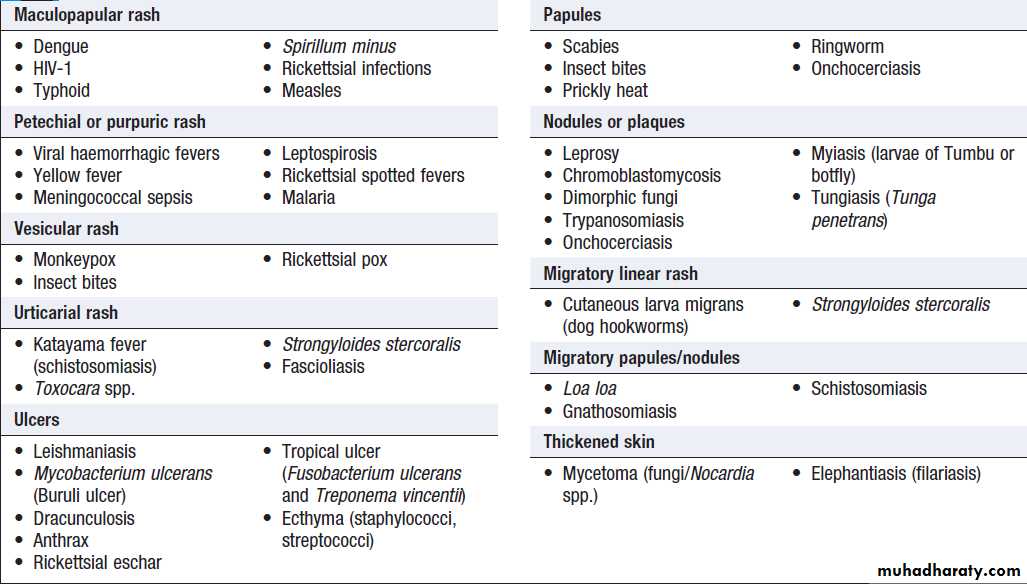
Rash in tropical travellers/residents
Systemic viral infections with exanthemMaternal antibody gives protection for the first 6–12 months of life
Measles
The WHO has set the objective of eradicating measles
globally using the live attenuated vaccine. However, vaccination of > 95% of the population is required to prevent outbreaks. Natural illness produces
life-long immunity.
Clinical features
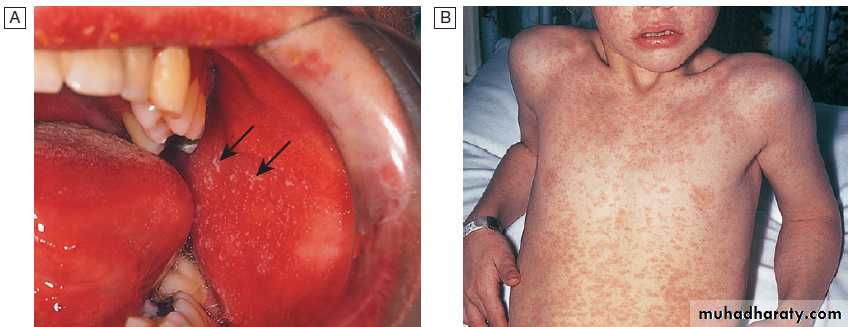
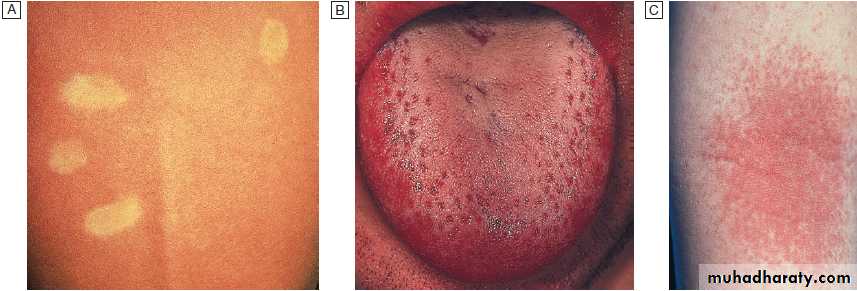
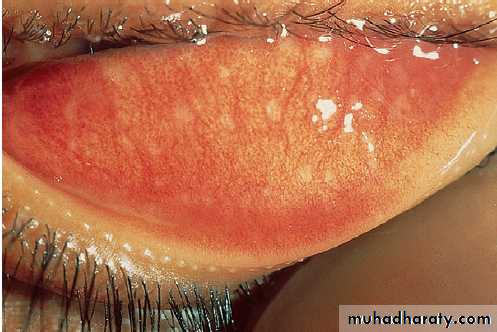
Infection is by respiratory droplets , incubation period of 6–19 days. A prodromal illness, 1–3 days before the rash, occurs, with upper respiratory symptoms, conjunctivitis and the presence of the pathognomonic Koplik’s spots, small white spots surrounded by erythema on the buccal mucosa .
As natural antibody develops, the maculopapular rash
appears, spreading from the face to the extremities. Generalised lymphadenopathy and diarrhoeaare common. Complications are more common in older
children and adults, and include otitis media, bacterial
pneumonia, transient hepatitis and clinical encephalitis
(approximately 0.1% of cases). A rare late complication is subacute sclerosing panencephalitis (SSPE), which occurs up to 7 years after infection. Diagnosis is clinical (although this has become unreliable in areas where measles is no longer common) and by detection of antibody (serum IgM, seroconversion or salivary IgM). Measles is a serious in the malnourished, immunocompromised, vitamin-deficient or in whom the typical rash may be missing.
In tuberculosis infection, measles suppresses cellmediated immunity and may exacerbate disease; for this reason, measles vaccination should be deferred until
after commencing antituberculous treatment.
Measles does not cause congenital malformation but may be more severe in pregnant women. Mortality clusters at the extremes of age, averaging 1 : 1000 in developed countries and up to 1 : 4 in developing countries, usually results from a bacterial superinfection, most often pneumonia, diarrhoeal disease or noma/cancrum oris, a gangrenous stomatitis and encephalitis.
Management and prevention
Normal immunoglobulin attenuates the disease in theimmunocompromised (regardless of vaccination status)
and in non-immune pregnant women, but must be given
within 6 days of exposure. Vaccination can be used in
outbreaks and vitamin A may improve the outcome in
uncomplicated disease. Antibiotic therapy is reserved for
bacterial complications. All children aged 12–15 months
should receive measles vaccination (as combined
measles, mumps and rubella (MMR), a live attenuated
vaccine), and a further MMR dose at age 4 years.
Measles.
A Koplik’s spots (arrows) seen on buccal mucosa in the early stages of clinical measles.B Typical measles rash.
Rubella (German measles)
Rubella causes exanthem in the non-immunised.
Clinical features
Rubella is spread by respiratory droplet, with infectivity
from up to 10 days before to 2 weeks after the onset of
the rash. The incubation period is 15–20 days. In childhood, most cases are subclinical, although clinical features may include fever, maculopapular rash spreading
from the face, and lymphadenopathy.
Complications are rare but include thrombocytopenia and hepatitis.
Encephalitis and haemorrhage are occasionally reported.
In adults, arthritis involving hands or knees is relatively
common, especially in women.
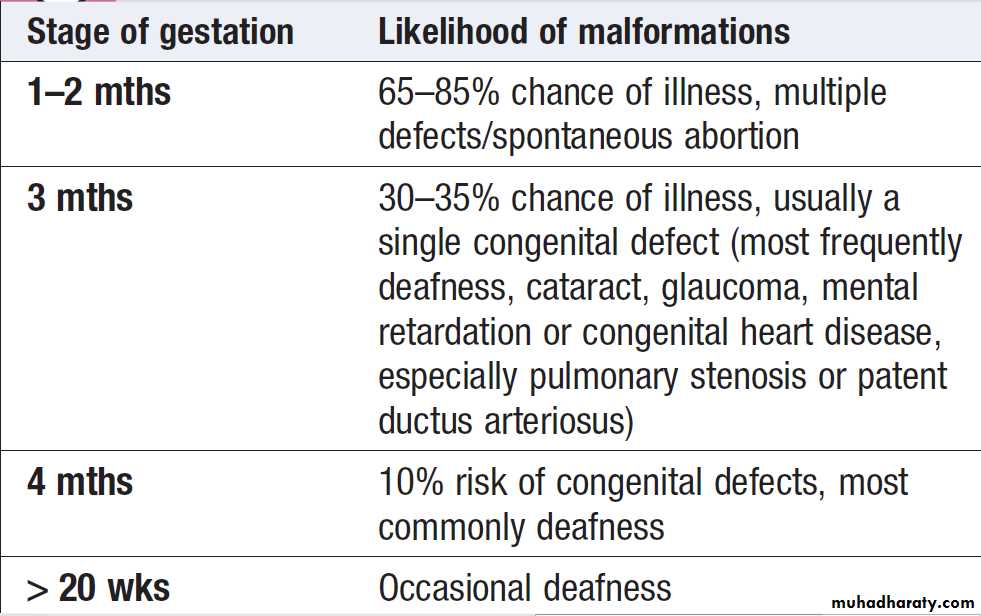
If transplacental infection takes place in the first trimester
or later, persistence of the virus is likely and severe congenital disease may result . Even if normal at birth, the infant has an increased incidence of other diseases developing later, e.g. diabetes mellitus. DiagnosisLaboratory confirmation is required if there has been contact with a pregnant woman. This is achieved either by detection of rubella IgM in serum or by IgG seroconversion. In the exposed pregnant woman, absence of rubella-specific IgG confirms the potential for congenital infection.
Prevention
All children should be immunised with MMR. In view of the risks of congenital rubella syndrome, all women of child-bearing age should also be tested for rubella and vaccinated if seronegative.
Rubella infection: risk of congenital malformation
Human herpesvirus 6 and 7
A lymphotropic virus that causes a childhood viral exanthem (exanthema subitum), rare cases of an infectious mononucleosis-like syndrome. Infection is almost universal.Transmission is via saliva.
Clinical features
Also known as roseola infantum or sixth disease . A high fever is followed by a maculopapular rash as the fever resolves. Fever and/or febrile convulsions may also occur without a rash. Rarely, older children or adults may develop an infectious mononucleosis-like illness, hepatitis or rash. In the immunocompromised, can cause fever, rash, hepatitis, pneumonitis, cytopenia or encephalitis.
Diagnosis and management
Exanthem subitum is usually a clinical diagnosis but canbe confirmed by antibody and/or DNA detection. The
disease is self-limiting.
Treatment with ganciclovir or foscarnet is used in immunocompromised hosts infected with HHV-6.
Herpesvirus hominis (herpes simplex, HSV)
HSV-1• Herpes labialis (‘cold sores’)
• Stomatitis, pharyngitis
• Corneal ulceration
• Finger infections (‘whitlows’)
• Eczema herpeticum
• Encephalitis
HSV-2
• Genital ulceration and neonatal infection (acquired during vaginal delivery)
• Acute meningitis or transverse myelitis.
• Rarely, encephalitis
Herpesvirus infections
Varicella zoster virus (VZV)
• Chickenpox (varicella)
• Shingles (herpes zoster)
Cytomegalovirus (CMV)
• Congenital infection
• Infectious mononucleosis (heterophileantibody-negative)
• Hepatitis
• Disease in immunocompromised patients: retinitis, encephalitis, pneumonitis, hepatitis, enteritis
• Fever with abnormalities in haematological parameters
Herpesvirus infections 'cont'd
Epstein–Barr virus (EBV)
• Infectious mononucleosis• Burkitt’s and other lymphomas
• Nasopharyngeal carcinoma
• Oral hairy leucoplakia (AIDS patients)
• Other lymphomas
Human herpesvirus 6 and 7 (HHV-6, HHV-7)
• Exanthem subitum
• Disease in immunocompromised patients
Human herpesvirus 8 (HHV-8)
• Kaposi’s sarcoma, primary effusion lymphoma,
• multicentric Castleman’s disease
Herpesvirus infections 'cont'd
Chickenpox (varicella) (VZV)
A dermotropic and neurotropic virus. VZV is spread by aerosol and direct contact. It is highly infectious to non-immune individuals. Manifestations are more severe in adults, pregnant women and the immunocompromised.
Clinical features
The IP 11–20 days, after which a vesicular eruption begins , often on mucosal surfaces first, followed by rapid dissemination in a centripetal distribution (most dense on trunk and sparse on limbs). New lesions occur every 2–4 days and each crop is associated with fever. The rash progresses from small pink macules to vesicles and pustules within
24 hours. Infectivity lasts from up to 4 days (usually 48 hours) before the lesions until the last vesicles crust over.
Due to intense itching, secondary bacterial infection from scratching is the most common complication . Self-limiting cerebellar ataxia and encephalitis are rare. Adults, pregnant and the immunocompromised are at increased risk of visceral involvement, pneumonitis, hepatitis or encephalitis. Maternal infection in early pregnancy carries a 3% risk of neonatal damage with abnormalities of eyes, CNS and limbs. Chickenpox within 5 days of delivery leads to severe neonatal varicella with visceral involvement and haemorrhage.
Diagnosis
primarily clinical. If necessary, this can be confirmed by detection of antigen (direct immunofluorescence) or DNA (PCR) of aspirated vesicular fluid. Serology is used to identify seronegative individuals at risk of infection.
Management and prevention
The benefits of antivirals for uncomplicated primaryVZV infection in children are marginal and treatment is
not required . Antivirals are, however, used
for uncomplicated chickenpox when the patient presents
within 24–48 hours of onset of vesicles, in all patients
with complications, and in those who are immunocompromised, including pregnant women, regardless of duration of vesicles . Human VZ immunoglobulin (VZIG) is used to attenuate infection in people who have had significant contact with VZV, are susceptible to infection (i.e. have no history of chickenpox or shingles and are seronegative for VZV IgG) and are at risk of severe disease (e.g. immunocompromised, steroid-treated or pregnant) .
Ideally, VZIG should be given within 7 days
of exposure, but it may attenuate disease even if givenup to 10 days afterwards. Susceptible contacts who
develop severe chickenpox after receiving VZIG should
be treated with aciclovir.
A live, attenuated VZV vaccine is available. Children receive one dose after 1 year of age and a second dose at 4–6 years of age; seronegative adults receive two doses at least 1 month apart.
The vaccine may also be used prior to planned
iatrogenic immunosuppression, e.g. before transplant.
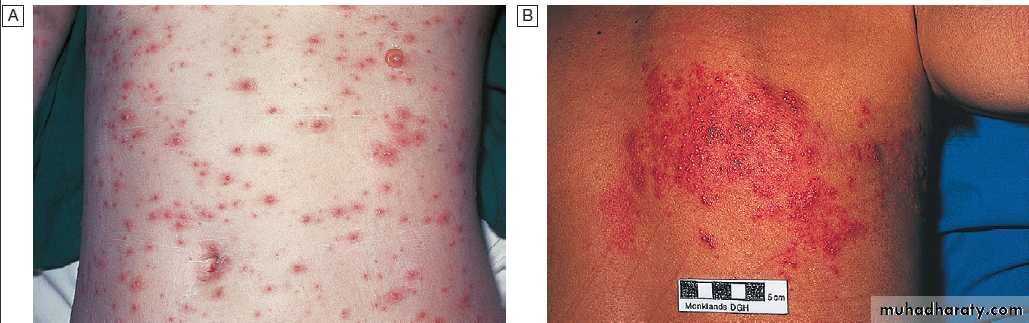
Varicella zoster virus infection.
A Chickenpox.B Shingles in a thoracic dermatome.
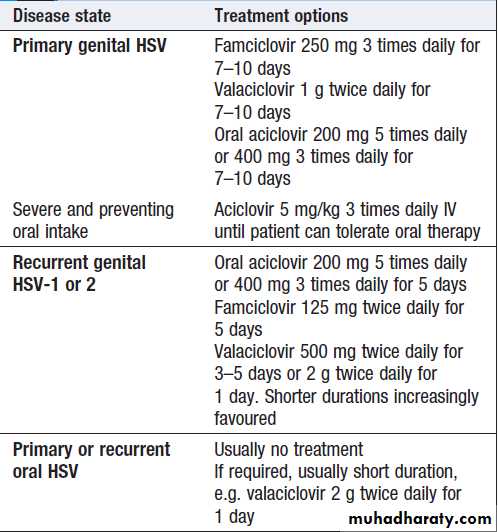
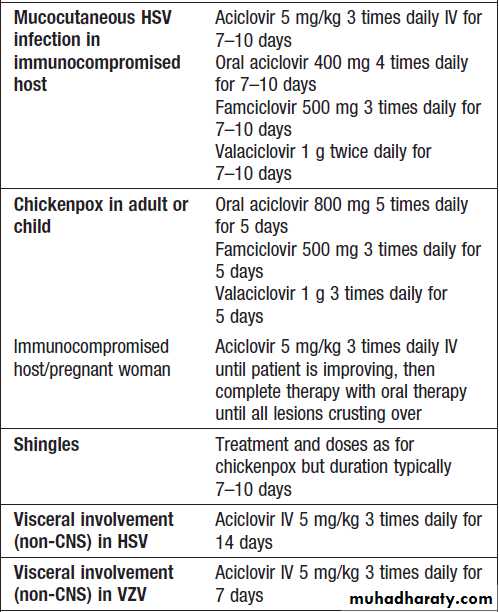
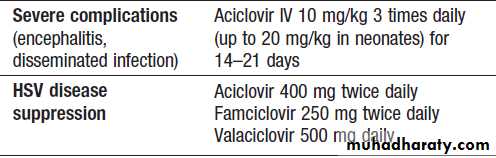
Therapy for herpes simplex and varicella zoster virus infection
Therapy for herpes simplex and varicella zoster virus infection 'cont'd
Indications for varicella zoster
immunoglobulin (VZIG) in adultsShingles (herpes zoster)
After initial infection, VZV persists in latent form in thedorsal root ganglion of sensory nerves and can reactivate
in later life.
Clinical features
Burning discomfort occurs in the affected dermatome,
where discrete vesicles appear 3–4 days later. This is associated with a brief viraemia, which can produce distant
satellite ‘chickenpox’ lesions. Occasionally, paraesthesia
occurs without rash (‘zoster sine herpete’). Severe disease,
a prolonged duration of rash, multiple dermatomal
involvement or recurrence suggests underlying
Immune deficiency, including HIV. Chickenpox may be contracted from a case of shingles but not vice versa.
Although thoracic dermatomes are most commonly
involved , the ophthalmic division of the trigeminal nerve is also frequently affected; vesicles may appear on the cornea and lead to ulceration. This condition can lead to blindness. Geniculate ganglion involvement causes the Ramsay Hunt syndrome of facial palsy, ipsilateral loss of taste and buccal ulceration, plus a rash in the external auditory canal. This may be mistaken for Bell’s palsy.
Bowel and bladder dysfunction occur with sacral nerve root involvement. The virus occasionally causes cranial nerve palsy, myelitis or encephalitis, cerebral angiitis leads to a stroke-like syndrome in association with shingles, especially in an ophthalmic distribution.
Post-herpetic neuralgia causes troublesome persistence of pain for 1–6 months or longer, following healing of the rash. It is more with advanced age.
Management
Early therapy with aciclovir or related agents has been
shown to reduce both early- and late-onset pain, especially in patients over 65 years . Postherpetic
neuralgia requires aggressive analgesia, along
with agents such as amitriptyline 25–100 mg daily or
gabapentin (commencing at 300 mg daily and building
slowly to 300 mg twice daily or more). Capsaicin cream
(0.075%) may be helpful. Although controversial,
corticosteroids have not been demonstrated to reduce
post-herpetic neuralgia to date.
Enteroviral exanthems
Coxsackie or echovirus infections can lead to a maculopapular eruption or roseola-like rash.Systemic viral infections without exanthem
Other systemic viral infections present with features
other than a rash suggestive of exanthem. Rashes may
occur but differ from exanthems or not primary feature.
Mumps
Mumps is a systemic viral infection characterised by
swelling of the parotid glands. Vaccination has reduced the incidence in children but incomplete coverage and waning immunity with time have led to outbreaks in young adults. Infection is spread by respiratory droplets.
Clinical features
The incubation period ,15–24 days. Classical tender parotid enlargement, which is bilateral in 75%, follows a prodrome of pyrexia and headache . Meningitis complicates up to 10% of cases. The CSF reveals a lymphocytic pleocytosis or, less commonly, neutrophils. Rare complications include encephalitis, transient hearing loss, labyrinthitis, ECG abnormalities, pancreatitis and arthritis.
Approximately 25% of post-pubertal males with mumps develop epididymo-orchitis but, although testicular atrophy occurs, sterility is unlikely. Oophoritis is less common. Abortion may occur if infection takes place in the first trimester of pregnancy.
Complications may occur in the absence of parotitis.
Diagnosis
The diagnosis is usually clinical. In atypical presentationswithout parotitis, serology for mumps-specific IgM
or IgG seroconversion (four-fold rise in IgG convalescent
titre) confirms the diagnosis. Virus can also be cultured
from urine in the first week of infection or detected
by PCR in urine, saliva or CSF.
Management and prevention
Treatment is with analgesia. There is no evidence that
corticosteroids are of value for orchitis. Mumps vaccine
is one of the components of the combined MMR vaccine.
Influenza
Acute systemic viral infection that primarily affects the respiratory tract. It is caused by influenza A virus or, in milder form, influenza B virus. Infection is seasonal, and variation in the haemagglutinin (H) and neuraminidase (N) glycoproteins on the surface of the virus leads to disease of variable intensity each year. Minor changes in haemagglutinin are known as ‘genetic drift’, whereas a switch in the haemagglutinin or neuraminidase antigen is termed ‘genetic shift’. Nomenclature of influenza strains is based on these glycoproteins, e.g. H1N1, H3N2 etc.Genetic shift results in the circulation of a new influenza
strain within a community to which few people are
immune, potentially initiating an influenza epidemic or
pandemic and there may be increased disease severity.
Clinical features
After an incubation period of 1–3 days, uncomplicated
leads to fever, malaise and cough. Viral pneumonia may occur, although pulmonary complications are most often due to superinfection with Strep. pneumoniae, Staph. aureus or other bacteria. Rare extrapulmonary manifestations include myositis, myocarditis, pericarditis and neurological complications (Reye’s syndrome in children, encephalitis or transverse myelitis).
Mortality is greatest in the elderly, those with medical
comorbidities and pregnant women. Recently, polymorphisms in the gene encoding an antiviral protein,
interferon-induced transmembrane protein 3 (IFITM3),
have been associated with more severe influenza.
Diagnosis
Acute infection is diagnosed by viral antigen or RNAdetection in a nasopharyngeal sample. The disease may
also be diagnosed retrospectively by serology.
Management and prevention
Management involves early microbiological identification
of cases and good infection control, with an emphasis
on hand hygiene and preventing dissemination of
infection by coughing and sneezing. Administration of
neuraminidase inhibitor, oral oseltamivir (75 mg twice
daily) or inhaled zanamivir (10 mg twice daily) for
5 days, can reduce the severity of symptoms if started
within 48 hours of symptom onset (or possibly later in
immunocompromised individuals).
These agents have superseded routine use of amantadine and rimantadine. Antiviral drugs can also be used as prophylaxis in highrisk individuals during the ‘flu’ season. Resistance can emerge to all of these agents and so updated local advice should be followed.
Prevention relies on seasonal vaccination of the
elderly and of individuals with chronic medical illnesses
which place them at increased risk of the complications
of influenza, such as chronic cardiopulmonary diseases
or immune compromise, as well as their health-care
workers. The vaccine composition changes each year to
cover the ‘predicted’ seasonal strains but vaccination
may fail when a new pandemic strain emerges.
Avian influenza
Avian influenza is caused by transmission of avian influenzaA viruses to humans. Avian viruses, such as H5N1,
possess alternative haemagglutinin antigens to seasonal
influenza strains. Most cases have had contact with sick
poultry, predominantly in South-east Asia, and personto-
person spread has been limited to date. Infections
with H5N1 viruses have been severe, with enteric features and respiratory failure. Treatment depends on
the resistance pattern but often involves oseltamivir.
Vaccination against seasonal ‘flu’ does not adequately
protect against avian influenza. There is a concern that
adaptation of an avian strain to allow effective person to-person transmission is likely to lead to a global pandemic of life-threatening influenza.
Infectious mononucleosis and Epstein–Barr virus (IM)
A clinical syndrome characterised by pharyngitis, cervical lymphadenopathy, fever and lymphocytosis. It is most often caused by Epstein–Barr virus (EBV) but other infections can
produce a similar clinical syndrome . EBV is a gamma herpesvirus. In developing countries, subclinical infection in childhood is virtually universal. In developed countries, primary infection may be delayed until adolescence or early adult life. Under these circumstances, about 50% of infections result in typical IM. The virus is usually acquired from asymptomatic excreters via saliva, either by droplet infection or environmental contamination in childhood, or by kissing among adolescents and adults. EBV is not highly contagious and isolation of cases is unnecessary.
Causes of infectious mononucleosis Syndrome
• Epstein–Barr virus infection• Cytomegalovirus
• Human herpesvirus-6 or 7
• HIV-1 primary infection
• Toxoplasmosis
Clinical features
EBV infection has a prolonged and undetermined incubation period, followed in some cases by a prodrome of fever, headache and malaise. This is succeeded by IMwith severe pharyngitis, which may include tonsillar
exudates and non-tender anterior and posterior
cervical lymphadenopathy. Palatal petechiae, periorbital
oedema, splenomegaly, inguinal or axillary lymphadenopathy, and macular, petechial or erythema multiforme rashes may occur.
In most cases, fever resolves over 2 weeks, and fatigue and other abnormalities settle over a further few weeks.
Death is rare but can occur due to respiratory
obstruction, haemorrhage from splenic rupture orthrombocytopenia, or encephalitis.
The diagnosis of EBV infection outside the usual age
in adolescence and young adulthood is more challenging.
In children under 10 years the illness is mild and
short-lived, but in adults over 30 years of age it can be
severe and prolonged. In both groups, pharyngeal
symptoms are often absent. EBV may present with jaundice,
as a PUO or with a complication.
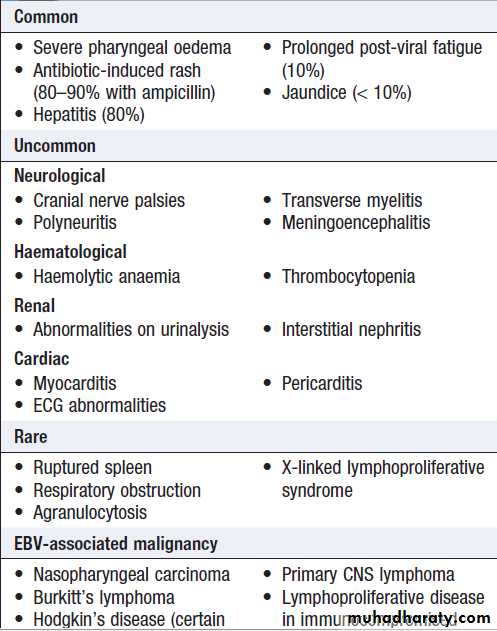
Complications of Epstein–Barr virus
infectionLong-term complications of EBV infection
Lymphoma complicates EBV infection in immunocompromised hosts, and some forms of Hodgkin’s disease are EBV-associated .The endemic form ofBurkitt’s lymphoma complicates EBV infection in areas
of sub-Saharan Africa where falciparum malaria is
endemic. Nasopharyngeal carcinoma is a geographically
restricted tumour seen in China and Alaska that is associated with EBV infection. X-linked lymphoproliferative
(Duncan’s) syndrome is a familial lymphoproliferative
disorder that follows primary EBV infection in boys
without any other history of immunodeficiency
; it is due to mutation of the SAP gene, causing failure of T-cell and NK-cell activation and inability to contain EBV infection.
Investigations
Atypical lymphocytes are common but also occur in other causes of IM, HIV infection, viral hepatitis, mumps andrubella . A ‘heterophile’ antibody is present during the acute illness and convalescence, which is detected by the Paul–Bunnell or ‘Monospot’ test. However, many children
and 10% of adolescents with IM do not produce
heterophile antibody at any stage.
