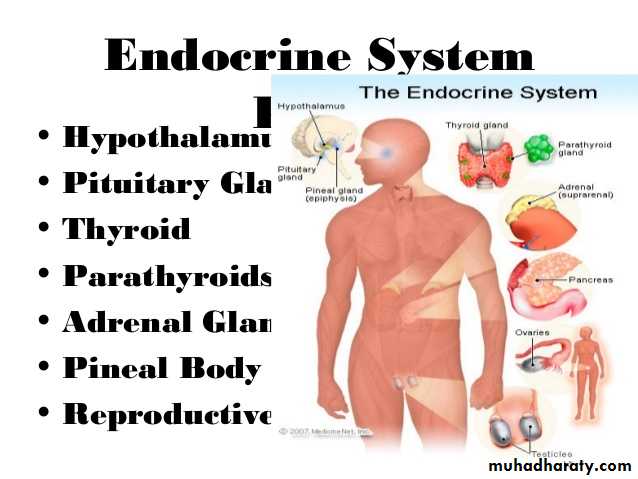
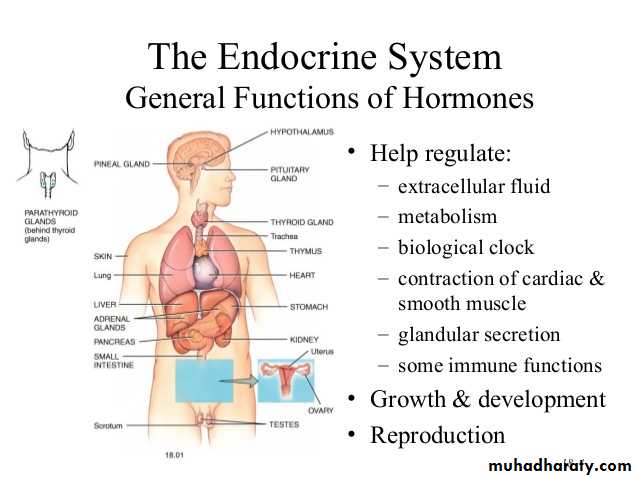
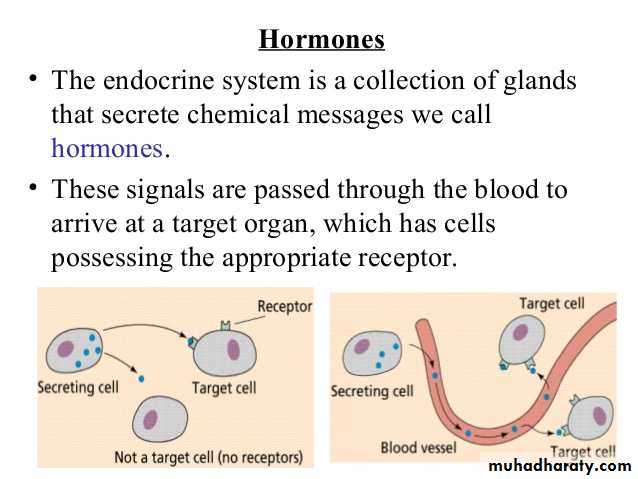
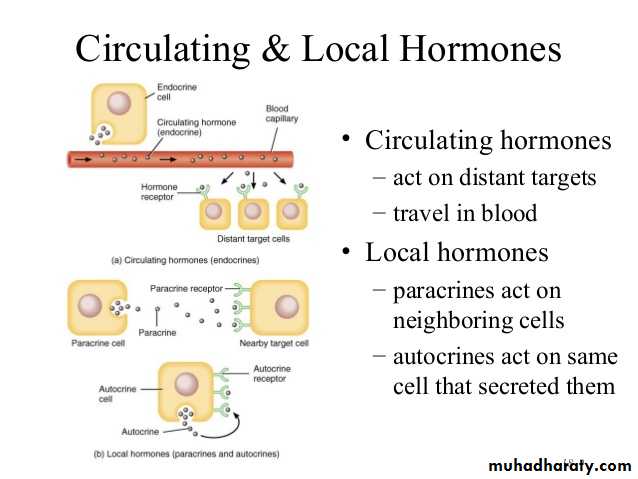
Hormones
Hormones in the Blood
Hormones circulate in the blood either free or boundSteroids and thyroid hormone are attached to plasma proteinsAll others circulate without carriersThe concentration of a circulating hormone reflects: Rate of releaseSpeed of inactivation and removal from the bodyClassification of hormones
Two main classes1. Amino acid-based hormones Amines, thyroxine, peptides, and proteins2. SteroidsSynthesized from cholesterolGonadal and adrenocortical hormonesPolypeptides
1- Insulin 8- Thyrotropin
9- ACTH 2- Glucagon
3- Somatotropin
4- FSH
5- LH
6- Vasopressin
7- Oxytosin
Steroids: Amino acid derivatives:
1- Estrogene 1- Epinephrine
2- Testosterone 2- Norepinephrine
3- Cortisol 3- Dopamine
4- Aldosterone 4- Thyroxine, T3, T4
5- Corticosterone 5- Melatonin
6- Progesterone 6- Serotonin
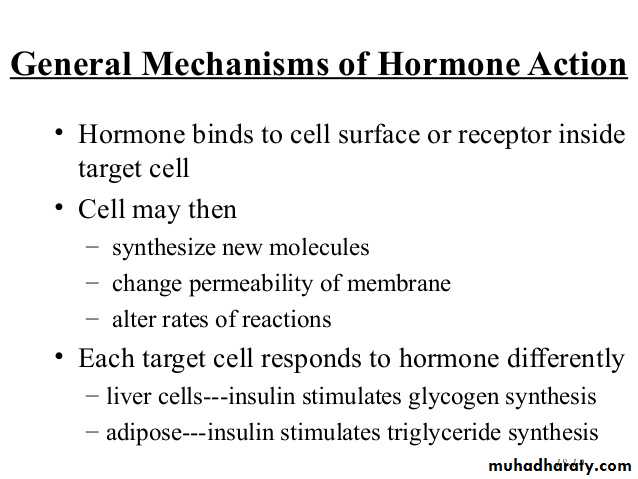
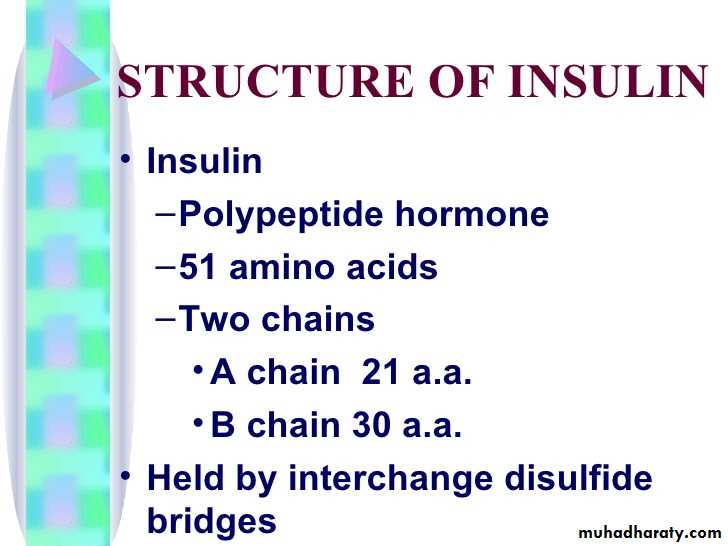
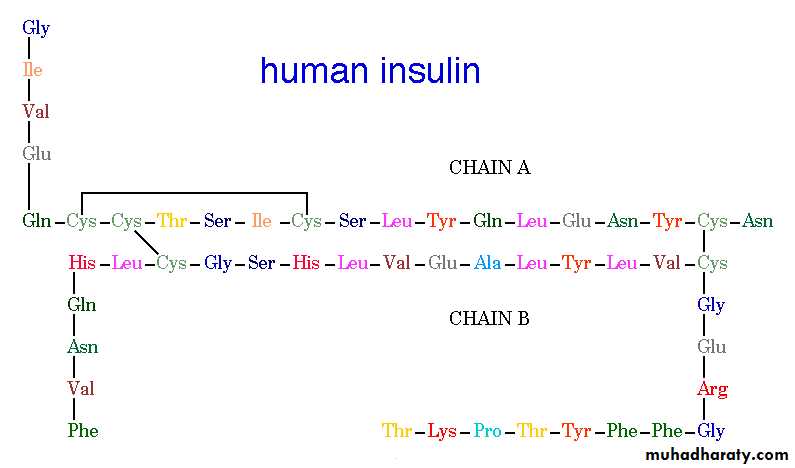
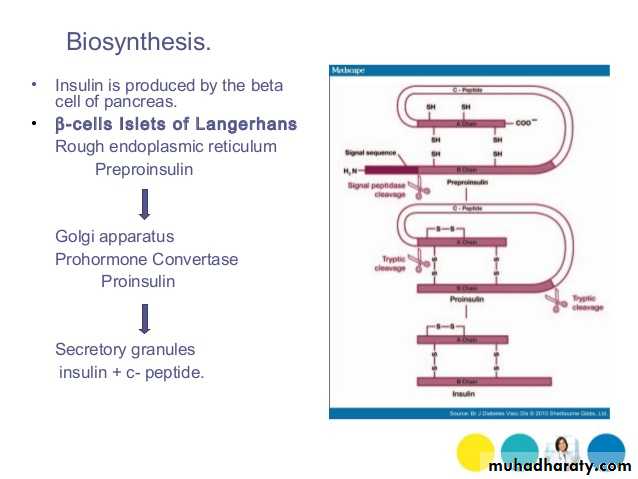
Insulin:
INSULIN – Hormone Associated with Energy Abundance
1.Effect on Carbohydrate Metabolism:A. Promotes Muscle Glucose Uptake and Metabolism -Storage of Glycogen in Muscle.
B. Promotes Liver Uptake, Storage and Use of Glucose Mechanisms:
a. inactivates liver phosphorylase
