Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
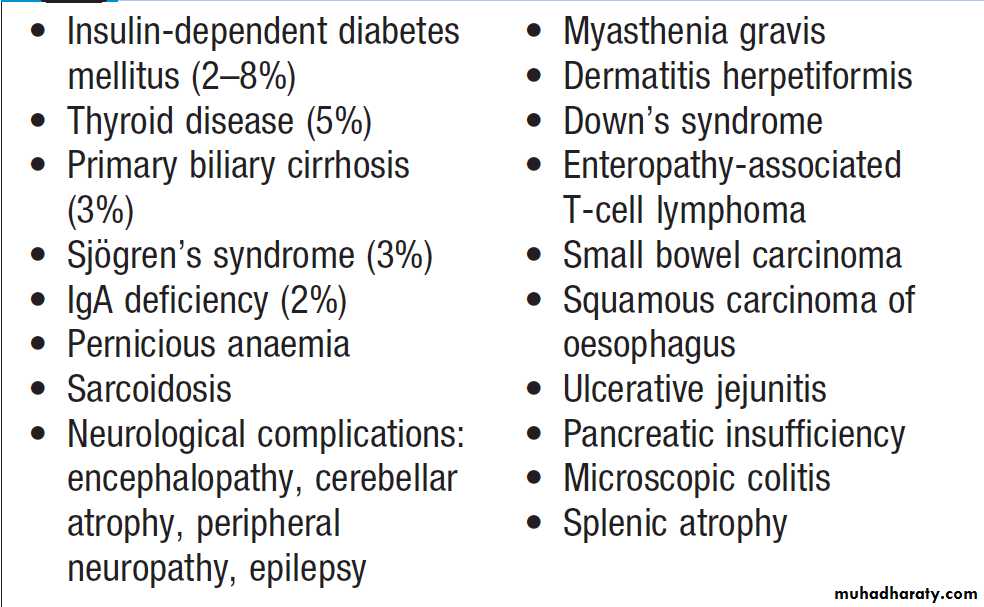
Alimentary tract and pancreatic diseaseGastric secretion
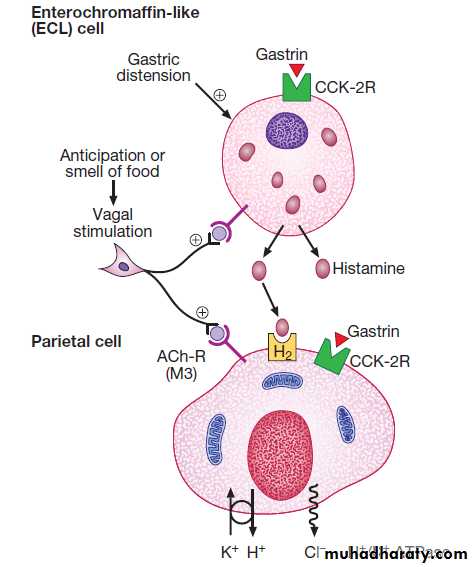
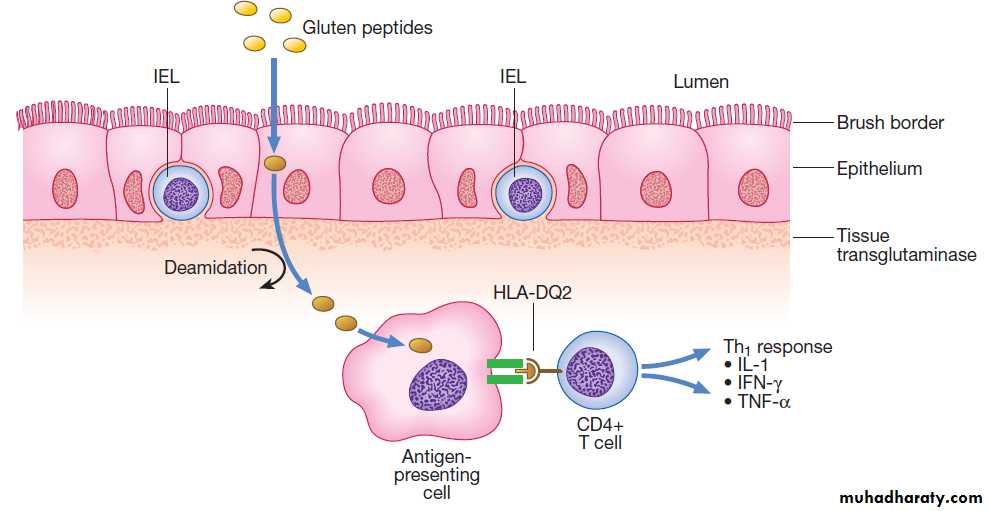
Gastrin secreted by G cells in the antrum (stimulates acid secretion and mucosal growth. Histamine and acetylcholine are the key stimulants of acid secretion) in response to food (protein) binds to cholecystokinin receptors (CCK-2R) on the surface of enterochromaffin-like (ECL) cells, which in turn release histamine. The histamine binds to H2 receptors on parietal cells and this leads to secretion of hydrogen ions, in exchange for potassium ions at the apical membrane of parietal cells. Hydrogen and chloride ions are secreted from parietal cells into the lumen of the stomach by a H-K ATPase (proton pump) . Parietal cells also express CCK-2R and it is thought that activation of these receptors by gastrin is involved in regulatory proliferation of parietal cells.Cholinergic (vagal) activity and gastric distension also stimulate acid secretion ,whilst somatostatin is secreted from D cells throughout the stomach, vasoactive intestinal polypeptide (VIP) and gastric inhibitory polypeptide (GIP) may inhibit it. The hydrochloric acid sterilises the upper GIT and converts pepsinogen (secreted by chief cells) to pepsin.
The parietal cells produce the glycoprotein intrinsic factor, in parallel with acid, necessary for B12 absorption.
Ghrelin, secreted from oxyntic glands, stimulates acid secretion ,appetite and gastric emptying.
Protective factors: Bicarbonate ions, stimulated by prostaglandins, mucins and trefoil factor family (TFF) peptides protect the gastroduodenal mucosa from the ulcerative properties of acid and pepsin.
Control of acid secretion. Gastrin released from antral G cells in response to food (protein) binds to cholecystokinin receptors (CCK-2R) on the surface of enterochromaffin-like (ECL) cells, which in turn release histamine. The histamine binds to H2 receptors on parietal cells and this leads to secretion of hydrogen ions, in exchange for potassium ions at the apical membrane. Parietal cells also express CCK-2R and it is thought that activation of these receptors by gastrin is involved in regulatory proliferation of parietal cells. Cholinergic (vagal) activity and gastric distension also stimulate acid secretion; somatostatin, vasoactive intestinal polypeptide (VIP) and gastric inhibitory polypeptide (GIP) may inhibit it.
(ACh-R = acetylcholine receptor; ATPase = adenosine triphosphatase)
Small intestine
The small bowel extends from the ligament of Treitz tothe ileocaecal valve . During fasting, a wave of peristaltic activity passes down the small bowel every
1–2 hours. Entry of food into the gastrointestinal tract
stimulates small bowel peristaltic activity.
Functions of the small intestine are:
• digestion (mechanical, enzymatic and peristaltic)• absorption – the products of digestion, water,
electrolytes and vitamins
• protection against ingested toxins
• immune regulation.
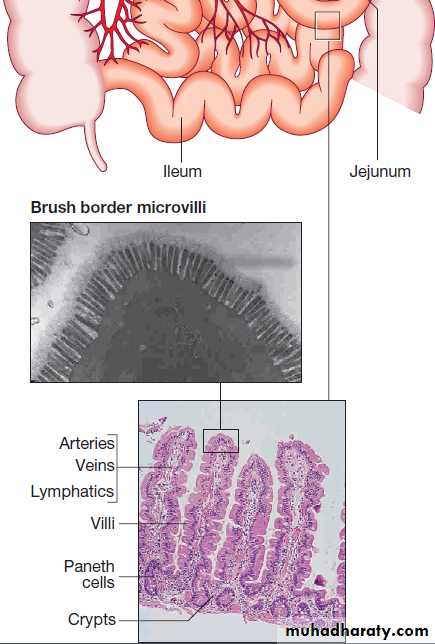
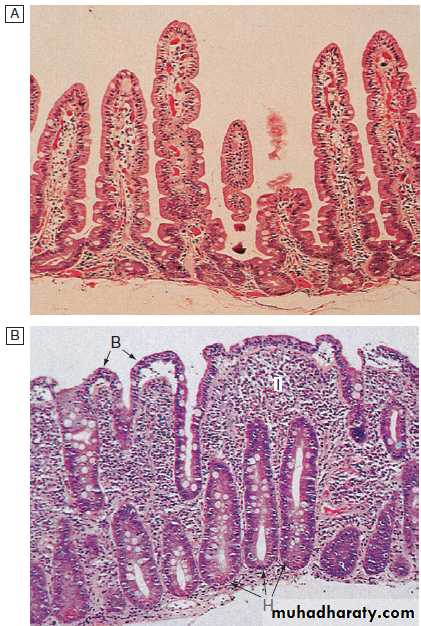
Small intestine: Epithelial cells are formed in crypts and differentiate as they migrate to the tip of the villi to form enterocytes (absorptive cells) and goblet cells.