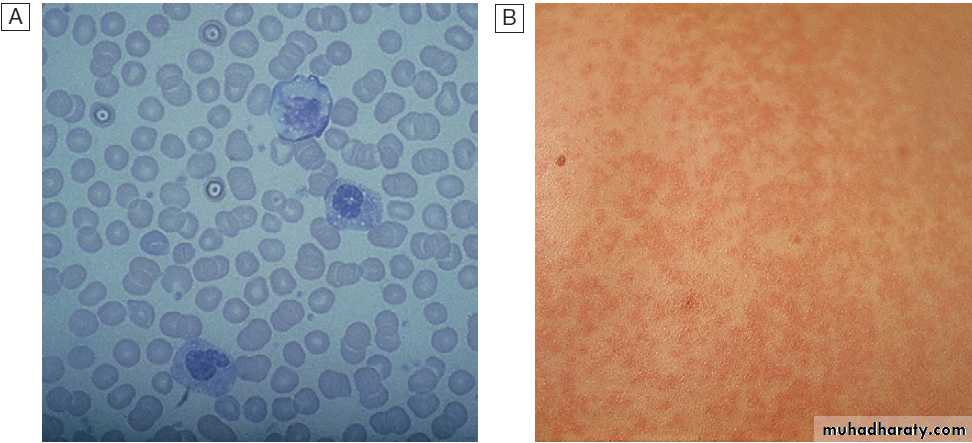
A Atypical lymphocytes in peripheral blood.
B Skin reaction to ampicillin.
Management
Treatment is largely symptomatic. If a throat cultureyields a β-haemolytic streptococcus, penicillin should be
given. Administration of ampicillin or amoxicillin in this
condition commonly causes an itchy macular rash and
should be avoided . When pharyngeal oedema is severe, a short course of corticosteroids, e.g.prednisolone 30 mg daily for 5 days.
Current antiviral drugs are not active against EBV.
Return to work or school is governed by physical
fitness rather than laboratory tests; contact sports should
be avoided until splenomegaly has resolved because of
the danger of splenic rupture. Unfortunately, about 10%
of patients with IM suffer a chronic relapsing syndrome.
Cytomegalovirus (CMV)
CMV like EBV, circulates readily among children. A second period among teenagers and young adults. Most childhood infections are acquired from asymptomatic excreters who shed virus in saliva, urine, semen and genital secretions. Sexual transmission and oral spread are common among adults, but infection may also be acquired by women caring for children with asymptomatic infections.Clinical features
Most infections are subclinical, some young develop an IM-like syndrome and some have a prolonged influenza-like illness. Physical signs resemble those of IM, but in CMV infections hepatomegaly is more common, while lymphadenopathy, splenomegaly, pharyngitis and tonsillitis occur less often. Jaundice is uncommon and usually mild.
Complications include meningoencephalitis, Guillain–Barré syndrome, autoimmune haemolytic anaemia, thrombocytopenia, myocarditis and skin eruptions, such as ampicillin-induced rash. Immunocompromised patients can develop hepatitis, oesophagitis, colitis, pneumonitis, retinitis, encephalitis and polyradiculitis. A primary CMV infection during pregnancy have about a 40% chance of passing CMV to the fetus, causing congenital infection and
disease at any stage of gestation. Features include
petechial rashes, hepatosplenomegaly and jaundice;
10% of infected infants will have long-term CNS sequelae,
such as microcephaly, cerebral calcifications, chorioretinitis
and deafness. Infections in the newborn usually
are asymptomatic or have features of an IM-like illness.
Investigations
Atypical lymphocytosis is not as prominent as in EBVinfection and heterophile antibody tests are negative.
LFTs are often abnormal, with an alkaline phosphatase
level raised out of proportion to transaminases. Serological
diagnosis depends on the detection of CMV-specific
IgM antibody plus a four-fold rise or seroconversion of
IgG. In the immunocompromised, antibody detection is
unreliable and detection of CMV in an involved organ
by PCR, culture or histopathology establishes the diagnosis. A positive culture of CMV in the blood may be
useful in transplant populations. Detection of CMV
in urine is not helpful in diagnosing infection, except in
neonates, since CMV is intermittently shed in the urine
throughout life following infection.
Management
Symptomatic treatment is required in the immunocompetent patient. Immunocompromised individuals are treated with ganciclovir 5 mg/kg IV twice daily or with oral valganciclovir 900 mg twice daily for at least 14 days.
Foscarnet or cidofovir is also used in CMV treatment
of immunocompromised patients who are resistant
to or intolerant of ganciclovir-based therapy.
Dengue
Dengue is a febrile illness caused by a flavivirus transmitted by mosquitoes. It is endemic in Asia, the Pacific,Africa and the Americas . Approximately 50 million infections occur annually and dengue is the most rapidly spreading mosquito-borne viral illness. The principal vector is the mosquito Aedes aegypti. There are four serotypes of dengue virus, all producing a similar clinical syndrome; type-specific immunity is life-long, but immunity against the other serotypes lasts only a few months.
Dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS) occur in individuals who are immune to one virus serotype and are then infected with another.
Prior immunity results in increased uptake of virus by cells expressing the antibody Fc receptor and increased T-cell activation with resultant cytokine release, causing capillary leak and disseminated intravascular coagulation .
Clinical features
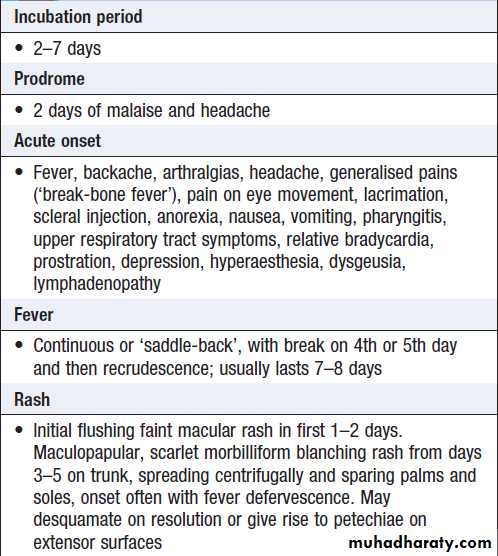
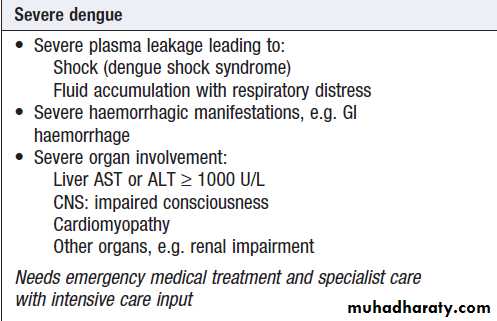
Are listed in Box . Asymptomatic infections are common, particularly in children, but the disease is more severe in infants and the elderly. The initial febrile phase is frequently followed by a rash as the fever settles. Laboratory features include leucopenia, neutropenia, thrombocytopenia and elevated ALT or AST. The period 3–7 days after onset of fever is termed the ‘critical’ phase, during which signs of DHF or DSS may develop.
In mild forms, petechiae occur in the arm when a blood pressure cuff is inflated to a point between systolic
and diastolic blood pressure and left for 5 minutes
(the positive ‘tourniquet test’) – a non-specific test of
capillary fragility and thrombocytopenia. As the extent
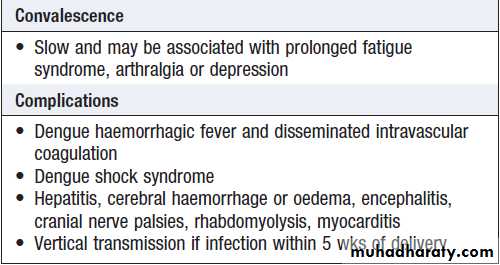
of capillary leak increases, there may be a raised haematocrit, tachycardia and hypotension, pleural effusions and ascites. This may progress to metabolic acidosis and multi-organ failure, including acute respiratory distress syndrome . Minor (petechiae, ecchymoses, epistaxis) or major (gastrointestinal or cerebrovascular) haemorrhage may occur.
Clinical features of dengue fever
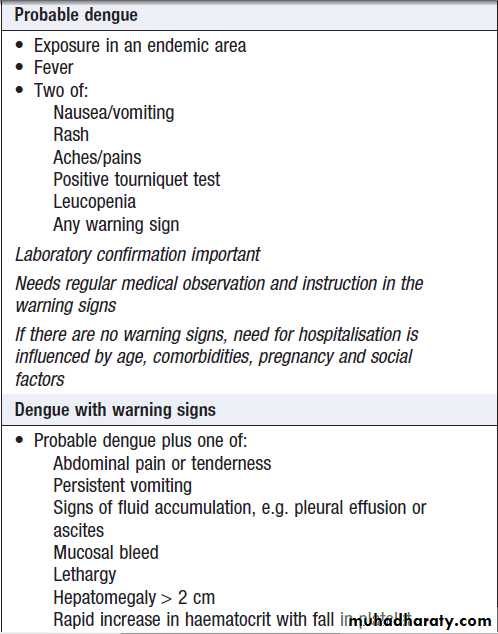
DiagnosisIn endemic areas, mild dengue must be distinguished
from other viral infections. The WHO recently revised
its clinical classification of dengue and is evaluating the
usefulness of these categories in guiding diagnosis and
treatment . The diagnosis can be confirmed
by seroconversion of IgM or a fourfold rise in IgG antibody
titres. Serological tests may detect cross-reacting
antibodies against other flaviviruses, including yellow
fever vaccine. IgM/IgG ratios may be used to distinguish
primary from secondary infection. Isolation of
dengue virus from blood or detection of dengue virus
RNA by PCR is available in specialist laboratories.
Commercial enzyme-linked immunosorbent assay (ELISA) kits to detect the NS1 viral antigen, although less sensitive than PCR, are becoming more widely available in endemic areas .
WHO-proposed clinical definition of dengue
Management and preventionTreatment is supportive, emphasising fluid replacement
and appropriate management of shock and organ dysfunction.
With intensive care support, mortality rates are
1% or less. Aspirin should be avoided due to bleeding
risk. Corticosteroids have not been shown to help.
No existing antivirals are effective.
Breeding places of Aedes mosquitoes should be abolished
and the adults destroyed by insecticides. There is
no licensed vaccine available .
Yellow fever
Yellow fever is a haemorrhagic fever of the tropics,caused by a flavivirus. It is a zoonosis of monkeys in
West and Central African, and South and Central American
tropical rainforests, where it may cause devastating
epidemics . Transmission is by tree-top mosquitoes Aedes africanus (Africa) and Haemagogus spp. (America). The infection is introduced to humans either by infected mosquitoes when trees are felled, or by monkeys raiding human settlements. In towns, yellow fever may be transmitted between humans by Aedes aegypti, which breeds efficiently in small collections of water. Overall mortality is around 15%.Humans are infectious during the
viraemic phase, which starts 3–6 days after the bite of
the infected mosquito and lasts for 4–5 days.
Clinical features
After an incubation period of 3–6 days, yellow fever is
often a mild febrile illness lasting less than 1 week, with
headache, myalgia, conjunctival erythema and bradycardia.
This is followed by fever resolution (defervescence),
but in some cases, fever recurs after a few hours
to days. In more severe disease, fever recrudescence
is associated with lower back pain, abdominal pain
and somnolence, prominent nausea and vomiting,
bradycardia and jaundice. Liver damage and DIC lead
to bleeding with petechiae, mucosal haemorrhages and
gastrointestinal bleeding. Shock, hepatic failure, renal
failure, seizures and coma may ensue.
Diagnosis
The differential diagnosis includes malaria, typhoid,viral hepatitis, leptospirosis, haemorrhagic fevers and
aflatoxin poisoning. Diagnosis confirmed by viral isolation from blood in the first 24 days of illness, the presence of IgM or a fourfold rise in IgG antibody titre. Leucopenia is characteristic. Liver biopsy should be avoided in life due to the risk of fatal bleeding.
Immunohistochemistry for viral antigens improves specificity. Management and prevention
Supportive, with meticulous attention to fluid and electrolyte balance, urine output and blood pressure.
Blood transfusions, plasma expanders and peritoneal dialysis may be necessary.
Patients should be isolated, as their blood and body products may contain virus particles.
A single vaccination with a live attenuated vaccine gives full protection for at least 10 years. Potential side effects include hypersensitivity, encephalitis and systemic features of yellow fever (viscerotropic disease) caused by the attenuated virus. Vaccination is not recommended in people who are significantly immunosuppressed. The risk of vaccine side-effects must be balanced against the risk of infection for less immunocompromised hosts, pregnant women and older patients. An internationally recognised certificate of vaccination is sometimes necessary when crossing borders.
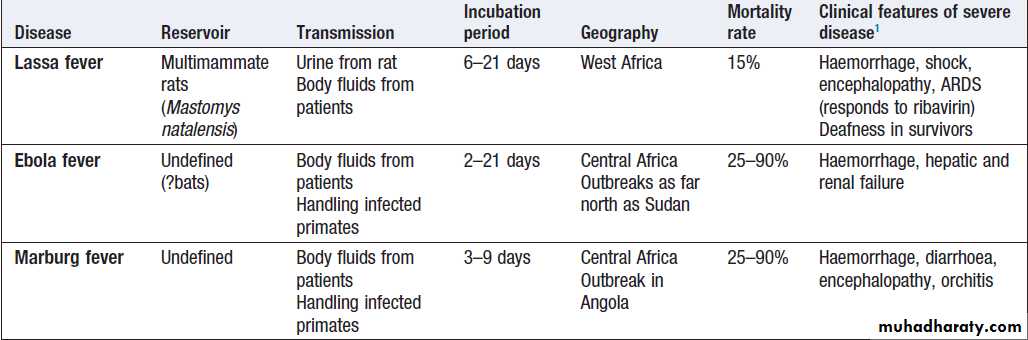
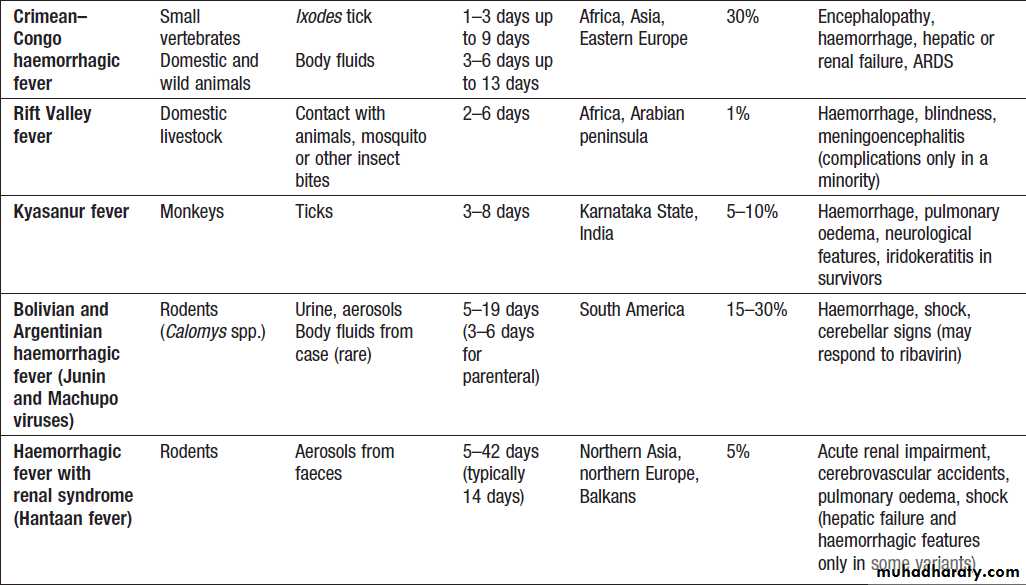
Viral haemorrhagic fevers (VHF)
zoonoses caused by several different viruses .They are geographically restricted and occur in rural settings or inhealth-care facilities. All of these viral illnesses, except
Ebola and Marburg, have mild self-limiting forms.
Mortality overall may be low, as 80% are asymptomatic, but in hospitalised cases mortality averages 15%.
Marburg has been documented less frequently, with
outbreaks in the Democratic Republic of Congo and
Uganda, but the largest outbreak to date involved 163
cases in Angola in 2005.
Mortality rates of Ebola and Marburg are high.
Clinical features
VHF present with non-specific fever, malaise, bodypains, sore throat and headache.
On examination, conjunctivitis, throat injection, an erythematous or petechial rash, haemorrhage, lymphadenopathy and bradycardia may be noted. The viruses cause endothelial dysfunction with capillary leak.
Bleeding is due to endothelial damage and platelet
dysfunction. Hypovolaemic shock and ARDS may
develop . Haemorrhage is a late feature of VHF.
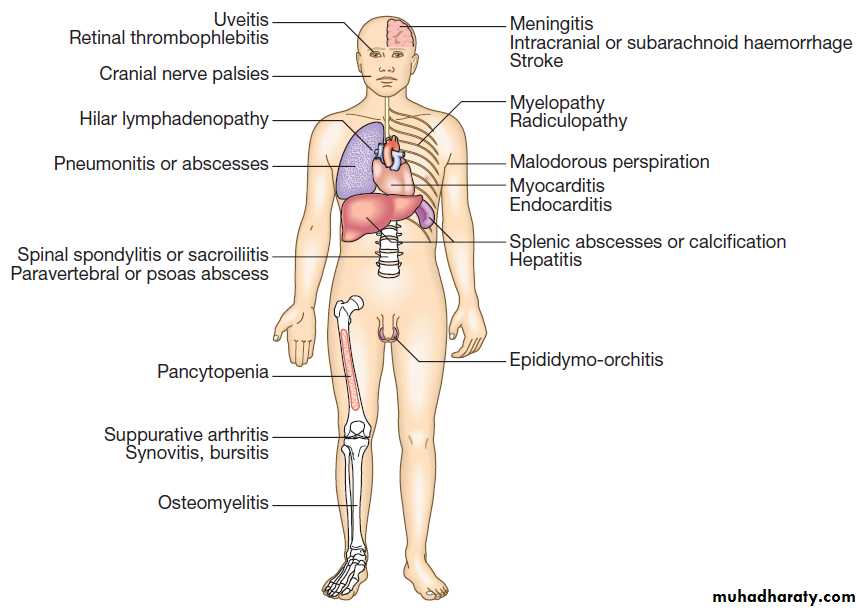
In Lassa fever, joint and abdominal pain is prominent.
A macular blanching rash may be present but bleeding is unusual, occurring in 20% of hospitalised patients. Encephalopathy may develop and deafness affects 30%.
The clue to the viral aetiology comes from the travel
and exposure history. Enquiry should be made about insect bites, hospital visits and attendance at ritual funerals (Ebola virus infection). For Lassa fever, retrosternal pain, pharyngitis and proteinuria have a positive predictive value of 80% in West Africa.
Investigations and management
Leucopenia, thrombocytopenia and proteinuria. In Lassa fever, an AST > 150 U/L is associated with a 50% mortality. It is important to exclude other causes of fever, especially malaria, typhoid and respiratory tract infections. The diagnosis of VHF must be considered in all febrile who present within 21 days of leaving an endemic area or who present with haemorrhage or organ failure.
A febrile patient from an endemic area within the incubation period, who has specific epidemiological
risk factors or who has signs of organ failure or haemorrhage, should be treated as being at high risk of VHF; appropriate infection control measures must be implemented and the patient transferred to a centre with biosafety level (BSL) 4 facilities. Individuals with a history of travel within 21 days and fever, but without the relevant epidemiological features or signs of VHF, are classified as
medium-risk and should have an initial blood sample
tested to exclude malaria. If this is negative, relevant
specimens (blood, throat swab, urine and pleural fluid,
if available) are collected and sent to an appropriate
reference laboratory for nucleic acid detection (PCR),
virus isolation, and serology.
If patients are still felt to be at significant risk of VHF or if infection is confirmed, they should be transferred to a specialised high-security infectious disease unit. All further laboratory tests should be performed at BSL4. Transport requires an ambulance with BSL3 facilities. In addition to general supportive measures, ribavirin is given intravenously (100 mg/kg, then 25 mg/kg daily for 3 days and 12.5 mg/kg daily for 4 days) when Lassa fever or South American haemorrhagic fevers are suspected.
Prevention
Ribavirin has been used as prophylaxis in close contacts
in Lassa fever but there are no formal trials of its efficacy.
Viral haemorrhagic fevers
Viral haemorrhagic fevers'cont'd
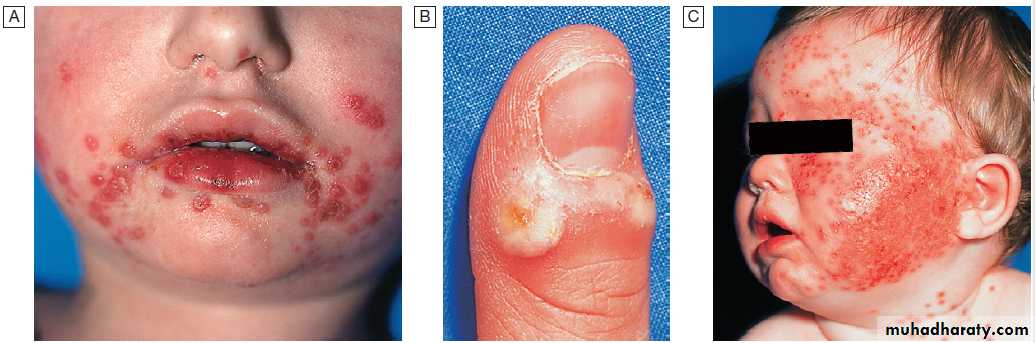
Viral infections of the skinHerpes simplex virus 1 and 2 Herpes simplex viruses (HSV) cause recurrent mucocutaneous infection; HSV-1 typically involves the mucocutaneous surfaces of the head and neck whilst HSV-2 predominantly involves the genital
mucosa , although there is overlap .
The seroprevalence of HSV-1 is 30– 100%, varying by socioeconomic status, while that of HSV-2 is 20–60%. Infection is acquired by inoculation of viruses shed by an infected individual on to a mucosal surface in a susceptible person. The virus infects sensory and autonomic neurons and establishes latent infection in the nerve ganglia.
Primary infection is followed by episodes of reactivation throughout life.
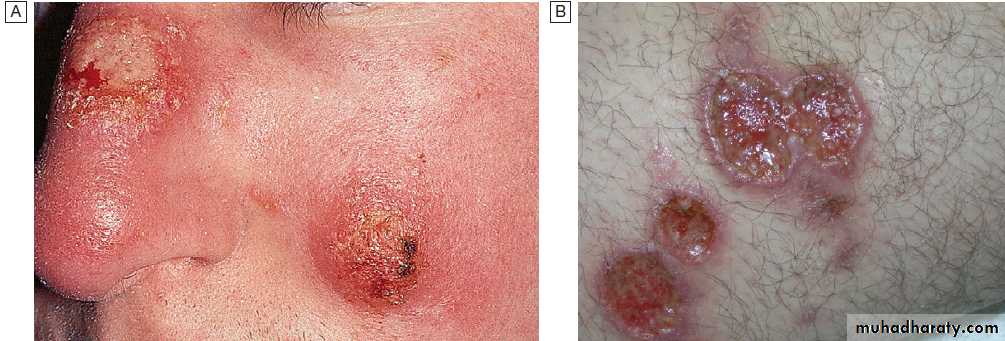
Cutaneous manifestations of herpes simplex virus-1 (HSV-1).
A Acute HSV-1. There were also vesicles in the mouth – herpetic stomatitis.
B Herpetic whitlow.
C Eczema herpeticum. HSV-1 infection spreads rapidly in eczematous skin.
Clinical features
Primary HSV-1 or 2 infection is more likely to be symptomatic later in life, causing gingivostomatitis, pharyngitis or painful genital tract lesions. The primary attack may be associated with fever and regionallymphadenopathy.
Recurrence
Recurrent attacks occur throughout life, most often in
association with concomitant medical illness, menstruation,
mechanical trauma, immunosuppression, psychological
stress or, for oral lesions, ultraviolet light exposure. HSV reactivation in the oral mucosa produces the classical
‘cold sore’ or ‘herpes labialis’. Prodromal hyperaesthesia is followed by rapid vesiculation, postulation and crusting.
Recurrent HSV genital disease is a common cause of recurrent painful ulceration . An inoculation lesion on the finger gives rise to a paronychia termed a ‘whitlow’ in contacts of patients with herpetic lesions .
It was formerly seen in health-care workers and dentists, but is prevented by protective gloves.
Complications
Disseminated cutaneous lesions can occur in individuals
with underlying dermatological diseases, such as eczema
(eczema herpeticum) . Herpes keratitis presents with pain and blurring of vision; characteristic dendritic ulcers are visible on slit-lamp examination and may produce corneal scarring and permanent visual impairment. Primary HSV-2 can cause meningitis or transverse myelitis.
HSV is the leading cause of sporadic viral encephalitis , this serious complication may occur following either primary or secondary disease, usually with HSV-1. A haemorrhagic necrotising temporal lobe cerebritis produces temporal lobe epilepsy and altered consciousness/coma. Without treatment, mortality is 80%. HSV is also implicated in the pathogenesis of Bell’s palsy with a lower motor neuron VII nerve palsy, although antivirals have not been demonstrated to improve outcome.
Neonatal HSV disease is usually associated with primary infection of the mother at term . In excess of two-thirds of cases develop disseminated disease with cutaneous lesions, hepatitis, pneumonitis and frequently encephalitis. Immunocompromised can develop visceral disease with oesophagitis, hepatitis, pneumonitis, encephalitis or retinitis.
Diagnosis
Differentiation from other vesicular eruptions is achieved by demonstration of virus in vesicular fluid, usually by direct immunofluorescence or PCR. HSV encephalitis is diagnosed by a positive PCR for HSV in CSF. Serology is of limited value, only confirming whether an individual has had previous infection.Management
The acyclic antivirals are the treatment of choice for HSV
infection . Therapy of localised disease must commence in the first 48 hours of clinical disease (primary or recurrent); thereafter it is unlikely to influence clinical outcome. Oral lesions in an immunocompetent individual may be treated with topical aciclovir.
Suspicion of HSV encephalopathy is an indication for immediate empirical antiviral therapy. Aciclovir resistance is encountered occasionally in immunocompromised hosts, in which case foscarnet is the treatment of choice.
Human herpesvirus 8
Human herpesvirus 8 (HHV-8) causes Kaposi’s sarcoma in both AIDS-related and endemic non-AIDS-related forms . HHV-8 is spread via saliva, and men who have sex with men have increased incidence of infection.
Seroprevalence varies widely, being highest in sub-Saharan Africa.
HHV-8 also causes two rare haematological malignancies: primary effusion lymphoma and multicentric Castleman’s
disease. Current antivirals are not effective.
Enterovirus infections
Hand, foot and mouth diseaseThis systemic infection is usually caused by Coxsackie
Or occasionally echoviruses. It affects children and occasionally adults, resulting in local or household
outbreaks, particularly in the summer months. A relatively
mild illness with fever and lymphadenopathy develops after an incubation period of approximately 10 days; 2–3 days later, a painful papular or vesicular rash appears on palmoplantar surfaces of hands and feet, with associated oral lesions on the buccal mucosa and tongue that ulcerate rapidly. A popular erythematous rash may appear on buttocks and thighs. Antiviral treatment is not available and management consists of symptom relief with analgesics.
Herpangina
This infection, caused by Coxsackie viruses, primarily
affects children and teenagers in the summer months. It
is characterised by a small number of vesicles at the
soft/hard palate junction, often associated with high
fever, an extremely sore throat and headache. The
lesions are short-lived, rupturing after 2–3 days and
rarely persisting for more than 1 week.
Treatment is with analgesics if required. Culture of the virus from vesicles or DNA detection by PCR differentiates herpangina from HSV.
Poxviruses
These DNA viruses are rare but potentially importantpathogens.
Smallpox (variola)
This severe disease, which has high mortality, was
eradicated worldwide by a global vaccination programme.