b. causes enhanced uptake of glucose from the blood by the liver cells (by increasing the activity of the enzyme glucokinase.
C. increases activity of enzyme glycogen synthase , that promote glycogen synthesis - Glucose is released from the liver between meals Lack of insulin activates Phosphorylase , which causes splitting of glycogen into glucose phosphate - Insulin promotes Conversion of Excess Glucose into fatty Acids and Inhibits Gluconeogenesis in the liver.
2. Effect on Fat Metabolism:
A. Insulin promotes fat synthesis and storage - storage of fat n the adipose cells
a. insulin inhibits the action of hormone-sensitive lipase.
b. insulin promotes glucose transport through the cell membrane into the fat cells.
B. Insulin deficiency causes increase metabolic use of fat causing
a . Lipolysis of storage fat and release of free fatty acids.
b. Increase plasma cholesterol and phospholipid.
c. Excess usage of fats during insulin lack causes ketosis and acidosis
3- Effect of Insulin on Protein Metabolism:
A. Insulin promotes protein synthesis and storage.
a. stimulates transport of amino acids into the cells ( valine, leucine, isoleucine, tyrosine, phenylalanine ).
b. increases the translation of messenger RNA, forming new proteins.
c. increases the rate of transcription of DNA genetic sequences in cell nuclei.
d. inhibits catabolism of proteins e. depresses the rate of gluconeogenesis.
B. Insulin Lack Causes Protein Depletion and Increased Plasma Amino Acids. protein wasting is one of the most serious of all effects of severe diabetes mellitus