Digestion and absorption
FatDietary lipids comprise long-chain triglycerides, cholesterol esters and lecithin. Lipids are insoluble in water and undergo lipolysis and incorporation into mixed micelles before they can be absorbed into enterocytes along with the fat-soluble vitamins A, D, K and E. The lipids are processed within enterocytes and pass via lymphatics into the systemic circulation.
Carbohydrates
Starch is hydrolysed by salivary and pancreatic amylases to alpha-limit dextrins containing 4–8 glucose molecules; to the disaccharide, maltose; and to the trisaccharide, maltotriose.Disaccharides are digested by enzymes fixed to the
microvillous membrane to form the monosaccharides,
glucose, galactose and fructose.
Glucose and galactose enter the cell by an energy-requiring process involving a carrier protein, and fructose enters by simple diffusion.
Protein
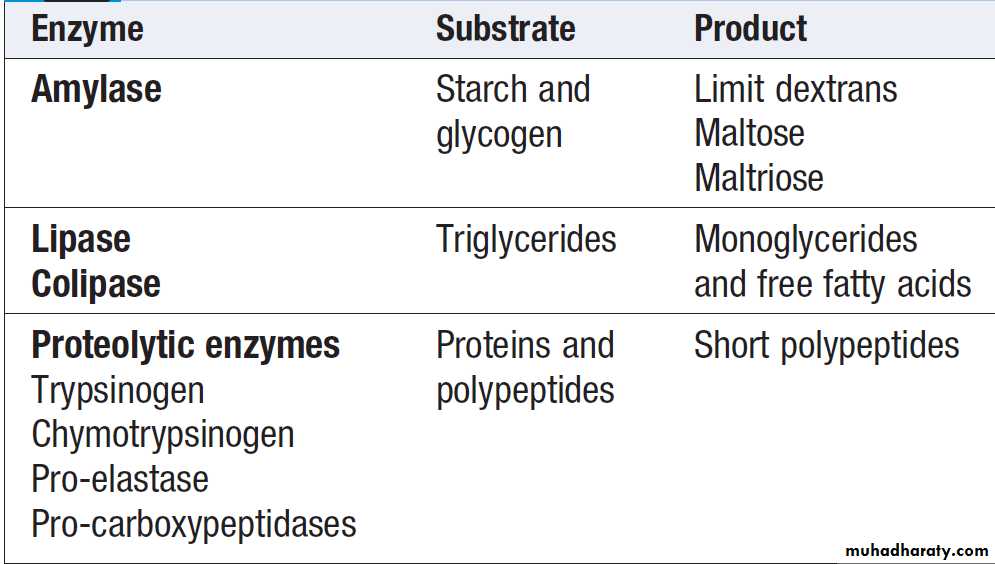
The steps involved in protein digestion are shown in Figure. Intragastric digestion by pepsin is quantitatively modest but important because the resulting polypeptides and amino acids stimulate CCK release from the mucosa of the proximal jejunum, which in turn stimulates release of pancreatic proteases, including trypsinogen, chymotrypsinogen, pro-elastases and procarboxypeptidases, from the pancreas.On exposure to brush border enterokinase, inert trypsinogen is converted to the active proteolytic enzyme trypsin, which activates the other pancreatic proenzymes.
Trypsin
digests proteins to produce oligopeptides, peptides and amino acids.Oligopeptides are further hydrolysed by
brush border enzymes to yield dipeptides, tripeptides and amino acids. These small peptides and the amino acids are actively transported into the enterocytes, where intracellular peptidases further digest peptides to amino acids. Amino acids are then actively transported across the basal cell membrane of the enterocyte into the portal circulation and the liver.
Water and electrolytes
Absorption and secretion of electrolytes and water occur throughout the intestine and are transported by
two pathways:
• the paracellular route, in which passive flow through
tight junctions between cells is a consequence of
osmotic, electrical or hydrostatic gradients
• the transcellular route across apical and basolateral
membranes by energy-requiring specific active
transport carriers (pumps).
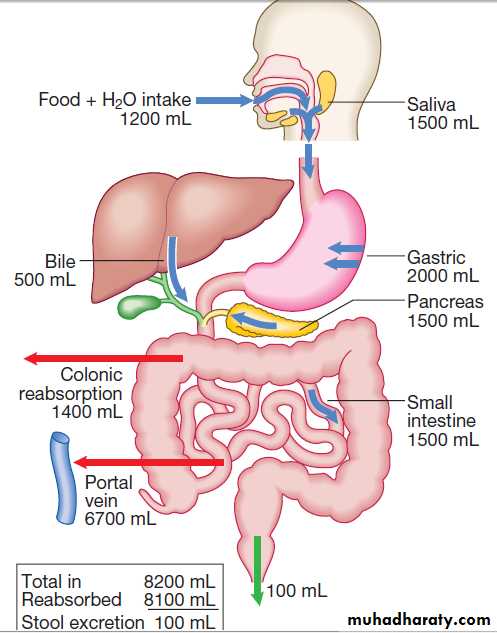
In healthy individuals, fluid balance is tightly controlled, such that only 100 mL of the 8 litres of fluid entering the GIT daily is excreted in stools .
Vitamins and trace elements
Water-soluble vitamins are absorbed throughout the intestine.
Intestinal defence mechanisms
Protective function of the small intestinePhysical defence mechanisms
There are several levels of defence in the small bowel (Fig).
Firstly, the gut lumen contains host bacteria, mucins and secreted antibacterial products, including defensins and immunoglobulins which help combat pathogenic infections.
Secondly, epithelial cells have relatively impermeable brush border membranes, and passage between cells is prevented by tight and adherens junctions.
These cells can react to foreign peptides (‘innate immunity’) using pattern recognition receptors found on cell surfaces (Toll receptors) or intracellularly.
Lastly, in the subepithelial layer, immune responses occur under control of the adaptive immune system in response to pathogenic compounds.
Immunological defence mechanisms
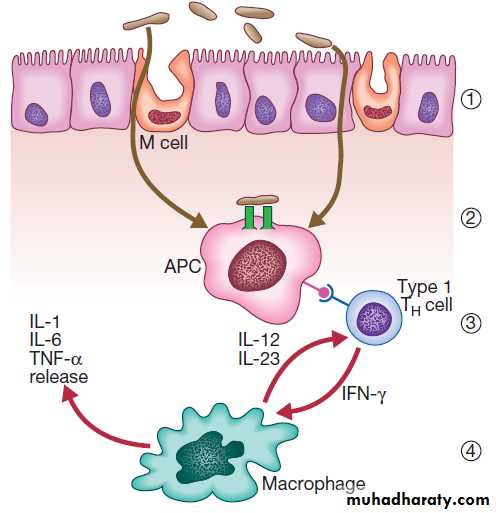
GI mucosa-associated lymphoid tissue constitutes 25% of the total lymphatic tissue of the body and is at the heart of adaptive immunity. Within Peyer’s patches, B lymphocytes differentiate to plasma cells following exposure to antigens, and these migrate to mesenteric lymph nodes, to enter the blood stream via the thoracic duct. The plasma cells return to the lamina propria of the gut through the circulation and release immunoglobulin A (IgA), which is transported into the lumen of the intestine. Intestinal T lymphocytes help localise plasma cells to the site of antigen exposure, producing inflammatory mediators. Macrophages phagocytose foreign materials and secrete cytokines, which mediate inflammation.