Interest in the disease has re-emerged due to its potential as a bioweapon. The virus is spread by the respiratory route or contact with lesions, and is highly infectious. The incubation period is 7–17 days. A prodrome with fever, headache and prostration leads, in 1–2 days, to the rash, which develops through macules and papules to vesicles and pustules, worst on the face and distal extremities.
Lesions in one area are all at the same stage
of development with no cropping (unlike chickenpox).Vaccination can lead to a modified course of disease
with milder rash and lower mortality.
If a case of smallpox is suspected, national public
health authorities must be contacted.
Electron micrography and DNA detection tests (PCR) are used to confirm smallpox or, using specific primers, an
alternative poxvirus.
Other poxviruses:
orf and molluscum contagiosum
Monkeypox
Despite the name, the animal reservoirs for this virus are
probably small squirrels and rodents. It causes a rare
zoonotic infection in communities in the rainforest belt
of Central Africa, producing a vesicular rash that is
indistinguishable from smallpox, but differentiated by
the presence of lymphadenopathy. Little person-toperson
transmission occurs. Outbreaks outside Africa
have been linked to importation of African animals as
exotic pets. Diagnosis is by electron micrography
and/or DNA detection (PCR).
Cowpox
Humans in contact with infected cows develop largevesicles, usually on the hands or arms and associated
with fever and regional lymphadenitis. The reservoir is
thought to be wild rodents, and the virus also produces
symptomatic disease in cats and a range of other animals.
Vaccinia virus
This laboratory strain is the basis of the existing vaccine to prevent smallpox. Widespread vaccination is no longer recommended due to the likelihood of local spread from the vaccination site (potentially life-threatening in those with eczema (eczema vaccinatum) or immune deficiency) and of encephalitis. However, vaccination may still be recommended for key medical staff.
Gastrointestinal viral infections
Norovirus (Norwalk agent)
Norovirus is the most common cause of infectious gastroenteritis in the UK and causes outbreaks in closed
communities, such as long-stay hospital wards, cruise
ships and military camps. Food handlers may also transmit
this virus, which is relatively resistant to decontamination
procedures. The incubation period is 24–48 hours.
High attack rates and prominent vomiting are characteristic. Diagnosis is by electron microscopy, antigen or DNA detection (PCR) in stool samples. The virus is
highly infectious and cases should be isolated and
environmental surfaces cleaned with detergents and
disinfected with bleach.
Astrovirus
Astroviruses cause diarrhoea in small children and occasionally in immunocompromised adults.Rotavirus
Rotaviruses are the major cause of diarrhoeal illness in
young children worldwide and cause 10–20% of deaths
due to gastroenteritis in developing countries. There are
winter epidemics in developed countries, particularly in
nurseries. Adults are less often infected but those in
close contact with cases may develop disease. The virus
infects enterocytes, causing decreased surface absorption.
The incubation period is 48 hours and patients
present with watery diarrhoea, vomiting, fever and
abdominal pain. Dehydration is prominent.
Diagnosis is aided by commercially available enzyme immunoassay kits, which require fresh or refrigerated stool samples. Immunity develops to natural infection. Monovalent and multivalent vaccines have been licensed in many countries and have now demonstrated efficacy in large trials in Africa and the Americas. Increased rates of
intussusception were observed with early rotavirus vaccines,
but the benefits of the recently licensed vaccines
outweigh this risk.
Hepatitis viruses (A–E)
Other viruses
Adenoviruses are frequently identified from stool culture
and implicated as a cause of diarrhoea in children. They
have also been linked to cases of intussusception.
Respiratory viral infections
Adenoviruses, rhinoviruses and enteroviruses(Coxsackie viruses and echoviruses) often produce nonspecific symptoms.
Parainfluenza and respiratory syncytial viruses cause upper respiratory tract disease, croup and bronchiolitis in small children and pneumonia in the immunocompromised. Respiratory syncytial virus also causes pneumonia in nursing home residents and may be associated with nosocomial pneumonia.
In recent years, metapneumovirus and bocavirus have
been identified as causes of upper and occasionally
lower respiratory tract infection.
They may also cause pneumonia in immunosuppressed individuals, such as recipients of allogeneic haematopoietic stem cell transplants.
The severe acute respiratory syndrome (SARS)
caused by the SARS coronavirus emerged as a major
respiratory pathogen during an outbreak in 2002–2003,
with 8000 cases and 10% mortality . In 2012, a
novel coronavirus, distantly related to the SARS coronavirus, caused several deaths connected with pneumonia and acute renal failure in patients originating from the Middle East.
Viral infections with neurological involvement
Japanese B encephalitis
This flavivirus is an important cause of endemic encephalitis
in Japan, China, Russia, South-east Asia, India and
Pakistan; outbreaks also occur elsewhere. There are
10 000–20 000 cases reported to the WHO annually. Pigs
and aquatic birds are the virus reservoirs and transmission
is by mosquitoes. Exposure to rice paddies is a
recognised risk factor.
Clinical features
The incubation period is 4–21 days. Most infections aresubclinical in childhood and 1% or less of infections
lead to encephalitis. Initial systemic illness with fever,
malaise and anorexia is followed by photophobia, vomiting, headache and changes in brainstem function.
Neurological features other than encephalitis include
meningitis, seizures, cranial nerve palsies, flaccid or
spastic paralysis, and extrapyramidal features. Mortality
with neurological disease is 25%.
Most children die from respiratory failure with infection of brainstem nuclei. Approximately 50% of survivors are left with neurological sequelae.
Investigations, management and prevention
Other infectious causes of encephalitis should be
excluded . There is neutrophilia and often
hyponatraemia. CSF analysis reveals lymphocytosis and
elevated protein. Serological testing may be helpful and
there is a CSF antigen test.
Treatment is supportive, anticipating and treating
complications. Vaccination for travellers to endemic
areas during the monsoon period is effective prophylaxis.
Some endemic countries include this vaccination
in their childhood schedules.
Human T-cell lymphotropic
virus type IHuman T-cell lymphotropic virus type I (HTLV-1) is a
retrovirus which causes chronic infection with development
of adult T-cell leukaemia/lymphoma or HTLV-1-
associated myelopathy (HAM) in a subset of those
infected . It is found mainly in Japan, the Caribbean, Central and South America, and the Seychelles. Myositis and uveitis may also occur with HTLV-1
infection. Serology confirms the diagnosis. Treatment is
usually supportive for asymptomatic patients but can
include zidovudine and interferon-alfa for leukaemia.
Viral infections with rheumatological involvement
Rheumatological syndromes characterise a variety of
viral infections ranging from exanthems, such as rubella
and parvovirus B19 , to blood-borne viruses,
such as HBV and HIV-1.
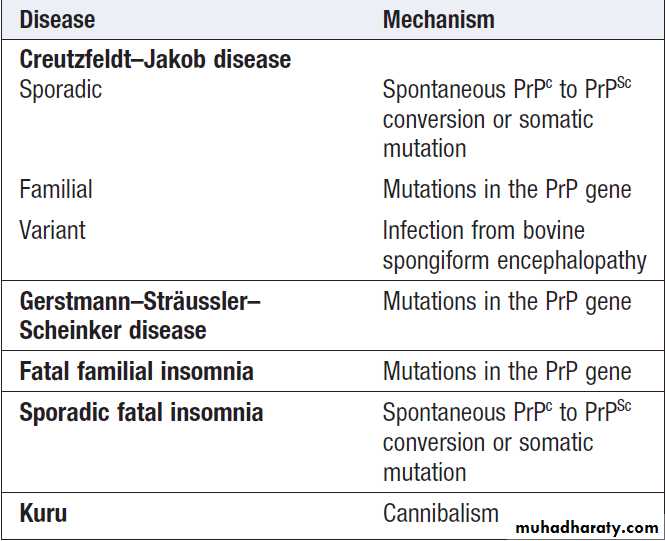
PRION DISEASES
Prions cause transmissible spongiform encephalopathiesin humans, sheep, cows and cats .The prion protein is not inactivated by cooking or conventional sterilisation, and transmission is thought to occur by consumption of infected CNS tissue or by inoculation (e.g. via depth EEG electrodes,
corneal grafts, cadaveric dura mater grafts and pooled
cadaveric growth hormone preparations). The apparent transmission of bovine spongiform encephalopathy (BSE) to humans following an outbreak of BSE in the UK beginning in the late 1980s has caused great concern, leading to precautionary measures, such as leucodepletion of all blood used for transfusion, and the mandatory use of disposable surgical instruments wherever possible.
Prion diseases affecting humans
BACTERIAL INFECTIONSBacterial infections of the skin, soft tissues and bones
Most infections of the skin, soft tissues and bone are
caused by either staphylococci (mainly Staph. aureus) or
streptococci (mainly Strep. pyogenes).
Staphylococcal infections
Staphylococci are usually found colonising the anterior
nares and skin. Traditionally, staphylococci were
divided into two groups according to their ability to
produce coagulase, an enzyme that converts fibrinogen
to fibrin in rabbit plasma, causing it to clot.
Staph. Aureus is coagulase-positive, and most other species coagulase negative. The coagulase test is now less commonly undertaken, with identification of Staph. aureus often achieved by other methods.
Staph. aureus is the main cause of staphy-infections. Staph. intermedius is another coagulase- positive staphylococcus, which causes infection following dog bites. Among coagulase-negative organisms, Staph. epidermidis is the predominant commensal organism of the skin, and can cause severe infections in those with central venous catheters or implanted prosthetic materials. Staph. saprophyticus is part of the normal vaginal flora and causes urinary tract infections in sexually active young women. Staphylococci are particularly dangerous if they gain access to the blood stream, having the potential to cause disease in many sites .
In any patient with staphylococcal bacteraemia, especially injection drug-users, the possibility of endocarditis must be considered . Growth of Staph. aureus in blood cultures should not be dismissed as a ‘contaminant’ unless all possible underlying sources have been excluded and repeated blood culture is negative. Any evidence of spreading cellulitis indicates the urgent need for an antistaphylococcal antibiotic, such as flucloxacillin. This is particularly true for mid-facial cellulitis, which can result in
cavernous sinus thrombophlebitis.
In addition, Staph. aureus can cause severe systemic
disease due to the effects of toxin produced at superficial
sites in the absence of tissue invasion by bacteria.
Infections caused by Staphylococcus aureus.
Skin infectionsStaphylococcal infections cause ecthyma, folliculitis,
furuncles, carbuncles, bullous impetigo and the scalded
skin syndrome .They may also be involved in necrotising infections of the skin and subcutaneous tissues .
Wound infections
Many wound infections are caused by staphylococci,
which may significantly prolong post-operative hospital
stays . Prevention involves careful attention to hand hygiene, skin preparation and aseptic technique, and the use of topical and systemic antibiotic prophylaxis.
Treatment is by drainage of any abscesses plus adequate
dosage of antistaphylococcal antibiotics. These
should be instituted early, particularly if prosthetic
implants of any kind have been inserted.
Manifestations of skin infection with Staph. aureus.
A Wound infection. B Cannula-related infection.Cannula-related infection
Staphylococcal infection associated with cannula sepsisand thrombophlebitis is an important and, unfortunately, extremely common reason for morbidity following hospital admission. The Visual Infusion Phlebitis (VIP) score is a useful way of monitoring cannulae .
Staphylococci have a predilection for plastic, rapidly forming a biofilm which remains as a source of bacteraemia as long as the plastic is in situ. Local poultice application may relieve symptoms but cannula removal and antibiotic treatment with flucloxacillin (or a glycopeptide if MRSA is suspected) are necessary.
How to assess an intravenous cannula
using the Visual Infusion Phlebitis score (VIP)How to assess an intravenous cannula using the Visual Infusion Phlebitis score (VIP) -'cont'd
Meticillin-resistant Staph. aureus
Resistance to meticillin, due to a penicillin-bindingprotein mutation, has been recognised in Staph. aureus
for more than 30 years. The recognition of resistance
to vancomycin/teicoplanin (glycopeptides) in either
glycopeptide intermediate Staph. aureus (GISA) or, rarely, vancomycin-resistant (VRSA) strains threatens
the ability to manage serious infections produced by
such organisms. Meticillin-resistant Staph. aureus
(MRSA) is now a major worldwide health care-acquired
pathogen, accounting for up to 40% of staphylococcal
bacteraemia in developed countries. Community-acquired
MRSA (c-MRSA) currently accounts for 50% of
all MRSA infections in the USA.
These organisms have also acquired other virulence factors, such as Panton– Valentine leukocidin (PVL), which can cause rapidly fatal infection in young people. Treatment should always be based on the results of antimicrobial susceptibility testing, since resistance to all these agents occurs.
Milder MRSA infections may be treated with clindamycin, tetracyclines or co-trimoxazole.
Severe, Glycopeptides (linezolid and Daptomycin ).
PVL-producing Staph. aureus infections should be treated with protein-inhibiting antibiotics (clindamycin, linezolid).
Staphylococcal toxic shock syndrome
Staphylococcal toxic shock syndrome (TSS) is a serious
and life-threatening disease associated with infection by
Staph. aureus, which produces a specific toxin (toxic
shock syndrome toxin 1, TSST1). It was commonly seen
in young women in association with the use of highly
absorbent intravaginal tampons but can occur with any
Staph. aureus infection involving a relevant toxin-producing
strain.
The toxin acts as a ‘superantigen’, triggering significant T-helper cell activation and massive cytokine release.
TSS has an abrupt onset with high fever, generalised
systemic upset (myalgia, headache, sore throat andvomiting), a generalised erythematous blanching rash
resembling scarlet fever, and hypotension. It rapidly
progresses over a matter of hours to multisystem
involvement with cardiac, renal and hepatic compromise,
leading to death in 10–20%.
Recovery is accompanied at 7–10 days by desquamation .
The diagnosis is clinical and may be confirmed in
menstrual cases by vaginal examination, the finding of
a retained tampon, and microbiological examination
by Gram stain demonstrating typical staphylococci. Subsequent culture and demonstration of toxin production
are confirmatory.
Management
Treatment is with immediate and aggressive fluid resuscitation and an intravenous antistaphylococcal antibiotic (flucloxacillin or vancomycin), usually with the addition of a protein synthesis inhibitor (e.g. clindamycin) to inhibit toxin production.
Intravenous immunoglobulin is occasionally added in the most severe cases on the basis of efficacy in streptococcal toxic shock.
Women who recover should be advised not to use tampons for at least 1 year and should also be warned that, due to an inadequate antibody response to TSST1, the condition can recur.
Streptococcal infections
Streptococci are nasopharyngeal and gut commensals,which appear as Gram-positive cocci in chains .They are classified by the haemolysis they produce on blood agar and by their serotypes . Some streptococci (e.g. Strep.
milleri group) defy simple classification.
Skin presentations of streptococcal infections
Group A streptococci (GAS) are the major cause of cellulitis, erysipelas and impetigo .
Groups C and G streptococci cause cellulitis, in elderly,
diabetic or immunocompromised patients in particular.
Group B streptococcal (GBS) infection is an increasing
problem at the extremes of age.
Streptococcal and related infections
Streptococcal and related infections 'cont'd
Streptococcal scarlet feverGroup A (or occasionally groups C and G) causing pharyngitis, tonsillitis may lead to scarlet fever, if the infecting strain produces a streptococcal pyrogenic exotoxin. Common in school age- children, scarlet fever can occur in young adults who have contact with young children. A diffuse erythematous rash occurs, which blanches on pressure , classically with circumoral pallor. The tongue, initially coated, becomes red and swollen (‘strawberry tongue’). The disease lasts about 7 days, the rash disappearing in 7–10 days, followed by a fine desquamation. Residual petechial lesions in the antecubital fossa may be seen (‘Pastia’s sign’). Treatment involves active therapy for the underlying infection (benzylpenicillin or orally available penicillin) plus symptomatic measures.
Clinical features of scarlet fever.
A Characteristic rash with blanching on pressure.B ‘Strawberry tongue’.
C Pastia’s sign: a petechial rash in the cubital fossa.
Streptococcal toxic shock syndrome
This is associated with severe group A (or occasionallygroup C or G) streptococcal skin infections producing
pyogenic exotoxin A. Like staphylococcal TSS toxin, these act as superantigens, stimulating a dramatic cytokine response. Initially, an influenza-like illness occurs with signs of localised infection. A faint erythematous rash, mainly on the chest, rapidly progresses to circulatory shock.
Without aggressive management, multi-organ failure will develop. Fluid resuscitation must be undertaken, with
parenteral antistreptococcal antibiotic therapy, usually
with benzylpenicillin and a protein inhibitor, such as
clindamycin, to inhibit toxin production. Intravenous
immunoglobulin is usually administered in addition.
Treponematoses
Syphilis
Endemic treponematoses
Yaws
Yaws is a granulomatous disease, mainly involving the
skin and bones; it is caused by Treponema pertenue, morphologically and serologically indistinguishable from
the causative organisms of syphilis and pinta.
It is important to establish the geographical origin and
sexual history of patients to exclude a false-positive
syphilis serology due to the endemic treponemal infections. Organisms are transmitted by bodily contact from a
patient with infectious yaws through minor abrasions of
the skin of another patient, usually a child.
After an incubation period of 3–4 weeks, a proliferative granuloma containing numerous treponemes develops at the site of inoculation.
This primary lesion is followed by secondary eruptions. In addition, there may be hypertrophic periosteal lesions of many bones, with underlying cortical rarefaction. Lesions of late yaws are characterised by destructive changes which closely resemble the osteitis and gummas of tertiary syphilis and which heal with much scarring and deformity. The disease disappears with improved housing and cleanliness. In few fields of medicine have chemotherapy and improved hygiene achieved such
dramatic success as in the control of yaws.
Pinta and bejel
Occur in poor rural populations with low standards of domestic hygiene. They have features in common, notably that they are transmitted by contact, usually within the family and not sexually, and in the case of bejel, through common eating and drinking utensils.• Pinta. Pinta is probably the oldest of the human
treponemal infections. The infection is confined to the skin. The early lesions are scaly papules on the skin. The late lesions are depigmented and disfiguring.
• Bejel. Bejel is the Middle Eastern name for non-venereal
syphilis Transmission is most commonly from the mouth of the mother or child and the primary mucosal lesion is seldom seen. The lesions resemble those of secondary and tertiary syphilis but cardiovascular and neurological disease is rare.
Diagnosis and treatment of yaws, pinta and bejel
Diagnosis of early stages
• Detection of spirochaetes in exudate of lesions by dark
ground microscopy
Diagnosis of latent and early stages
• Positive serological tests, as for syphilis
Treatment of all stages
• Single intramuscular injection of 1.2 g long-acting (e.g.
benzathine) benzylpenicillin.
Systemic bacterial infections
BrucellosisBrucellosis is an enzootic infection (i.e. endemic in animals). Although six species of Brucella Gram-negative
bacilli are known, only four are important to humans:
B. melitensis (goats, sheep and camels) , B. abortus (cattle ),
B. suis (pigs) and B. canis (dogs).
B. melitensis causes the most severe disease; B. suis is often associated with abscess formation. Infected animals may excrete Brucella. in their milk for prolonged periods, and human infection is acquired by ingesting contaminated dairy products (unpasteurised milk), uncooked meat or offal. Animal urine, faeces, vaginal discharge may act as sources of infection through abraded skin or via splashes and aerosols to the respiratory tract and conjunctiva.
Clinical features
Brucella spp. are intracellular organisms that survive forlong periods within the reticulo-endothelial system. This
explains many of the clinical features, including the
chronicity of disease and tendency to relapse, even after
antimicrobial therapy. Acute illness is characterised by a high swinging temperature, rigors, lethargy, headache, joint and muscle pains, and scrotal pain. Occasionally, there is delirium, abdominal pain and constipation. Physical signs are non-specific, e.g. enlarged lymph nodes. Enlargement of the spleen may lead to hypersplenism and
thrombocytopenia.
Localised infection , occurs in about 30% of patients, is more likely if diagnosis and treatment are delayed.
Clinical features of brucellosis.
DiagnosisDefinitive diagnosis depends on the isolation of the
organism. Blood cultures are positive in 75–80% of infections caused by B. melitensis and 50% of those caused by B. abortus. Bone marrow culture should not be used routinely but may increase the diagnostic yield, particularly
if antibiotics have been given before specimens are
taken. CSF culture in neurobrucellosis is positive in
about 30% of cases. The laboratory should be alerted to
a suspected diagnosis of brucellosis, as the organism has
a propensity for infecting laboratory workers and must
be cultured at an enhanced containment level.
Serology may also aid diagnosis. In endemic areas, a
single high antibody titre of more than 1/320 or a fourfoldrise in titre is needed to support a diagnosis of acute
infection. The test usually takes several weeks to become
positive but should eventually detect 95% of acute
infections.
Management
Aminoglycosides show synergistic activity with tetracyclines
against brucellae. Treatment regimens for different
forms of brucellosis are outlined in Box .
Treatment of brucellosis
Borrelia infectionsBorrelia are flagellated spirochaetal bacteria which infect
humans after bites from ticks or lice. They cause a variety
of human infections worldwide .
Lyme disease
Lyme disease (named after the town of Old Lyme in
Connecticut, USA) is caused by B. burgdorferi, which
occurs in the USA, Europe, Russia, China, Japan and
Australia. The reservoir of infection is ixodid (hard) ticks that feed on a variety of large mammals, particularly deer. Birds may spread ticks over a wide area. The organism is transmitted to humans via the bite of infected ticks; larval, nymphal and adult forms are all capable of spreading infection.
Clinical features
There are three stages of disease. Progression may bearrested at any stage.
• Early localised disease. The characteristic feature is a
skin reaction around the site of the tick bite, known
as erythema migrans. Initially, a red ‘bull’s eye’ macule or papule appears 2–30 days after the bite. It then enlarges peripherally with central clearing, and may persist for months. The lesion is not pathognomonic of Lyme disease since similar lesions can occur after tick bites in areas where Lyme disease does not occur. Other acute manifestations, such as fever, headache and regional lymphadenopathy, may develop with or without the rash.
• Early disseminated disease. Dissemination occurs via
the blood stream and lymphatics. There may be a
systemic reaction with malaise, arthralgia, and
occasionally metastatic areas of erythema migrans .
Neurological involvement may follow weeks or months after infection. Common features include lymphocytic meningitis, cranial nerve palsies (especially unilateral or bilateral facial nerve palsy) and peripheral neuropathy.
painful radiculopathy, Carditis.
• Late disease. Late manifestations include arthritis,
polyneuritis and encephalopathy.
Diagnosis
The diagnosis of early Lyme borreliosis is often clinical.Culture from biopsy material is not generally available.
Antibody detection is frequently negative early in the
course of the disease, but sensitivity increases to 90–
100% in disseminated or late disease. Immunofluorescence
or ELISA can give false-positive reactions in a number of conditions, including other spirochaetal infections, infectious mononucleosis, rheumatoid arthritis and systemic lupus erythematosus (SLE). Immunoblot (Western blot) techniques are more specific.
Microorganism DNA detection by PCR has been applied to blood, urine, CSF, and biopsies of skin and synovium.
Rash of erythema migrans in Lyme disease with
metastatic secondary lesions.
Management
Recent evidence suggests that asymptomatic patientswith positive antibody tests should not be treated.
However, erythema migrans always requires therapy
because organisms may persist and cause progressive
disease, even if the skin lesions resolve. Standard therapy
consists of a 14-day course of doxycycline (200 mg daily)
or amoxicillin (500 mg 3 times daily). Some 15% of
patients with early disease will develop a mild Jarisch–
Herxheimer reaction (JHR) during the first 24 hours of
therapy . In pregnant women and small children, or in those allergic to amoxicillin and doxycycline, 14-day treatment with cefuroxime axetil (500 mg twice daily) or erythromycin (250 mg 4 times daily) may be used.
Disseminated disease and arthritis require therapy
for a minimum of 28 days. Neuroborreliosis is treated with parenteral β-lactam antibiotics for 3–4 weeks; the cephalosporins may be superior to penicillin in this situation.Prevention
Protective clothing and insect repellents should be used
in tick-infested areas. Since the risk of borrelial transmission is lower in the first few hours of a blood feed, prompt removal of ticks is advisable. Unfortunately, larval and nymphal ticks are tiny and may not be noticed. Where risk of transmission is high, a single 200 mg dose of doxycycline, given within 72 hours of exposure, has been shown to prevent erythema migrans.
A recombinant OspA vaccine was developed but withdrawn due to side-effects.
Louse-borne relapsing fever
The human body louse, Pediculus humanus, causes itching.
Borreliae (B. recurrentis) are liberated from infected lice
when they are crushed during scratching, which also
inoculates the borreliae into the skin. The borreliae multiply in the blood, where they are abundant in the febrile phases, and invade most tissues, especially the liver, spleen and meninges.
Hepatitis and thrombocytopenia are common. Onset is sudden with fever, a tachycardia, headache, generalised aching, injected conjunctivae and, frequently, a petechial rash, epistaxis and herpes labialis. As the disease progresses, the liver and spleen frequently become tender and palpable, and jaundice is common.
There may be severe serosal and intestinal haemorrhage, mental confusion and meningism. The fever ends in crisis between the 4th and 10th days, often associated with profuse sweating, hypotension, and circulatory and cardiac failure. There may be no further fever but, in a proportion of patients, after an afebrile period of about 7 days, there are one or more relapses, which are usually milder and less prolonged. In the absence of specific treatment, the mortality rate is up to 40%, especially among the elderly and malnourished.
Investigations and management
The organisms are demonstrated in the blood during
fever either by dark ground microscopy of a wet film or
in Wright–Giemsa stained thick and thin films.
The problems of treatment are to eradicate the organism, to minimise the severe JHR which inevitably follows successful chemotherapy, and to prevent relapses. The safest treatment is procaine penicillin 300 mg IM, followed the next day by 0.5 g tetracycline. Tetracycline
alone is effective and prevents relapse, but may give rise
to a worse reaction. Doxycycline 200 mg once orally in
place of tetracycline has the advantage of also being
curative for typhus, which often accompanies epidemics
of relapsing fever. JHR is best managed in a highdependency unit with expert nursing and medical care.
The patient, clothing and all contacts must be freed
from lice, as in epidemic typhus.
Leptospirosis
Microbiology and epidemiology
Leptospirosis is one of the most common zoonotic diseases, favoured by a tropical climate and flooding during the monsoon but occurring worldwide.
Leptospires are tightly coiled, thread-like organisms about 5–7 μm in length, which are actively motile; each end is bent into a hook.
The organisms persist indefinitely in the convoluted tubules of the kidney and are shed into the urine in massive numbers, but infection is asymptomatic in the host. The most frequent hosts are rodents, especially the common rat (Rattus norvegicus). Leptospires can enter their human hosts through intact skin or mucous membranes, but entry is facilitated by cuts and abrasions.
Prolonged immersion in contaminated water will also favour invasion, as the spirochaete can survive in water for months. Leptospirosis is common in the tropics and also in freshwater sports enthusiasts.
Clinical features
After a relatively brief bacteraemia, invading organisms
are distributed throughout the body, mainly in kidneys,
liver, meninges and brain.
The incubation period averages 1–2 weeks.
Four main clinical syndromes can be discerned and clinical features can involve multiple organ systems .
Bacteraemic leptospirosis
Bacteraemia with any serogroup can produce a nonspecificillness with high fever, weakness, muscle pain and tenderness (especially the calf and back), intense headache and photophobia, and sometimes diarrhea and vomiting. Conjunctival congestion is the only notable physical sign. The illness comes to an end after about 1 week, or else merges into one of the other forms of infection.
Aseptic meningitis
Classically associated with L. canicola infection, this
illness is very difficult to distinguish from viral meningitis.
The conjunctivae may be congested but there are
no other differentiating signs. Laboratory clues include
a neutrophil leucocytosis, abnormal LFTs, and the occasional
presence of albumin and casts in the urine.
Icteric leptospirosis (Weil’s disease)
Fewer than 10% of symptomatic infections result in
severe icteric illness. Weil’s disease is a dramatic life-threatening event, characterised by fever, haemorrhages, jaundice and renal impairment. Conjunctival hyperaemia is a frequent feature. The patient may have a
transient macular erythematous rash, but the characteristic
skin changes are purpura and large areas of bruising.
