
1
L1
History
1. Early 1900s: Paul Erlich -Concepts of
Chemotherapy and selective toxicity Salvarsan
for Syphilis
2. Fleming (1928) and Florey and Chain (1939) –
penicillin
3. 1935: Gehard Domagk - sulfa drugs
4. 1944: Waksman - streptomycin
Chemotherapy:
is the use of chemical agents (either synthetic or natural) to destroy infective agents
(microorganisms’ i.e bacteria, fungus and viruses, protozoa, and helminthes) and to inhibit the
growth of malignant or cancerous cells. Characterised by selective toxicity depends on the
existence of exploitable biochemical difference between the parasite and the host cell.
Antimicrrobials:
are chemical agents (synthetic/natural) used for the treatment of infections by suppressing or
destroying the causative microorganisms (bacteria, mycobacteria, fungi, protozoa, or viruses).
Antibiotics:
are substances produced by various species of microorganisms (bacteria, fungi, actinomycetes)
that suppress the growth of other microorganisms.
Classification of antibacterial drugs
1) Agents
ⱴ Bacteriostatic agents: Agents that inhibit the growth of the microorganisms by
producing reversible changes. This delay in the growth will give the immune system the
chance to get rid of the microorganism.
ⱴ Bactericidal agents: Agents that kill the microorganism.
(Being a bactericidal or a bacteriostatic agent depends on the mechanism of action of the
antibacterial agent and on its concentration.)
Antibiotics
2
2) Spectrum
ⱴ Narrow spectrum: The range of activity for agents that kill the micro-organism is
small. It affects 1-2 classes of microorganisms only. For example, Penicillin-G
affects G+ve organisms and Neisseriae.
ⱴ Broad spectrum: The range of activity extends to many micro-organisms. For
example, Tetracyclines depress G+ve, Gve, Rickettsiae and Chlamydiae. Separation
between narrow and broad spectrum activity is not clear due to the emergence of
many resistant strains due to the overuse of these antibiotics. Broad spectrum
antibiotics should be restricted to treatment of specific infections caused by a few
organisms or even a single species of organism. The property of broad specification
should not be confused with a free license for broad-nonspecific use.
Classification according to chemical structure
ⱴ Beta-lactam ( penicillins and cephalosporins)
ⱴ Aminoglycosides
ⱴ Tetracyclins
ⱴ Chloramphenicol
ⱴ Macrolids
ⱴ Quinolines
ⱴ sulfanomides
Classification according to mode of action
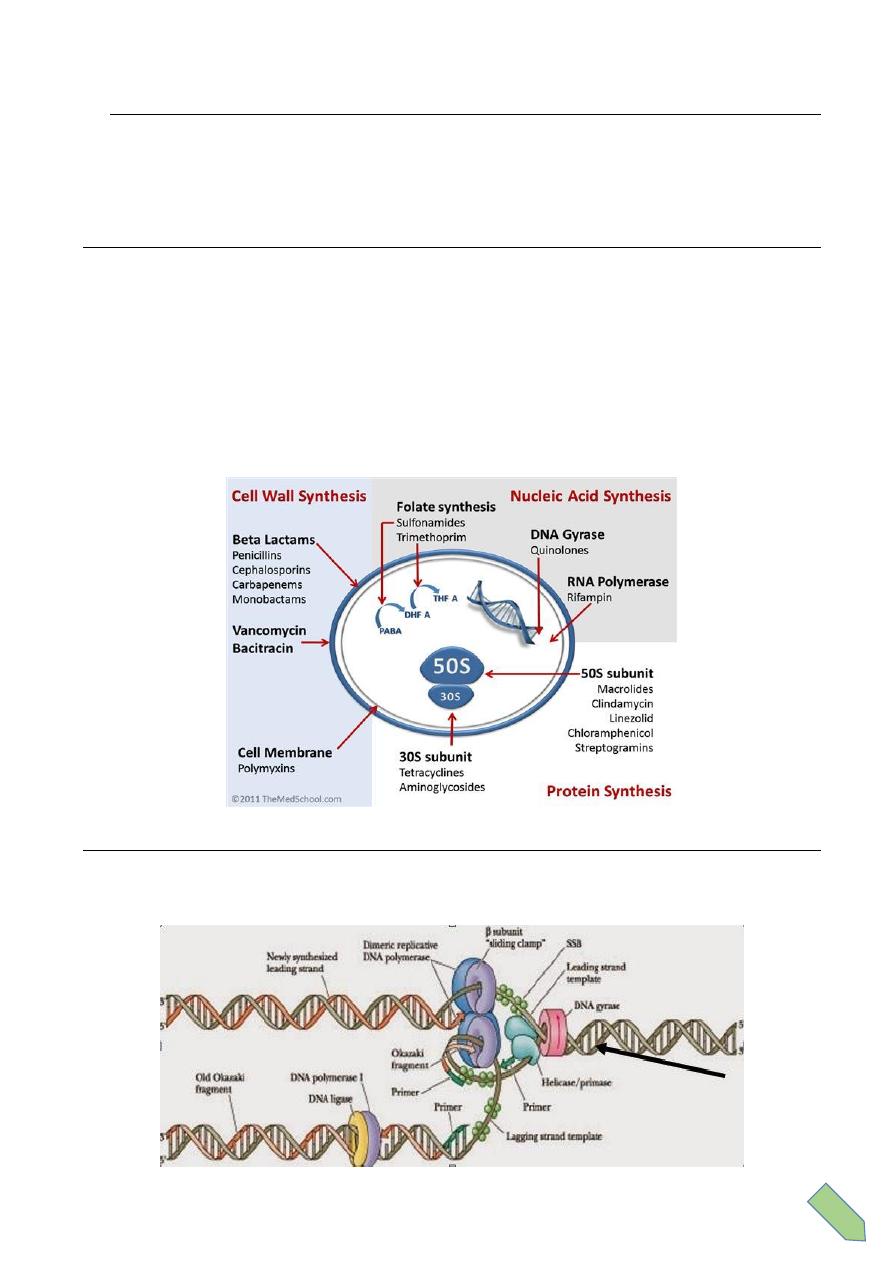
Mechanisms of antimicrobial drug action:
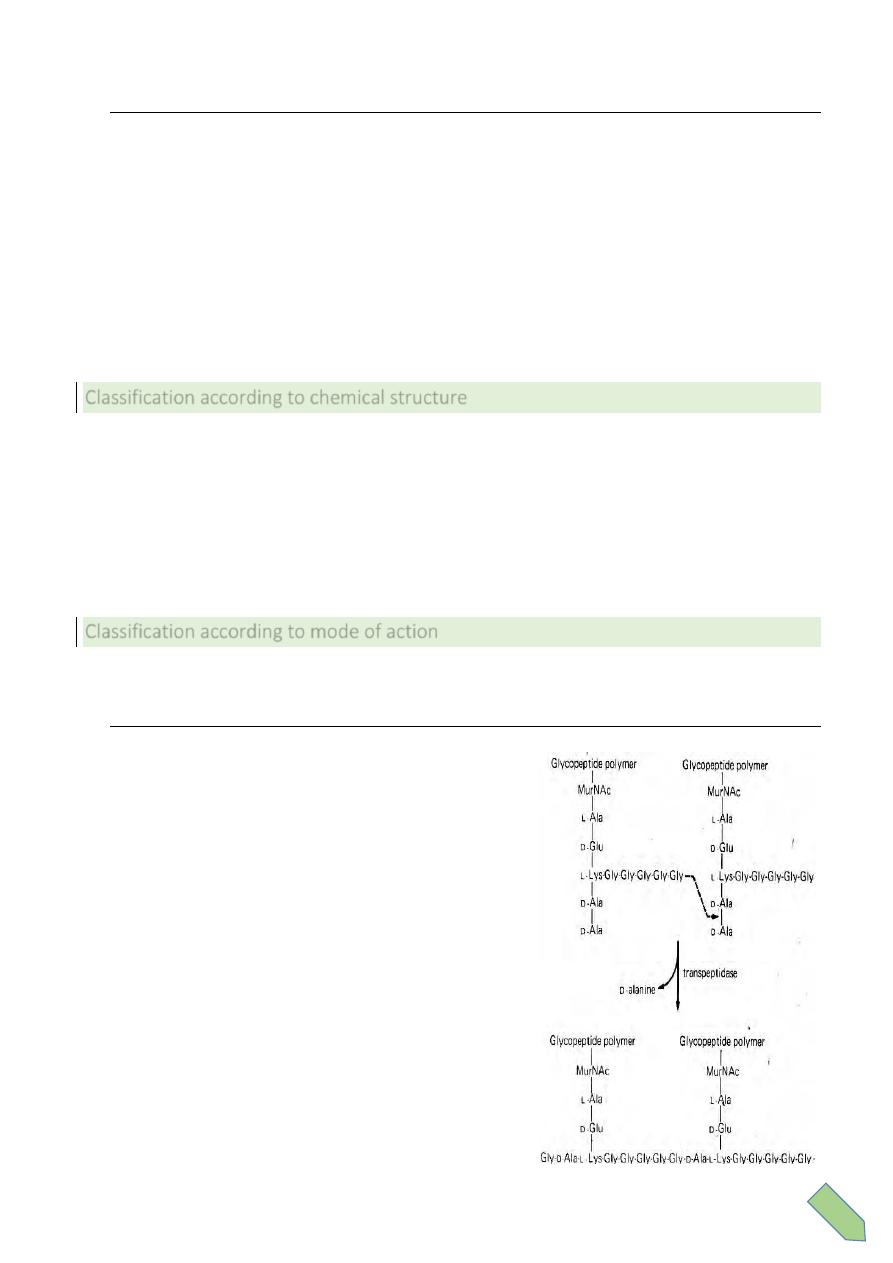
1. Inhibition of cell wall synthesis ( beta-lactams)
Beta lactams contain β- ring which is an analogue of
D-alanyl-D-alanine on peptide side chain of