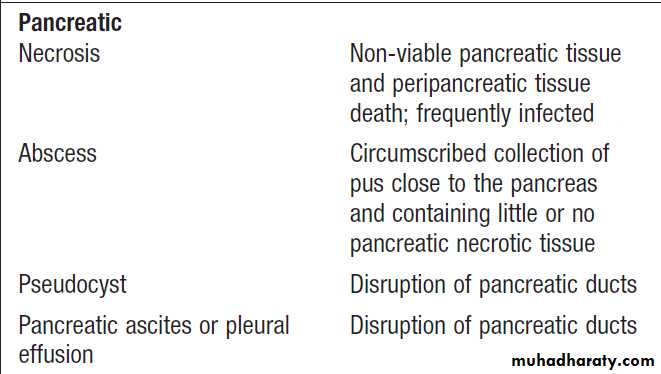
Pancreas
The exocrine pancreas is necessary for the digestion of fat, protein and carbohydrate. Proenzymes are secreted from pancreatic acinar cells in response to circulating GI hormones and are activated by trypsin. Bicarbonate-rich fluid is secreted from ductular cells to produce an optimum alkaline pH for enzyme activity.Pancreatic enzymes
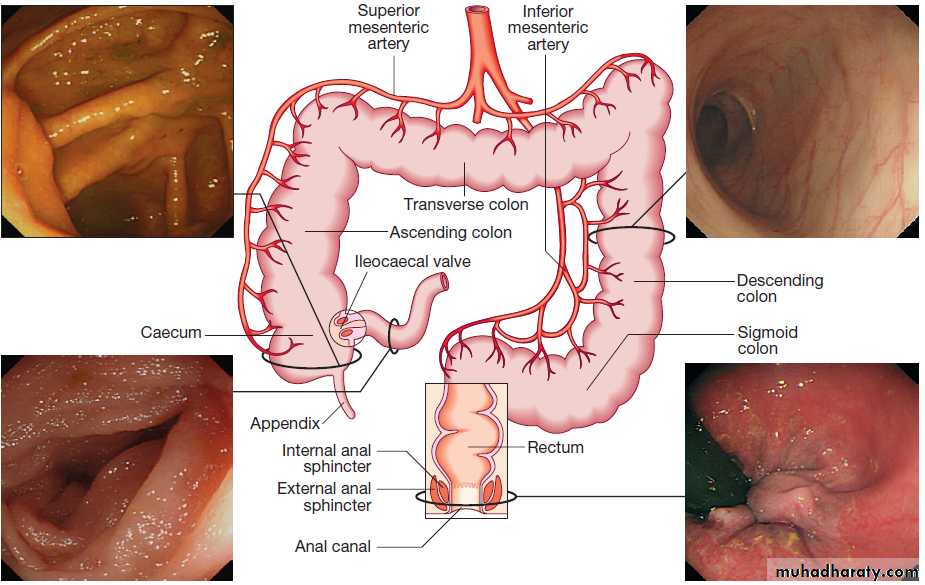
ColonAbsorbs water and electrolytes. Acts as a storage organ. Two types of contraction occur. The first is segmentation (ring contraction), which leads to mixing but not propulsion; this promotes absorption of water and electrolytes. Propulsive (peristaltic contraction) waves occur several times a day and propel faeces to the rectum. All activity is stimulated after meals, in response to release of motilin and CCK. Faecal continence depends upon maintenance of the anorectal angle and tonic contraction of the external anal sphincters.
On defecation, there is relaxation of the anorectal muscles, increased intra-abdominal pressure , contraction of abdominal muscles, and relaxation of the anal sphincters.
Control of gastrointestinal function
Secretion, absorption, motor activity, growth and differentiation of the gut are all modulated by a combination of neuronal and hormonal factors. The CNS, the autonomic system (ANS) and the enteric nervous system (ENS) interact to regulate gut function. The ANS comprises:• parasympathetic pathways (vagal and sacral efferent), which are cholinergic, and increase smooth muscle tone and promote sphincter relaxation
• sympathetic pathways, which release noradrenaline
(norepinephrine), reduce smooth muscle tone and
stimulate sphincter contraction.
The parasympathetic system generally stimulates motility and secretion, while the sympathetic system generally acts in an inhibitory manner.
The normal colon, rectum and anal canal.
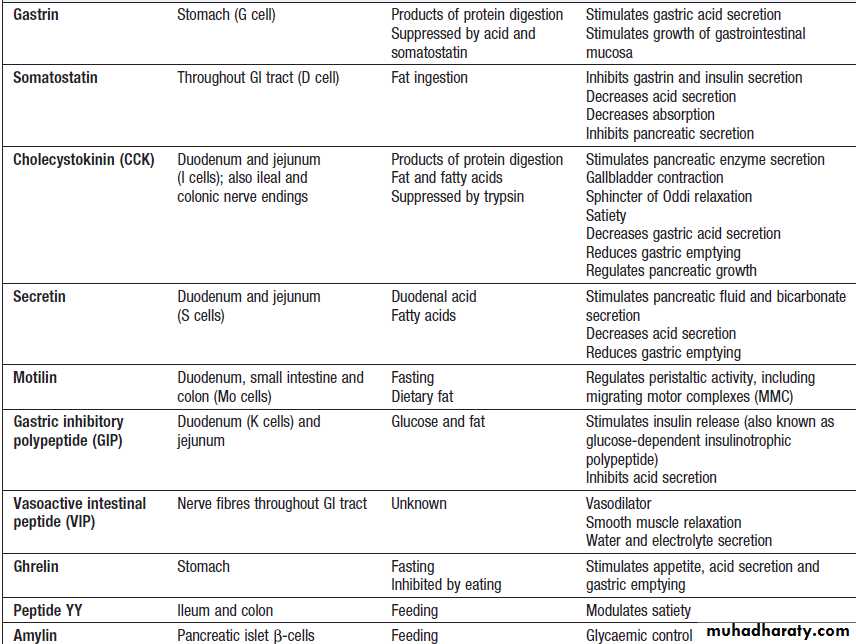
Gut hormones and peptides
INVESTIGATION OF GASTROINTESTINAL DISEASE
ImagingPlain X-rays
Of the abdomen are useful in the diagnosis of intestinal obstruction or paralytic ileus, where dilated loops of bowel and (in the erect position) fluid levels. Calcified lymph nodes, gallstones and renal stones can also be detected. Chest X- ray (performed with the patient in erect position) is useful in the diagnosis of suspected perforation, as it shows subdiaphragmatic free air.
Contrast studies
X-rays with contrast medium are usually performed to assess not only anatomical abnormalities but also motility.
Water-soluble contrast is used to opacify bowel prior to abdominal CT and in cases of suspected perforation. The double contrast technique improves mucosal visualisation by using gas to distend the barium-coated intestinal surface. Contrast studies are useful for detecting filling defects, such as tumours, strictures, ulcers and motility disorders, but are inferior to endoscopic procedures and more sophisticated cross-sectional imaging techniques, such as CT and MRI.
Ultrasound, CT and MRI
US, CT and MRI are key tests in the evaluation of intra-abdominal disease. They are noninvasive and offer detailed images of the abdominal contents.
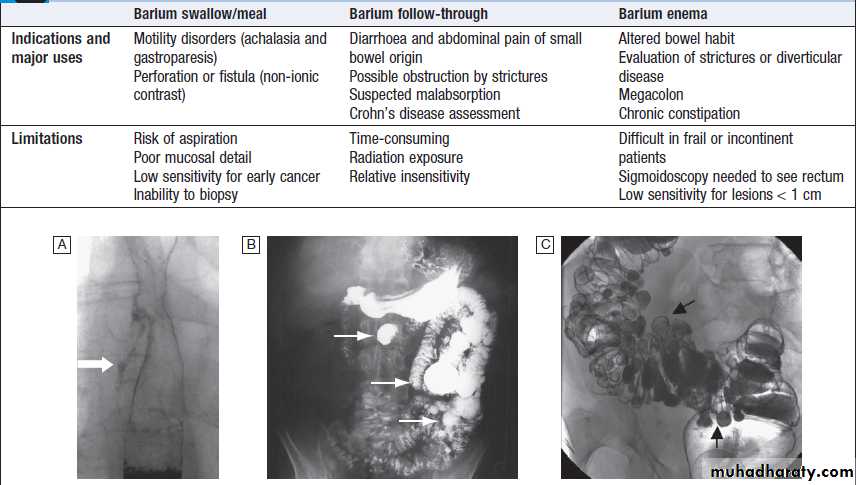
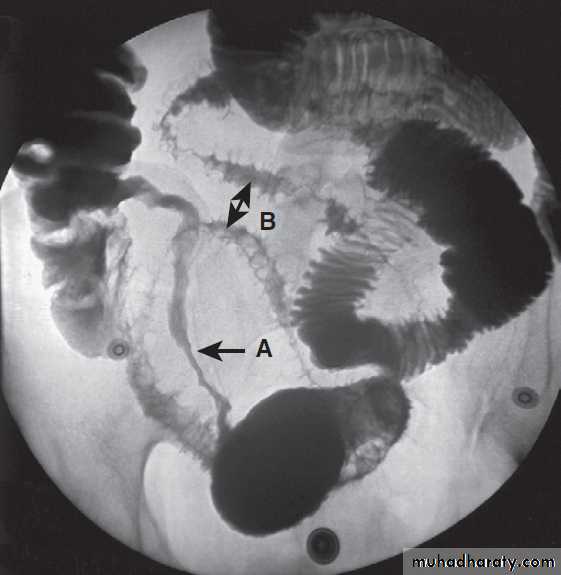
Contrast radiology in the investigation of gastrointestinal disease
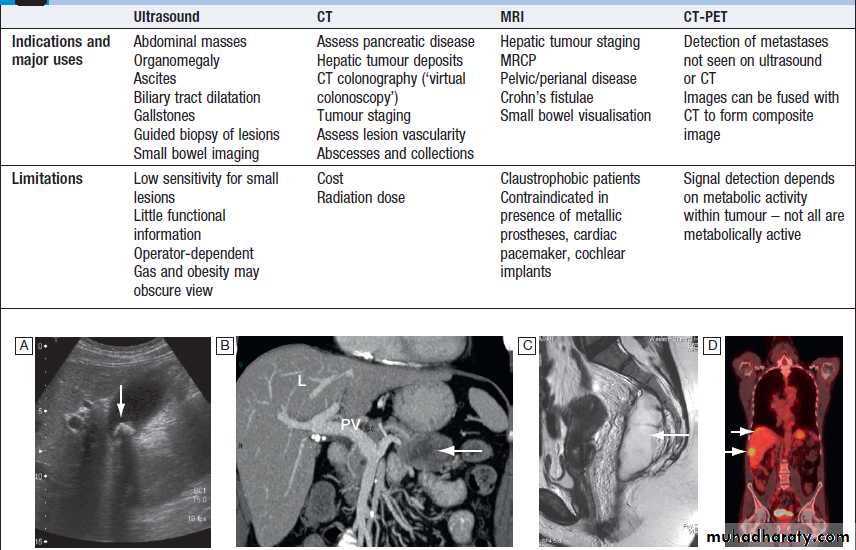
A Non-ionic contrast swallow shows leakage of contrast (arrow) into the mediastinum following stricture dilatation. B Barium follow-through. There are multiple diverticula (arrows) in this patient with jejunal diverticulosis. C Barium enema showing severe diverticular disease. There is tortuosity and narrowing of the sigmoid colon with multiple diverticula (arrows).Imaging in gastroenterology :Examples of ultrasound, CT and MRI. A Ultrasound showing large gallstone (arrow) with acoustic shadowing. B Multidetector coronal CT showing large solid and cystic malignant tumour in the pancreatic tail (arrow). (PV = portal vein; L = liver) C Pelvic MRI showing large pelvic abscess (arrow) posterior to the rectum in a patient with Crohn’s disease. D Fused CT-PET image showing two liver metastases (arrows).