C. Insulin and growth hormone interact synergistically to promote growth. Insulin promotes protein formation and prevents degradation of proteins.
Glucagon: Secreted from A cells or α- cells in the islets of langerhans of pancreas. It is also secreted from A cells of stomach and L cells of intestine. It is polypeptide with a molecular weight of 3.485, it contains 29 amino acids. Its half- life of glucacon is 3 to 6 minutes.
HGH (human growth 3- Somatotropinhormone)
It’s a protein based poly-pepitideProduced by the pituitary gland in the brain.Used to stimulate growth, and cell reproduction and regeneration in humans and other animals.Growth hormone is a Protein hormone made of ~ 190 amino acids, made in Anterior Pituitary by somatotroph cells.
Direct Effects of GH
Binds to adipocytes and causes them to break down triglycerides and prevents them from accumulating fat in the bloodReleases insulin-like growth factor-1 (IGF-1)
Indirect Effects of GH
Stimulates:Cartilage cells (chrondrocytes) growthMyoblasts growth and differentiation Amino Acid uptakeProtein synthesis
Other Effects
Keeps blood glucose at healthy levelsPrevents insulin from absorbing glucose in tissueCauses creation of glucose in the liver In mature cows, it stimulates lactation
GH Secretion
Growth Hormone-Releasing Hormone: causes production and release of GH 1-
Somatostatin: inhibits release of GH.