peptidoglycan à inhibits transpeptidase from
crosslinking Peptidoglycan + binds penicillin binding
proteins à activation of autolysins
Bacitracin,
Vancomycin,
Cycloserine:
Block
peptidoglycan synthesis
3
2.
Cell membrane function inhibitors (polypeptides):
Polymxin B: interact with phospholipids to increase permeability and decrease osmotic
integrity and leakage on intracellular components
3. Inhibition of protein synthesis (tetracycline, aminoglycosides, macrolides,
chloramphenicol)
a. Tetracyclins: reversible binding to the 30s subunit of ribosome ànd inhibit binding of
aminoacyl tRNA to ribosome à inhibition of protein synthesis
b. Aminoglycosides: irreversible binding to the 30s subunit of ribosome and inhibit protein
synthesis and causes misreading of mRNA
c. Macrolids: reversibly binds 50s subunit of the ribosome à inhibits translocation during
protein synthesis
d. Chloramphenicol: binds to 50s subunit of the ribosome à inhibits transpeptidation
during protein synthesis
4- Inhibition of nucleic acid synthesis ( quinolines, rifampicin)
a. Quinolines: inhibits DNA gyrase à inhibition of DNA replication.
b. Rifampicin: inhibits DNA- dependent RNA polymerase à inhibition of RNA synthesis

4
5. Antimetabolites ( sulfonamides, trimethoprim)
a. sulfonamides: competes with p-aminobenzoic acid for binding to the enzyme
dihydropteroate synthetase à no folic acid synthesis à no nitrogenous base synthesis
b. Trimethoprim: dihydrofolate reductase inhibitor
Using and avoiding antibacterial drugs
Antibacterial are valuable drugs if used appropriately. They are very effective in treating
infections if used in appropriate doses, at appropriate intervals and for the appropriate
period of time against sensitive microorganisms.
Anti-infective agents should be used only when:
a. A significant infection has been diagnosed or is strongly suspected.
b. An established indication for prophylactic therapy exists.
Abuse of these agents causes superinfections, cross-sensitivity and cross resistance, resulting
in inappropriate treatment and in consequent adverse reaction in addition to wastage of
money.
They should not be used in the following cases:
1. To treat all infections (e.g. viral infections or nonspecific inflammation).
2. For minor infections (e.g. superficial bruises).
3. For cases need surgical opening and draining such as abscess
Superinfection (suprainfection):
Is the appearance of both microbiological and clinical evidence of a new infection with
pathogenic microorganisms or fungi not sensitive to the used drugs during antimicrobial
treatment of a primary disease. The body's natural resistance is compromised, making it
more susceptible to secondary infections by more dangerous strains.