In severe cases there may be epistaxis, haematemesis
and melaena, or bleeding into the pleural, pericardial
or subarachnoid spaces. Thrombocytopenia, probably
related to activation of endothelial cells with platelet
adhesion and aggregation, is present in 50% of cases.
Jaundice is deep and the liver is enlarged, but there is
usually little evidence of hepatic failure or encephalopathy.Renal failure, primarily caused by impaired renal
perfusion and acute tubular necrosis, manifests as oliguria
or anuria, with the presence of albumin, blood and
casts in the urine. Weil’s disease may also be associated with myocarditis, encephalitis and aseptic meningitis. Uveitis and iritis may appear months after apparent clinical recovery.
Pulmonary syndrome
It is characterised by haemoptysis, patchy lung infiltrates on chest X-ray, and respiratory failure. Total bilateral lung consolidation and ARDS with multi-organ dysfunction may develop, with a high mortality (over 50%).
Clinical syndromes of
leptospirosis. (ARDS = acute respiratory distresssyndrome)
Diagnosis
A polymorphonuclear leucocytosis is accompanied insevere infection by thrombocytopenia and elevated
blood levels of creatine kinase. In jaundiced patients,
there is hepatitis and the prothrombin time may be prolonged.
The CSF in leptospiral meningitis shows a variable
cellular response, a moderately elevated protein
level and normal glucose content.
Acute renal failure due to interstitial nephritis is common.
In the tropics, dengue, malaria, typhoid fever, typhus and infection are important differential diagnoses.
Definitive diagnosis of leptospirosis depends upon
isolation of the organism, serological tests or the detection of specific DNA. In general, however, it is probably under-diagnosed.• Blood cultures are most likely to be positive if taken
before the tenth day of illness. Special media are
required and cultures may have to be incubated for
several weeks.
• Leptospires appear in the urine during the second
week of illness, and in untreated patients may be
recovered on culture for several months.
• Serological tests are diagnostic if seroconversion or
a fourfold increase in titre is demonstrated. The
microscopic agglutination test (MAT) is the test of
choice and can become positive by the end of the
first week. IgM ELISA and immunofluorescent
techniques are, however, easier to perform, while
rapid immunochromatographic tests are specific but
of only moderate sensitivity in the first week of
illness.
• Detection of leptospiral DNA by PCR is possible in
blood in early symptomatic disease, and in urine from the eighth day of illness and for many months thereafter.
Management and prevention
Blood transfusion for haemorrhage and careful attentionto renal failure, the usual cause of death, are especially
important. Renal failure is potentially reversible with
adequate support, such as dialysis. The optimal antimicrobial regimen has not been established. Most
infections are self-limiting. Therapy with either oral
doxycycline (100 mg twice daily for 1 week) or IV penicillin (900 mg 4 times daily for 1 week) is effective but may not prevent the development of renal failure. Parenteral ceftriaxone (1 g daily) is as effective as penicillin. A Jarisch–Herxheimer reaction may occur during treatment but is usually mild. Uveitis is treated with a combination of systemic antibiotics and local corticosteroids.
Plague
Plague is caused by Yersinia pestis, a small Gram-negativebacillus that is spread between rodents by their fleas. If
domestic rats become infected, infected fleas may bite
humans. In the late stages of human plague, Y. pestis may be expectorated and spread between humans by droplets, causing ‘pneumonic plague’. Epidemics of plague, such as the ‘Black Death’, have occurred since ancient times. It is often said that the first sign of plague is the appearance of dead rats. Y. pestis is a potential bioweapon because of its capacity for mass production and aerosol transmission, and the high fatality rate associated with pneumonic plague.
Clinical features
Organisms inoculated through the skin are taken rapidly
to the draining lymph nodes. If the infection is not contained, septicaemia ensues and necrotic, purulent or haemorrhagic lesions develop in many organs. Oliguria and shock follow, and DIC may result in widespread haemorrhage. Inhalation of Y. pestis causes alveolitis. The incubation period is 3–6 days, but shorter in pneumonic. Bubonic plague
In this, the most common form, onset is usually sudden, with a rigor, high fever, dry skin and severe headache. Soon, aching and swelling at the site of the affected lymph nodes begin. The groin is the most common site made up of the swollen lymph nodes and surrounding tissue.
Some infections are relatively mild but, in the majority toxaemia quickly increases, with a rapid pulse, hypotension and mental confusion. The spleen is usually palpable.
Septicaemic plague
Those not exhibiting a bubo usually deteriorate rapidly
and have a high mortality. The patient is toxic and may have gastrointestinal symptoms, such as nausea, vomiting,
abdominal pain and diarrhoea. DIC may occur, manifested
by bleeding from various orifices or puncture sites, along with ecchymoses. Hypotension, shock, renal failure and ARDS may lead to further deterioration. Meningitis, pneumonia and expectoration of bloodstained
sputum containing Y. pestis may complicate septicaemic,
or occasionally bubonic, plague.
Pneumonic plague
Following primary infection in the lung, the onset ofdisease is very sudden, with cough and dyspnoea. The
patient soon expectorates copious blood-stained, frothy,
highly infective sputum, becomes cyanosed and dies.
Chest radiology reveals bilateral infiltrates which may
be nodular and progress to an ARDS-like picture. Investigations
The organism may be cultured from blood, sputum and
bubo aspirates. For rapid diagnosis, Gram, Giemsa and
Wayson’s stains (the latter containing methylene blue)
are applied to smears from these sites. Y. pestis is seen
as bipolar staining coccobacilli, sometimes referred to as
having a ‘safety pin’ appearance.
Smears are also subjected to antigen detection by immunofluorescence, using Y. pestis F1 antigen-specific antibodies. The diagnosis may be confirmed by seroconversion or a single high titre (> 128) of anti-F1 antibodies in serum. DNA detection by PCR is under evaluation. Plague is a notifiable disease under international health regulations .
Management
Treatment must be started as soon as, or even before, samples have been collected for laboratory diagnosis. Streptomycin (1 g twice daily) or gentamicin (1 mg/kg 3 times daily) is the drug of choice. Tetracycline (500 mg 4 times daily) and chloramphenicol (12.5 mg/kg 4 times daily) are alternatives. Fluoroquinolones (ciprofloxacin and levofloxacin) may be effective.
Treatment may also be needed for acute circulatory failure, DIC and hypoxia.
Prevention and infection controlRats and fleas should be controlled, avoid handling and skinning wild animals in endemic areas. The patient should be isolated for the first 48 hours. Attendants must wear gowns, masks and gloves. Exposed symptomatic or
asymptomatic who have been in close contact with pneumonic plague should receive post-exposure antibiotic prophylaxis (doxycycline 100 mg or ciprofloxacin 500 mg twice daily) for 7 days. A formalin-killed vaccine is available for those at
occupational risk but offers little protection against
pneumonic plague. A recombinant subunit vaccine
(protein antigens F1 + V) is in development.
Typhoid and paratyphoid (enteric) fevers
Typhoid and paratyphoid fevers, transmitted by the faecal–oral route. Enteric fevers are caused by infection with Salmonella typhi and S. paratyphi A and B.After a few days of bacteraemia, the bacilli localise,
mainly in the lymphoid tissue of the small intestine,
resulting in typical lesions in the Peyer’s patches and
follicles. These swell at first, then ulcerate and usually
heal. After clinical recovery, about 5% of patients become
chronic carriers (i.e. continue to excrete the bacteria after
1 year); the bacilli may live in the gallbladder for months
or years and pass intermittently in the stool and, less
commonly, in the urine.
Clinical features
Typhoid fever
The incubation period is typically about 10–14 days but can be longer, and the onset may be insidious. The temperature rises in a stepladder fashion for 4 or 5 days with malaise, increasing headache, drowsiness and aching in the limbs. Constipation may be caused by swelling of lymphoid tissue around the ileocaecal junction, although in
children diarrhoea and vomiting may be prominent
early in the illness. The pulse is often slower than would
be expected from the height of the temperature, i.e. a
relative bradycardia. At the end of the first week, a rash may appear on the upper abdomen and on the back as sparse, slightly raised, rose-red spots, fade on pressure.
It is usually visible only on white skin. Cough and epistaxis
occur. Around the 7th–10th day, the spleen becomespalpable. Constipation is then succeeded by diarrhoea
and abdominal distension with tenderness. Bronchitis
and delirium may develop. If untreated, by the end of
the second week the patient may be profoundly ill.
Paratyphoid fever
The course tends to be shorter and milder than that of
typhoid fever and the onset is often more abrupt with
acute enteritis. The rash may be more abundant and the
intestinal complications less frequent.
Clinical features of typhoid fever
ComplicationsHaemorrhage from, or a perforation of, the ulcerated Peyer’s may occur at the end of the second week or during the third week of the illness. A drop in temperature to normal or subnormal levels may be falsely reassuring in patients with haemorrhage. Complications may involve almost any viscus or system because of the septicaemia present during the first week. Bone and joint infection is common in children with sickle-cell disease.
Investigations
In the first week, diagnosis may be difficult because, in
this invasive stage with bacteraemia, the symptoms are
those of a generalised infection without localising features. There is a leucopenia. Blood culture is the most important diagnostic method.
Complications of typhoid fever
ManagementAntibiotic therapy must be guided by in vitro sensitivity
testing. Chloramphenicol (500 mg 4 times daily), ampicillin
(750 mg 4 times daily) and co-trimoxazole (2 tablets or IV equivalent twice daily) are losing their effect due to resistance in many areas of the world, especially India and South-east Asia. The fluoroquinolones are the drugs of choice (e.g. ciprofloxacin 500 mg twice daily) if the organism is susceptible, but resistance is common, especially in the Indian subcontinent and also in the UK.
Extended-spectrum cephalosporins (ceftriaxone and
cefotaxime) are useful alternatives but have a slightly
increased treatment failure rate.
Azithromycin (500 mg once daily) is an alternative when fluoroquinolone resistance is present but has not been validated in severe disease.
Treatment should be continued for 14 days.
Pyrexia may persist for up to 5 days after the start of
specific therapy.
Even with effective chemotherapy, there is still a danger of complications, recrudescence of the disease and the development of a carrier state.
Chronic carriers were formerly treated for 4 weeks
with ciprofloxacin but may require an alternative agent
and duration, as guided by antimicrobial sensitivity
testing. Cholecystectomy may be necessary.
Prevention
Improved sanitation and living conditions reduce theincidence of typhoid. Travellers to countries where
enteric infections are endemic should be inoculated with
one of the three available typhoid vaccines (two inactivated injectable and one oral live attenuated).
Actinomycete infections
NocardiosisNocardiosis is an uncommon Gram-positive bacterial
infection caused by aerobic actinomycetes of the genus
Nocardia found in the soil. Infection occurs most frequently
by direct traumatic inoculation or occasionally
via inhalation or ingestion. Nocardiosis can result in
localised cutaneous ulcers or nodules, most often in the
lower limbs. Chronic destructive infection in tropical
countries can result in actinomycetoma, involving soft
tissues with occasional penetration to the bone. Actinomycetoma may also be caused by other bacteria, and a similar clinical syndrome, termed eumycetoma, is
caused by fungi .
Systemic Nocardia infection, most likely in immunocompromised individuals, results in suppurative disease with lung and brain abscesses.
On microscopy, Nocardia spp. appear as long, filamentous,
branching Gram-positive rods which are also
weakly acid-fast. They are easily grown in culture but
require prolonged incubation.
Treatment is guided by susceptibility testing. Systemic
infection typically requires combinations of ceftriaxone,
meropenem, amikacin and co-trimoxazole, often
for 6–12 months or longer. Abscesses are drained surgically when this is feasible.
Gastrointestinal bacterial infections
Staphylococcal food poisoningStaph. aureus transmission takes place via the hands of
food handlers to foodstuffs such as dairy products,
including cheese, and cooked meats. Inappropriate storage of these foods allows growth of the organism and production of one or more heat-stable enterotoxins which cause the symptoms.
Nausea and profuse vomiting develop within 1–6 hours.
Diarrhoea may not be marked. The toxins that cause the syndrome act as ‘superantigens’, inducing a significant neutrophil leucocytosis that may be clinically misleading.
Superantigens are secreted proteins (exotoxins)
that exhibit highly potent lymphocyte-transforming(mitogenic) activity directed towards T lymphocytes.
Most cases settle rapidly but severe dehydration can
occasionally be life-threatening.
Antiemetics and appropriate fluid replacement are
the mainstays of treatment.
Suspect food should be cultured for staphylococci and demonstration of toxin production.
The public health authorities should be notified
if food vending is involved.
Bacillus cereus food poisoning
Ingestion of the pre-formed heat-stable exotoxins of B.
cereus causes rapid onset of vomiting and some diarrhoea
within hours of food consumption, which resolves
within 24 hours. Fried rice and freshly made sauces are
frequent sources; the organism grows and produces
enterotoxin during storage . If viable bacteria
are ingested and toxin formation takes place within
the gut lumen, then the incubation period is longer
(12–24 hours) and watery diarrhoea and cramps are the
predominant symptoms.
The disease is self-limiting but can be quite severe.
Clostridium perfringens
food poisoningSpores of C. perfringens are widespread in the guts of
large animals and in soil. If contaminated meat products
are incompletely cooked and stored in anaerobic conditions,
C. perfringens spores germinate and viable organisms
multiply to give large numbers. Subsequent reheating of the food causes heat-shock sporulation of the organisms, during which they release an enterotoxin. Clostridial enterotoxins are potent and most people who ingest them will be symptomatic , diarrhoea and cramps ccur some 6–12 hours following ingestion.
The illness is usually self-limiting.
Campylobacter jejuni infection
This infection is essentially a zoonosis, although contaminated water may be implicated. The most
common sources of the infection are chicken, beef and
contaminated milk products. Campylobacter infection is now the most common cause of bacterial gastroenteritis in the
UK. The incubation period is 2–5 days. Colicky abdominal
pain with nausea, vomiting and significant diarrhoea, frequently containing blood. The majority affect fit young adults and are self-limiting after 5–7 days. About 10–20% will have prolonged symptomatology, occasionally meriting
treatment with antibiotics such as erythromycin, as
many organisms are resistant to ciprofloxacin.
Campylobacter spp. have been linked to Guillain–Barré syndrome and postinfectious reactive arthritis .
Salmonella spp. infection
Salmonella serotypes other than S. typhi and S. paratyphiof which there are more than 2000, two serotypes are most important worldwide: S. enteritidis phage type 4 and S. typhimurium. Transmission is by contaminated water or food, particularly poultry, egg products and related fast foods, direct person-to-person. The incubation period is 12–72 hours and the predominant feature is diarrhoea,
sometimes with passage of blood. Vomiting may be present at the outset. Approximately 5% of cases are bacteraemic.
Reactive (post-infective) arthritis occurs in 2%.
Antibiotics are not indicated for uncomplicated Salmonella gastroenteritis . However, evidence of bacteraemia is a clear indication for antibiotic therapy.
Escherichia coli infection
Many serotypes of E. coli are present in the human gut .Production of disease depends on either colonisation with a new or previously unrecognized strain, or the acquisition by current colonizing bacteria of a particular pathogenicity factor for mucosal attachment or toxin production. At least five different clinico-pathological patterns of diarrhoea are associated with specific strains of E. coli with characteristic virulence factors.
Enterotoxigenic E. coli (ETEC)
Cause the majority of cases of travellers’ diarrhoea. The organisms produce either a heat-labile or a heat-stable enterotoxin, causing marked secretory diarrhoea and vomiting after 1–2 days’ incubation. The illness is usually mild and self-limiting after 3–4 days.
Entero-invasive E. coli very similar to Shigella dysentery and is caused by invasion and destruction of colonic mucosal cells. No enterotoxin is produced. Acute watery diarrhoea, abdominal cramps and some scanty blood-staining of the stool are common. The symptoms are rarely severe and are usually self-limiting.
Clostridium difficile infection (CDI)
C. difficile is the most commonly diagnosed cause ofantibiotic-associated diarrhoea and is an occasional constituent of the normal intestinal flora. C. difficile is capable of producing two toxins (A and B), usually follows antimicrobial therapy, which alters the composition of the gastrointestinal flora and may result in colonisation with
C. difficile if the patient is exposed to C. difficile spores. The combination of toxin production and the ability to produce environmentally stable spores accounts for the clinical features and transmissibility of CDI.
A hypervirulent strain of C. difficile, ribotype 027, has emerged, which produces more toxin than other C. difficile strains and thus more severe disease.
Clinical features
Disease manifestations range from diarrhoea to life-threatening pseudomembranous colitis. Around 80% ofcases occur in people over 65 years of age, many of
whom are frail with comorbid diseases. Symptoms
usually begin in the first week of antibiotic therapy but
can occur at any time up to 6 weeks after treatment has
finished. The onset is often insidious, with lower abdominal
pain and diarrhoea which may become profuse and
watery. The presentation may resemble acute ulcerative
colitis with bloody diarrhoea, fever and even toxic dilatation and perforation. Ileus is also seen in pseudomembranous colitis.
Investigations
C. difficile can be isolated from stool culture in 30% of
patients with antibiotic-associated diarrhoea and over
90% of those with pseudomembranous colitis, but also
from 5% of healthy adults and up to 20% of elderly
patients in residential care. The diagnosis of CDI therefore rests on detection of toxins A or B in the stool.
Current practice in the UK is to screen stool from patients
with a compatible clinical syndrome by detection either
of glutamate dehydrogenase (GDH), an enzyme produced
by C. difficile, or of C. difficile nucleic acid (e.g. by
PCR); if screening is positive, a C. difficile toxin ELISA or
a tissue culture cytotoxicity assay is performed.
Clostridium difficile infection.
Colonoscopic view showing numerous adherent ‘pseudomembranes’.The rectal appearances at sigmoidoscopy may be characteristic, with erythema, white plaques or an adherent pseudomembrane . Appearances may also resemble those of ulcerative colitis. In some
cases, the rectum is spared and abnormalities are observed in the proximal colon. Patients who are ill may require abdominal and erect chest X-rays to exclude perforation or toxic dilatation.
CT may be useful when the diagnosis is in doubt.
Management
The precipitating antibiotic should be stopped ,patient isolated. Supportive therapy with intravenous fluids. CDI is treated with antibiotics. First-line therapy are metronidazole (500 mg orally 3 times daily for 10 days) or vancomycin (125 mg orally 4 times daily for 7–10 days).
Although vancomycin is more effective than metronidazole against hypervirulent C. difficile strains (e.g. ribotype 027), it is more expensive and may drive the emergence of vancomycin resistance in other organisms (e.g. enterococci, Staph. aureus). For these reasons, some authorities reserve its use for relapse (15–30% of patients), failure of initial response or severe infection.
A new agent, fidaxomicin, is associated with a lower relapse rate than vancomycin. IV immunoglobulin and/or corticosteroids are sometimes given in the most severe or refractory cases and faecal transplantation is also emerging as a therapy in relapsing patients.
Yersinia enterocolitica infection
This organism, commonly found in pork, causes mild tomoderate gastroenteritis and can produce significant
mesenteric adenitis after an incubation period of
3–7 days. It predominantly causes disease in children but adults may also be affected.
The illness resolves slowly, with 10–30% of cases complicated by persistent arthritis or Reiter’s syndrome .
Cholera
Cholera, caused by Vibrio cholerae serotype O1, toxin-mediated bacterial cause of acute watery diarrhoea. The enterotoxin activates adenylate cyclase in the intestinal epithelium, inducing net secretion of chloride and water.V. cholerae O1 has two biotypes,
classical and El Tor, and each of these has two
distinct serotypes, Inaba and Ogawa. The seventh pandemic, due to the El Tor biotype, began in 1961 and spread via the Middle East to become endemic in Africa. There are recurrent outbreaks and epidemics in Africa, often related to flooding. El Tor is more resistant to commonly used antimicrobials than classical Vibrio, and causes prolonged carriage in 5% of infections.
Infection spreads via the stools or vomit of symptomatic
patients or subclinical cases. Organisms survive for up to 2 weeks in fresh water and 8 weeks in salt water.Transmission is through infected drinking water, shellfish and food contaminated by flies, or hands of carriers.
Clinical features
Severe diarrhoea without pain or colic begins suddenly
and is followed by vomiting. Following the evacuation
of normal gut faecal contents, typical ‘rice water’ material
is passed, consisting of clear fluid with flecks of mucus.
Classical cholera produces enormous loss of fluid and
electrolytes, leading to intense dehydration with muscular
cramps.
Shock and oliguria develop but mental clarity remains.
Death from acute circulatory failure may occur rapidly unless fluid and electrolytes are replaced. The majority of infections, however, cause mild illness with slight diarrhoea. Occasionally, a very intense illness,
‘cholera sicca’, occurs, with loss of fluid into dilated bowel, killing the patient before typical gastrointestinal symptoms appear.
Diagnosis and management
Clinical diagnosis is easy during an epidemic. Otherwise,
should be confirmed bacteriologically. Stool dark-field microscopy shows the typical ‘shooting star’ motility of V. cholerae. Rectal swab or stool cultures allow identification. Cholera is notifiable diseas. Maintenance of circulation by replacement of water and electrolytes is paramount .
Ringer-Lactate is the best fluid for replacement. Vomiting
usually stops once the patient is rehydrated, and fluidshould then be given orally up to 500 mL hourly.
Early intervention with oral rehydration solutions that include resistant starch, based on either rice or cereal, shortens the duration of diarrhoea and improves prognosis. Total fluid requirements may exceed 50 L over a period of 2–5 days. Accurate records are greatly facilitated by the use of a ‘cholera cot.
Three days treatment with tetracycline 250 mg
4 times daily, a single dose of doxycycline 300 mg or
ciprofloxacin 1 g in adults reduces the duration of excretion of V. cholerae and the total volume of fluid needed for replacement.
Prevention
Strict personal hygiene is vital and drinking watershould come from a clean piped supply or be boiled.
Flies must be denied access to food. Parenteral vaccination with a killed suspension of V. cholerae provides
some protection. Oral vaccines containing killed V. cholerae
and the B subunit of cholera toxin are available but
are of limited efficacy.
In epidemics, public education and control of water
sources and population movement are vital. Mass
single-dose vaccination and treatment with tetracycline
are valuable. Disinfection of discharges and soiled clothing,
and scrupulous hand-washing by medical attendants
reduce the danger of spread.
Bacillary dysentery (shigellosis)
Gram-negative rods, invade the colonic mucosa. There are four main groups: Sh. dysenteriae, flexneri, boydii and sonnei. In the tropics, bacillary dysentery is usually caused by Sh. flexneri, whilst in the UK most cases are caused by Sh. sonnei. Shigellae are often resistant to multiple antibiotics. The organism only infects humans and its spread is facilitated by its low infecting dose of around 10 organisms. Spread may occur via contaminated food or flies, but transmission by unwashed hands after defecation is by far the most important . Outbreaks occur in mental hospitals, schools and closed institutions. dysentery is a constant accompaniment of wars and catastrophes, which bring crowding and poor sanitation. Shigella may spread rapidly amongst men who have sex with men.
Clinical features
Disease severity varies from mild Sh. sonnei infectionsthat may escape detection to more severe Sh. flexneri
infections, while those due to Sh. dysenteriae may be fulminating and cause death within 48 hours.
In a moderately severe illness, the patient complains
of diarrhoea, colicky abdominal pain and tenesmus.
Stools are small, and after a few evacuations contain
blood and purulent exudate with little faecal material.
Fever, dehydration and weakness occur, with tenderness
over the colon.
Arthritis or iritis may occasionally complicate bacillary dysentery (Reiter’s syndrome ), associated with HLA-B27.
Management and prevention
Oral rehydration therapy or, if diarrhoea is severe, intravenous replacement of water and electrolyte loss is necessary.Antibiotic therapy with ciprofloxacin (500 mg
twice daily for 3 days) is effective in known shigellosis
and appropriate in epidemics. The use of antidiarrhoeal
medication should be avoided.
The prevention of faecal contamination of food and
milk and the isolation of cases may be difficult, except
in limited outbreaks. Hand-washing is very important.
Respiratory bacterial infections
Diphtheria
Infection with Corynebacterium diphtheriae occurs most
commonly in the upper respiratory tract and is usually
spread by droplet infection. Infection may also complicate
skin lesions. The organisms remain localised at the site of infection but release of a soluble exotoxin damages the heart muscle and the nervous system. Diphtheria has been eradicated from many parts of the world by mass vaccination. The disease is notifiable.
Clinical features
The average incubation period is 2–4 days. The disease
begins insidiously with a sore throat . Despite
modest fever, there is usually marked tachycardia.
The diagnostic feature is the ‘wash-leather’ elevated, greyish-green membrane on the tonsils. It has a well-defined edge, is firm and adherent, and is surrounded by a zone of inflammation. There may be swelling of the neck
(‘bull neck’) and tender enlargement of the lymph nodes.
In the mildest infections, especially in the presence of a
high degree of immunity, a membrane may never
appear and the throat is merely slightly inflamed. With anterior nasal infection there is nasal discharge, frequently blood-stained. In laryngeal diphtheria, a husky voice and high-pitched cough signal potential respiratory obstruction requiring urgent tracheostomy. If infection spreads to the uvula, fauces and nasopharynx, the patient is gravely ill.
Death from acute circulatory failure may occur within
the first 10 days. About 25% of survivors of the early toxaemia may later develop myocarditis, arrhythmias or cardiac failure. These are usually reversible, with no permanent damage other than heart block in survivors.Neurological involvement occurs in 75% of cases.
After tonsillar or pharyngeal diphtheria, it usually starts
after 10 days with palatal palsy.
Paralysis of accommodation often follows, manifest by difficulty in reading small print.
Generalised polyneuritis with weakness and
paraesthesia may follow in the next 10–14 days.
Recovery from such neuritis is always ultimately complete.
Clinical features of diphtheria
ManagementA clinical diagnosis of diphtheria must be notified. Treatment should begin once appropriate swabs have been taken before waiting for microbiological confirmation.
Antitoxin is produced from hyperimmune horse serum. It neutralises circulating toxin but has no effect on toxin already fixed to tissues, so it must be injected IM without awaiting the result of a throat swab. However, reactions to this foreign protein include a potentially lethal immediate anaphylactic reaction and a ‘serum sickness’ with fever, urticarial and joint pains, which occurs 7–12 days after injection. A careful history of previous horse serum injections or allergic reactions should be taken and a small test injection of serum should be given half an hour before the
full dose in every patient.
Adrenaline solution must be available to deal with any immediate type of reaction (0.5–1.0 mL of 1/1000 solution IM). An
antihistamine is also given. In a severely ill patient, the
risk of anaphylactic shock is outweighed by the mortal
danger of diphtheritic toxaemia, and up to 100 000 U of
antitoxin are injected intravenously if the test dose has
not given rise to symptoms. For disease of moderate severity, 16 000–40 000 U IM will suffice, and for mild cases 4000–8000 U. Penicillin (1200 mg 4 times daily IV) or amoxicillin (500 mg 3 times daily) should be administered for 2 weeks to eliminate C. diphtheriae. Patients allergic to penicillin can be given erythromycin. Due to poor immunogenicity of primary infection, all sufferers should be immunised with diphtheria toxoid following recovery.
Patients must be managed in strict isolation and attended by staff with a clearly documented immunization history until three swabs 24 hours apart are culture-negative.
Prevention
Active immunisation should be given to all children. If
diphtheria occurs in a closed community, contacts
should be given erythromycin, which is more effective
than penicillin in eradicating the organism in carriers.
All contacts should also be immunised or given a
booster dose of toxoid.
Booster doses are required every 10 years to maintain immunity.
Pneumococcal infection
Strep. pneumoniae (the pneumococcus) is the leading
cause of community-acquired pneumonia globally
and one of the leading causes of infection-related
mortality. Otitis media, meningitis and sinusitis are also
frequently due to Strep. pneumoniae. Occasional patients
present with bacteraemia without obvious focus.