Endoscopy
Videoendoscopes provide high-definition imaging and accessories can be passed down the endoscope to allow both diagnostic and therapeutic procedures.Upper gastrointestinal endoscopy
This is performed under light intravenous benzodiazepine
sedation, or using only local anaesthetic throat
spray after the patient has fasted for at least 4 hours.
With the patient in the left lateral position, the entire
oesophagus (excluding pharynx), stomach and first two
parts of duodenum can be seen.
Upper gastrointestinal endoscopy
*These are ‘relative’ contraindications; in experienced hands, endoscopycan be safely performed.
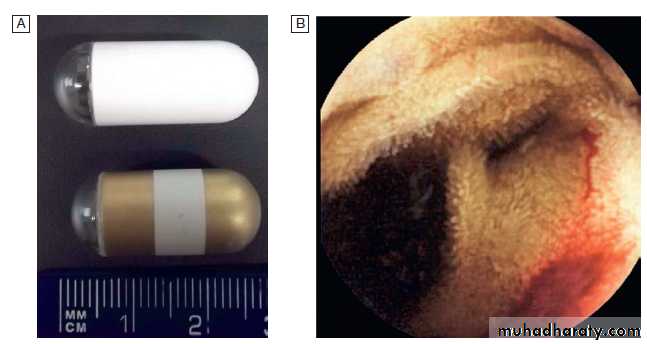
Wireless capsule endoscopy.
A Examples of capsules.B image of bleeding jejunal vascular malformation.
Capsule endoscopy
Capsule endoscopy uses a capsule containing an imaging device, battery, transmitter and antenna; as it traverses the small intestine, it transmits images to a battery-powered recorder worn on a belt round the patient’s waist. After approximately 8 hours, the capsule is excreted. Images from the capsule are analysed as a video sequence and it is usually possible to localise the segment of small bowel in which lesions are seen.
Abnormalities detected usually require enteroscopy for
confirmation and therapy.
Double balloon enteroscopy
Double balloon enteroscopyUses a long endoscope with a flexible overtube. Sequential and repeated inflation and deflation of balloons on the tip of the overtube and enteroscope allow the operator to push and pull along the entire length of the small intestine to the terminal ileum, in order to diagnose or treat small bowel lesions detected by capsule endoscopy or other imaging modalities.
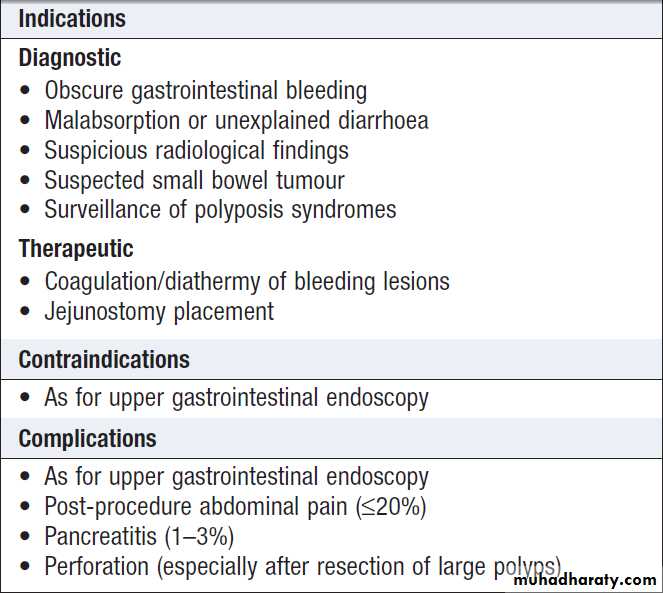
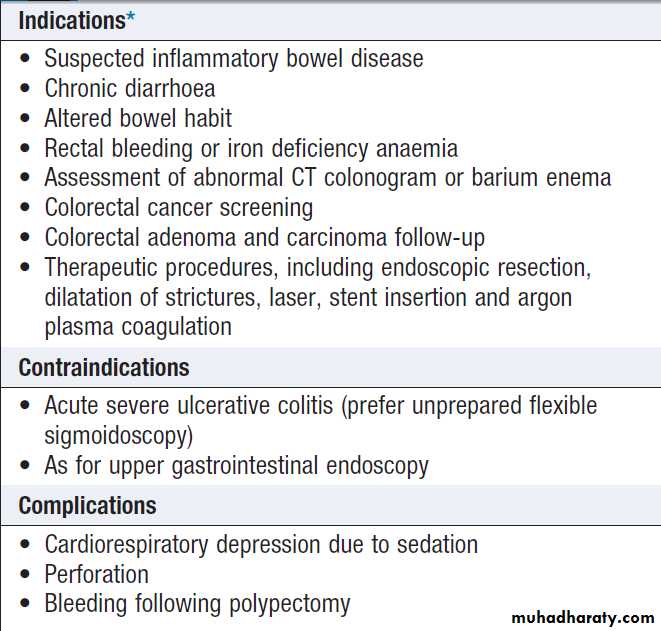
Colonoscopy
*Colonoscopy is not useful in the investigation of constipationSigmoidoscopy and colonoscopy
a 20 cm rigid plastic sigmoidoscope or in the endoscopy suite using a 60 cm flexible colonoscope following bowel preparation. Allow accurate detection of haemorrhoids, ulcerative colitis and distal colorectal neoplasia is possible. After full bowel cleansing, it is possible to examine the entire colon and the terminal
ileum using a longer colonoscope.
Magnetic resonance cholangiopancreatography
(MRCP)has largely replaced endoscopic retrograde cholangiopancreatography (ERCP) in the evaluation of obstructive jaundice .
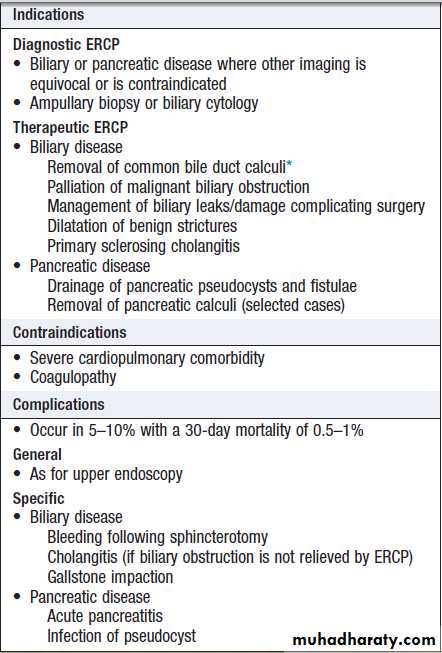
Endoscopic retrograde cholangiopancreatography
( ERCP)
Using a side-viewing duodenoscope, it is possible to
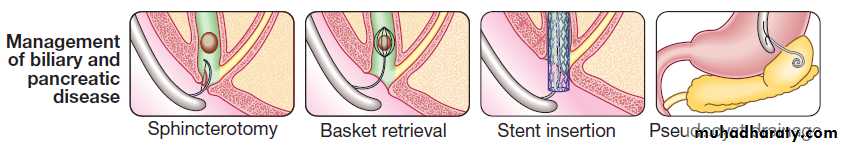
cannulate the main pancreatic duct and common bile duct. Nowadays, ERCP is mainly used in the treatment of a range of biliary and pancreatic diseases that have been identified by other techniques such as MRCP, EUS and CT.