5
Bacterial resistance:
Antimicrobial agents are loosing their effectiveness because of the spread of drugresistant
strains. Therefore, there might come a time when such agents are no longer useful to combat
diseases.
Mechanisms of resistance to antibiotics
1. Production of enzymes that inactivate the drug (eg. β -lactamase, which inactivates
beta lactam antibiotics; acetyl transferases, which inactivate chloramphenicol; kinases
and other enzymes, which inactivate aminoglycosides.
2. Alteration of the drug-binding site: this occurs with penicillins, aminoglycosides and
erythromycin.
3. Reduction of drug uptake by the bacterium: eg. Tetracyclines
4. Alteration of enzymes: eg. Dihydrofolate reductase becomes insensitive to
trimethoprim.
Reasons for antibiotic resistance
1. Misuse of antibiotics selects for resistance mutants. Misuse includes:
a. Using outdated, weakened antibiotics
b. Using antibiotics for viral infection like common cold and other inappropriate
conditions
c. Use of antibiotics in animal feed
d. Failure to complete the prescribed regiment
Selecting anti-infective agent:
a. The spectrum of activity of the antiinfective agent: It should be active against
the causative pathogen. This can be known by carrying the susceptibility tests or by a
good clinical experience in treating a given syndrome that will help in suggesting a
potential effective agent.
b. Patient factors: These factors play a very important role in the selection of a specific
anti-infective agent, determination of the appropriate drug dosage and route of
administration,...etc. Those factors include:
1) History of drug allergy or adverse
reactions. Anaphylaxis or reactions due to immunoglobulin E (IgE) may be life
threatening when taking penicillins.
2) Age: A drug’s pharmacokinetic properties vary widely in patients of different age
groups.
3) Underlying disease: -A pre-existing kidney and liver disease, CNS disorder.
Neuromuscular disorders.
4) Immunological status: Patients with impaired immune system require a bactericidal
agent rather than a bacteriostatic one.
5) Pregnancy and lactation.
6
6) Genetic traits.
Combination therapy in special situations
ⱴ Extend the antimicrobial spectrum especially in Initial empiric therapy
ⱴ Mixed infections or severe infections:
ⱴ To prevent the emergence of resistance:
Duration of therapy:
ⱴ Acute cases: Treatment of acute uncomplicated infections generally should continue
until the patient has been a febrile and asymptomatic for at least 72 hours (minimum
5 days in most cases). Other cases as in Strep. throat (Streptococcal pharyngitis)
should be treated for 7-10 days. Some infections require a proof of eradication by
culture.
ⱴ Chronic cases: Treatment of chronic infections (e.g., endocarditis, osteomyelitis) may
require a longer duration (4 to 6weeks), with a follow-up culture analysis afterwards.
Cell wall inhibitors:
Beta lactams (penicillins and cephalosporins)
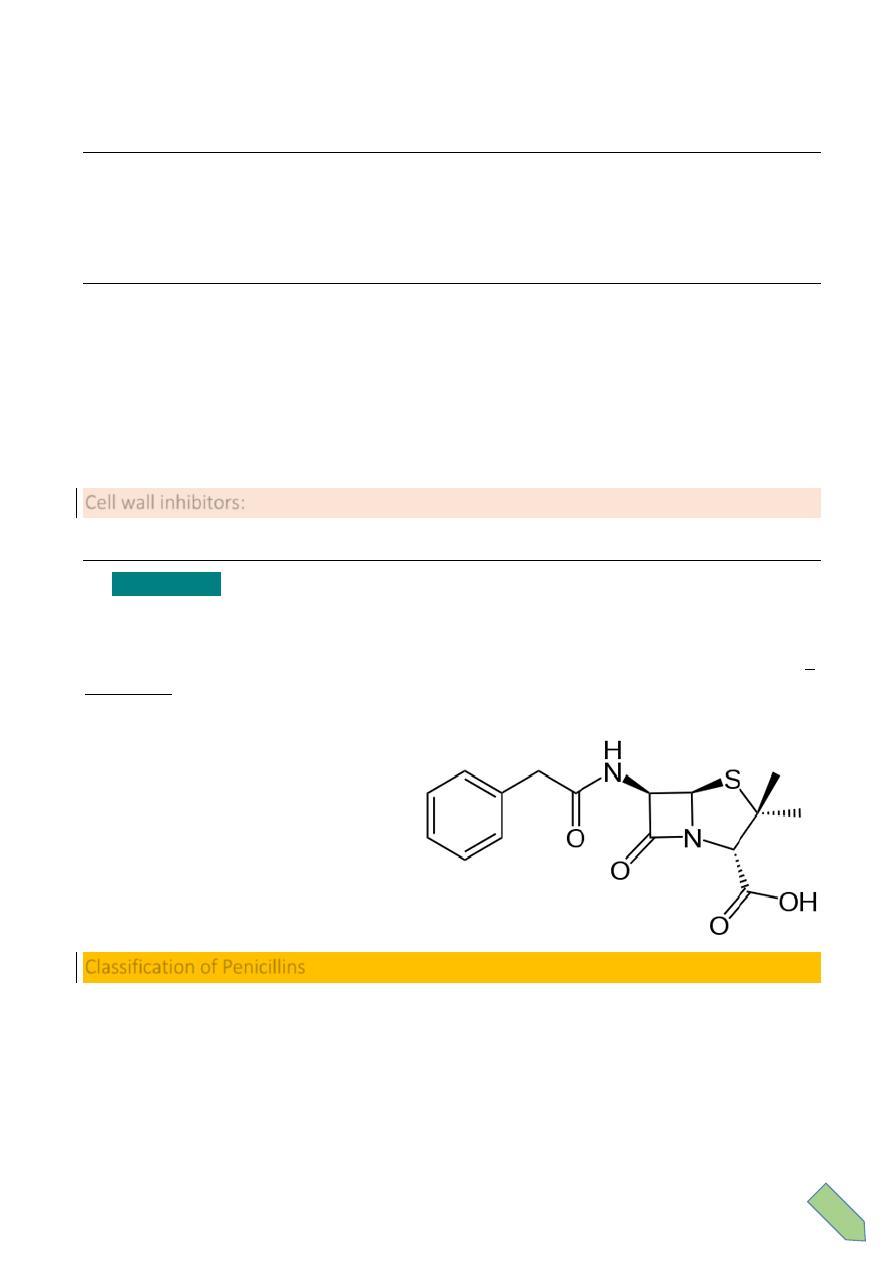
ⱴ Penicillins:
Chemical structure:
Three components: A thiazolidine ring, the β-lactam ring (responsible for their activity), and a
side chain (determines in large part to antibacterial spectrum and pharmacologic properties
of a particular penicillin)
Classification of Penicillins
ⱴ Natural Penicillins
- Penicillin G (benzylpenicillin)
- Penicillin G benzathine,
- phenoxymethyl penicillin)
ⱴ Penicillinase-Resistant Penicillins
- Methicillin, Cloxacillin, Oxacillin, Nafcillin, Dicloxacillin

7
ⱴ Extended-Spectrum Penicillins
- Ampicillin , Amoxicillin,
ⱴ Anti-Pseudomonal Pencillins
- Carbenicillin, Ticarcillin, Piperacillin
ⱴ ß-Lactamase Combinations
- Ampicillin- Cloxacillin
- Amoxillin-clavulanic acid,
- Ampicillin-sulbactam,
- Ticarcillin-clavulanic acid
Natural penicillins:
Benzylpenicillin & Phenoxymethyl penicillin
These two are known as the natural penicillins. They are the first two penicillins that were
discovered and are still in use. Natural penicillins are narrow spectrum antibiotics and are
only active against facultative gram-positive cocci, rods and gram-negative cocci. Several
anaerobic gram-negative rods are sensitive to penicillin, with the notable exception of
Bacteroides fragilis.