2- Grehlin: secreted from stomach, binds to somatotrophs and causes secretion of GH.
Estrogen:
Estrogen is the primary female sex hormone as well as a medication. It is responsible for the development and regulation of the female reproductive system and secondary sex characteristics. Estrogen may also refer to any substance, natural or synthetic, that mimics the effects of the natural hormone. Estrogens are used as medications as part of some oral contraceptives, in hormone replacement therapy for postmenopausal, hypogonadal, and transgender women, and in the treatment of certain hormone-sensitive cancers like prostate cancer and breast cancer. Estrogens are synthesized in all vertebratesas well as some insects. Their presence in both vertebrates and insects suggests that estrogenic sex hormones have an ancient evolutionary history. The three major naturally occurring forms of estrogen in women are estrone (E1), estradiol (E2), and estriol (E3). Another type of estrogen called estetrol (E4) is produced only during pregnancy. Quantitatively, estrogens circulate at lower levels than androgens in both men and women. While estrogen levels are significantly lower in males compared to females, estrogens nevertheless also have important physiological roles in males. Like all steroid hormones, estrogens readily diffuse across the cell membrane. Once inside the cell, they bind to and activate estrogen receptors (ERs) which in turn modulate the expression of many genes. Additionally, estrogens bind to and activate rapid-signaling membrane estrogen receptors (mERs), such as GPER GPR30). )
Testosterone:
Testosterone is the primary male sex hormone and an anabolic steroid. In men, testosterone plays a key role in the development of male reproductive tissues such as the testis and prostate, as well as promoting secondary sexual characteristics such as increased muscle and bone mass, and the growth of body hair. In addition, testosterone is essential for health and well-being, and for the prevention of osteoporosis. Insufficient levels of testosterone in men may lead to abnormalities including frailty and bone loss.Testosterone is also used as a medication to treat male hypogonadism and certain types of breast cancer. Since testosterone levels gradually decrease as men age, synthetic testosterone is sometimes prescribed to older men to counteract this deficiency. Testosterone is a steroid from the androstane class containing a keto and hydroxyl groups at the three and seventeen positions respectively. It is biosynthesized in several steps from cholesterol and is converted in the liver to inactive metabolites. It exerts its action through binding to and activation of the androgen receptor. In humans and most other vertebrates, testosterone is secreted primarily by the testicles of males and, to a lesser extent, the ovaries of females. Small amounts are also secreted by the adrenal glands. On average, in adult males, levels of testosterone are about 7–8 times as great as in adult females. As the metabolic consumption of testosterone in males is greater, the daily production is about 20 times greater in men. Females are also more sensitive to the hormone.Cortisol: is a steroid hormone, in the glucocorticoid class of hormones. When used as a medication, it is known as hydrocortisone. It is produced in humans by the zona fasciculata of the adrenal cortex within the adrenal gland. It is released in response to stress and low blood-glucose concentration. It functions to increase blood sugar through gluconeogenesis, to suppress the immune system, and to aid in the metabolism of fat, protein, and carbohydrates. It also decreases bone formation.
Health effects
Metabolic response
In the early fasting state, cortisol stimulates gluconeogenesis (the formation of glucose), and activates antistress and anti-inflammatory pathways. Cortisol also plays an important, but indirect, role in liver and muscle glycogenolysis, the breaking down of glycogen to glucose-1-phosphate and glucose. This is done through its passive influence on glucagon.[clarification needed] Additionally, cortisol facilitates the activation of glycogen phosphorylase, which is necessary for epinephrine to have an effect on glycogenolysis. In the late fasting state, the function of cortisol changes slightly and increases glycogenesis. This response allows the liver to take up glucose not being used by the peripheral tissue and turn it into liver glycogen stores to be used if the body moves into the starvation state. Elevated levels of cortisol, if prolonged, can lead to proteolysis (breakdown of proteins) and muscle wasting.