Endoscopic retrograde
cholangiopancreatography (ERCP)*Laparoscopic surgery is preferred in fit individuals who also require
cholecystectomy.
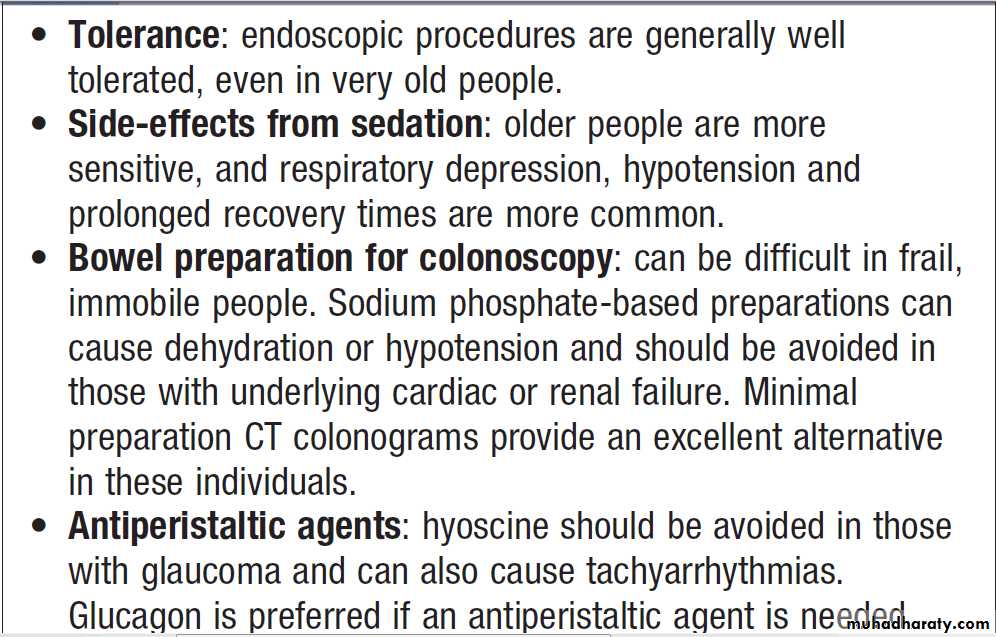
Endoscopy in old age
Reasons for biopsy or cytological examination
HistologyBiopsy material obtained during endoscopy or percutaneously can provide useful information
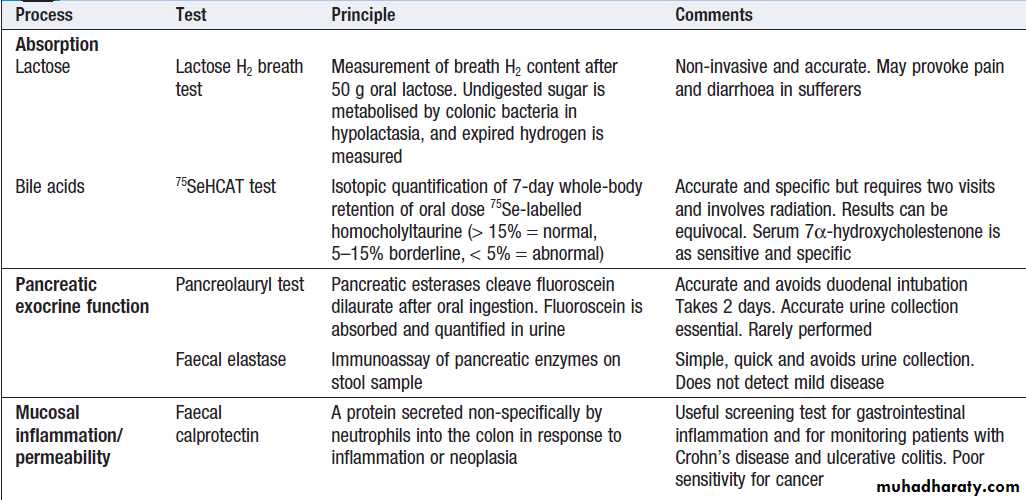
Tests of function
A number of dynamic tests can be used to investigateaspects of gut function, including digestion, absorption,
inflammation and epithelial permeability. In the
assessment of suspected malabsorption, blood tests
(full blood count, ESR, C-reactive protein (CRP), folate, vitamin B12, iron status, albumin, calcium and phosphate) are essential, and endoscopy is undertaken to obtain mucosal biopsies. Faecal calprotectin is very sensitive at detecting mucosal inflammation.
Dynamic tests of gastrointestinal function
Oesophageal motilityA barium swallow can give useful information about
oesophageal motility. Videofluoroscopy, with joint
assessment by a speech and language therapist and a
radiologist, may be necessary in difficult cases. Oesophageal manometry , often in conjunction with 24-hour pH measurements, is of value in diagnosing cases of refractory gastro- oesophageal reflux, achalasia and non-cardiac chest pain.
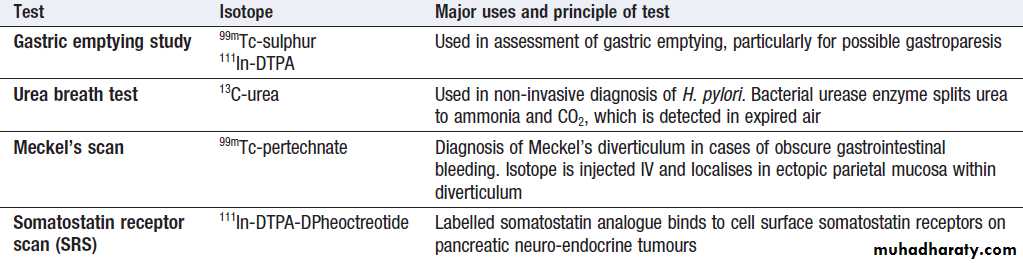
Gastric emptying
This involves administering a test meal containing solids and liquids labelled with different radioisotopes and measuring the amount retained in the stomach
Afterwards. It is useful in the investigation of suspected delayed gastric emptying (gastroparesis).
Commonly used radioisotope tests in gastroenterology
Colonic and anorectal motilityA plain abdominal X-ray taken on day 5 after ingestion of inert plastic pellets on days 1–3 gives an estimate of whole gut transit time. The test is useful in the evaluation of chronic constipation, when the position of any retained pellets can be observed, and helps to differentiate cases of slow transit from those due to obstructed defecation.
Radioisotope tests
Different radioisotope are used , information is obtained, such as the localisation of a Meckel’s diverticulum, rate of gastric emptying. Yet others are tests of infection and rely on the presence of bacteria to hydrolyse a radio-labelled test substance followed by detection of the radioisotope in expired air, such as the urea breath test for H. pylori.
PRESENTING PROBLEMS IN GI DISEASE
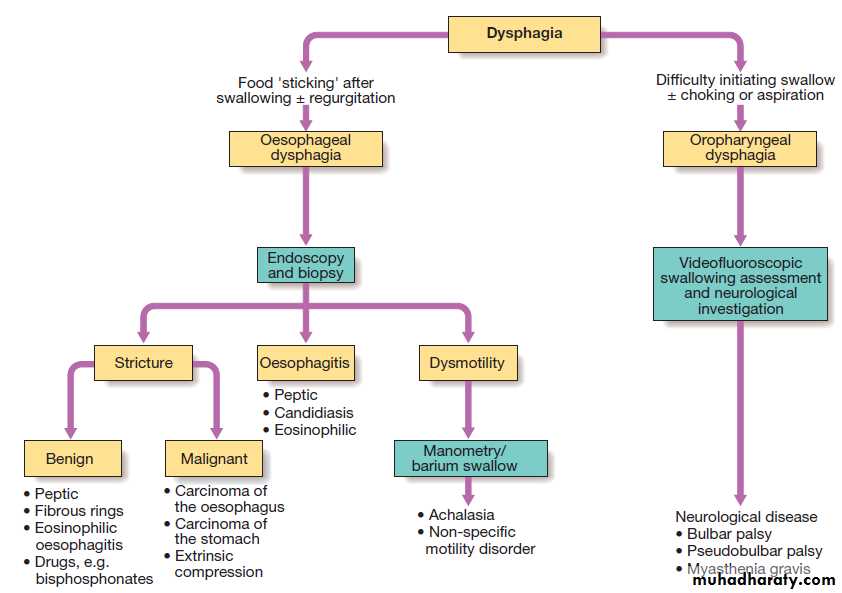
Dysphagia is defined as difficulty in swallowing. It may
coexist with heartburn or vomiting but should be distinguished from both globus sensation (in which
anxious people feel a lump in the throat without organic cause) and odynophagia (pain during swallowing, usually from gastro- oesophageal reflux or candidiasis).