Benzyl penicillin
ⱴ Is the drug of choice in streptococcal, pneumococcal, gonococcal, and eningococcal
infections.
ⱴ It is also used in anthrax, diphtheria, gas gangrene, leptospirosis, syphilis, tetanus,
yaws, and in the treatment of lyme disease in children.
ⱴ It is inactivated by the gastric fluids, and absorption from the gut is low; therefore it is
given by injection.
ⱴ In addition to the use of Pen. G as sodium or potassium salts (soluble Pen. G), it is also
available in two other salts that are commonly used.
They are:
a- Procaine penicillin: a sparingly soluble salt of benzylpenicillin. It is used in
intramuscular
depot preparations that provide therapeutic tissue concentrations for up to 24 hrs. It
is the preferred choice for the treatment of syphilis, but neurosyphilis requires special
consideration.
b- b- Benzathine penicillin: a benzylpenicillin salt with a very low solubility, giving a
prolonged action after intramuscular injection. Its duration of action is 20 days.
Phenoxymethyl penicillin (Pen. V)
Has a similar antibacterial spectrum as Pen. G, but it is less active. It is gastric acid stable
so it is suitable for oral administration. It should not be used for serious infections because
absorption can be unpredictable and plasma concentrations are variable.

8
Indications:
Benzylpenicillin mainly indicated for the treatment for:
ⱴ throat infections
ⱴ otitis media
ⱴ Streptococcal endocarditis
meningococcal and pneumococcal meningitis (if caused by susceptible
microorganism)
ⱴ prophylactic agent after limb amputation.
ⱴ Also it is used in combination with other agents if more than one organism are
suspected.
Phenoxymethyl penicillin
ⱴ Is indicated principally for
respiratory tract infections in children, for Streptococcal tonsillitis and for continuing
treatment after one or more injections of Pen. G when clinical response has begun.
ⱴ It should not be used for meningococcal or gonococcal infections.
ⱴ It is used prophylactically against rheumatic fever following streptococcal infections.
Contraindications:
They are contraindicated in the case of hypersensitivity to any of the penicillins or
cephalosporins.
Procaine penicillin is also contraindicated in the case of hypersensitivity
to procaine or any other “caine type” local anesthetic.
Penicillinase resistance penicillins (Antistaphylococcal penicillins)
Cloxacillin, dicloxacillin, flucloxacillin and methicillin
Most staphylococci are now resistant to benzylpenicillin because they produce
penicillinases.
ⱴ Cloxacillin and flucloxacillin are not affected by such enzymes, so they are effective
in infections caused by penicillin resistant staphylococci, but they are less potent than
Pen. G against penicillin sensitive microorganisms. and generally ineffective against
G-ve bacteria and methicillin resistant staphylococci.
The only difference between cloxacillin and flucloxacillin is that flucloxacillin has a
higher bioavailability than cloxacillin after oral administration.
Another two examples.
ⱴ Methicillin is toxic and didn’t used medicinally
These drugs used in combination with ampicillin: ampicillin with cloxacillin or
flucloxacillin and amoxicillin with cloxacillin.
9
ⱴ Cloxacillin: prepared as capsules and suspension. Flucloxacillin: Not available in a
separate formulation, but as combination with ampicilin in capsules.
Indication
:
Cloxacillin and flucloxacillin are indicated for the treatment of infections caused by
penicillinase producing Staphylococci. Methicillin is toxic and not used medicinally.
Extended-Spectrum Penicillins also called (aminopenicillins):
Including ampicillin, amoxicillin and (bacampicillin and pivampicillin), which are esters of
ampicillin.
ⱴ Aminopenicillins are active against some G+ve and G-ve organisms but inactivated by
penicillinases, including those produced by Staphylococcus aureus, and by common G-
ve bacilli such as Escherichia coli.
The majority of Staphylococci, 50% of E. coli strains and 15% of Haemophilus
influenzae strains are now resistant.
ⱴ Amoxicillin is a derivative of ampicillin that differs only by one hydroxyl group. Unlike
ampicillin it can be given 3 times daily without regard to food. Ampicilline is given 4
times daily and its absorption affected by the presence of food in the stomach, so it
should be taken one hour before or two hours after the meal).
Indication:
They are principally indicated for the treatment of chronic bronchitis and mild ear infections,
both of which are usually due to Streptococcus pneumoniae and Haemophilus influenzae.
They are also indicated for: urinary-tract infections, otitis media, sinusitis, chronic bronchitis,
invasive salmonellosis, and gonorrhea.
Amoxicillin is also used for typhoid fever and endocarditis prophylaxis.