Asplenic individuals are at risk of fulminant pneumococcal disease with purpuric rash. Increasing rates of penicillin resistance have been reported around the world for Strep. pneumoniae, although they remain relatively low in the UK. Strains with high-level resistance causing meningitis require treatment with glycopeptides rather than with penicillins or cephalosporins.
Vaccination of infants with the protein conjugate
pneumococcal vaccine decreases Strep. pneumoniae infection in infants and in their relatives.The polysaccharide pneumococcal vaccine is used in individuals predisposed to Strep. pneumoniae infection and the elderly, but only modestly reduces pneumococcal bacteraemia and does not prevent pneumonia.
All asplenic individuals should receive vaccination against Strep. pneumoniae.
Anthrax
Anthrax is an endemic zoonosis in many countries; itcauses human disease following inoculation of the
spores of Bacillus anthracis. B. anthracis was the first recognised bacterial pathogen described by Koch and
became the model pathogen for ‘Koch’s postulates’ .
It is a Gram-positive organism with a central
spore. The spores can survive for years in soil. Infection
is commonly acquired from contact with animals, particularly herbivores.
The ease of production of B. anthracis spores makes this infection a candidate for biological warfare or bioterrorism. B. anthracis produces a number of toxins which mediate the clinical features of disease.
Clinical features
These depend on the route of entry of the anthrax spores.
Cutaneous anthrax
This skin lesion is associated with occupational exposure
to anthrax spores during processing of hides and bone products. Spores are inoculated into exposed skin. A single lesion develops as an irritable papule on an oedematous haemorrhagic base. This progresses to a depressed black eschar. Despite extensive oedema, pain is infrequent.
Gastrointestinal anthrax
This is associated with the ingestion of contaminated meat products. The caecum is the seat of the infection, which produces nausea, vomiting, anorexia and fever, followed in 2–3 days by severe abdominal pain and bloody diarrhoea. Toxaemia and death can develop rapidly.
Inhalational anthrax
This form is extremely rare but has been associated with bioterrorism. Without rapid and aggressive therapy at the onset of symptoms, the mortality is 50–90%. Fever, dyspnoea, cough, headache and symptoms of septicaemia.Typically, the chest X-ray shows only widening of
the mediastinum and pleural effusions, which are haemorrhagic. Meningitis may occur.
Management
B. anthracis can be cultured from skin swabs from lesions.
Skin lesions are readily curable with early antibiotic .
Treatment is with ciprofloxacin (500 mg twice daily) until penicillin susceptibility is confirmed; the regimen can then be changed to benzylpenicillin 2.4 g IV given 6 times daily or phenoxymethylpenicillin500–1000 mg 4 times daily administered for 10 days.
The addition of an aminoglycoside may improve the outlook in severe disease. In view of concerns about concomitant inhalational exposure, particularly in the era of bioterrorism, a further 2-month course of ciprofloxacin 500 mg twice daily or doxycycline 100 mg twice daily orally is added.
Prophylaxis with ciprofloxacin (500 mg twice daily) is recommended for anyone at high risk of exposure to anthrax spores.
Bacterial infections with neurological involvement
Infections affecting the CNS, including bacterialmeningitis, botulism and tetanus .
Mycobacterial infections
Leprosy
Leprosy (Hansen’s disease) is a chronic granulomatous
disease affecting skin and nerves, and is caused by Mycobacterium leprae, a slow-growing mycobacterium which cannot be cultured in vitro. The clinical manifestations are determined by the degree of the patient’s cell-mediated immunity towards M. leprae .
High levels of CMI with elimination of leprosy bacilli produces tuberculoid leprosy, whereas absent CMI results in lepromatous leprosy. The complications of leprosy are due to nerve damage, immunological reactions and bacillary infiltration. Leprosy patients are frequently stigmatised and using the word ‘leper’ is inappropriate.
Epidemiology and transmission
Some 4 million people have leprosy and around 750 000new cases are detected annually. About 70% of the
world’s leprosy patients live in India.
Untreated lepromatous patients discharge bacilli
from the nose. Infection occurs through the nose, followed by haematogenous spread to skin and nerve.
The incubation period is 2–5 years for tuberculoid cases
and 8–12 years for lepromatous cases. Leprosy incidence
peaks at 10–14 years, and is more common in males and
in those with close household exposure to leprosy cases.
Pathogenesis
M. leprae has a predilection for infecting Schwann cellsand skin macrophages. In tuberculoid leprosy, effective
CMI controls bacillary multiplication (‘paucibacillary’)
but organised epithelioid granulomas are formed. In
lepromatous leprosy, there is abundant bacillary multiplication (‘multibacillary’). Between these two extremes is a continuum, varying from patients with moderate CMI (borderline tuberculoid) to patients with little cellular response (borderline lepromatous).
Delayed hypersensitivity reactions produce type 1 (reversal) reactions, while immune complexes contribute to type 2 (erythema nodosum leprosum) reactions.
HIV/leprosy co-infected patients have typical lepromatous
and tuberculoid leprosy skin lesions.
Leprosy: mechanisms of damage and tissue affected. Mechanisms under the broken line are characteristic of disease near the lepromatous end of the spectrum, and those under the solid line are characteristic of the tuberculoid end. They overlap in the centre where, in addition, instability predisposes to type 1 lepra reactions. At the peak in the centre, neither bacillary growth nor cell-mediated immunity has the upper hand
(BL = borderline lepromatous; BT = borderline tuberculoid).
Clinical features
• Skin. The most common skin lesions are macules orplaques. Tuberculoid patients have few, hypopigmented lesions . In lepromatous leprosy, papules, nodules or diffuse infiltration of the skin occur. The earliest lesions are
ill defined; gradually, the skin becomes infiltrated
and thickened. Facial skin thickening leads to the
characteristic leonine facies .
• Anaesthesia. In skin lesions, the small dermal sensory and autonomic nerve fibres are damaged, causing local sensory loss and loss of sweating within that area. Anaesthesia may also occur in the distribution of a damaged large peripheral nerve. A ‘glove and stocking’ sensory neuropathy is also common in lepromatous leprosy.
• Nerve damage. Peripheral nerve trunks are affected
at ‘sites of predilection’. These are the ulnar (elbow),median (wrist), radial (humerus), radial cutaneous (wrist), common peroneal (knee), posterior tibial and sural nerves (ankle), facial nerve (zygomatic arch) and great auricular nerve (posterior triangle of the neck). All these nerves should be examined for enlargement and tenderness and tested for motor and sensory function.
The CNS is not affected.
• Eye involvement. Blindness is a devastating complication for a patient with anaesthetic hands and feet. Eyelid closure is impaired when the facial nerve is affected. Damage to the trigeminal nerve causes anaesthesia of the cornea and conjunctiva.
The cornea is then susceptible to trauma and ulceration.
• Other features. Many organs can be affected. Nasal
collapse occurs secondary to bacillary destruction
of the bony nasal spine. Diffuse infiltration of the testes causes testicular atrophy and the acute orchitis that occurs with type 2, results in azoospermia and hypogonadism. Leprosy reactions
Leprosy reactions are events superimposed on the cardinal features shown in Box .
• Type 1 (reversal) reactions. These occur in 30% of
borderline (BT, BB or BL) and are delayed hypersensitivity reactions. Skin lesions become erythematous .Peripheral nerves become tender and painful, with sudden loss of nerve function. These ay occur spontaneously, after starting treatment and also after completion of multidrug therapy.
• Type 2 (erythema nodosum leprosum, ENL) reactions.
These are partly due to immune complex depositionand occur in BL and LL patients who produce antibodies and have a high antigen load. They manifest with malaise, fever and crops of small pink nodules on the face and limbs. Iritis and episcleritis are common. Other signs are acute neuritis, lymphadenitis, orchitis, bone pain, dactylitis, arthritis and proteinuria.
ENL may continue intermittently for several years.
Borderline cases
In borderline tuberculoid (BT) cases, skin lesions are
more numerous than in tuberculoid (TT) cases, and
there is more severe nerve damage and a risk of type 1
reactions. In borderline leprosy (BB) cases, skin lesions are numerous and vary in size, shape and distribution.
In borderline lepromatous (BL) cases, there are
widespread small macules in the skin and widespreadnerve involvement; both type 1 and type 2 reactions
occur. Pure neural leprosy (i.e. without skin lesions) occurs
principally in India and accounts for 10% of patients.
There is asymmetrical involvement of peripheral nerve
trunks and no visible skin lesions. On nerve biopsy, all
types of leprosy have been found.
Clinical features of leprosy.
A Tuberculoid leprosy. Single lesion with a well-defined active edge and anaesthesia within the lesion.B Lepromatous leprosy. Widespread nodules and infiltration, with loss of the eyebrows.
This man also has early collapse of the nose.
C Borderline tuberculoid leprosy with severe nerve damage. This boy has several well-defined, hypopigmented, macular, anaesthetic lesions. He has severe nerve damage affecting both ulnar and median nerves bilaterally and severe burns.
D Reversal (type 1) reactions. Erythematous, oedematous lesions.
Cardinal features of leprosy
Clinical characteristics of the polar forms of leprosy
Reactions in leprosy
Investigations
The diagnosis is clinical, made by finding a cardinal signof leprosy and supported by finding acid-fast bacilli in
slit-skin smears or typical histology in a skin biopsy.
Slit-skin smears are obtained by scraping dermal
material on to a glass slide. The smears are then stained
for acid-fast bacilli, the number counted per high-power
field and a score derived on a logarithmic scale (0–6): the
bacterial index (BI). Smears are useful for confirming the
diagnosis and monitoring response to treatment.
Neither serology nor PCR testing for M. leprae DNA is sensitive or specific enough for diagnosis.
Management
The principles of treatment are outlined in Box . Allleprosy patients should be given multidrug treatment
(MDT) with an approved first-line regimen (Box).
Rifampicin is a potent bactericidal for M. leprae but
should always be given in combination with other antileprotics, since a single-step mutation can confer resistance.
Dapsone is bacteriostatic. It commonly causes mild
haemolysis and rarely anaemia. Clofazimine is a red,
fat-soluble crystalline dye, weakly bactericidal for M.
leprae.
Skin discoloration (red to purple-black) and ichthyosis are troublesome side-effects, particularly on pale skins.
New drugs that are bactericidal for M. leprae have
been identified, notably the fluoroquinolones pefloxacin
and ofloxacin, minocycline and clarithromycin. These
agents are now established second-line drugs. Minocycline
causes a grey pigmentation of skin lesions.
Although single-dose treatment is less effective than
the conventional 6-month treatment for paucibacillary
leprosy, it is an operationally attractive field regimen
and has been recommended for use by the WHO.
Lepra reactions are treated as shown in Box .
Chloroquine can also be used.
Patient education
Educating leprosy patients about their disease is vital.Patients should be reassured that, after 3 days of chemotherapy, they are not infectious and can lead a normal social life. It should be emphasised that gross deformities are not inevitable.
Patients with anaesthetic hands or feet need to take
special care to avoid and treat burns and other minor
injuries. Good footwear is important. Physiotherapy
may be required to maintain range of movement of
affected muscles and neighbouring joints.
Prevention and control
The previous strategy of centralised leprosy control
campaigns has now been superseded by integrated programmes, with primary health-care workers in many
countries now responsible for case detection and provision
of MDT. It is not yet clear how successful this will
be, especially in the time-consuming area of disability
prevention.
BCG vaccination has been shown to give good but
variable protection against leprosy; adding killed M.
leprae to BCG does not enhance protection.
Principles of leprosy treatment
Modified WHO-recommended multidrug
therapy (MDT) regimens in leprosyRickettsial and related intracellular bacterial infections
Rickettsial fevers
The rickettsial fevers are the most common tick-borne infections. It is important to ask potentially infected patients about contact with ticks, lice or fleas.
There are two main groups of rickettsial fevers: spotted fevers and typhus .
Features of rickettsial infections
Pathogenesis
The rickettsiae are intracellular Gram-negative organisms,parasitise the intestinal canal of arthropods. Infection is usually conveyed to humans through the skin from the excreta of arthropods, but the saliva of some biting vectors is infected. The organisms multiply in capillary endothelial cells, producing lesions in the skin, CNS, heart, lungs, liver, kidneys and skeletal muscles. Endothelial proliferation, associated with a perivascular reaction, may cause thrombosis and purpura. In epidemic typhus, the brain is the target organ; in scrub typhus, the cardiovascular system and lungs in particular are attacked. An eschar, a black necrotic crusted sore, is often found in tick- and mite-borne typhus . This is due to vasculitis following immunological recognition of the inoculated organism. Regional lymph nodes often enlarge.
Spotted fever group
Rocky Mountain spotted feverRickettsia rickettsii is transmitted by tick bites. It is widely
distributed and increasing in western and south-eastern
states of the USA.
The incubation period is about 7 days. The rash appears
on about the third or fourth day of illness, looking at first
like measles, but in a few hours a typical maculopapular
eruption develops. The rash spreads in 24–48 hours from
wrists, forearms and ankles to the back, limbs and chest,
and then to the abdomen, where it is least pronounced.
Larger cutaneous and subcutaneous haemorrhages may
appear in severe cases. The liver and spleen become
palpable. At the extremes of life, the mortality is 2–12%.
Typhus group
Scrub typhus fever
Caused by Orientia tsutsugamushi (formerly Rickettsia tsutsugamushi), transmitted by mites. It occurs in the Far East. In many patients, one eschar or more develops, surrounded by an area of cellulitis .
and enlargement of regional lymph nodes. The incubation
period is about 9 days.
Mild or subclinical cases are common. The onset of
symptoms is usually sudden, with headache (often
retro-orbital), fever, malaise, weakness and cough.
In severe illness, the general symptoms increase, with
apathy and prostration. An erythematous maculopapularrash often appears on about the 5th–7th day and
spreads to the trunk, face and limbs, including the palms
and soles, with generalised painless lymphadenopathy.
The rash fades by the 14th day. The temperature rises
rapidly and continues as a remittent fever (i.e. the difference between maximum and minimum temperature
exceeds 1°C), remaining above normal with sweating
until it falls on the 12th–18th day. In severe infection, the
patient is prostrate with cough, pneumonia, confusion
and deafness. Cardiac failure, renal failure and haemorrhage may develop. Convalescence is often slow and tachycardia may persist for some weeks.
Epidemic (louse-borne) typhus
Epidemic typhus is caused by R. prowazekii and is transmitted by infected faeces of the human body louse,
usually through scratching the skin. Patients suffering
from epidemic typhus infect the lice, which leave when
the patient is febrile. In conditions of overcrowding, the
disease spreads rapidly. It is prevalent in parts of Africa,
especially and in the South American and Afghanistan. Large epidemics have occurred in Europe, usually as a sequel to war. The incubation period is usually 12–14 days.
There may be a few days of malaise but the onset is
more often sudden, with rigors, fever, frontal headaches,pains in the back and limbs, constipation and bronchitis.
The face is flushed and cyanotic, the eyes are congested
and the patient becomes confused. The rash appears on
the 4th–6th day. In its early stages, it disappears on pressure but soon becomes petechial with subcutaneous
mottling. It appears first on the anterior folds of the
axillae, sides of the abdomen or backs of hands, then on
the trunk and forearms. The neck and face are seldom
affected. During the second week, increase in severity. Sores develop on the lips. The tongue becomes dry, brown, shrunken and tremulous. The spleen is palpable, the pulse feeble and the patient stuporous and delirious.
The temperature falls rapidly at the end of the
second week and the patient recovers gradually. In fatalcases, the patient usually dies in the second week from
toxaemia, cardiac or renal failure, or pneumonia.
Endemic (flea-borne) typhus
Flea-borne or ‘endemic’ typhus caused by R. typhi is
endemic worldwide. Humans are infected when the
faeces or contents of a crushed flea, which has fed on an
infected rat, are introduced into the skin. The incubation
period is 8–14 days.
The symptoms resemble those of a mild louse-borne typhus. The rash may be scanty and transient.
Investigation of rickettsial infection
Routine blood investigations are not diagnostic but
malaria must be excluded by blood film examination
in most cases, and there is usually hepatitis and thrombocytopenia.
Diagnosis is made on clinical grounds
and response to treatment, and may be confirmed by
antibody detection or PCR in specialised laboratories.
Differential diagnoses include malaria, typhoid, meningococcal sepsis and leptospirosis.
Management of rickettsial fevers
The different rickettsial fevers vary greatly in severitybut all respond to tetracycline 500 mg 4 times daily,
doxycycline 200 mg daily or chloramphenicol 500 mg
4 times daily for 7 days. Louse-borne typhus and scrub
typhus can be treated with a single dose of 200 mg doxycycline, repeated for 2–3 days to prevent relapse.
Chloramphenicol- and doxycycline-resistant strains of
O. tsutsugamushi have been reported from Thailand and
patients here may need treatment with rifampicin.
Nursing care is important, especially in epidemic typhus. Sedation may be required for delirium and blood transfusion for haemorrhage. Relapsing fever and typhoid are common intercurrent infections in epidemic typhus, and pneumonia in scrub typhus. They must be sought and treated. Convalescence is usually protracted, especially in older people.
To prevent rickettsial infection, lice, fleas, ticks and
mites need to be controlled with insecticides.
Q fever
Q fever occurs worldwide and is caused by the rickettsia-like organism Coxiella burnetii, an obligate intracellular organism that can survive in the extracellular environment. Cattle, sheep and goats are important reservoirs and the organism is transmitted by inhalation of aerosolised particles. An important characteristic of C. burnetii is its antigenic variation, called phase variation, due to a change of lipopolysaccharide (LPS). When isolated from animals or humans, C. burnetii expresses phase I antigen and is very infectious (a single bacterium is sufficient to infect a human). In culture, there is an antigenic
shift to the phase II form, which is not infectious. This
antigenic shift can be measured and is valuable for the
differentiation of acute and chronic Q fever.
Clinical features
The incubation period is 3–4 weeks. The initial symptomsare non-specific with fever, headache and chills; in
20% of cases, a maculopapular rash occurs. Other presentations include pneumonia and hepatitis.
Chronic Q fever may present with osteomyelitis, encephalitis and endocarditis.
Investigations and management
Diagnosis is usually serological and the stage of the
infection can be distinguished by isotype tests and
phase-specific antigens.
Phase I and II IgM titres peak at 4–6 weeks.
In chronic infections, IgG titres to phase I and II antigens may be raised.
Prompt treatment of acute Q fever with doxycycline
reduces fever duration.Treatment of Q fever endocarditis is problematic, requiring prolonged therapy with doxycycline and rifampicin or ciprofloxacin with hydroxychloroquine; even then, organisms are not always eradicated.
Valve surgery is often required .
Bartonellosis
This group of diseases are caused by intracellular Gramnegative bacilli closely related to the rickettsiae, which have been discovered to be important causes of ‘culture-negative’ endocarditis. They are found in many domestic pets, such as cats, although for several the host is
ill defined . The principal human pathogens are Bartonella quintana, B. henselae and B. bacilliformis.
Bartonella infections are associated with the following:
• Trench fever. This is a relapsing fever with severe leg
pain and is caused by B. quintana. The disease is not
fatal but is very debilitating.
• Bacteraemia and endocarditis in the homeless.
Endocarditis due to B. quintana or henselae is
associated with severe damage to the heart valves.
Cat scratch disease. B. henselae causes this common benign lymphadenopathy in children and young adults. A vesicle or papule develops on the head, neck or arms after a cat scratch. The lesion resolves spontaneously but there may be regional lymphadenopathy that persists for up to 4 months
before also resolving spontaneously.
• Bacillary angiomatosis. This is an HIV-associated disease caused by B. quintana or henselae .
• Oroya fever and verruga peruana (Carrion’s disease).
This is endemic in areas of Peru. It is a biphasic disease caused by B. bacilliformis and is transmitted by sandflies of the genus Phlebotomus. Fever, haemolytic anaemia and microvascular thrombosis with end-organ ischaemia are features. It is frequently fatal if untreated.
Clinical diseases caused by Bartonella spp.
Investigations and management
Bartonellae can be grown from the blood but this
requires prolonged incubation using enriched media.
Serum antibody detection is possible.
Bartonella species are susceptible to β-lactams,
rifampicin, erythromycin and tetracyclines. Antibiotic
use is guided by clinical need.
Cat scratch disease usually resolves spontaneously but Bartonella endocarditis requires valve replacement and combination antibiotic therapy.
Chlamydial infections
TrachomaTrachoma is a chronic keratoconjunctivitis caused by
Chlamydia trachomatis, and is the most common cause of
avoidable blindness. The classic trachoma environment
is dry and dirty, causing children to have eye and nose
discharges. Transmission occurs through flies, on fingers
and within families.
In endemic areas, the disease is most common in children.
Chlamydial infections
Organism Disease causedChlamydia trachomatis Trachoma
Lymphogranuloma venereum
Cervicitis, urethritis, proctitis
Chlamydia psittaci Psittacosis
Chlamydophila
(Chlamydia) pneumoniaeAtypical pneumonia
Acute/chronic sinusitis
Pathology and clinical features
The onset is usually insidious. Infection may be asymptomatic, lasts for years. The conjunctiva of the upper lid is first affected with vascularisation and cellular infiltration. Early symptoms include conjunctival irritation and blepharospasm. The early follicles are characteristic , but clinical differentiation from conjunctivitis due to other viruses may be difficult. Scarring causes inversion of the lids (entropion) so that the lashes rub against the cornea (trichiasis). The cornea becomes vascularized and opaque. The problem may not be detected until vision begins to fail. InvestigationsIntracellular inclusions may be demonstrated in conjunctival
scrapings by staining with iodine or immunofluorescence.
Chlamydia may be isolated in chick embryo or cell culture.
Trachoma. Trachoma is characterised by hyperaemia and
numerous pale follicles.management
Intracellular inclusions may be demonstrated in conjunctivalscrapings by staining with immunofluorescence. Chlamydia may be isolated in chick embryo or cell culture. A single dose of azithromycin (20 mg/kg) has been shown to be superior to 6 weeks tetracycline ointment twice daily for individuals in mass treatment. Deformity and scarring of the lids, and corneal opacities, ulceration and scarring require surgical treatment after control of local infection.
Prevention
Personal and family cleanliness should be improved. Proper care of the eyes of newborn and children is essential. Family contacts should be examined. The WHO is promoting the SAFE strategy for trachoma control (surgery, antibiotics, facial cleanliness and environmental improvement).
Systemic protozoal infections
Malaria
Malaria in humans is caused by Plasmodium falciparum,
P. vivax, P. ovale, P. malariae and the predominantly
simian parasite, P. knowlesi. It is transmitted by the bite
of female anopheline mosquitoes and occurs throughout
the tropics and subtropics.
Recent estimates have put the number of episodes of clinical malaria at 515 million cases per year, with two-thirds of these occurring in sub-Saharan Africa,
especially amongst children and pregnant women.
Following previous WHO-sponsored campaigns focusing on prevention and effective treatment, the incidence of malaria was greatly reduced between 1950 and 1960, but since 1970 there has been resurgence. Furthermore, P. falciparum has now become resistant to chloroquine and sulfadoxine-pyrimethamine.
Pathogenesis
Life cycle of the malarial parasiteThe female anopheline mosquito becomes infected
when it feeds on human blood containing gametocytes,
the sexual forms of the malarial parasite .Development in the mosquito takes from 7 to 20 days, and results in sporozoites accumulating in the salivary glands and being inoculated into the human blood stream. Sporozoites disappear from human blood within half an hour and enter the liver. After some days, merozoites leave the liver and invade red blood cells, where further asexual cycles of multiplication take place, producing schizonts. Rupture of the schizont releases more merozoites into the blood and causes fever, the periodicity of which depends on the species of parasite.
P. vivax and P. ovale may persist in liver cells as
dormant forms, hypnozoites, capable of developing intomerozoites months or years later. Thus the first attack of clinical malaria may occur long after the patient has left the endemic area.
P. falciparum and P. malariae have no persistent exoerythrocytic phase but recrudescence of fever may result from multiplication in red cells which have not been eliminated by treatment and immune processes .
Pathology
Red cells infected with malaria are prone to haemolysis.
This is most severe with P. falciparum, which invades red
cells of all ages but especially young cells;
P. vivax and P. ovale invade reticulocytes, and
P. malariae normoblasts, so that infections remain lighter.
Anaemia may be profound and is worsened by dyserythropoiesis, splenomegaly and depletion of folate stores. In P. falciparum, red cells containing trophozoites adhere to vascular endothelium in postcapillary venules in brain, kidney, liver, lungs and gut by the formation of ‘knob’ proteins. They also form ‘rosettes’ and rouleaux with uninfected red cells. The vessels become congested, resulting in widespread organ damage ,exacerbated by rupture of schizonts, liberating toxic and antigenic substances. falciparum does not grow well in red cells that contain haemoglobin F, C or especially S.
Haemoglobin S heterozygotes (AS) are protected against the lethal complications of malaria.
P. vivax cannot enter red cells that lack the Duffy group.
Malarial parasites: life cycle. Hypnozoites(*) are present only in P. vivax and P. ovale infections.
Relationships between life cycle of
parasite and clinical features of malariaClinical features
The clinical features of malaria are non-specific and thediagnosis must be suspected in anyone returning from
an endemic area who has features of infection.
P. falciparum infection This is the most dangerous of the malarias and patients are either ‘killed or cured’. The onset is often insidious, with malaise, headache and vomiting. Cough and mild diarrhoea are also common.
The fever has no particular pattern. Jaundice is common due to haemolysis and hepatic dysfunction. The liver and spleen enlarge and may become tender. Anaemia develops rapidly, as does thrombocytopenia.
A patient with falciparum malaria, apparently not seriously ill, may rapidly develop dangerous complications . Cerebral malaria is manifested by confusion, seizures or coma, usually without localising signs.
Children die rapidly without any special symptoms other than fever.
Immunity is impaired in pregnancy and the parasite can preferentially bind to a placental protein known as chondroitin sulphate A. Abortion and intrauterine growth retardation from parasitisation of the maternal side of the placenta are frequent.
Previous splenectomy increases the risk of severe malaria.
P. vivax and P. ovale infection
In many cases, the illness starts with several days ofcontinued fever before the development of classical
bouts of fever on alternate days. Fever starts with a rigor.
The patient feels cold and the temperature rises to about
40°C. After half an hour to an hour, the hot or flush
phase begins. It lasts several hours and gives way to
profuse perspiration and a gradual fall in temperature.
The cycle is repeated 48 hours later. Gradually, the
spleen and liver enlarge and may become tender.
Anaemia develops slowly. Relapses are frequent in the
first 2 years after leaving the malarious area and infection
may be acquired from blood transfusion.
P. malariae infection
Bouts of fever every third day. Parasitaemia may persistfor many years, with the occasional recrudescence of
fever or without producing any symptoms.
Chronic P. malariae infection causes glomerulonephritis and longterm nephrotic syndrome in children.
Investigations
Giemsa-stained thick and thin blood films should be
examined. In the thick film, erythrocytes are lysed, releasing all blood stages of the parasite. This, as well as the fact that more blood is used in thick films, facilitates the diagnosis of low-level parasitaemia. A thin film is essential to confirm the diagnosis, to identify the species of parasite and, in P. falciparum infections, to quantify the parasite load (percentage of infected erythrocytes).
P. falciparum parasites may be very scanty, especially in patients who have been partially treated. With P. falciparum, only ring forms are normally seen in the early stages ,with the other species, all stages of the erythrocytic cycle may be found. Gametocytes appear after about 2 weeks, persist after treatment and are harmless, except that they are the source by which more mosquitoes become infected.
Immunochromatographic tests for malaria antigens,
such as OptiMal® (which detects the Plasmodium lactate
dehydrogenase of several species) and ParasightF®
(which detects the P. falciparum histidine-rich protein 2),
are extremely sensitive and specific for falciparum malaria but less so for other species.
The QBC Malaria Test is a fluorescence microscopy-based malaria diagnostic test which is also widely used.
DNA detection (PCR) is used mainly in research and
is useful for determining whether a patient has a recrudescence of the same malaria parasite or a re-infection with a new parasite.