Dysphagia can occur due to problems in the oropharynx
or oesophagus . Drooling, dysarthria, hoarseness and cranial nerve or other neurological signs may be present. Oesophageal disorders cause dysphagia by obstructing the lumen or by affecting motility.
Patients with oesophageal disease complain of food ‘sticking’ after swallowing.
Investigations
Dysphagia should always be investigated urgently.Endoscopy is the investigation of choice because it
allows biopsy and dilatation of strictures. If no abnormality is found, then barium swallow with videofluoroscopic swallowing assessment is indicated to detect major motility disorders. In some, oesophageal manometry is required.
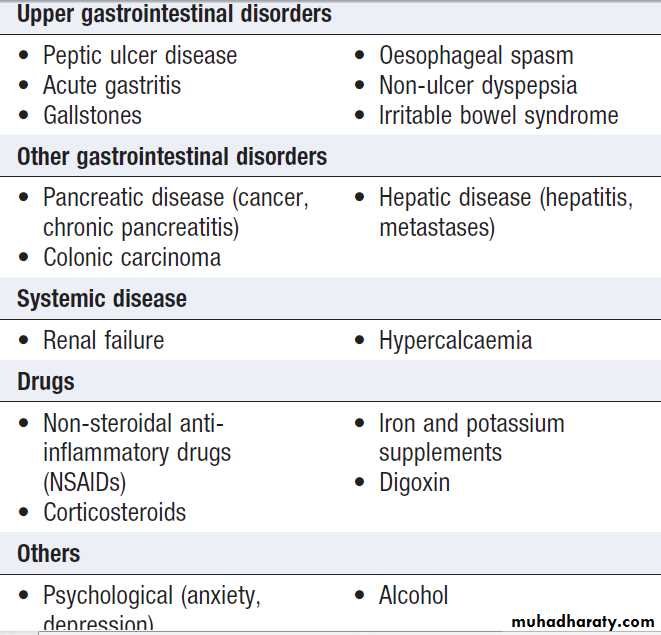
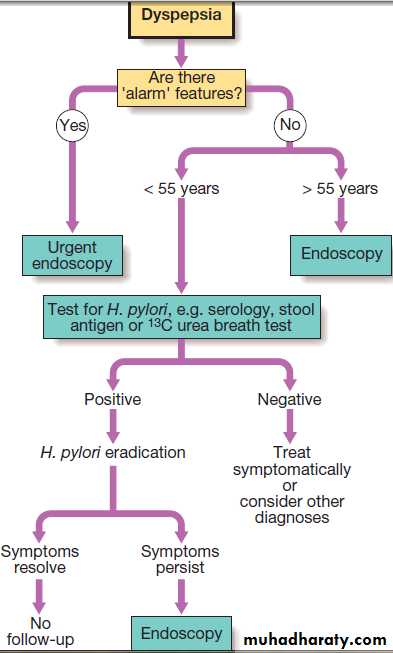
Dyspepsia symptoms such as discomfort, bloating and nausea.
Heartburn and other ‘reflux’ symptoms are separate entities, a careful history is important to detect ‘alarm’ features requiring urgent investigation and to detect atypical symptoms which might be due to problems outside the gastrointestinal tract.
Investigation of dysphagia.
Causes of dyspepsia
Alarm features in dyspepsia
Investigation of dyspepsia
Heartburn and regurgitationHeartburn describes retrosternal, burning discomfort,
often rising up into the chest and sometimes accompanied by regurgitation of acidic or bitter fluid into the throat. These symptoms often occur after meals, on lying down or with bending, straining or heavy lifting. They are classical of gastro-oesophageal reflux but up to 50% of patients present with other symptoms, such as chest pain, belching, halitosis, chronic cough or sore throats.
In young patients with typical symptoms and a good
response to dietary changes, antacids or acid suppression,
investigation is not required, but in patients over
55 years of age, those with alarm symptoms or atypical
features, urgent endoscopy is necessary.
Vomiting
Vomiting is a complex reflex involving both autonomic and somatic neural pathways. Synchronous contraction of the diaphragm, intercostal muscles and abdominal muscles raises intra-abdominal pressure and, combined
with relaxation of the lower oesophageal sphincter,
results in forcible ejection of gastric contents.
It is important to distinguish true vomiting from regurgitation and to elicit whether the vomiting is acute or chronic (recurrent), as the underlying causes may differ.
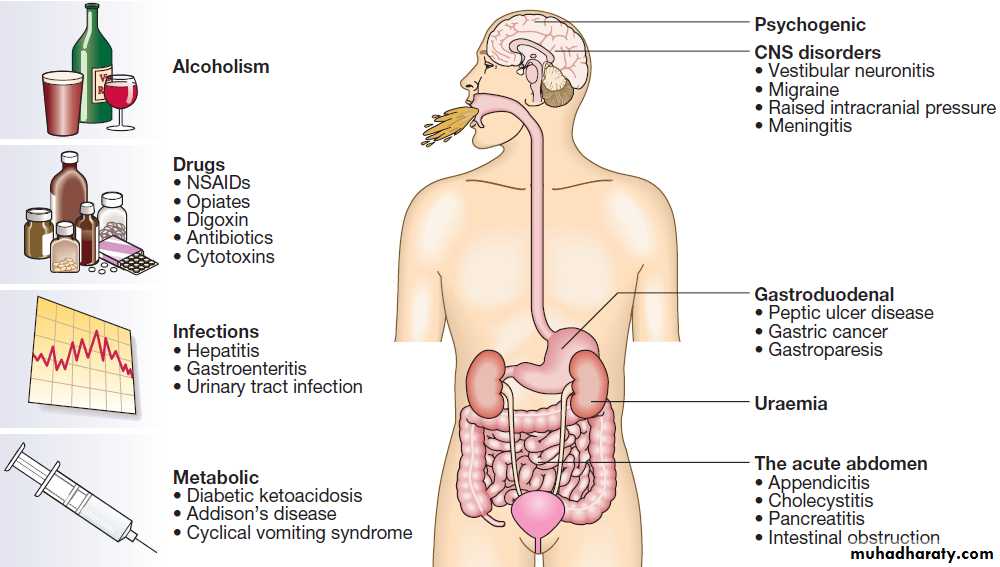
Causes of vomiting
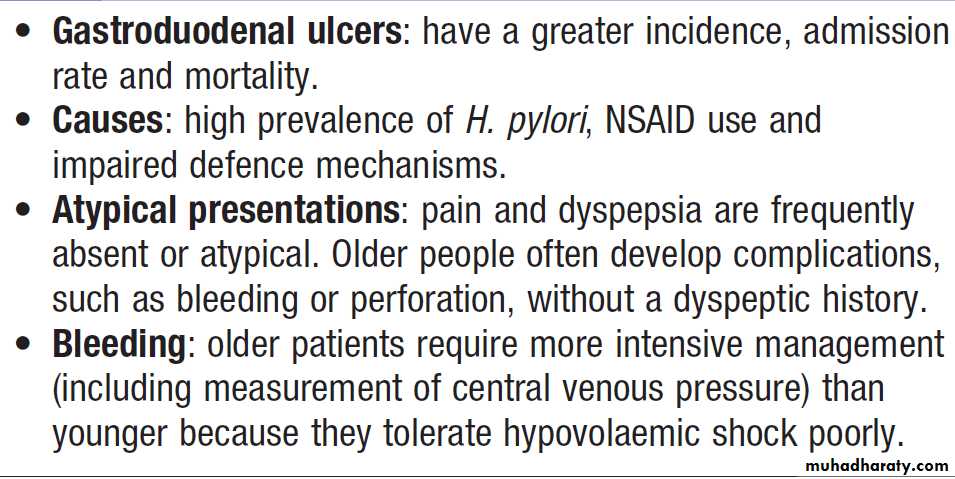
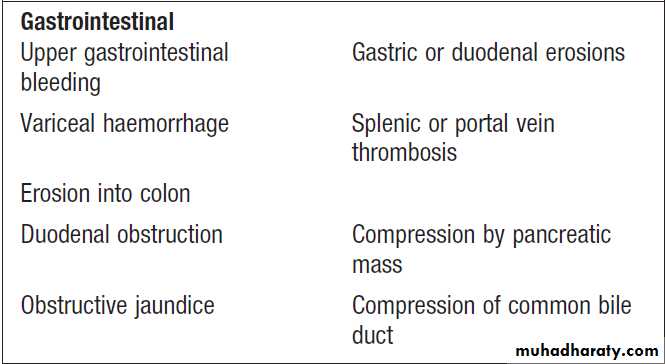
Gastrointestinal bleedingAcute upper gastrointestinal haemorrhage
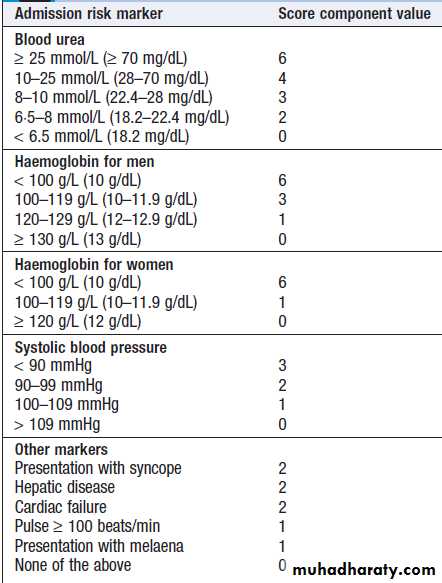
This is the most common gastrointestinal emergency. The mortality of patients admitted to hospital is about 10% but there is some evidence that outcome is better when patients are treated in specialised units. Risk scoring systems have been developed to stratify risk of needing endoscopic therapy or a poor outcome .The advantage of the Blatchford score is that it may be used before
endoscopy to predict the need for intervention to treat
bleeding. Low scores (2 or less) are associated with
a very low risk of adverse outcome.