Antipseudomonals
Include both carboxypenicillins (carbenicillin and ticarcillin) and ureidopenicillins (piperacillin,
azlocillin, and mezlocillin).
ⱴ Antipseudomonal penicillins are similar to the aminopenicillins in structure but have
either a carboxyl group or urea group instead of the amine.
ⱴ The major advantage of carboxypenicillins is their activity against Pseudomonas
aeruginosa (one of the major pathogens responsible for nosocomial pneumonia) and
certain indole-positive Proteus species that are resistant to aminopenicillins. Ticarcillin
is stronger against P. aeruginosa and Enterobacter species than carbenicillin.
ⱴ Against anaerobes and Gram-positive organisms, carboxypenicillins generally have the
same spectrum of activity as penicillin G. However, they are substantially weaker in
comparison with penicillin G.

10
ⱴ Ureidopenicillins have greater activity against P. aeruginosa compared to carbenicillin
and ticarcillin. Piperacillin is the most potent of the extended-spectrum penicillins
against Pseudomonas. The spectrum of piperacillin and mezlocillin is extended to
include Klebsiella, Enterobacter, Citrobacter.
ⱴ All antipseudomonals are destroyed by β-lactamases.
ⱴ The extended-spectrum penicillins are not used in the treatment of infections caused
by Gram-positive bacteria because penicillin G and aminopenicillins are more potent
against these organisms.
ⱴ Antipseudomonals penicillins may be used in combination with Aminoglycosides.
Note:
The natural penicillins was originally used as units. International Unite= 0.6 microgram
(0.0006 milligram) of penicillin ( 1 mg = 1670 IU) . Synthetic and semisynthetic penicillins in
mg …
Side effects of Penicillins:
- Hypersensitivity and anaphylactic shock
Hypersensitivity is one of the most important adverse reactions to penicillins. The
frequency of allergic reactions to all penicillins ranges from 0.7% to 10%. The
manifestations of penicillin allergy include maculopapular or morbilliform rash, fever,
urticaria, exfoliative dermatitis, swelling of the throat, difficulty breathing,
eosinophilia, serum sickness, Stevens-Johnson syndrome, and anaphylactic shock
(0.004% to 0.015%)
- Pain in injection
- GI: diarrhea, nausea, vomiting, pseudo membranous colitis (rare).
- CNS: convulsive seizures.
- Skin: pruritus, urticaria, or other skin eruptions.
- Hematological: hemolytic anemia, thrombocytopenia, purpura, eosinophilia,
leukopenia, agranulocytosis.
- Others: superinfections
-
First generation
ⱴ Cefazolin IM, IV,
ⱴ Cephalothin IM, IV,
ⱴ Cephapirin IM, IV,
ⱴ Cefadroxil IV, PO
ⱴ Cephalexin PO,
ⱴ Cephradine PO
11
Activity
1. Staph. aureus - excellent activity against b-lactamase-producing strains
Not effective against methicillin-resistant Staph. aureus & epidermidis
2. Streptococci - excellent activity versus Streptococcus sp. Not effective against
penicillin-resistant Strep. Pneumoniae
3. Other Gm + bacteria - excellent activity except for Enterococcus sp.
4. Moderate activity against gram negative bacteria. Susceptible organisms include: E.
coli, Proteus mirabilis Indole + Proteus sp. (many strains resistant) , Haemophilus
influenzae (some strains resistant), Neisseria sp. (some gonococci resistant)
Indication
1. Upper respiratory tract infections due to Staph. and Strep.
2. Lower respiratory tract infections due to susceptible bacteria e.g. Strep. pneumoniae
in penicillin-allergic patient
3. Uncomplicated urinary tract infections (Cephalexin)
4. Surgical prophylaxis for orthopedic and cardiovascular operations (cefazolin preferred
because of longer half-life)
5. Staphylococcal infections of skin and skin structure
Second Generation
ⱴ Cefuroxime IM, IV
ⱴ Cefuroxime axetil PO
ⱴ Cefamandole IM, IV
ⱴ Cefaclor PO
ⱴ Cefoxitin IM, IV
ⱴ Cefdinir PO
ⱴ Cefonicid IM, IV
ⱴ Cefprozil PO
ⱴ Cefotetan IM, IV
ⱴ Cefmetazole IV
Activity
1. Expanded activity against gram negative bacilli. Still have excellent activity against
gram positive (Staph. and Strep.) bacteria.
Activity for Gram negative bacteria
Neisseria sp. (some gonococci resistant)
H. influenzae (including some ampicillin-resistant strains)
Moraxella catarrhalis (some resistance esp. to cefaclor),
E. coli, Proteus mirabilis,
Indole + Proteus (some strains resistant) Morganella morganii (some strains
resistant),
Klebsiella pneumonia,
Serratia sp. (many strains resistant)
12
2. Anaerobic infections - (Cefoxitin & Cefotetan only)
moderate activity against Bacteroides fragilis group.
Good activity for other Bacteroides sp., Peptostreptococcus, Fusobacterium,
Clostridium sp.
Indications
1. Community-acquired pneumonia - Cefuroxime is widely used for empiric therapy.
2. Skin and soft tissue infection
3. Urinary tract infections
4. Upper respiratory tract infections (otitis media, sinusitis).
5. Mixed aerobic & anaerobic infections
6. Surgical prophylaxis - Cefoxitin or cefotetan are widely used in cases where mixed
aerobic & anaerobic infections may occur, esp. intra-abdominal, colorectal, and
gynecologic operations. For cardiovascular and orthopedic procedures, cefuroxime
and others may be used, but cefazolin is cheaper and appears to work well.