Management
Mild P. falciparum malaria
Since P. falciparum is now resistant to chloroquine and
sulfadoxine-pyrimethamine (Fansidar) almost worldwide,
an artemisinin-based treatment is recommended.
Co-artemether (CoArtem® or Riamet®) contains artemether and lumefantrine and is given as 4 tablets at 0,
8, 24, 36, 48 and 60 hours.
Alternatives are quinine by mouth (600 mg of quinine salt 3 times daily for 5–7 days), together with or followed by either doxycycline (200 mg once daily for 7 days) or clindamycin
(450 mg 3 times daily for 7 days) or atovaquone-proguanil
(Malarone®, 4 tablets once daily for 3 days).
Doxycycline should not be used in pregnancy and artemether should be avoided in early pregnancy.
WHO policy in Africa is moving towards always
using artemisinin-based combination therapy (ACT),
e.g. co-artemether or artesunate-amodiaquine. In India
and other areas, artesunate (200 mg orally daily for
3 days) and mefloquine (1 g orally on day 2 and 500 mg
orally on day 3) may be used. Unfortunately, artemisinin
resistance has now been reported in Cambodia.
Features of P. falciparum infection.
Severe manifestations/complications of falciparum malaria and their immediate management
Severe manifestations/complications of falciparum malaria and their immediate management'cont'd
Severe manifestations/complications of falciparum malaria and their immediate management'cont'd
Severe manifestations/complications of falciparum malaria and their immediate management'cont'd
Complicated P. falciparum malaria
Severe malaria should be considered in any non-immunepatient with a parasite count > 2% and is a medical emergency (see Box). Management includes early and appropriate antimalarial chemotherapy, active treatment of complications, correction of fluid, electrolyte and acid–base balance. The treatment of choice is IV artesunate
2.4 mg/kg IV at 0, 12 and 24 hours and then once daily for 7 days. However, as soon as the patient has recovered sufficiently to swallow tablets, oral artesunate 2 mg/kg once daily is given instead of IV therapy, to complete a total cumulative dose of 17– 18 mg/kg.
Rectal administration of artesunate is also being developed to allow administration in remote rural areas.
Quinine salt can also be used and is started with a
loading dose infusion of 20 mg/kg over 4 hours, up to a maximum of 1.4 g. This is followed by maintenance doses of 10 mg/kg quinine salt given as 4-hour infusions 2–3 times daily, up to a maximum of 700 mg per dose, until the patient can take drugs orally.The loading dose should not be given if the patient has received quinine, quinidine or mefloquine during the previous 24 hours. Patients should be monitored by ECG, with special attention to QRS duration and QT interval. Mefloquine should not be used for severe malaria since no parenteral form is available.
Exchange transfusion has not been tested in randomised
controlled trials but may be beneficial for nonimmune
patients with persisting high parasitaemias
(> 10% circulating erythrocytes).
Management of non-falciparum malaria
P. vivax, P. ovale and P. malariae infections should betreated with oral chloroquine: 600 mg chloroquine base,
followed by 300 mg base in 6 hours, then 150 mg base
twice daily for 2 more days. Some chloroquine resistance
has been reported from Indonesia. Late relapses can be prevented by prescribing antimalarial drugs in suppressive doses. However, ‘radical cure’ is now achieved in most patients with P. vivax or P. ovale malaria using a course of primaquine (15 mg daily for 14 days), which destroys the hypnozoite phase in the liver. Haemolysis may develop in those who are G6PD-deficient. Cyanosis due to the formation of methaemoglobin in the red cells is more common but not dangerous.
Prevention
Clinical attacks of malaria may be preventable with
chemoprophylaxis using chloroquine, atovaquone plus
proguanil (Malarone), doxycycline or mefloquine. Box.
gives the recommended doses for protection of the
non-immune. The risk of malaria in the area to be visited
and the degree of chloroquine resistance guide the recommendations for prophylaxis.
Fansidar should not be used for chemoprophylaxis, as deaths have occurred from agranulocytosis
or Stevens– Johnson syndrome .
Mefloquine is useful in areas of multiple drug resistance. Experience shows it to be safe for at least 2 years, but there are several contraindications to its use .
Mefloquine should be started 2–3 weeks before travel to give time for assessment of side-effects.
Chloroquine should not be taken continuously as a prophylactic for > 5 years without regular ophthalmic examination, as it may cause irreversible retinopathy.
Pregnant and lactating women may take proguanil or chloroquine safely.
Prevention also involves advice about the use of
high-percentage diethyltoluamide (DEET), covering up
extremities when out after dark, and sleeping under
permethrin-impregnated mosquito nets .
Malaria control in endemic areas
Successful programmes have involved a combination ofvector control, including indoor residual spraying, use
of long-lasting insecticide-treated bed nets (ITNs) and
intermittent preventative therapy (IPT; repeated dose of
prophylactic drugs in high-risk groups, such as children
and pregnant women) .
Development of a fully protective malaria vaccine is
still some way off, which is not surprising, considering
that natural immunity is incomplete and not long-lived.
There is, however, some evidence that vaccination can
reduce the incidence of severe malaria in populations.
Trial vaccines are being evaluated in Africa.
Chemoprophylaxis of malaria1
Prevention of malaria
BabesiosisThis is caused by a tick-borne intra-erythrocytic protozoon
parasite. There are more than 100 species of Babesia,
all of which have an animal reservoir, typically either
rodents or cattle, and are transmitted to humans via the
tick vector Ixodes scapularis. Most cases of babesiosis in
the USA are due to B. microti and most in Europe to
B. divergens. Patients present with fever and malaise
1–4 weeks after a tick bite. Illness may be complicated
by haemolytic anaemia. Severe illness is seen in
splenectomised patients. The diagnosis is made by
blood-film examination.
Treatment is with quinine and clindamycin.
African trypanosomiasis
(sleeping sickness)
African sleeping sickness is caused by trypanosomes
conveyed to humans by the bites of infected tsetse flies, and is unique to sub-Saharan Africa. Trypanosoma brucei gambiense trypanosomiasis has a wide distribution in West and Central Africa and accounts for 90% of reported cases.
T. brucei rhodesiense trypanosomiasis is found in parts of East and Central Africa. No animal reservoir has been identified for T. gambiense. T. rhodesiense has a large reservoir in numerous wild animals and transmission takes place in the shade of woods bordering grasslands.
Clinical features
A bite by a tsetse fly is painful and commonly becomesinflamed, but if trypanosomes are introduced, the site
may again become painful and swollen about 10 days
later (‘trypanosomal chancre’) and the regional lymph
nodes enlarge (‘Winterbottom’s sign’).
Within 2–3 weeks of infection, the trypanosomes invade the blood stream.
The disease is characterised by
an early haematolymphatic
stage and a late encephalitic stage, in which the parasite crosses the blood–brain barrier and chronic
encephalopathy develops.
Rhodesiense infections
The disease is more acute and severe than in gambiense, within days or a few weeks, the patient is usually severely ill and may develop pleural effusions and myocarditis orHepatitis, a petechial rash. The patient may die before there are signs of involvement of the CNS.
Gambiense infections
The disease usually runs a slow course over months or years, with irregular bouts of fever and enlargement of lymph nodes, firm, discrete, rubbery and painless, and prominent in the posterior triangle of the neck. The spleen and liver may become palpable. Without treatment, the CNS is invaded, headache and changed behaviour, insomnia by night and sleepiness by day, mental confusion and eventually tremors, pareses, coma and death.
Investigations
Trypanosomiasis should be considered in any febrile
patient from an endemic area. In rhodesiense infections,
thick and thin blood films, stained as for the detection
of malaria, will reveal trypanosomes. The trypanosomes
may be seen in the blood or from puncture of the primary
lesion in the earliest stages of gambiense infections, but it
is usually easier to demonstrate them by aspiration
of a lymph node. Concentration methods include buffy coat microscopy. The diagnosis is often made by demonstration of antibodies using a simple, rapid screening card agglutination trypanosomiasis test (CATT), followed by parasitological confirmation.
If the CNS is affected, the cell count (> 20 × 109 leucocytes per litre) and protein content of the CSF are increased and the glucose is diminished. A very high level of serum IgM or IgM in the CSF is suggestive of trypanosomiasis.
Recognition of CNS involvement is critical.
Management
Unfortunately, therapeutic options for African trypanosomiasis are limited and most of the antitrypanosomal drugs are toxic and expensive. The prognosis is good if treatment is begun early, before the brain has been invaded. At this stage, IVsuramin, after a test dose of 100–200 mg, should be given for rhodesiense infections (1 g on days 1, 3, 7, 14 and 21). For gambiense infections, Im or IVpentamidine 4 mg/kg for 10 days is given .
Once the nervous system is affected, treatment with
melarsoprol (an arsenical) is effective for both East andWest African diseases. It is used in a dose of 2–3.6 mg/
kg/day IV for the first course and 3.6 mg/kg/day thereafter.
Three 3-day treatment courses are given, separated
by 7 days and by 10–21 days. Melarsoprol should be
given with prednisolone 1 mg/kg up to 40 mg started
1–2 days before, continued during and tapered after
treatment to reduce side-effects.
Treatment-related mortality with melarsoprol is 4–12% due to reactive encephalopathy.
For CNS infections due to gambiense, eflornithine (DFMO), an irreversible inhibitor of ornithine decarboxylase (100 and 150 mg/kg IV 4 times daily for 14 days for adults and children, respectively), is considered to be a safer and cost-effective option.
Combinations of eflornithine (400 mg daily for 7 days)
with oral nifurtimox (15 mg/kg daily for 15 days) have
been shown to decrease relapses, deaths and drug toxicity.
Prevention
In endemic gambiense areas, various measures may be
taken against tsetse flies, and field teams help to detect
and treat early human infection.
In rhodesiense areas, control is difficult.
Drugs used to treat human African trypanosomiasis
American trypanosomiasis(Chagas’ disease)
Chagas’ disease occurs widely in South and Central
America. The cause is Trypanosoma cruzi, transmitted to
humans from the faeces of a reduviid (triatomine) bug,
in which the trypanosomes have a cycle of development
before becoming infective to humans. These bugs live in
wild forests in crevices, burrows and palm trees. It lives in the mud and wattle walls and emerges at night to feed and defecate on the sleeping occupants.
Infected faeces are rubbed in through the conjunctiva,
mucosa of mouth or nose, or abrasions of the skin. In some
areas, blood transfusion accounts for about 5% of cases.
Congenital transmission occasionally occurs.
Pathology
The trypanosomes migrate via the blood stream, develop
into amastigote forms in the tissues and multiply intracellularly by binary fission. In the acute phase (primarily cell-mediated), inflammation of parasitised as well as non-parasitised cardiac muscles and capillaries occurs, resulting in acute myocarditis. In the chronic phase,
focal myocardial atrophy, signs of chronic passive congestion and thromboembolic phenomena, cardiomegaly
and apical cardiac aneurysm are salient findings. In the
digestive form of disease, focal myositis and discontinuous
lesions of the intramural myenteric plexus, predominantly
in the oesophagus and colon, are seen.
Clinical features
Acute phaseClinical manifestations of the acute phase are seen in
only 1–2% of individuals who are infected before the age
of 15 years. Young children (1–5 years) are most commonly affected. The entrance of T. cruzi through an
abrasion produces a dusky-red firm swelling and
enlargement of regional lymph nodes. A conjunctival
lesion, although less common, is characteristic; the unilateral
firm, reddish swelling of the lids may close the
eye and constitutes ‘Romaٌa’s sign’. In a few patients,
an acute generalised infection soon appears, with a transient morbilliform or urticarial rash, fever, lymphadenopathy and enlargement of the spleen and liver.
In a small minority of patients, acute myocarditis and heart failure or neurological features, including personality changes and signs of meningoencephalitis, may be seen.
The acute infection may be fatal to infants.
Chronic phase
About 50–70% of infected patients become seropositive .
They have a normal lifespan with no symptoms, but are a natural reservoir for the disease and maintain the life cycle of parasites . After a latent period of several years, 10–30% of chronic cases develop cardiomyopathy characterised by cardiac dilatation, arrhythmias, partial or complete heart block and sudden death. In nearly 10% of patients, damage to Auerbach’s plexus results in dilatation of various parts of the alimentary canal, especially the colon and oesophagus, so-called ‘mega’ disease.
Dilatation of the bile ducts and bronchi is also a recognised sequela.
Autoimmune processes may be responsible for much ofthe damage. There are geographical variations of the
basic pattern of disease. Reactivation of Chagas’ disease
can occur in patients with HIV if the CD4 count falls
lower than 200 cells/mm3 ,this can cause spaceoccupying lesions with a presentation similar to Toxoplasma encephalitis, encephalitis, meningoencephalitis
or myocarditis.
Investigations
T. cruzi is easily detectable in a blood film in the acuteillness. In chronic disease, it may be recovered in up to
50% of cases by xenodiagnosis, in which infection-free,
laboratory-bred reduviid bugs are allowed to feed on the
patient; subsequently, the hind gut or faeces of the bug
are examined for parasites. Parasite DNA detection by
PCR in the patient’s blood is a highly sensitive method
for documentation of infection and, in addition, can be
employed in faeces of bugs used in xenodiagnosis tests
to improve sensitivity.
Antibody detection is also highly sensitive.
Management and prevention
Parasiticidal agents are used to treat the acute phase,
congenital disease and early chronic phase (within
10 years of infection). Nifurtimox is given orally. The
dose, which has to be carefully supervised to minimise
toxicity while preserving parasiticidal activity, is 10 mg/
kg divided into three equal doses, daily orally for
60–90 days. The paediatric dose is 15 mg/kg daily. Cure
rates of 80% in acute disease are obtained. Benznidazole
is an alternative, given at a dose of 5–10 mg/kg daily
orally, in two divided doses for 60 days; children receive
10 mg/kg daily. Both nifurtimox and benznidazole are
toxic, with adverse reaction rates of 30–55%.
Specific drug treatment of the chronic form is now increasingly favoured, but, in the cardiac or digestive ‘mega’ diseases, it does not reverse established tissue damage.
Surgery may be needed.
Preventative measures include improving housing
and destruction of reduviid bugs by spraying of houses
with insecticides.
Blood donors should be screened.
Toxoplasmosis
Toxoplasma gondii is an intracellular parasite. The sexual
phase of the parasite’s life cycle occurs in the
small intestinal epithelium of the domestic cat. Oِcysts
are shed in cat faeces and are spread to intermediate
hosts (pigs, sheep and also humans) through widespread
contamination of soil. Oِcysts may survive in
moist conditions for weeks or months. Once they are
ingested, the parasite transforms into rapidly dividing
tachyzoites through cycles of asexual multiplication.
This leads to the formation of microscopic tissue cysts
containing bradyzoites, which persist for the lifetime of
the host. Cats become infected or re-infected by ingesting
tissue cysts in prey such as rodents and birds.
Human acquisition of infection occurs via Oِcysts
cyst contaminated soil, salads and vegetables, or by theingestion or tasting of raw or undercooked meats containing tissue cysts. Sheep, pigs and rabbits are the most common meat sources.
Outbreaks of toxoplasmosis have
been linked to the consumption of unfiltered water. In
developed countries, toxoplasmosis is the most common
protozoal infection; around 22% of adults in the UK are
seropositive. Most primary infections are subclinical;
however, toxoplasmosis is thought to account for about 15% of heterophile antibody-negative glandular fever .
In India or Brazil, approximately 40–60% of
pregnant females are seropositive for T. gondii. In HIV-1
infection (p. 402), toxoplasmosis is an important opportunistic infection with considerable morbidity and mortality.
Generalised toxoplasmosis has been described after accidental laboratory infection with highly virulent strains.
Life cycle of Toxoplasma gondii.
Clinical featuresIn most immunocompetent individuals, including children
and pregnant women, the infection goes unnoticed.
In approximately 10% of patients, it causes a self-limiting
illness, most common in adults aged 25–35 years.
The most common presenting feature is painless lymphadenopathy, either local or generalised. In particular, the cervical nodes are involved, but mediastinal, mesenteric
or retroperitoneal groups may be affected. The spleen is
seldom palpable.
Most patients have no systemic symptoms, but some complain of malaise, fever, fatigue, muscle pain, sore throat and headache.
Complete resolution usually occurs within a few months, although symptoms and lymphadenopathy tend to fluctuate
unpredictably and some patients do not recover completely
for a year or more.
Very infrequently, patients may develop encephalitis, myocarditis, polymyositis, pneumonitis or hepatitis. Retinochoroiditis is nearly always the result of congenital infection but has also been reported in acquired disease.
Congenital toxoplasmosis
Acute toxoplasmosis, mostly subclinical, affects 0.3–1%
of pregnant women, with an approximately 60% transmission rate to the fetus, which rises with increasing
gestation. Seropositive females infected 6 months before
conception have no risk of fetal transmission.
Congenital disease affects approximately 40% of infected fetuses, and is more likely and more severe with infection early in gestation .
Many fetal infections are subclinical at birth but long-term sequelae include
retinochoroiditis, microcephaly and hydrocephalus.
Investigations
In contrast to immunocompromised patients, in whomthe diagnosis often requires direct detection of parasites,
serology is often used in immunocompetent individuals. The Sabin–Feldman dye test (indirect fluorescent antibody
test), which detects IgG antibody, is most commonly
used. Recent infection is indicated by a fourfold
or greater increase in titre when paired sera are tested
in parallel. Peak titres of 1/1000 or more are reached
within 1–2 months of the onset of infection, and the dye
test then becomes an unreliable indicator of recent infection.
The detection of significant levels of Toxoplasma specific IgM antibody may be useful in confirming acute infection.
A false-positive result or persistence of IgM antibodies for years after infection makes interpretation difficult; however,
negative IgM antibodies virtually rule out acute infection.
During pregnancy, it is critical to differentiate
between recent and past infection; the presence of
high-avidity IgG antibodies excludes infection acquired
in the preceding 3–4 months.
If necessary, the presence of Toxoplasma organisms in
a lymph node biopsy can be sought by staining sections
histochemically with T. gondii antiserum, or by the use
of PCR to detect Toxoplasma-specific DNA.
Management
In immunocompetent, uncomplicated is self-limiting and responds poorly to antimicrobial therapy. Treatment with pyrimethamine, sulfadiazine and folinic acid is therefore usually reserved for rare cases of severe or progressive disease, and for infection in immunocompromised patients.A pregnant with established recent infection, spiramycin (3 g daily in divided doses) should be given until term. Once fetal infection is established, treatment with sulfadiazine and pyrimethamine plus calcium folinate is recommended (spiramycin does not cross the placental barrier). The cost/benefit of routine screening and treatment in pregnancy is being debated. There is insufficient evidence to determine the effects on mother or baby treatment for women who seroconvert in pregnancy.Leishmaniasis
Leishmaniasis is caused by unicellular, flagellate, intracellular protozoa belonging to the genus Leishmania(order Kinetoplastidae). There are 21 leishmanial species
that cause several diverse clinical syndromes, which can
be placed into three broad groups:
• Visceral leishmaniasis (VL, kala-azar)
• Cutaneous leishmaniasis (CL)
• Mucosal leishmaniasis (ML).
Epidemiology and transmission
Although most clinical syndromes are caused by
zoonotic transmission of parasites from animals (chiefly
canine and rodent reservoirs) to humans through phlebotomine sandfly vectors , humans are the
only known reservoir (anthroponotic) in major VL foci
in the Indian subcontinent and for transmission of leishmaniasis between injection drug-users .
The life cycle of Leishmania is shown in Figure .
Flagellar promastigotes (10–20 μm) are introduced by
the feeding female sandfly, are taken up by neutrophils, which undergo apoptosis and are then engulfed by macrophages, in which the parasites transform into amastigotes (2–4 μm; Leishman–Donovan body).
These multiply, ultimately causing lysis of the
macrophages and infection of other cells. Sandflies pickup amastigotes when feeding on infected patients or
animal reservoirs. In the sandfly, the parasite transforms
into a flagellar promastigote, which multiplies by binary
fission in the gut of the vector and migrates to the proboscis
to infect a new host. Sandflies live in hot and humid climates in the cracks and crevices of mud or straw houses and lay eggs in organic matter.
People living in such conditions are more prone to acquiring the disease. Female sandflies bite during the night and preferentially feed on animals; humans are incidental hosts.
Transmission of leishmaniasis.
A Zoonotic transmission.
B Anthroponotic transmission.
C Anthroponotic transmission in the injection drug-user.
Life cycle of Leishmania.
Visceral leishmaniasis (kala-azar)VL is caused by the protozoon Leishmania donovani
complex (comprising L. donovani, L. infantum and L.
chagasi). India, Sudan, Bangladesh and Brazil account for
90% of cases of VL, while other affected regions include the Mediterranean, East Africa, China, Arabia, Israel and
other South American countries (Fig.). In addition
to sandfly transmission, VL has also been reported to
follow blood transfusion, and disease can present unexpectedly in immunosuppressed patients – for example, after renal transplantation and in HIV infection.
The great majority of people infected remain asymptomatic. In visceral diseases, the spleen, liver, bone
marrow and lymph nodes are primarily involved.
Clinical features
The incubation period weeks to months (occasionally several years). The first sign is high fever, usually accompanied by rigor and chills. Fever decreases over time, and become afebrile for intervening periods ranging from weeks to months. This is followed by a relapse of fever, often of lesser intensity. Splenomegaly develops quickly in the first few weeks and becomes massive. Moderate hepatomegaly occurs later. Lymphadenopathy is seen. Blackish discoloration of the skin, from which the disease derived its name, kala-azar (the Hindi word for ‘black fever’), is a feature of advanced illness. Pancytopenia ,severe anaemia develops rapidly, and can result in congestive cardiac failure.
Thrombocytopenia, compounded by hepatic dysfunction, may result in bleeding from the retina, GIT and nose.
In advanced illness, hypoalbuminaemia may manifest as pedal oedema, ascites and anasarca. As the disease advances, there is profound immunosuppression and secondary infections are very common, include tuberculosis, pneumonia, amoebic or bacillary dysentery, herpes zoster and chickenpox. Skin infections, boils, cellulitis and scabies are common. Without adequate treatment, most VL patients die.
Investigations
Pancytopenia is dominant.Polyclonal hypergammaglobulinaemia, IgG followed by IgM. Hypoalbuminaemia.A mastigotes (Leishman–Donovan bodies) in splenic smears is efficient means of diagnosis, with 98% sensitivity , however, it carries a risk of serious haemorrhage in inexperienced hands.
Safer methods, bone marrow or lymph node smears, are not as sensitive. Parasites may be demonstrated in buffy coat smears, especially in immunosuppressed. Sensitivity can be improved by culturing the aspirate material or by PCR for DNA detection and species identification. Serodiagnosis, by ELISA or immunofluorescence antibody. Management
Pentavalent antimonials
Antimony (Sb) compounds were the first drugs to be used for the treatment of leishmaniasis and remain the mainstay of treatment in most parts of the world,available as sodium stibogluconate (100 mg/mL) .The daily dose is 20 mg/kg body weight, given either IV or IM for 28–30 days.
Side-effects are common and include arthralgias, myalgias, raised hepatic transaminases, pancreatitis and ECG changes (T wave inversion).Severe cardiotoxicity, manifest by concave ST segment elevation, prolongation of QTc and ventricular dysrhythmias and death .
Amphotericin B
Given once daily or on alternate days 0.75–1.00 mg/kg for 15–20 doses, is used as the first-line in regions where there is a significant level of Sb unresponsiveness. It has a cure rate of nearly 100%. Infusion-related side-effects, e.g. high fever with rigor, thrombophlebitis, diarrhoea and vomiting, are extremely common. Serious adverse events, renal or hepatic toxicity, hypokalaemia and thrombocytopenia, are not uncommon. Lipid formulations are less toxic.
Paromomycin is an aminoglycoside that has undergone
trials in India and Africa, and is highly effective ifgiven intramuscularly at 11 mg/kg body weight of
paromomycin base, daily for 3 weeks. No significant
auditory or renal toxicity is seen. The drug has been
approved in India for the treatment of VL.
Pentamidine isethionate was used to treat Sbrefractory
patients with VL. However, declining efficacy
and serious side-effects, such as type 1 diabetes mellitus,
hypoglycaemia and hypotension, have led to it being
abandoned.
Multidrug therapy of VL is likely to be used
increasingly to prevent emergence of drug resistance .
Splenic smear showing numerous intracellular, and a few extracellular, amastigotes.
Differential diagnosisThis includes malaria, typhoid, tuberculosis, schistosomiasis
and many other infectious and neoplastic conditions,
some of which may coexist with VL. Fever,
splenomegaly, pancytopenia and non-response to antimalarial therapy may provide clues before specific laboratory diagnosis is made.
Response to treatment
A good response results in abatement of fever, a feelingof well-being, gradual decrease in spleen size, weight
gain and recovery of blood counts. Patients should be
followed regularly for a period of 6–12 months, as a
small minority may experience a relapse of the disease
during this period, irrespective of the treatment regimen.
Combination therapy for visceral leishmaniasis
Post-kala-azar dermal leishmaniasis
After treatment and apparent recovery from the visceral
disease in India and Sudan, some patients develop
dermatological manifestations due to local parasitic
infection.
Clinical features
In India, dermatological changes occur in a small minority
of patients 6 months to at least 3 years after the initial
infection. They are seen as macules, papules, nodules
(most frequently) and plaques, which have a predilection
for the face, especially the area around the chin. The
face often appears erythematous . Hypopigmented
macules can occur over all parts of the body
and are highly variable in extent and location.
There are no systemic symptoms and no spontaneous healing.
In Sudan, approximately 50% of patients with VLdevelop post-kala-azar dermal leishmaniasis (PKDL),
experiencing skin manifestations concurrently with VL
or within the following 6 months. In addition to the dermatological features described above, a measles-like
micropapular rash (Fig.) may be seen all over the
body. In Sudan, children are more frequently affected
than in India. Spontaneous healing occurs in about
three-quarters of cases within 1 year.
Post-kala-azar dermal leishmaniasis.
A In India, with macules, papules, nodules and plaques. From Sundar S, et al. 2006B In Sudan, with micronodular rash.
Investigations and management
The diagnosis is clinical, supported by demonstration ofscanty parasites in lesions by slit-skin smear and culture.
Immunofluorescence and immunohistochemistry may
demonstrate the parasite in skin tissues. In the majority
of patients, serological tests (direct agglutination test or
k39 strip tests) are positive.
Treatment of PKDL is difficult. In India, Sb for
120 days, several courses of amphotericin B infusions, or
miltefosine for 12 weeks is required. In Sudan, Sb for
2 months is considered adequate. In the absence of a
physical handicap, most patients are reluctant to complete
the treatment. PKDL patients are a human reservoir,
and focal outbreaks have been linked to patients
with PKDL in areas previously free of VL.
Prevention and control
Sandflies are extremely sensitive to insecticides, and
vector control through insecticide spray is very important.
Mosquito nets or curtains treated with insecticides
will keep out the tiny sandflies. In endemic areas with
zoonotic transmission, infected or stray dogs should be
destroyed.
In areas with anthroponotic transmission, early diagnosis
and treatment of human infections, to reduce the
reservoir and control epidemics of VL, is extremely
important. Serology is useful in diagnosis of suspected
cases in the field. No vaccine is currently available.
Cutaneous and mucosal leishmaniasis
Cutaneous leishmaniasisCL (oriental sore) occurs in both the Old World and the
New World (the Americas). In the Old World, CL is mild. It is found around the Mediterranean basin, throughout the Middle East and Central Asia as far as Pakistan, and in sub-Saharan West Africa and Sudan . The causative organisms for Old World zoonotic CL are L. major, L. tropica and L. aethiopica . In recent years, there has been an increase in the incidence of zoonotic CL in both the Old and the New World due to urbanisation and deforestation which led to peridomestic transmission.
Types of Old World cutaneous leishmaniasis
Pathogenesis
Inoculated parasites are taken up by dermal macrophages,in which they multiply and form a focus for lymphocytes, epithelioid cells and plasma cells. Self healing may occur with necrosis of infected macrophages, or the lesion may become chronic with ulceration of the overlying epidermis. Clinical features
The incubation period is typically 2–3 months (2 weeks to 5 years). In all types of CL, the common feature is development of a papule followed by ulceration of the skin with raised borders, usually at the site of the bite of the vector. Lesions, single or multiple, start as small red papules that increase gradually in size, reaching 2–10 cm in diameter. A crust forms, overlying an ulcer with a granular base .