[6] Several studies have shown that cortisol can have a lipolytic effect (promote the breakdown of fat). Under some conditions, however, cortisol may somewhat suppress lipolysis.Immune response. Cortisol prevents the release of substances in the body that cause inflammation. It is used to treat conditions resulting from over activity of the B-cell-mediated antibody response. Examples include inflammatory and rheumatoid diseases, as well as allergies. Low-potency hydrocortisone, available as a nonprescription medicine in some countries, is used to treat skin problems such as rashes and eczema. It inhibits production of interleukin (IL)-12, interferon (IFN)-gamma, IFN-alpha, and tumor-necrosis-factor (TNF)-alpha by antigen-presenting cells (APCs) and T helper (Th)1 cells, but upregulates IL-4, IL-10, and IL-13 by Th2 cells. This results in a shift toward a Th2 immune response rather than general immunosuppression. The activation of the stress system (and resulting increase in cortisol and Th2 shift) seen during an infection is believed to be a protective mechanism which prevents an over activation of the inflammatory response. Cortisol can weaken the activity of the immune system. It prevents proliferation of T-cells by rendering the interleukin-2 producer T-cells unresponsive to interleukin-1 (IL-1), and unable to produce the T-cell growth factor (IL-2). Cortisol also has a negative-feedback effect on interleukin-1. Though IL-1 is useful in combating some diseases, endotoxic bacteria have gained an advantage by forcing the hypothalamus to increase cortisol levels (forcing the secretion of corticotropin-releasing hormone, thus antagonizing IL-1). The suppressor cells are not affected by glucosteroid response-modifying factor, so the effective setpoint for the immune cells may be even higher than the setpoint for physiological processes (reflecting leukocyte redistribution to lymph nodes, bone marrow, and skin). Rapid administration of corticosterone (the endogenous type I and type II receptor agonist) or RU28362 (a specific type II receptor agonist) to adrenalectomized animals induced changes in leukocyte distribution. Natural killer cells are affected by cortisol. Cortisol stimulates many copper enzymes (often to 50% of their total potential), probably to increase copper availability for immune purposes.:337 This includes lysyl oxidase, an enzyme that cross-links collagen, and elastin. 334 Especially valuable for immune response is cortisol's stimulation of the superoxide dismutase, since this copper enzyme is almost certainly used by the body to permit superoxides to poison bacteria.
Other effects:
1- Metabolism:
Glucose: Cortisol counteracts insulin, contributes to hyperglycemia-causing hepatic gluconeogenesisand inhibits the peripheral use of glucose (insulin resistance) by decreasing the translocation of glucose transporters (especially GLUT4) to the cell membrane. However, cortisol increases glycogen synthesis (glycogenesis) in the liver. The permissive effect of cortisol on insulin action in liver glycogenesis is observed in hepatocyte culture in the laboratory, although the mechanism for this is unknown.
Bone and collagen: Cortisol reduces bone formation, favoring long-term development of osteoporosis (progressive bone disease). It transports potassium out of cells in exchange for an equal number of sodium ions. This can trigger the hyperkalemia of metabolic shock from surgery. Cortisol also reduces calcium absorption in the intestine.
Collagen is an important component of connective tissue. It is vital for structural support and is found in muscles, tendons, and joints, as well as throughout the entire body. Cortisol down-regulates the synthesis of collagen.
Amino acid: Cortisol raises the free amino acids in the serum by inhibiting collagen formation, decreasing amino acid uptake by muscle, and inhibiting protein synthesis. Cortisol (as opticortinol) may inversely inhibit IgA precursor cells in the intestines of calves. Cortisol also inhibits IgA in serum, as it does IgM; however, it is not shown to inhibit IgE.
Wound healing: Cortisol and the stress response have known deleterious effects on the immune system. High levels of perceived stress and increases in cortisol have been found to lengthen wound-healing time in healthy, male adults. Those who had the lowest levels of cortisol the day following a 4 mm punch biopsy had the fastest healing time. In dental students, wounds from punch biopsies took an average of 40% longer to heal when performed three days before an examination as opposed to biopsies performed on the same students during summer vacation. This is in line with previous animal studies that show similar detrimental effects on wound healing, notably the primary reports showing that turtles recoil from cortisol.
Electrolyte balance: Cortisol acts as a diuretic, increasing water diuresis, glomerular filtration rate, and renal plasma flow from the kidneys, as well as increasing sodium retention and potassium excretion. It also increases sodium and water absorption and potassium excretion in the intestines.
Sodium: Cortisol promotes sodium absorption through the small intestine of mammals. Sodium depletion, however, does not affect cortisol levelsso cortisol cannot be used to regulate serum sodium. Cortisol's original purpose may have been sodium transport. This hypothesis is supported by the fact that freshwater fish use cortisol to stimulate sodium inward, while saltwater fish have a cortisol-based system for expelling excess sodium.