Third Generation
ⱴ Cefotaxime IM, IV
ⱴ Cefixime PO
ⱴ Ceftizoxime IM, IV
ⱴ Ceftibuten PO
ⱴ Ceftriaxone IM, IV
ⱴ Cefpodoxime axetil PO
ⱴ Ceftazidime IM, IV
ⱴ Cefoperazone IM, IV
Activity
Prototype drugs are Cefotaxime (IV) and Cefixime (oral). Ceftazidime (for Ps aeruginosa
1) Further expansion of Gm negative spectrum to include
E. Coli
Proteus mirabilis (indole –) Proteus vulgaris (indole +)
Klebsiella pneumonia
Morganella morganii,
Providencia retgerri,
Citrobacter freundii,
Serratia marcescens,
Pseudomonas aeruginosa, (Ceftazidime)
Enterobacter
Stenotrophomonas maltophilia (Cefoperazone & Ceftazidime only) Acinetobacter
2) In general, activity toward Gm + bacteria is reduced
Indications:
1. Gram negative septicemia & other serious Gm – infections
2. Pseudomonas aeruginosa infections (Ceftazidime - 90% effective)

13
3. Gram negative meningitis - Cefotaxime, Ceftriaxone, Cefepime. For empiric therapy add
vancomycin ± rifampin to cover resistant Strep. Pneumoniae
4. Gonorrhea - Single shot of Ceftriaxone is drug of choice. Oral cefixime and ceftibuten are
also OK.
5. Complicated urinary tract infections, pyelonephritis
6. Osteomyelitis - Ceftriaxone in home health care situations
7. Lyme disease - ceftriaxone in home health care situations
Fourth generation
cefepime IM, IV
Activity
- With similar activity against gram-positive organisms as first-generation
cephalosporins.
- Bu they are more active against G –ve and have a greater resistance to beta-
lactamases than the third generation cephalosporins.
Indications:
1. Pneumonia: Treatment of moderate-to-severe pneumonia
2. Febrile Neutropenia: Empiric therapy in febrile neutropenic patients.
3. Urinary Tract Infections.
4. Treatment of uncomplicated skin and skin structure infections.
5. Treatment of complicated intra-abdominal infections; use in combination with
metronidazole
Fifth generation
Ceftaroline IV
Activity
- Enhanced coverage of G+ve organisms: MRSA, S. pneumonia, and E. faecalis …
- Similar gram negative coverage to third- and fourth-generation agents , does not
cover Pseudomonas, limited anti-anaerobes activity.
Indications:
1. Community-acquired bacterial pneumonia
2. Indicated for acute bacterial skin and skin structure infections, including MRSA
Pharmacokinetics:
Cephalexin, cephradine, cefaclor, cephadroxil, cefuroxime axetil and cefixime are
absorbed after oral administration and can be given orally.
Most of the remaining cephalosporins are given parenterally.
Cefixime is the only third generation cephalosporin available in oral dosage form.

14
They are well distributed in the body. Adequate CSF concentrations are mainly
achieved with third generation cephalosporins.
Cephalosporins cross the placenta and they are found in higher concentration in
synovial fluid and pericardial fluid.
Cephalosporins are variably bound to plasma proteins, cefazolin is maximally bound
(85%).
They are mainly excreted in urine by glomerular filtration and tubular secretion.
Probenecid decreases the tubular secretion and increase plasma level of
cephalosporins.
Dose adjustment is necessary in chronic renal failure.
Cephalothin, cephapirin and cefotaxime are metabolized and their metabolites are
excreted in urine.
Cephoperazone and ceftriaxone are mainly excreted in bile, therefore can safely be
given in CRF without dosage adjustments.
Side effects of cephalosporins:
ⱴ Hypersensitivity reactions to cepalosporins are the most common side effects,
reaction appear to be identical to those caused by penicillin.
ⱴ More commonly maculopapular rash develops, usually after several days of therapy
immediate reactions such as anaphylaxis, bronchospasm and urticaria are also
observed.
ⱴ There is cross reactivity between penicillin and cephalosporins due to structural
resemblance.
ⱴ Cephalosporins should be avoided in patients allergic to penicillin.
ⱴ Cephalosporins may produce nephrotoxicity, that is more common with cephaloridine
(it is not used now a days).
ⱴ High dose of cephalothin produces renal tubular necrosis.
ⱴ Risk of nephrotoxicity is increased when cephalosporins are administered with other
potential nephrotoxic agents (Aminoglycosides and Vancomycin).
ⱴ Diarrhea occurs frequently with cefoperazone because of greater biliary excretion.
ⱴ Pain and local irritation occurs after I/M injection.
ⱴ Some cephalosporins like cefamandole, moxalactam, cefoperazone, cefotetan
frequently produce hypoprothrombinemia & bleeding disorders that can be prevented
by administration of vitamin K.
ⱴ Cefamandole, cefoperazone, cefotetan and moxalactam can also cause severe
disulfiram like reaction.
ⱴ Alcohol and alcohol containing medication must be avoided.
ⱴ Superinfections are observed mainly with second and third generation cephalosporins.
ⱴ Thrombophlebitis is observed after I/V injections

15
β-lactamase Inhibition
1. Sulbactam (ampicillin-sulbactam).
2. Clavulanic acid (amoxicillin-clavulanate, Augmentin; ticarcillin-clavulanate, Timentin).
3. Tazobactam (pipercillin-tazobactam, Zosyn).
Monobactams
Aztreonam
- Aztreonam- a monocyclic β−lactam relatively resistant to β−lactamases. Spectrum
similar to gentamycin. Active against Gram negative rods and resist β-lactamase.
- It is effective in treating Gram-negative urinary tract infections, lower respiratory tract,
skin, intraabdominal, gynecologic infections and septicemia.
- It is administered either IV or IM.
- It is excreted in the urine.
- Has a low immunogenic potential. A safe alternative in patients allergic to penicillins
and cephalosporins.
Carbapenems:
Carbapenems are synthetic β-lactams. Imipenem, meropenem and ertapenem are the
available drugs of this group.
Imipenem
1. Unique pharmacologic problem: After imipenem is removed from the circulation by
glomerular filtration and secreted, it is metabolized by a renal peptidase which is
located on the brush border of the proximal renal tubules. The metabolites are
nephrotoxic.
2. To overcome this problem, a specific peptidase inhibitor, cilastin, was synthesized,
which totally blocks the metabolism of imipenem in the kidney, thus blocking toxicity.
Cilastin has no antimicrobial activity.
3. Compound drug is imipenem-cilastin combination (Primaxin).
4. Particular toxicity is seizures, primarily in renal failure or in the face of ongoing or
preceding brain injury.
Other Inhibitors of Cell-Wall Synthesis
Vancomycin
Vancomycin, a glycopeptide, is active only against gram-positive bacteria, including ß-
lactamase producing staphylococci and those resistant to nafcillin and methicillin
Vacomycin is an inhibitor of bacterial cell wall synthesis by preventing peptidoglycan
elongation and cross-linking.