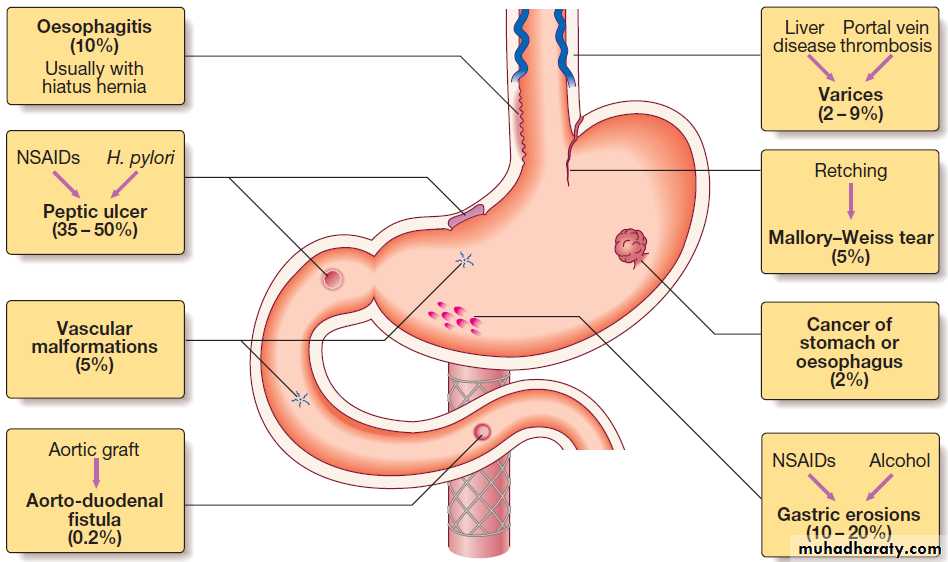
Causes of acute upper gastrointestinal haemorrhage.
Clinical assessment
Haematemesis is red with clots when bleeding is rapidand profuse, or black (‘coffee grounds’) when less
severe. Syncope may occur and is due to hypotension
from intravascular volume depletion. Symptoms of
anaemia suggest chronic bleeding.
Melaena is the passage of black, tarry stools containing altered blood; it is usually caused by bleeding from the upper GI tract, although haemorrhage from the right side of the colon is occasionally responsible. The characteristic colour and smell are the result of the action of digestive enzymes and bacteria upon haemoglobin. Severe acute upper gastrointestinal bleeding can sometimes cause maroon or bright red stool.
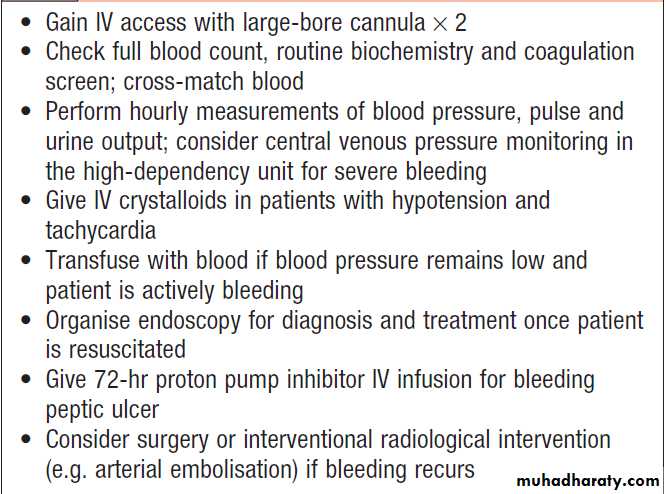
Management
1. Intravenous access.The first step is to gain IV access.2. Initial clinical assessment
• Define circulatory status. tachycardia, hypotension , oliguria, cold , sweating, and may be agitated.
• Seek evidence of liver disease ,Jaundice, cutaneous stigmata, hepatosplenomegaly and ascites.
• Identify comorbidity, cardiorespiratory, cerebrovascular or renal disease is important, because these may be worsened by acute bleeding and they increase the hazards of endoscopy and surgical operations. These factors can be combined using the Blatchford score. A score of < 3 is associated with a good prognosis, while progressively higher scores are associated with poorer outcomes.
3. Basic investigations
• Full blood count. Chronic or subacute bleeding leads to anaemia, but the haemoglobin concentration may be normal after sudden, major bleeding until haemodilution occurs. Thrombocytopenia may be a clue to the presence of hypersplenism in chronic liver disease.• Urea and electrolytes. For evidence of renal failure. The blood urea rises as the absorbed products of luminal blood are metabolised by the liver; an elevated blood urea with normal creatinine concentration implies severe bleeding.
• Liver function tests. For evidence of chronic liver disease.
• Prothrombin time. liver disease or anticoagulated patients.
• Cross-matching. 2 units of blood cross-matched.
4. Resuscitation
IV crystalloid fluids should be given to raise the blood pressure, and blood should be transfused when the patient is actively bleeding with low blood pressure and tachycardia. Comorbidities should be managed as appropriate. Patients with suspected chronic liver disease should receive broad-spectrum antibiotics. Central venous pressure (CVP) monitoring may be useful in severe bleeding, particularly in patients with cardiac disease.
5. Oxygen
This should be given to all patients in shock.
6. Endoscopy
This should be carried out after adequate resuscitation,
ideally within 24 hours.
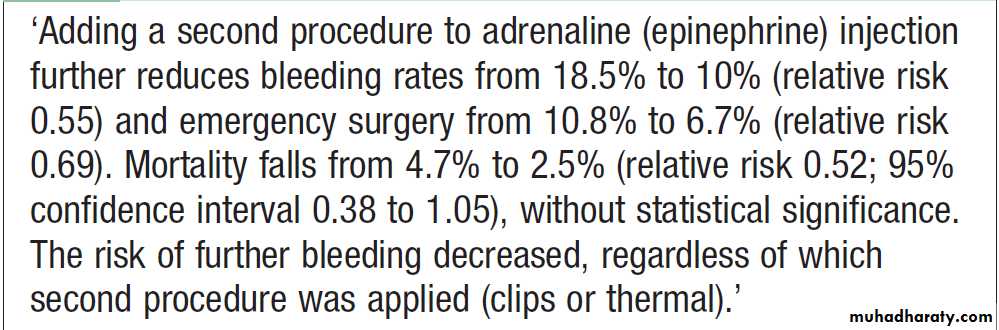
Patients who are found to have major endoscopic stigmata of recent haemorrhage can be treated endoscopically using a thermal or mechanical modality, such as a ‘heater probe’ or endoscopic clips, combined with injection of dilute adrenaline into the bleeding point (‘dual therapy’).
This may stop active bleeding and, combined
with intravenous proton pump inhibitor (PPI) therapy, prevent rebleeding, thus avoiding the need for surgery .
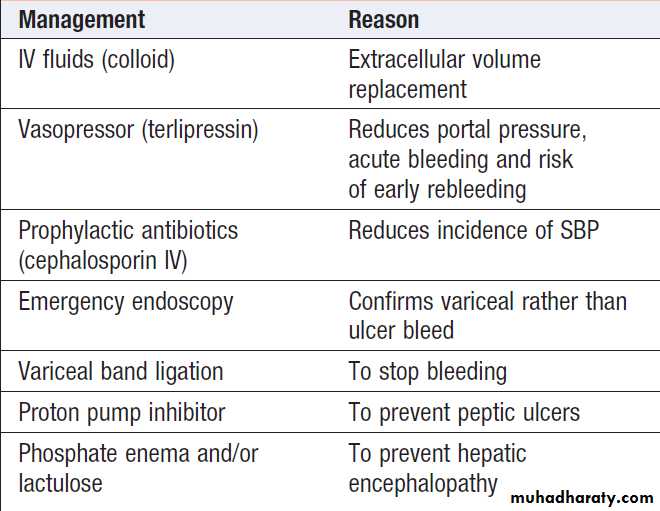
Patients found to have bled from varices should be treated by band ligation.