There can be satellite lesions, especially in L. major and occasionally in L. tropica infections. Regional lymphadenopathy, pain, pruritus and secondary bacterial infections may occur.
If immunity is good, there is usually spontaneous healing in L. tropica, L. major and L. mexicana lesions. In some patients with anergy to Leishmania, the skin lesions of L. aethiopica, L. mexicana and L. amazonensis infections progress to the development of diffuse CL; this is characterised by spread of the infection from the initial ulcer, face, to involve the whole body in the form of non-ulcerative nodules.
Cutaneous leishmaniasis.
A Papule.B Ulcer.
Mucosal leishmaniasis
In L. (V.) brasiliensis complex infections, cutaneous lesions may be followed by mucosal spread of the disease simultaneously or even years later. Young men with chronic lesions are particularly at risk, and between 2% and 40% of infected persons develop ‘espundia’, metastatic lesions in the mucosa of the nose or mouth. This is characterised by thickening and erythema of the nasal mucosa, typically starting at the junction of the nose and upper lip. Later, ulceration develops. The lips, soft palate, fauces and larynx may also be destroyed, leading to considerable suffering and deformity. There is no spontaneous healing, and death may result from severe respiratory tract infections due to massive destruction of the pharynx.Investigations in CL and ML
CL is often diagnosed on clinical characteristics. However, parasitological confirmation is important. Amastigotes can be demonstrated on a slit-skin smear with Giemsa staining; alternatively, they can be cultured from the sores early during the infection. Culture of fine needle aspiration material has been reported to be the most sensitive method. The leishmanin skin test measures delayed-type hypersensitivity to killed Leishmania organisms. A positive test is defined as induration of more than 5 mm 48 hours after intradermal injection.The test is positive, except in diffuse CL and during active VL. PCR is used increasingly for diagnosis and speciation, which is useful in selecting therapy.
Management of CL and ML
Small lesions may self-heal or are treated by freezing
with liquid nitrogen or curettage. There is no ideal antimicrobial therapy. Treatment should be individualised
on the basis of the causative organism, severity, availability of drugs, tolerance and local resistance patterns.
In CL, topical application of paromomycin 15% plus
methylbenzethonium chloride 12% is beneficial. Intralesional antimony (Sb: 0.2–0.8 mL/lesion) up to 2 g
seems to be rapidly effective in suitable cases; it is well
tolerated and economic, and is safe. In ML, and in CL when the lesions are multiple or in a disfiguring site, it is better to treat with parenteral Sb in a dose of 20 mg/kg/day (usually given for 20 days for CL and 28 days for ML), or with conventional or liposomal amphotericin B.
Sb is also indicated to prevent the development of
mucosal disease. Refractory CL or ML should be treated with an amphotericin B preparation.Two to four doses of pentamidine (2–4 mg/kg), administered on alternate days, are effective.
In ML, 8 injections of pentamidine (4 mg/kg on alternate days) cure the majority of patients. Ketoconazole (600 mg daily for 4 weeks) has shown some potential against L. mexicana infection. In
Saudi Arabia, fluconazole (200 mg daily for 6 weeks)
reduced healing times and cured 79% of patients. In India, itraconazole (200 mg daily for 6 weeks) produced good results.
Prevention of CL and ML
Personal protection against sandfly bites is important.
No effective vaccine is yet available
Gastrointestinal protozoal infections
AmoebiasisAmoebiasis is caused by Entamoeba histolytica, which is
spread between humans by its cysts. It is one of the
leading parasitic causes of morbidity and mortality in
the tropics and is occasionally acquired in other countries,
such as the UK. Two non-pathogenic Entamoeba
species (E. dispar and E. moshkovskii) are morphologically
identical to E. histolytica, and are distinguishable only by
molecular techniques, isoenzyme studies or monoclonal
antibody typing. However, only E. histolytica causes
amoebic dysentery or liver abscess.
The life cycle of the amoeba is shown in Figure .
Amoebiasis.
A The life cycle of Entamoeba histolytica.B The chocolate-brown appearance of aspirated material from an amoebic liver abscess.
Pathology
Cysts of E. histolytica are ingested in water or uncookedfoods contaminated by human faeces. Infection may
also be acquired through anal/oral sexual practices. In
the colon, trophozoite forms emerge from the cysts. The
parasite may invade the mucous membrane of the large
bowel, producing lesions that are maximal in the
caecum but found as far down as the anal canal. These
are flask-shaped ulcers, varying greatly in size and surrounded by healthy mucosa.
A localised granuloma (amoeboma), presenting as a palpable mass in the rectum or a filling defect in the colon on radiography, is a rare complication which should be differentiated from colonic carcinoma.
Amoebic ulcers may cause severe haemorrhage but rarely perforate the bowel wall.
Amoebic trophozoites can emerge from the vegetativecyst from the bowel and be carried to the liver in a
portal venule. They can multiply rapidly and destroy the
liver parenchyma, causing an abscess .
The liquid contents at first have a characteristic pinkish
colour, which may later change to chocolate-brown
(like anchovy sauce).
Cutaneous amoebiasis,
though rare, causes progressive genital, perianal or peri-abdominal surgical wound ulceration.
Clinical features
Intestinal amoebiasis – amoebic dysentery Most amoebic infections are asymptomatic. The incubation period of amoebiasis ranges from 2 weeks to many years, followed by a chronic course with abdominal pains and two or more unformed stools a day.
Offensive diarrhoea alternating with constipation, and blood or mucus in the stool, are common. There may be abdominal pain, especially right lower quadrant (which may simulate acute appendicitis).
A dysenteric presentation with passage of blood, simulating bacillary dysentery or ulcerative colitis, occurs particularly in older people, in the puerperium and with superadded pyogenic infection of the ulcers.
Amoebic liver abscess
The abscess is usually found in the right hepatic lobe.There may not be associated diarrhoea. Early symptoms
may be local discomfort only and malaise; later, a swinging temperature and sweating may develop, usually
without marked systemic symptoms or associated cardiovascular signs. An enlarged, tender liver, cough and
pain in the right shoulder are characteristic, but symptoms
may remain vague and signs minimal. A large abscess may penetrate the diaphragm and rupture into the lung, from where its contents may be coughed up through a hepaticobronchial fistula. Rupture into the pleural or peritoneal cavity, or rupture of a left lobe abscess in the pericardial sac, is less common but more serious.
Investigations
The stool and any exudate should be examined at onceunder the microscope for motile trophozoites containing
red blood cells. Movements cease rapidly as the stool
preparation cools. Several stools may need to be examined in chronic amoebiasis before cysts are found. Sigmoidoscopy may reveal typical flask-shaped ulcers,
which should be scraped and examined immediately for
E. histolytica. In endemic areas, one-third of the population
are symptomless passers of amoebic cysts.
An amoebic abscess of the liver is suspected on clinical
grounds; there is often a neutrophil leucocytosis and
a raised right hemidiaphragm on chest X-ray. Confirmation is by ultrasonic scanning.
Aspirated pus from an amoebic abscess has the characteristic anchovy sauce or chocolate-brown appearance but only rarely contains free amoebae .
Serum antibodies are detectable by immunofluorescence
in over 95% with hepatic amoebiasis and intestinal amoeboma, but in only about 60% of dysenteric amoebiasis. DNA detection by PCR has been shown to be useful in diagnosis.
Management
Intestinal and early hepatic amoebiasis responds quickly
to oral metronidazole (800 mg 3 times daily for 5–10 days) or other long-acting nitroimidazoles like tinidazole or ornidazole (both in doses of 2 g daily for 3 days). Nitazoxanide (500 mg twice daily for 3 days) is an alternative drug.
Either diloxanide furoate or paromomycin, in doses of 500 mg orally 3 times daily for 10 days after treatment, should be given to eliminate luminal cysts. If a liver abscess is large or threatens to burst, or if the response to chemotherapy is not prompt, aspiration is required and is repeated if necessary. Rupture of an abscess into the pleural cavity, pericardial sac or peritoneal cavity necessitates immediate aspiration or surgical drainage. Small serous effusions resolve without drainage.
Prevention
Personal precautions against contracting amoebiasis
consist of not eating fresh, uncooked vegetables or
drinking unclean water.
Giardiasis
Infection with Giardia lamblia is found worldwide and iscommon in the tropics. It particularly affects children,
tourists and immunosuppressed individuals, and is the
parasite most commonly imported into the UK. In cystic
form, it remains viable in water for up to 3 months and
infection occurs by ingesting contaminated water.
Its flagellar trophozoite form attaches to the duodenal and jejunal mucosa, causing inflammation.
Clinical features and investigations
After an incubation period of 1–3 weeks, there is diarrhoea, abdominal pain, weakness, anorexia, nausea
and vomiting. On examination, there may be abdominal
distension and tenderness.
Stools obtained at 2–3-day intervals should be examined
for cysts. Duodenal or jejunal aspiration by endoscopy
gives a higher diagnostic yield. The ‘string test’
may be used, in which one end of a piece of string is
passed into the duodenum by swallowing and retrieved
after an overnight fast; expressed fluid is then examined
for the presence of G. lamblia trophozoites. A number of stool antigen detection tests are available. Jejunal biopsy specimens may show G. lamblia on the epithelial surface.
Management
Treatment is with a single dose of tinidazole 2 g, metronidazole 400 mg 3 times daily for 10 days, or nitazoxanide 500 mg orally twice daily for 3 days.
Cryptosporidiosis
Cryptosporidium spp. are coccidian protozoal parasites ofhumans and domestic animals. Infection is acquired by
the faecal–oral route through contaminated water supplies.
The incubation period is approximately 7–10 days
and is followed by watery diarrhoea and abdominal
cramps. The illness is usually self-limiting, but in
immunocompromised patients, especially those with
HIV, the illness can be devastating, with persistent
severe diarrhoea and substantial weight loss .
INFECTIONS CAUSED BY HELMINTHS
Helminths (Greek helmins, meaning worm) include three groups of parasitic worm , large multicellular organisms with complex tissues and organs.
Classes of helminth that parasitise humans
Intestinal human nematodesDiseases are caused by adult nematodes living in the
human gut. There are two types:
• the hookworms, which have a soil stage in which
they develop into larvae that then penetrate the host
• a group of nematodes which survive in the soil
merely as eggs that have to be ingested for their life
cycle to continue. The geographical distribution of hookworms is limited by the larval requirement for warmth and humidity. Soil-transmitted nematode infections can be
prevented by avoidance of faecal soil contamination
(adequate sewerage disposal) or skin contact (wearing
shoes), and by strict personal hygiene.
Ancylostomiasis (hookworm)
Ancylostomiasis is caused by parasitisation with Ancylostoma duodenale or Necator americanus. The complex life cycle is shown in Figure . The adult hookworm is1 cm long and lives in the duodenum and upper jejunum.
Eggs are passed in the faeces. In warm, moist, shady soil, the larvae develop into rhabditiform and then the infective filariform stages; they then penetrate human skin
and are carried to the lungs.
After entering the alveoli, they ascend the bronchi, are swallowed and mature in the small intestine, reaching maturity 4–7 weeks after infection.
The worms attach themselves to the mucosa
of the small intestine by their buccal capsule (Fig.)
and withdraw blood. The mean daily loss of blood from
one A. duodenale is 0.15 mL and from N. americanus
0.03 mL.
Hookworm infection is one of the main causes of
anaemia in the tropics and subtropics. A. duodenale is
endemic in the Far East and Mediterranean coastal
regions, and is also present in Africa, while N. americanus
is endemic in Africa, and Central and South America, as well as in the Far East.
Life cycle of Ancylostoma.
Ancylostoma duodenale. Electron micrograph showing the ventral teeth.
Clinical featuresAn allergic dermatitis, usually on the feet (ground itch),
may be experienced at the time of infection. The passage
of the larvae through the lungs in a heavy infection
causes a paroxysmal cough with blood-stained sputum,
associated with patchy pulmonary consolidation and
eosinophilia. When the worms have reached the small
intestine, vomiting and epigastric pain resembling peptic ulcer disease may occur. Sometimes, frequent loose stools are passed. The degree of iron and protein deficiency which develops depends not only on the load of worms but also on the nutrition of the patient and especially on the iron stores. Anaemia with high-output cardiac failure may result. The mental and physical development of children may be retarded in severe infection.
Investigations
There is eosinophilia. The characteristic ovum can be
recognised in the stool. If hookworms are present in
numbers sufficient to cause anaemia, faecal occult blood
testing will be positive and many ova will be present.
Management
A single dose of albendazole (400 mg) is the treatment
of choice. Alternatively, mebendazole 100 mg twice
daily for 3 days may be used.
Anaemia and heart failure associated with hookworm infection respond well to oral iron, even when severe; blood transfusion is rarely required.
Strongyloidiasis
Strongyloides stercoralis is a very small nematode (2 mm× 0.4 mm) which parasitises the mucosa of the upper
part of the small intestine, often in large numbers,
causing persistent eosinophilia.
The eggs hatch in the bowel but only larvae are passed in the faeces.
In moist soil, they moult and become the infective filariform
larvae.
After penetrating human skin, they undergo a
development cycle similar to that of hookworms, except
that the female worms burrow into the intestinal mucosa
and submucosa.
Some larvae in the intestine may develop into filariform larvae, which may then penetrate the mucosa or the perianal skin and lead to autoinfection and persistent infection. Patients with Strongyloides infection persisting for more than 35 years have been described.
Clinical features
The classic triad of symptoms consists of abdominal pain, diarrhoea and urticaria. Cutaneous manifestations, either urticaria or larva currens (a highly characteristic pruritic, elevated, erythematous lesion advancing along the course of larval migration), are characteristic and occur in 66%. Systemic strongyloidiasis (the Strongyloides hyperinfection syndrome), with dissemination of larvae throughout the body, occurs in association with immune suppression (intercurrent disease, HIV and HTLV-1infection, corticosteroid treatment).
Patients present with severe, generalised abdominal pain, abdominal distension and shock. Massive larval invasion of the lungs causes cough, wheeze and dyspnoea; cerebral involvement has manifestations ranging from subtle neurological signs to coma. Gram-negative sepsis frequently complicates the picture.
Investigations
There is eosinophilia. Serology (ELISA) is helpful but
definitive diagnosis depends upon finding the larvae.
The faeces should be examined microscopically for
motile larvae; excretion is intermittent and so repeated
examinations may be necessary. Larvae can also be
found in jejunal aspirate or detected using the string test.
Larvae may also be cultured from faeces.
Management
A course of two doses of ivermectin (200 μg/kg), administeredon successive days, is effective. Alternatively,albendazole is given orally in a dose of 15 mg/kg body
weight twice daily for 3 days. A second course may be
required. For the Strongyloides hyperinfection syndrome,
ivermectin is given at 200 μg/kg for 5–7 days.
Clinical features of strongyloidiasis
Ascaris lumbricoides (roundworm)
This pale yellow nematode is 20–35 cm long. Humansare infected by eating food contaminated with mature
ova. Ascaris larvae hatch in the duodenum, migrate
through the lungs, ascend the bronchial tree, are swallowed
and mature in the small intestine. This tissue
migration can provoke both local and general hypersensitivity reactions, with pneumonitis, eosinophilic granulomas, bronchial asthma and urticaria.
Clinical features
Intestinal ascariasis symptoms ranging from occasional vague abdominal pain through to malnutrition. The large size of the adult worm and its tendency to aggregate and migrate can result in obstructive complications.
Tropical and subtropical areas are endemic for ascariasis, and in these areas it causes up to 35% of all intestinal obstructions, most commonly in the terminal ileum. Obstruction can be complicated further by intussusception, volvulus, haemorrhagic infarction and perforation. Other complications include blockage of the bile or pancreatic duct and obstruction of the appendix by adult worms. Investigations
The diagnosis is made microscopically by finding ova in
the faeces. Adult worms are frequently expelled rectally
or orally. Occasionally, the worms are demonstrated
by a barium examination. There is eosinophilia.
Management
A single dose of albendazole (400 mg), pyrantelpamoate (11 mg/kg; maximum 1 g), ivermectin (150–
200 μg/kg) or mebendazole (100 mg twice daily for
3 days) is effective for intestinal ascariasis. Patients
should be warned that they might expel numerous
whole, large worms. Obstruction due to ascariasis
should be treated with nasogastric suction, piperazine
and intravenous fluids.
Prevention
Community chemotherapy programmes have been
used to reduce Ascaris infection. The whole community
can be treated every 3 months for several years.
Alternatively, schoolchildren can be targeted; treating
them lowers the prevalence of ascariasis.
Enterobius vermicularis
(threadworm)
This helminth is common throughout the world and
affects mainly children. After the ova are swallowed,
development takes place in the small intestine, but the
adult worms are found chiefly in the colon.
Clinical features
The gravid female worm lays ova around the anus,
causing intense itching, especially at night. The ova are
often carried to the mouth on the fingers and so
re-infection or human-to-human infection takes place.
In females, the genitalia may be involved. The adult worms may be seen moving on the buttocks or in the stool.
Investigations
Ova are detected by applying the adhesive surface ofcellophane tape to the perianal skin in the morning. This
is then examined on a glass slide under the microscope.
A perianal swab, moistened with saline, is an alternative.
Management
A single dose of mebendazole (100 mg), albendazole
(400 mg), pyrantel pamoate (11 mg/kg) or piperazine
(4 g) is given and may be repeated after 2 weeks to
control auto-reinfection. If infection recurs in a family,
each member should be treated as above. During this
period all nightclothes and bed linen are laundered. Fingernails kept short and hands washed carefully before meals.
Trichuris trichiura (whipworm)
Infections with whipworm are common all over the
world under unhygienic conditions. Infection is contracted
by the ingestion of earth or food contaminated with ova which have become infective after lying for 3 weeks or more in moist soil. The adult worm is 3–5 cm long and has a coiled anterior end resembling a whip. Whipworms inhabit the caecum, lower ileum, appendix, colon and anal canal. There are usually no symptoms, but intense infections in children may cause persistent diarrhoea or rectal prolapse, and growth retardation. The diagnosis is readily made by identifying ova in faeces. Treatment is with mebendazole in doses of 100 mg twice daily or albendazole 400 mg daily for 3 days for patients with light infections, and for 5–7 days for those with heavy infections.
Tissue-dwelling human nematodes
Filarial worms are tissue-dwelling nematodes. Thelarval stages are inoculated by biting mosquitoes or flies,
each specific to a particular filarial species. The larvae
develop into adult worms (2–50 cm long) which, after
mating, produce millions of microfilariae (170–320 μm
long) that migrate in blood or skin. The life cycle is
completed when the vector takes up microfilariae while
feeding on humans. In the insect, ingested microfilariae
develop into infective larvae for inoculation in humans,
normally the only host.
Disease is due to the host’s immune response to the worms (both adult and microfilariae), particularly dying worms, and its pattern and severity vary with the site and stage of each species . The worms are
long-lived; microfilariae survive 2–3 years and adult
worms 10–15 years. The infections are chronic and worst in individuals constantly exposed to re-infection.
Pathogenicity of filarial infections
depending on site and stage of wormsLymphatic filariasis
Infection with the filarial worms Wuchereria bancrofti andBrugia malayi is associated with clinical outcomes
ranging from subclinical infection to hydrocele and
elephantiasis. W. bancrofti is transmitted by night-biting culicine or anopheline mosquitoes in most areas .The
adult worms, 4–10 cm in length, live in the lymphatics,
and the females produce microfilariae which circulate in
large numbers in the peripheral blood, usually at night.
The infection is widespread in tropical Africa, in coastal areas of Asia, also in North and South America. B. malayi usually causes less severe disease than bancrofti.
Wuchereria bancrofti and Brugia malayi.
Life cycle oforganisms and pathogenesis of lymphatic filariasis.
Pathology
Several factors contribute to the pathogenesis of lymphatic
filariasis. Toxins released by the adult worm cause lymphangiectasia; this vessels leads to lymphatic dysfunction and the chronic clinical manifestations of lymphatic filariasis, lymphedema and hydrocele.
Death of the adult worm results in acute filarial lymphangitis. Lymphatic obstruction persists after death of the adult worm. Secondary bacterial infections cause tissue destruction.
The host response to microfilariae is central to the pathogenesis of tropical pulmonary eosinophilia.
Clinical features
Acute filarial lymphangitis presents with fever, pain,tenderness and erythema along the course of inflamed
lymphatic vessels. Inflammation of the spermatic cord,
epididymis and testis is common. The whole episode
lasts a few days but may recur several times a year.
Temporary oedema becomes more persistent and regional lymph nodes enlarge. Progressive enlargement, coarsening, corrugation, fissuring and bacterial infection of the skin and subcutaneous tissue develop gradually, causing irreversible ‘elephantiasis’. The scrotum may reach an enormous size. Chyluria and chylous effusions are milky and opalescent; on standing, fat globules rise to the top.
The acute lymphatic manifestations of filariasis must
be differentiated from thrombophlebitis and infection.The oedema and lymphatic obstructive changes must be
distinguished from congestive cardiac failure, malignancy,
trauma and idiopathic abnormalities of the lymphatic
system. Silicates absorbed from volcanic soil can
also cause non-filarial elephantiasis.
Tropical pulmonary eosinophilia is a complication
seen mainly in India and is likely to be due to microfilariae
trapped in the pulmonary capillaries and destroyed by allergic inflammation. Patients present with paroxysmal
cough, wheeze and fever. If untreated, this may progress to debilitating chronic interstitial lung disease.
Investigations
In the earliest stages of lymphangitis, the diagnosis is
made on clinical grounds, supported by eosinophilia
and sometimes by positive filarial serology. Filarial
infections cause the highest eosinophil counts of all
helminthic infections. Microfilariae can be found in the peripheral blood at night, and either are seen moving in a wet blood film or are detected by microfiltration of a sample of lysed blood. They are usually present in hydrocele fluid, which may occasionally yield an adult filaria. By the time elephantiasis develops, microfilariae become difficult to find. Calcified filariae may sometimes be demonstrable by radiography. Movement of adult worms can be seen on scrotal ultrasound.
PCR-based tests for detection of W. bancrofti and B. malayi DNA from blood have been developed.
Indirect fluorescence and ELISA detect antibodies in
over 95% of active cases and 70% of established elephantiasis.
The test becomes negative 1–2 years after cure.
Serological tests cannot distinguish the different filarial infections. Highly sensitive and specific immunochromatographic card tests for detection of circulating W. bancrofti antigen are now commercially available; fingerprick blood taken at any time of the day can be used for these.
In tropical pulmonary eosinophilia, serology is strongly positive and IgE levels are massively elevated, but circulating microfilariae are not found. The chest X-ray shows miliary changes or mottled opacities. Pulmonary function tests show a restrictive picture.
Management
Treatment of the individual is aimed at reversing andhalting disease progression. Diethylcarbamazine (DEC,
2 mg/kg orally 3 times daily for 12 days, or as a single
dose) kills microfilariae and adult worms. Most adverse
effects seen with DEC treatment are due to the host
response to dying microfilariae, and the reaction intensity
is directly proportional to the microfilarial load. The
main symptoms are fever, headache, nausea, vomiting,
arthralgia and prostration. These usually occur within
24–36 hours of the first dose of DEC. Antihistamines or
corticosteroids may be required to control these allergic
phenomena. Combining albendazole (400 mg) with
ivermectin (200 μg/kg) in a single dose, with or without
DEC (300 mg), is also highly effective in clearing the parasites.
Treatment of Wolbachia with doxycycline (200 mg/day) for 4–8 weeks provides additional benefit by eliminating the bacteria; this leads to interruption of parasite embryogenesis. For tropical pulmonary eosinophilia,
DEC for 14 days is the treatment of choice.
Chronic lymphatic pathology
Experience in India and Brazil shows that active management of chronic lymphatic pathology can alleviate symptoms. Patients should be taught meticulous skin care of
their lymphoedematous limbs to prevent secondary bacterial and fungal infections. Tight bandaging, massage
and bed rest with elevation of the affected limb may help
to control the lymphoedema.
Prompt diagnosis and antibiotic therapy of bacterial cellulitis are important in preventing further lymphatic damage and worsening of existing elephantiasis. Plastic surgery may be indicated in established elephantiasis. Great relief can be obtained by removal of excess tissue but recurrences are probable unless new lymphatic drainage is established. Hydroceles and chyluria can be repaired surgically.
Prevention
Treatment of the whole population in endemic areas
with annual single-dose DEC (6 mg/kg), either alone or
in combination with albendazole or ivermectin, can
reduce filarial transmission. This mass treatment should
be combined with mosquito control programmes.
Zoonotic nematodes
Trichinosis (trichinellosis)Trichinella spiralis is a nematode that parasitises rats and
pigs, and is only transmitted to humans if they eat partially
cooked infected pork, usually as sausage or ham.
Bear meat is another source. Symptoms result from invasion
of intestinal submucosa by ingested larvae, which
develop into adult worms, and the secondary invasion
of striated muscle by fresh larvae produced by these
adult worms. Outbreaks have occurred in the UK, as
well as in other countries where pork is eaten.
Clinical features
The clinical features of trichinosis are determined by the
larval numbers. A light infection with a few worms may
be asymptomatic; a heavy infection causes nausea and
diarrhoea 24–48 hours after the infected meal. A few
days later, the symptoms associated with larval invasion predominate: there is fever and oedema of the face,
eyelids and conjunctivae; invasion of the diaphragm
may cause pain, cough and dyspnoea; and involvement
of the muscles of the limbs, chest and mouth causes stiffness,
pain and tenderness in affected muscles. Larval migration may cause acute myocarditis and encephalitis. An eosinophilia is usually found after the second week. An intense infection may prove fatal but those who survive recover completely.
Investigations
Commonly, a group of people who have eaten infectedpork from a common source develop symptoms at about
the same time. Biopsy from the deltoid or gastrocnemius
muscle after the third week of symptoms may reveal
encysted larvae. Serological tests are also helpful.
Management
Treatment is with albendazole (400 mg twice daily for
8–14 days) or mebendazole (200–400 mg three times
daily for 3 days, followed by 400–500 mg three times
daily for 10 days). Given early in the infection, this may
kill newly formed adult worms in the submucosa and
thus reduce the number of larvae reaching the muscles.
Corticosteroids are necessary to control the serious
effects of acute inflammation.
Cutaneous larva migrans (CLM)
Is the most common linear lesion seen in travellers . Intensely pruritic, linear, serpiginous lesions result from the larval
migration of the dog hookworm (Ancylostoma caninum).
The track moves across the skin at a rate of 2–3 cm/day.
Although the larvae of dog hookworms frequently infect humans, they do not usually develop into the adult form. The most common site for CLM is the foot but elbows, breasts and buttocks may be affected. Most patients with CLM have recently visited a beach where the affected part was exposed.
The diagnosis is clinical.
Treatment may be local with 12-hourly application of 15% thiabendazole cream, or systemic with a single dose of albendazole (400 mg) or ivermectin (150–200 μg/kg).
Cutaneous larva migrans.
Trematodes (flukes)leaf-shaped worms are parasitic to humans and animals. Schistosomiasis
Schistosomiasis is one of the most important causes of
morbidity in the tropics. There are five species of the
genus Schistosoma which commonly cause disease in
humans: S. haematobium, S. mansoni, S. japonicum, S.
mekongi and S. intercalatum. S. haematobium was discovered by Theodor Bilharz in Cairo in 1861. Schistosome eggs have been found in Egyptian mummies dated 1250 BC. The ovum is passed in the urine or faeces of infected individuals and gains access to fresh water, where the ciliated miracidium inside it is liberated; it enters intermediate host, freshwater snail, in which it multiplies.