16
The resistance to the antibacterial action of vancomycin is due to a modification of its
peptidoglycan binding site, a modification that reduces binding affinity.
Vancomycin acts synergistically with gentamicin and streptomycin (aminoglycosides) against
E. faecium and E. faecalis isolates not resistant to aminoglycosides.
Vancomycin is not absorbed after oral administration. Slow I.V infusion is employed for
treatment of systemic infections or prophylaxis. Oral administration, is only employed for the
treatment of antibiotic induced colitis due to Closteridium difficile when metronidazole has
proven ineffective.
Major Clinical Use
- Sepsis
- Endocarditis due to methicillin resistant staphylococci
- Methicillin-susceptible Staph isolates would be more effectively treated with
methicillin than vancomycin.
- Treatment alternative enterococcal endocarditis.: Vancomycin with gentamycin: for
patient allergic to penicillin.
- Vancomycin incombination with cefotaxime, ceftriaxone or rifampim: appropriate for
treatment of mennigitis when the suspected infecting agent is thought/known to be
highly penicillin resistant.
Adverse effect:
Fever and chills
Phlebitis at the infusion site. Flushing and shock results from histamine release.
Dose related hearing loss has occurred in patients with renal failure. Ototoxicity more
common when vancomycin is administered with another drug that can also produce these
effects.
Protein synthesis inhibitors
Aminoglycosides
ⱴ -The aminoglycosides include streptomycin, neomycin, kanamycin, amikacin,
gentamicin, tobramycin, sisomicin, netilmicin, and others.
ⱴ Structure—All aminoglycosides consist of central six-membered aminocyclitol ring
linked to two or more aminosugar residues by glycosidic bonds.
Mechanism of Action
Aminoglycosides are irreversible inhibitors of protein synthesis, but the precise mechanism
for bactericidal activity is not known. The initial event is passive diffusion via porin channels
across the outer membrane.

17
Inside the cell, aminoglycosides bind to specific 30S-subunit ribosomal proteins.
Protein synthesis is inhibited by aminoglycosides in at least three ways
(1) interference with the initiation complex of peptide formation;
(2) misreading of mRNA, which causes incorporation of incorrect amino acids into the
peptide and results in a nonfunctional or toxic protein
(3) breakup of polysomes into nonfunctional monosomes. These activities occur more or
less simultaneously, and the overall effect is irreversible and lethal for the cell.
Spectrum of Activity and clinical uses:
Clinical Uses
- Aminoglycosides are mostly used against gram-negative enteric bacteria [Klebsiella
species: an aminoglycoside (gentamicin + an antipseudomonal penicillin), Yersinia
pestis, Francisella tularensis, and brucella species (gentamicin or streptomycin +
doxycycline). Pseudomonas aeuroginosa: infections in immunocompromised
patients and in burn victims: (tobramycin + anti-pseudomonal penicillin)].
- Streptomycin is used for tuberculosis.
- Aminoglycosides used in combination with a β-lactam antibiotic to extend coverage
to include potential gram-positive pathogens and to take advantage of the
synergism between these two classes of drugs.
- -Penicillin+aminoglycoside combinations also are used to achieve bactericidal
activity in treatment of enterococcal endocarditis and to shorten duration of therapy
for viridans streptococcal and some patients with staphylococcal endocarditis.
Mechanisms of Resistance
Three principal mechanisms have been established:
1. production of a transferase enzyme or other enzymes inactivates the aminoglycoside
by adenylylation, acetylation, or phosphorylation.
2. There is impaired entry of aminoglycoside into the cell.
3. The receptor protein on the 30S ribosomal subunit may be deleted or altered by
mutation.
Pharmacokinetics
- Aminoglycosides are absorbed very poorly from the intact gastrointestinal tract, and
almost the entire oral dose is excreted in feces after oral administration. However, the
drugs may be absorbed if ulcerations are present.
- After intramuscular injection, aminoglycosides are well absorbed, giving peak
concentrations in blood within 30–90 minutes. Aminoglycosides are usually
administered intravenously as a 30- to 60-minute infusion; after a brief distribution
phase, this results in serum concentrations that are identical with those following
intramuscular injection.

18
- The normal half-life of aminoglycosides in serum is 2–3 hours, increasing to 24–48 hours
in patients with significant impairment of renal function.
- Even after parenteral administration, concentrations of aminoglycosides are not high in
most tissues.
- They are poorly penetrated CNS, in the presence of active inflammation, however,
cerebrospinal fluid levels reach 20% of plasma levels, and in neonatal meningitis, the
levels may be higher. Intrathecal injection is required for high levels in cerebrospinal
fluid.
- All aminoglycosides are rapidly excreted into the urine, predominantly by glomerular
filtration. Accumulation occurs in patients with renal impairment, it requires dose
modification.
- All aminoglycosides are ototoxic and nephrotoxic. Ototoxicity and nephrotoxicity are
more likely to be encountered when therapy is continued for more than 5 days, at
higher doses, in the elderly, and in the setting of renal insufficiency. Concurrent use with
loop diuretics (eg, furosemide, ethacrynic acid) or other nephrotoxic antimicrobial
agents (eg, vancomycin or amphotericin) can potentiate nephrotoxicity and should be
avoided if possible. Ototoxicity can manifest either as auditory damage, resulting in
tinnitus and high-frequency hearing loss initially, or as vestibular damage, evident by
vertigo, ataxia, and loss of balance.
- Nephrotoxicity results in rising serum creatinine levels or reduced creatinine clearance,
although the earliest indication often is an increase in trough serum aminoglycoside
concentrations. Neomycin, kanamycin, and amikacin are the most ototoxic agents.
- Streptomycin and gentamicin are the most vestibulotoxic. Neomycin, tobramycin, and
gentamicin are the most nephrotoxic.
- In very high doses, aminoglycosides can produce a curare like effect with neuromuscular
blockade that results in respiratory paralysis. This paralysis is usually reversible by
calcium gluconate (given promptly) or neostigmine.
- Hypersensitivity occurs infrequently.
Streptomycin
Streptomycin was isolated from a strain of Streptomyces griseus.