Potassium
A sodium load augments the intense potassium excretion by cortisol. Corticosterone is comparable to cortisol in this case. For potassium to move out of the cell, cortisol moves an equal number of sodium ions into the cell.[18] This should make pH regulation much easier (unlike the normal potassium-deficiency situation, in which two sodium ions move in for each three potassium ions that move out—closer to the deoxycorticosterone effect
Stomach and kidneys[edit]
Cortisol stimulates gastric-acid secretion. Cortisol's only direct effect on the hydrogen-ion excretion of the kidneys is to stimulate the excretion of ammonium ions by deactivating the renal glutaminase enzyme.Cortisol works with epinephrine (adrenaline) to create memories of short-term emotional events; this is the proposed mechanism for storage of flash-bulb memories, and may originate as a means to remember what to avoid in the future. However, long-term exposure to cortisol damages cells in the hippocampus; this damage results in impaired learning. Furthermore, cortisol inhibits memory retrieval of already stored information.
Sleep, stress, and mood[edit]
Diurnal cycles of cortisol levels are found in humans. In humans, the amount of cortisol present in the blood undergoes diurnal variation; the level peaks in the early morning (around 8 am) and reaches its lowest level at about midnight-4 am, or three to five hours after the onset of sleep. Information about the light/dark cycle is transmitted from the retina to the paired suprachiasmatic nuclei in the hypothalamus. This pattern is not present at birth; estimates of when it begins vary from two weeks to nine months of age.Changed patterns of serum cortisol levels have been observed in connection with abnormal ACTH levels, mood disorders such as major depressive disorder, anxiety disorders, psychological stress, and physiological stressors such as hypoglycemia, illness, fever, trauma, surgery, fear, pain, physical exertion, or temperature extremes. Cortisol levels may also differ for individuals with autism or Asperger's syndrome. Also, significant individual variation is seen, although a given person tends to have consistent rhythms.
Effects during pregnancy: During human pregnancy, increased fetal production of cortisol between weeks 30 and 32 initiates production of fetal lung surfactant to promote maturation of the lungs. In fetal lambs, glucocorticoids (principally cortisol) increase after about day 130, with lung surfactant increasing greatly, in response, by about day 135,[40] and although lamb fetal cortisol is mostly of maternal origin during the first 122 days, 88% or more is of fetal origin by day 136 of gestation.[41] Although the timing of fetal cortisol concentration elevation in sheep may vary somewhat, it averages about 11.8 days before the onset of labor.[42] In several livestock species (e.g. cattle, sheep, goats, and pigs), the surge of fetal cortisol late in gestation triggers the onset of parturition by removing the progesterone block of cervical dilation and myometrial contraction. The mechanisms yielding this effect on progesterone differ among species. In the sheep, where progesterone sufficient for maintaining pregnancy is produced by the placenta after about day 70 of gestation,[43][44] the prepartum fetal cortisol surge induces placental enzymatic conversion of progesterone to estrogen. (The elevated level of estrogen stimulates prostaglandin secretion and oxytocin receptor development.)
Exposure of fetuses to cortisol during gestation can have a variety of developmental outcomes, including alterations in prenatal and postnatal growth patterns. In marmosets, a species of New World primates, pregnant females have varying levels of cortisol during gestation, both within and between females. Infants born to mothers with high gestational cortisol during the first trimester of pregnancy had lower rates of growth in body mass indices than infants born to mothers with low gestational cortisol (about 20% lower). However, postnatal growth rates in these high-cortisol infants was more rapid than low-cortisol infants later in postnatal periods, and complete catch-up in growth had occurred by 540 days of age. These results suggest that gestational exposure to cortisol in fetuses has important potential fetal programming effects on both pre- and postnatal growth in primates.
Synthesis and release
Cortisol is produced in the human body by the adrenal gland in the zona fasciculata, the second of three layers comprising the adrenal cortex. The cortex forms the outer "bark" of each adrenal gland, situated atop the kidneys. The release of cortisol is controlled by the hypothalamus, a part of the brain. The secretion of corticotropin-releasing hormone by the hypothalamustriggers cells in the neighboring anterior pituitary to secrete another hormone, the adrenocorticotropic hormone (ACTH), into the vascular system, through which blood carries it to the adrenal cortex. ACTH stimulates the synthesis of cortisol, glucocorticoids, mineralocorticoids, and dehydroepiandrosterone..