Large numbers of fork-tailed cercariae are then liberated into the water, where they may survive for 2–3 days. Cercariae can penetrate the skin or the mucous membrane of the mouth of humans. They transform into schistosomulae and moult as they pass through the lungs; thence they are carried by the blood stream to the liver, and so to the portal vein, where they mature. The male worm is up to 20 mm in length and the more slender cylindrical female, usually enfolded longitudinally by the male, is rather longer . Within 4–6 weeks
of infection, they migrate to the venules draining the
pelvic viscera, where the females deposit ova.
Schistosoma. A Life cycle.
B Scanning electron micrograph of adult schistosome worms, showing the larger male worm embracing the thinner female.Pathology
This depends on the species and the stage of infection.Most disease is due to the passage of eggs
through mucosa and to the granulomatous reaction to
eggs deposited in tissues. The eggs of S. haematobium pass mainly through the wall of the bladder, but may also involve rectum, seminal vesicles, vagina, cervix and uterine tubes.
S. mansoni and S. japonicum eggs pass
mainly through the wall of the lower bowel or are carried
to the liver. The most serious, although rare, site of ectopic
deposition of eggs is in the CNS. Granulomas are composed of macrophages, eosinophils, and epithelioid and giant cells around an ovum.
Later, there is fibrosis and eggs calcify, often in sufficient numbers to become radiologically visible. Eggs of S. haematobium may leave the vesical plexus and be carried directly to the lung. Those of S. mansoni and S. japonicum may also reach the lungs after the development of portal hypertension and consequent portasystemic collateral circulation.
In both circumstances, egg deposition in the pulmonary vasculature, and the resultant host response, can lead to the development of pulmonary hypertension.
Pathogenesis of schistosomiasis
Clinical featuresRecent travellers, especially those overlanding through
Africa, may present with allergic manifestations and
eosinophilia; residents of schistosomiasis-endemic areas
are more likely to present with chronic urinary tract
pathology or portal hypertension.
During the early stages of infection, there may be
itching lasting 1–2 days at the site of cercarial penetration (‘swimmer’s itch’).
After a symptom-free period of 3–5 weeks, acute schistosomiasis (Katayama syndrome) may present with allergic manifestations, such as urticaria, fever, muscle aches, abdominal pain, headaches, cough and sweating. On examination, hepatomegaly, splenomegaly, lymphadenopathy and pneumonia may be present.
These allergic phenomena may be severe in infections with S. mansoni and S. japonicum, but are rare with S. haematobium. The features subside after 1–2 weeks.
Chronic schistosomiasis is due to egg deposition and
occurs months to years after infection. The symptoms
and signs depend upon the intensity of infection and the
species of infecting schistosome .
Schistosoma haematobium
Humans are the only natural hosts of S. haematobium,
which is highly endemic in Egypt and East Africa, and
occurs throughout Africa and the Middle East). Infection can be acquired after a brief exposure, such as swimming in freshwater lakes in Africa.
Painless terminal haematuria is usually the first and
most common symptom. Frequency of micturition
follows, due to bladder neck obstruction. Later, the
disease may be complicated by frequent urinary tract
infections, bladder or ureteric stone formation, hydronephrosis, and ultimately renal failure with a contracted calcified bladder. Pain is often felt in the iliac fossa or in the loin, and radiates to the groin.
In endemic areas, there is a strong epidemiological association of S. haematobium infection with squamous cell carcinoma of the bladder. Disease of the seminal vesicles may lead to haemospermia.
Females may develop schistosomal papillomas of the vulva, and schistosomal lesions of the cervix may be mistaken for cancer. Intestinal symptoms may follow involvement of the bowel wall.
Ectopic worms cause skin or spinal cord lesions.
The severity of S. haematobium infection varies greatly,
and many with a light infection are asymptomatic.However, as adult worms can live for 20 years or more
and lesions may progress, these patients should always
be treated.
Schistosoma mansoni
S. mansoni is endemic throughout Africa, the Middle
East, Venezuela, Brazil and the Caribbean .
Characteristic symptoms begin 2 months or more
after infection. They may be slight, no more than malaise,
or consist of abdominal pain and frequent stools which
contain blood-stained mucus.
With severe advanced disease, increased discomfort from rectal polyps may be experienced.
The early hepatomegaly is reversible, but portal hypertension may cause massive splenomegaly,
fatal haematemesis from oesophageal varices, or progressive ascites . Liver function is initially preserved because the pathology is fibrotic rather than cirrhotic. S. mansoni and other schistosome predispose to the carriage of Salmonella, in part because Salmonella may attach to the schistosomes and in part because shared antigens on schistosomes may induce immunological tolerance to Salmonella.
Schistosoma japonicum, S. mekongi and S. intercalatum
In addition to humans, the adult worm of S. japonicum
infects the dog, rat, field mouse, water buffalo, ox, cat,
pig, horse and sheep.
Investigations
There is marked eosinophilia. ELISAare useful as screening tests. In S. haematobium infection, dipstick urine testing shows blood and albumin. The eggs can be found by microscopic examination of the centrifuged deposit of terminal stream urine . US is useful for assessing the UT; bladder wall thickening, hydronephrosis and bladder calcification can be detected. Cystoscopy reveals ‘sandy’ patches, bleeding mucosa and later distortion. In a heavy infection with S. mansoni or S. japonicum, the characteristic egg with its lateral spine can usually be found in the stool. When the infection is light or of long duration, a rectal biopsy can be examined. Sigmoidoscopy may show inflammation or bleeding. Biopsies examined for ova.Ova of Schistosoma haematobium in urine. Note the terminal spike.
ManagementThe object of specific treatment is to kill the adult schistosomes and so stop egg-laying. Praziquantel (20 mg/
kg orally twice daily for 1 day) is the drug of choice for all forms of schistosomiasis. The drug produces parasitological cure in 80% and over 90% reduction in egg counts in the remainder. Side effects are uncommon but include nausea and abdominal pain. Praziquantel therapy in early infection reverses pathologies such as hepatomegaly and bladder wall thickening and granulomas. Surgery may be required to deal with residual lesions such as ureteric stricture, small fibrotic urinary bladders, or granulomatous masses in the brain or spinal cord. Removal of rectal papillomas by diathermy may provide symptomatic relief.
Prevention
So far, no satisfactory single means of controlling schistosomiasis has been established. The life cycle is terminated if the ova in urine or faeces are not allowed tocontaminate fresh water containing the snail host. The
provision of latrines and of a safe water supply, however,
remains a major problem in rural areas throughout the
tropics. Annual mass treatment of the population helps prevent S. haematobium and S. mansoni infection, but this method has so far had little success with S. japonicum. Targeting the intermediate host, the snail, presents many difficulties and has not, on its own, proved successful on any scale. For personal protection, contact with infected water must be avoided.
Cestodes (tapeworms)
Cestodes are ribbon-shaped worms which inhabit the
intestinal tract. They have no alimentary system and
absorb nutrients through the tegumental surface. The
anterior end, or scolex, has suckers for attaching to the
host. From the scolex, a series of progressively developing
segments arise, the proglottides, which may continue
to show active movements when shed. Cross-fertilisation
takes place between segments. Ova, present in large
numbers in mature proglottides, remain viable for weeks,
and during this period, they may be consumed by the
intermediate host. Larvae liberated from the ingested
ova pass into the tissues, forming larval cysticerci.
Tapeworms cause two distinct patterns of disease,
either intestinal infection or systemic cysticercosis. Taenia saginata (beef tapeworm), Taenia asiaticaand Diphyllobothrium latum (fish tapeworm) cause only
intestinal infection, following human ingestion of intermediate hosts that contain cysticerci (the larval stage of the tapeworm). Taenia solium causes intestinal infection
if a cysticerci-containing intermediate host is ingested,
and cysticercosis (systemic infection from larval migration)
if ova are ingested. Echinococcus granulosus (dog tapeworm) does not cause human intestinal infection,
but causes hydatid disease (which is analogous to cysticercosis) following ingestion of ova and subsequent
larval migration.
Cysticercosis.
Life cycle of Taenia solium.Intestinal tapeworm
Humans acquire tapeworm by eating undercooked beef infected with the larval stage of T. saginata, undercooked pork containing the larval stage of T. solium or T. asiatica, or undercooked freshwater fish containing larvae of D. latum. Usually, only one adult tapeworm is present in the gut but up to ten have been reported. The ova of all the three Taenia are indistinguishable microscopically.However, examination of scolex and proglottides can differentiate: T. solium has a rostellum and two rows of hooklets on the scolex, and discharges multiple proglottides (3–5) attached together with lower degrees of uterine branching (approximately 10); T. saginata has
only four suckers in its scolex, and discharges single proglottids with greater uterine branching (up to 30); T. asiatica has a rostellum without hooks on its scolex, and is difficult to differentiate from T. saginata, except that there are fewer uterine branches (16–21).
Taenia saginata
The adult worm may be several metres long and produceslittle or no intestinal upset, but knowledge of its presence, by noting segments in the faeces or on underclothing, may distress the patient. Ova may be found in the stool. Praziquantel is the drug of choice; niclosamide or nitazoxanide is an alternative. Prevention depends on efficient meat inspection and the thorough cooking of beef.
Taenia solium
T. solium, the pork tapeworm, is common in central
Europe, South Africa, South America and parts of Asia.
It is not as large as T. saginata. The adult worm is found
only in humans following the eating of undercooked
pork containing cysticerci.
Intestinal infection is treated with praziquantel (5–10 mg/kg) or niclosamide (2 g), both as a single dose, or alternatively with nitazoxanide (500 mg twice daily for 3 days). These are followed by a mild laxative (after 1–2 hours) to prevent retrograde intestinal autoinfection. Cooking pork well prevents intestinal infection. Great care must be taken while attending a patient harbouring an adult worm to avoid ingestion of ova or segments.
Taenia asiatica
T. asiatica is a newly recognised species of Taenia,
restricted to Asia. It is acquired by eating uncooked meat
or viscera of pigs. Clinical features and treatment are
similar to those of T. saginata.
Cysticercosis
Human cysticercosis is acquired by ingesting T. soliumtapeworm ova, from either contaminated fingers or food.
The larvae are liberated from eggs in the stomach, penetrate the intestinal mucosa and are carried to many parts of the body, where they develop and form cysticerci, 0.5–1 cm cysts that contain the head of a young worm. They do not grow further or migrate. Common locations are the subcutaneous tissue, skeletal muscles and brain.
Clinical features
When superficially placed, cysts can be palpated under
the skin or mucosa as pea-like ovoid bodies. Here they
cause few or no symptoms, and will eventually die and
become calcified. Heavy brain infections, especially in children, may cause features of encephalitis.
More commonly, however, cerebral signs do not occur until the larvae die, 5–20 years later. Epilepsy, personality changes, staggering gait or signs of hydrocephalus are the most common features.
Investigations
Calcified cysts in muscles can be recognised radiologically.
In the brain, however, less calcification takes place
and larvae are only occasionally visible by plain X-ray;
usually CT or MRI will show them. Epileptic fits starting
in adult life suggest the possibility of cysticercosis if the
patient has lived in or travelled to an endemic area. The
subcutaneous tissue should be palpated and any nodule
excised for histology. Radiological examination of the
skeletal muscles may be helpful. Antibody detection is
available for serodiagnosis.
Management and prevention
Albendazole, 15 mg/kg daily for a minimum of 8 days,
has now become the drug of choice for parenchymal
neurocysticercosis. Praziquantel is another option,
50 mg/kg in three divided doses daily for 10 days. Prednisolone, 10 mg 3 times daily, is also given for 14 days, starting 1 day before the albendazole or praziquantel. In addition, anti-epileptic drugs should be given until the reaction in the brain has subsided. Operative intervention is indicated for hydrocephalus. Studies from India and Peru suggest that most small, solitary cerebral cysts will resolve without treatment.
Echinococcus granulosus (Taenia echinococcus) and hydatid disease
Dogs are the definitive hosts of the tiny tapewormE. granulosus. The larval stage, a hydatid cyst, normally
occurs in sheep, cattle, camels from contaminated pastures or water. By handling a dog or drinking contaminated water, humans may ingest eggs .The embryo is liberated from the ovum in the small intestine and gains access to the blood stream and thus to the liver. The resultant cyst grows very slowly, sometimes intermittently. It is composed of an enveloping fibrous pericyst, laminated hyaline membrane (ectocyst) and inner germinal layers (endocyst) which gives rise to daughter cysts, or germinating cystic brood capsule in which larvae (protoscolices) develop.
Over time, some cysts may calcify and become non-viable. The disease is common in the Middle East, North and East Africa, Australia and Argentina.
Foci of infection persist in the UK in rural Wales and
Scotland. E. multilocularis, which has a cycle between
foxes and voles, causes a similar but more severe infection, ‘alveolar hydatid disease’, which invades the
liver like cancer.
Hydatid disease. A Life cycle of Echinococcus granulosus. B Daughter cysts removed at surgery. C Within the daughter cysts are the protoscolices.
Clinical features
A hydatid cyst is typically acquired in childhood and
may, after growing for some years, cause pressure
symptoms. These vary, depending on the organ or tissue
involved. In nearly 75% of patients with hydatid disease,
the right lobe of the liver is invaded and contains a single
cyst . In others, a cyst may be found in lung,
bone, brain or elsewhere.
Investigations
The diagnosis depends on the clinical, radiological and
ultrasound findings in a patient who has lived in close
contact with dogs in an endemic area. Complement fixation and ELISA are positive in 70–90% of patients.
Management and prevention
Hydatid cysts should be excised wherever possible.Great care is taken to avoid spillage and cavities are
sterilised with 0.5% silver nitrate or 2.7% sodium chloride.
Albendazole (400 mg twice daily for 3 months)
should also be used. The drug is now often combined
with PAIR (percutaneous puncture, aspiration, injection
of scolicidal agent and re-aspiration) to good effect.
Praziquantel (20 mg/kg twice daily for 14 days) also
kills protoscolices perioperatively. Prevention is difficult in situations where there is a close association with dogs and sheep. Personal hygiene, satisfactory disposal of carcasses, meat inspection and deworming of dogs can greatly reduce the prevalence of disease.
FUNGAL INFECTIONS
Fungal infections, or mycoses, are classified as
superficial, subcutaneous or systemic (deep), depending on the degree of invasion of the host. They are also classified by the kind of fungus that causes the infection, which may be a filamentous fungus (mould) or a yeast, or may vary between these two forms, depending on the environmental Conditions .
Fig. Classification of medically important fungi. Fungal classification is based on simple morphological characteristics. Pneumocystis jirovecii
(carinii) is morphologically distinct from other fungi and does not fit into this classification. *Although Candida albicans exists in a number of forms, including filamentous (hyphae and pseudohyphae), it is generally encountered in its yeast form, so is classified in this category.
Superficial mycoses
Candidiasis (thrush)Superficial candidiasis is caused by Candida spp., mainly
C. albicans. Manifestations include oropharyngeal
and vaginal candidiasis (‘thrush’), intertrigo and chronic paronychia. Superficial candidiasis often follows antibiotic therapy. Intertrigo is characterised by inflammation in skin folds with surrounding ‘satellite lesions’. Chronic paronychia is associated with frequent wetting of the hands. Superficial candidiasis is treated mainly with topical azoles , oral azoles being reserved for refractory or recurrent disease.
Severe oropharyngeal and oesophageal candidiasis is a consequence of CD4+ T lymphocyte depletion/dysfunction,
as in HIV infection .
Recurrent vaginal or penile candidiasis may be a manifestation of diabetes mellitus.
Rarely, mutations in the autoimmune regulator gene,
AIRE, cause a syndrome of chronic mucocutaneous candidiasis .
Mycetoma
Mycetoma is a chronic suppurative infection of the deep
soft tissues and bones, most commonly of the limbs but
also of the abdominal or chest wall or head.
It is caused by either aerobic or anaerobic branching Gram-positive bacilli, Actinomycetales (actinomycetoma – 60%),
or by true fungi, Eumycetes (eumycetoma – 40%).
Actinomycetomas are caused by Actinomadura, Nocardia and Streptomyces spp.
Both groups produce characteristically coloured grains,
the colour depending on the organism (black grains –eumycetoma, red and yellow grains – actinomycetoma,
white grains – either). The disease occurs mostly in the
tropics and subtropics.
Clinical features
The disease is acquired by inoculation (e.g. from a thorn)
and most commonly affects the foot (Madura foot).
Mycetoma begins as a painless swelling at the implantation
site, which becomes chronic and progressive, grows
and spreads steadily within the soft tissues, eventually
extending into bone. Nodules develop under the epidermis
and these rupture, revealing sinuses through which
grains (fungal colonies) may be discharged.
Sinuses heal with scarring, while fresh sinuses appear elsewhere.
Deeper tissue invasion and bone involvement are less
rapid and extensive in eumycetoma than actinomycetoma.
There is little pain and usually no fever or
lymphadenopathy, but there is progressive disability.
Investigations
Diagnosis is confirmed by demonstration of fungal grains in pus, and/or histopathological examination of tissue. Culture is necessary for species identification and susceptibility testing. Serological tests are not available.
Management
Eumycetoma is usually treated with a combination ofsurgery and antifungal therapy. Antifungal susceptibility
testing, if available, is recommended, although
clinical outcome does not necessarily correspond to in
vitro test results. Itraconazole and ketoconazole (both
200–400 mg/day) are used most commonly. Success has
also been reported with terbinafine monotherapy, and
refractory cases have responded to both voriconazole
and posaconazole. Amphotericin B is not generally considered effective. Therapy is continued for 6–12 months
or longer. In extreme cases, amputation may be required.
Systemic mycoses
AspergillosisAspergillosis is an opportunistic systemic mycosis,
which affects predominantly the respiratory tract. Candidiasis
Systemic candidiasis is an opportunistic mycosis caused
by Candida spp. The most common cause is C. albicans.
Other agents include C. dubliniensis, C. glabrata, C. krusei,
C. parapsilosis and C. tropicalis. Species distribution varies
geographically. Candida species identification often enables prediction of susceptibility to fluconazole: C.
krusei is universally resistant, many C. glabrata isolates
have reduced susceptibility or are resistant, and other
species are mostly susceptible.
Candidiasis is usually an endogenous disease that originates from oropharyngeal, genitourinary or skin colonisation, although nosocomial spread has been reported.
Syndromes of systemic candidiasis
Acute disseminated candidiasis
This usually presents as candidaemia (isolation of
Candida spp. from the blood). The main predisposing
factor is the presence of a central venous catheter. Other
major factors include recent abdominal surgery, total
parenteral nutrition (TPN), recent antibiotic therapy and
localised Candida colonisation.
Up to 40% of cases will have ophthalmic involvement, with characteristic retinal ‘cotton wool’ exudates. As this is a sight-threatening condition, candidaemic patients should be assessed by detailed ophthalmoscopy. Skin lesions (non-tender pink/red nodules) may be seen. Although predominantly a disease of intensive care and surgical patients, acute disseminated candidiasis and/or Candida endophthalmitis is seen occasionally in injection drug-users,
thought to be due to candidal contamination of citric
acid or lemon juice used to dissolve heroin.
Chronic disseminated candidiasis
(hepatosplenic candidiasis)In this condition, a neutropenic patient has a persistent
fever, despite antibacterial therapy. The fever persists,
even though there is neutrophil recovery, and is associated
with the development of abdominal pain, raised
alkaline phosphatase and multiple lesions in abdominal
organs (liver, spleen and/or kidneys) on imaging. Other manifestations Renal tract candidiasis, osteomyelitis, septic arthritis, peritonitis, meningitis and endocarditis are all well recognised, and are usually sequelae of acute disseminated disease.
Diagnosis and treatment of these conditions
require specialist mycological advice.
Management
Blood cultures positive for Candida spp. must never be
ignored. Acute disseminated candidiasis is treated with
antifungal therapy, removal of any in-dwelling central
venous catheter. Candidaemia should be treated initially with an echinocandin with subsequent adjustment (usually to IV or oral fluconazole) guided by clinical response, species identification and susceptibility testing. Treatment should continue for a minimum of 14 days. Other appropriate therapies include voriconazole and amphotericin B formulations. Chronic disseminated candidiasis requires prolonged treatment over several months with fluconazole or other agents, depending on species and clinical response. The duration of the condition may be reduced by adjuvant therapy with systemic corticosteroids.
Cryptococcosis
Cryptococcosis is a systemic mycosis caused by twoenvironmental yeast species, Cr. neoformans and Cr.
gattii. Cr. neoformans is distributed worldwide and is
primarily an opportunistic pathogen, most commonly
associated with HIV infection. Cryptococcosis is acquired by inhalation of yeasts. These may disseminate to any organ, most commonly the CNS and skin. The manifestations are most severe in immunocompromised individuals.
Disseminated cryptococcosis (sepsis with cryptococci present in the blood stream or at multiple sites) is largely restricted to immunocompromised patients. CNS manifestations of cryptococcosis include meningitis and cryptococcoma , the latter more likely with Cr. gattii infection.
Manifestations of pulmonary cryptococcosis range from
severe pneumonia (in immunocompromised patients) toasymptomatic disease with single or multiple pulmonary
nodules, sometimes exhibiting cavitation (in
immunocompetent patients). Cryptococcal nodules may
mimic other causes of lung pathology, such as tuberculosis or malignancy, and diagnosis is often made by
histopathology and/or culture.
Treatment of severe cryptococcosis is the same as
for cryptococcal meningitis . Mild pulmonary disease is usually treated with fluconazole, although, for asymptomatic nodules, resection of the lesions is
likely to be sufficient.
Cryptococcal disease. A 23-year-old HIV-positive male developed headache and left-sided weakness.
A MRI scan of the brain showed a space-occupying lesion (arrow) with surrounding oedema.
B Histopathological examination of the lesion stained with Grocott’s silver stain showed encapsulated yeasts. Cryptococcus neoformans was cultured.
Mucormycosis
Mucormycosis is a severe but uncommon opportunisticsystemic mycosis caused by a number of ‘mucoraceous’
moulds, most commonly Lichtheimia (formerly Absidia)
spp., Rhizomucor spp., Mucor spp. and Rhizopus spp.
Disease patterns include rhinocerebral/craniofacial,
pulmonary, cutaneous and systemic disease. All are
characterised by the rapid development of severe tissue
necrosis, which is almost always fatal if left untreated.
The most common predisposing factors are profound
immunosuppression from neutropenia and/or haematopoietic stem cell transplantation, uncontrolled diabetes mellitus, iron chelation therapy with desferrioxamine and severe burns.
Definitive diagnosis is by culture, but histopathological
confirmation is required as the fungi may beenvironmental contaminants.
Treatment requires a combination of antifungal therapy and surgical débridement, with correction of predisposing factor(s) if possible.
High-dose lipid-formulated amphotericin B is used most commonly. Posaconazole is active against many mucoraceous moulds in vitro and may be used as
a second-line agent or as oral ‘step-down’ therapy.
Histoplasmosis
Histoplasmosis is a primary systemic mycosis caused by
the dimorphic fungus Histoplasma capsulatum. H. capsulatum
var. capsulatum is endemic to east-central USA
east and South-east Asia, and Africa.
Habitat
The primary reservoir of H. capsulatum is soil enriched
by bird and bat droppings, in which the fungus remains
viable for many years. Infection is by inhalation of dust
from such soil. Natural infections are found in bats,
which represent a secondary reservoir of infection (via
bat faeces). Histoplasmosis is a specific hazard for
explorers of caves and people who clear out bird roosts.
Pathology
The organism is inhaled in the form of conidia (spores)or hyphal fragments and transforms to the yeast phase
during infection. Conidia or yeasts are phagocytosed by
alveolar macrophages and neutrophils, and this may be
followed by haematogenous dissemination to any organ.
Subsequent development of a T-lymphocyte response
brings the infection under control, resulting in a latent
state in most exposed individuals.
Clinical features
Disease severity depends on the quantity of spores
inhaled and the immune status of the host. In most cases,
infection is asymptomatic. Pulmonary symptoms are the
most common disease presentation, with fever, nonproductive cough and an influenza-like illness.
Erythema nodosum, myalgia and joint pain are common,
and chest radiography may reveal a pneumonitis with
hilar or mediastinal lymphadenopathy.
Patients with pre-existing lung disease, such as
chronic obstructive pulmonary disease (COPD) oremphysema, may develop chronic pulmonary histoplasmosis.
The predominant features of this condition,
which may easily be mistaken for tuberculosis, are fever,
cough, dyspnoea, weight loss and night sweats. Radiological findings include fibrosis, nodules, cavitation and hilar/mediastinal lymphadenopathy.
Other disease patterns include a visceral form with liver and splenic invasion, and disseminated disease.
Acute disseminated histoplasmosis is seen in association
with immunocompromise, including HIV infection.Features include fever, pancytopenia, hepatosplenomegaly,
lymphadenopathy and often a papular skin
eruption. Chronic disseminated disease presents with
fever, anorexia and weight loss.
Cutaneous and mucosal lesions, lymphadenopathy, hepatosplenomegaly and meningitis may also develop.
Investigations
In areas where the disease occurs, histoplasmosis should
be suspected in every undiagnosed infection in which
there are pulmonary signs, enlarged lymph nodes,
hepatosplenomegaly or characteristic cutaneous/bony
lesions. Radiological examination in long-standing cases
may show calcified lesions in the lungs, spleen or other
organs. In the more acute phases of the disease, single
or multiple soft pulmonary shadows with enlarged tracheobronchial nodes are seen on chest X-ray.
Laboratory diagnosis is by direct detection (histopathology
or antigen detection), culture and serology;although antigen detection is the most effective method,
it is not widely available. Antibody is detected by complement fixation testing or immunodiffusion; the
pattern of antibody production is complex and the
results require specialist interpretation. Histoplasma
antigen may be detectable in blood or urine. Culture is
definitive but slow (up to 12 weeks). Histopathology
may show characteristic intracellular yeasts. Diagnosis
of subcutaneous or bony infection is mainly by histopathological examination and/or culture.
Management
Mild pulmonary disease does not require treatment.
However, if prolonged, it may be treated with itraconazole. More severe pulmonary disease is treated with an amphotericin B formulation for 2 weeks, followed by itraconazole for 12 weeks, with methylprednisolone
added for the first 2 weeks of therapy if there is hypoxia
or ARDS. Chronic pulmonary histoplasmosis is treated
with itraconazole (prescribed as the oral solution) for
12–24 months, and disseminated histoplasmosis with an
amphotericin B formulation followed by itraconazole.
Lipid formulations of amphotericin B are preferred, but
their use is subject to availability. A solitary bony lesion may require local surgical treatment only.
Coccidioidomycosis
This is a primary systemic mycosis caused by the dimorphicfungi Coccidioides immitis and C. posadasii, found in
the south-western USA, and Central and South America.
The disease is acquired by inhalation of conidia
(arthrospores). In 60% of cases it is asymptomatic, but in
the remainder it affects the lungs, lymph nodes and skin.
Rarely (in approximately 0.5%), it may spread haematogenously to bones, adrenal glands, meninges and
other organs.
Pulmonary coccidioidomycosis has two forms:
primary and progressive. If symptomatic, primary coccidioidomycosis presents with cough, fever, chest pain,
dyspnoea and (commonly) arthritis and a rash (erythema
multiforme). Progressive disease presents with systemic
upset (e.g. fever, weight loss, anorexia) and features of
lobar pneumonia, and may resemble tuberculosis.
Coccidioides meningitis (which may be associated
with CSF eosinophils) is the most severe disease manifestation; it is fatal if untreated, and requires life-long
suppressive therapy with antifungal azoles.
Investigations and management
Diagnosis is by direct detection (histopathological examination of infected tissue), culture of infected tissue orfluids, or antibody detection. IgM may be detected after
1–3 weeks of disease by precipitin tests.
IgG appears later and is detected with the complement fixation test.
Change in IgG titre may be used to monitor clinical progress.
Treatment depends on specific disease manifestations, and ranges from regular clinical re-assessment without antifungal therapy (in mild pulmonary, asymptomatic cavitary or single nodular disease) to high-dose treatment with an antifungal azole, which may be continued indefinitely (e.g. in meningitis). Amphotericin B is used in diffuse pneumonia, disseminated disease and, intrathecally, in meningitis. Posaconazole has been used successfully in refractory disease.