Clinical Uses
ⱴ Mycobacterial Infections:
ⱴ Streptomycin is mainly used as a second-line agent for treatment of tuberculosis. The
dosage is 0.5–1 g/d (7.5–15 mg/kg/d for children), which is given intramuscularly or
intravenously. It should be used only in combination with other agents to prevent
emergence of resistance.
ⱴ Nontuberculous Infections:
ⱴ In plague, tularemia, and sometimes brucellosis, streptomycin, 1 g/d (15 mg/kg/d for
children), is given intramuscularly in combination with an oral tetracycline.

19
ⱴ Penicillin plus streptomycin is effective for enterococcal endocarditis and 2-week
therapy of viridans streptococcal endocarditis.
ⱴ Gentamicin has largely replaced streptomycin for these indications.
ⱴ Streptomycin remains a useful agent for treating enterococcal infections, however,
because approximately 15% of enterococcal isolates that are resistant to gentamicin
(and therefore resistant to netilmicin, tobramycin, and amikacin) will be susceptible to
streptomycin.
Adverse Reactions
- Fever, skin rashes, and other allergic manifestations may result from hypersensitivity to
streptomycin. This occurs most frequently with prolonged contact with the drug either
in patients who receive a prolonged course of treatment (eg, for tuberculosis).
- Pain at the injection site is common but usually not severe. The most serious toxic effect
with streptomycin is disturbance of vestibular function-vertigo and loss of balance.
- Vestibular toxicity tends to be irreversible.
- Streptomycin given during pregnancy can cause deafness in the newborn and,
therefore, is relatively contraindicated.
Gentamicin
Gentamicin is isolated from Micromonospora purpurea . It is effective against both gram-
positive and gram-negative organisms.
C1a component of gentamicin.
Antimicrobial Activity
Gentamicin sulfate, 2–10 mcg/mL, inhibits in vitro many strains of staphylococci and
coliforms and other gram-negative bacteria. It is active alone, but also as a synergistic
companion with β-lactam antibiotics, against Escherichia coli , Proteus , Klebsiella
pneumoniae, Enterobacter, Serratia , Stenotrophomonas , and other gram-negative rods that
may be resistant to multiple other antibiotics. Like all aminoglycosides, it has no activity
against anaerobes.
Clinical Uses
- Intramuscular or Intravenous Administration
- Gentamicin is used mainly in severe infections (eg, sepsis and pneumonia) caused by
gram-negative bacteria that are likely to be resistant to other drugs, especially P
aeruginosa , Enterobacter sp, Serratia marcescens , Proteus sp, Acinetobacter sp, and
Klebsiella sp.
- It usually is used in combination with a second agent because an aminoglycoside alone
may not be effective for infections outside the urinary tract. For example, gentamicin
should not be used as a single agent to treat staphylococcal infections because
resistance develops rapidly. Aminoglycosides also should not be used for single-agent

20
therapy of pneumonia because penetration of infected lung tissue is poor and local
conditions of low pH and low oxygen tension contribute to poor activity. Gentamicin 5–
6 mg/kg/d traditionally is given intravenously in three equal doses, but once daily
administration is just as effective for some organisms and less toxic.
- Gentamicin, in combination with a cell wall-active antibiotic, is also indicated in the
treatment of endocarditis caused by grampositive bacteria (streptococci, staphylococci,
and enterococci).
- The synergistic killing achieved by combination therapy may achieve bactericidal activity
necessary for cure or allow for the shortening of the duration of therapy.
- The doses of gentamicin used for synergy against gram-positive bacteria are lower than
traditional doses.
- Typically the drug is administered at a dose of 3 mg/kg/day in three divided doses. Peak
levels should be approximately 3 mcg/mL, while trough levels should be < 1 mcg/mL.
There are limited data to support administering the 3-mg/kg dose as a single daily
injection in the treatment of streptococcal endocarditis.
- Topical and Ocular Administration
- Creams, ointments, and solutions containing 0.1–0.3% gentamicin sulfate have been
used for the treatment of infected burns, wounds, or skin lesions and in attempts to
prevent intravenous catheter infections.
- Intrathecal Administration
- Meningitis caused by gram-negative bacteria has been treated by the intrathecal
injection of gentamicin sulfate, 1–10 mg/d.
Adverse Reactions
Nephrotoxicity is usually reversible and mild. It occurs in 5–25% of patients receiving
gentamicin for longer than 3–5 days
Ototoxicity, which tends to be irreversible, manifests itself mainly as vestibular
dysfunction.
Occurs in 1–5% patient receiving gentamicin for more than 5 days.
Hypersensitivity reactions to gentamicin are uncommon.
Tobramycin
Tobramycin has almost the same antibacterial spectrum as gentamicin with a few exceptions.
Gentamicin is slightly more active against S marcescens , whereas tobramycin is slightly more
active against P aeruginosa ; Enterococcus faecalis is susceptible to both gentamicin and
tobramycin, but E faecium is resistant to tobramycin.
Kinetic and side effects, like gentamicin
Amikacin
Amikacin is a semisynthetic derivative of kanamycin; it is less toxic than the parent molecule.
It is resistant to many enzymes that inactivate gentamicin and tobramycin, and it therefore
can be used against some microorganisms resistant to the latter drugs. Many gram-negative
21
bacteria, including many strains of Proteus , Pseudomonas , Enterobacter , and Serratia , are
sensitive.
Netilmicin
Netilmicin shares many characteristics with gentamicin and tobramycin, netilmicin may be
active against some gentamicin-resistant and tobramycin-resistant bacteria. Dose and side
effects are similar to gentamicin.
Neomycin and kanamycin
Neomycin and kanamycin are closely related.
Neomycin and kanamycin are now limited to topical and oral use. Neomycin is too toxic for
parenteral use. Parenteral administration of kanamycin has also been largely abandoned.
In preparation for elective bowel surgery, 1 g of neomycin is given orally every 6–8 hours for
1–2 days, often combined with 1 g of erythromycin base. This reduces the aerobic bowel
flora with little effect on anaerobes.
Paromomycin
Paromomycin has recently been shown to be effective against visceral leishmaniasis when
given parenterally.
Spectinomycin
Spectinomycin is no longer available for use.
It is active in vitro against many gram-positive and gram-negative organisms, but it is used
almost solely as an alternative treatment for drug-resistant gonorrhea or gonorrhea in
penicillin-allergic patients.