7. Monitoring
Patients closely observed, with hourly measurements of pulse, blood pressure and urine output.
8. Surgery
indicated when endoscopic haemostasis fails to stop bleeding and if rebleeding occurs on one occasion in an elderly, frail patient, or twice in a younger, fitter patient.If available, angiographic embolisation is an effective alternative to surgery in frail patients.
Duodenal ulcers treated by under-running, with or without pyloroplasty. Under-running for gastric ulcers can also be carried out (a biopsy taken to exclude carcinoma).
Local excision may be performed, but when neither is possible, partial gastrectomy is required. Following surgery, should be treated with H. pylori eradication if they test positive for it, and should avoid NSAIDs.
Successful eradication should be confirmed by urea breath or faecal antigen testing.
Modified Blatchford score: risk
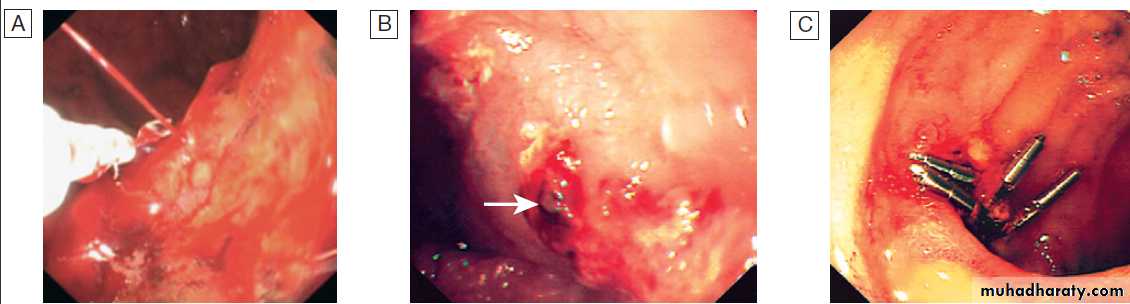
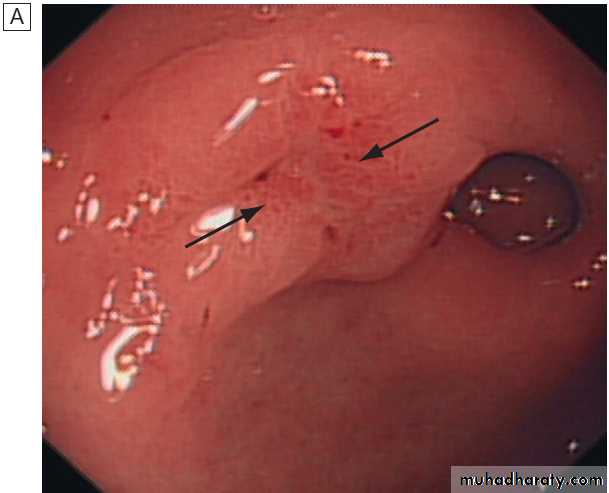
stratification in acute upper GI bleedingMajor stigmata of recent haemorrhage and endoscopic treatment. A Active arterial spurting from a gastric ulcer. An endoscopic
clip is about to be placed on the bleeding vessel. When associated with shock, 80% of cases will continue to bleed or rebleed.
B ‘Visible vessel’ (arrow).
In reality, this is a pseudoaneurysm of the feeding artery seen here in a pre-pyloric peptic ulcer. It carries a 50% chance of rebleeding.
C Haemostasis is achieved after endoscopic clipping of the bleeding vessel in the duodenum.
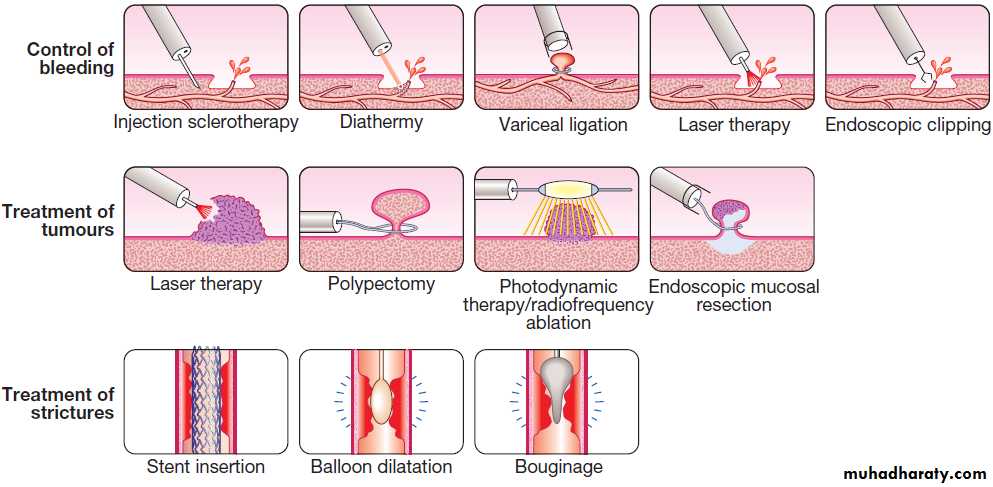
Examples of therapeutic techniques in endoscopy
Single versus dual modality endoscopic therapy in high-risk bleeding ulcers
Adjunctive drug therapy for bleeding ulcers
Emergency management of acute non- variceal upper gastrointestinal haemorrhage
Emergency management of variceal bleeding
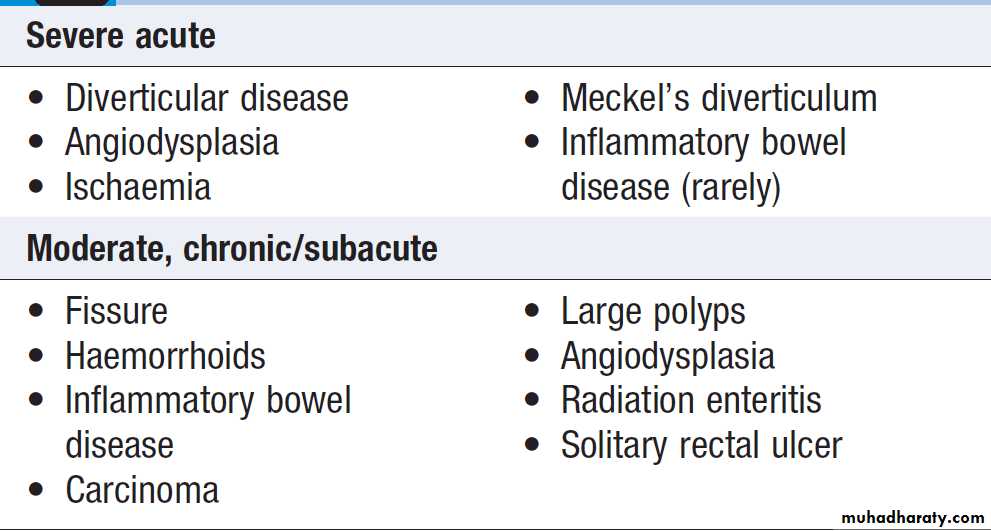
Causes of lower gastrointestinal bleeding
Lower gastrointestinal bleeding
This may be due to haemorrhage from the colon, anal canal or small bowel.Severe acute lower gastrointestinal bleeding
Profuse red or maroon diarrhoea and shock. Diverticular disease is the most common cause (erosion of an artery within the mouth of a diverticulum). Bleeding almost always stops spontaneously, but if it does not, the diseased segment of colon should be resected after confirmation by angiography or colonoscopy. Angiodysplasia is a disease of the elderly, in which vascular malformations develop in the proximal colon. Bleeding can be profuse; it usually stops spontaneously but commonly recurs. Diagnosis is often difficult. Colonoscopy may reveal vascular spots and, in the acute phase,visceral angiography can show bleeding.
In some, diagnosis is only achieved by laparotomy with on-table colonoscopy. The treatment of choice is endoscopic thermal ablation, but resection of the affected bowel may be required if bleeding continues. Bowel ischaemia due to occlusion of the inferior mesenteric artery can present with abdominal colic and rectal bleeding, considered in elderly (generalised atherosclerosis). The diagnosis is made at colonoscopy. Resection is required in the presence of peritonitis. Meckel’s diverticulum with ectopic gastric epithelium may ulcerate and erode into a major artery.
The diagnosis should be considered in children or adolescents who present with profuse or recurrent lower GI bleeding. A Meckel’s 99mTc-pertechnate scan is sometimes positive but the diagnosis is commonly made only by laparotomy, at which time the diverticulum is excised.
Subacute or chronic lower gastrointestinal bleeding
This can occur at all ages and is usually due to haemorrhoids (bleeding is bright red and occurs during or after defecation) or anal fissure, fresh rectal bleeding and anal pain occur during defecation. Proctoscopy can be used to make the diagnosis but subjects who have altered bowel habit and those who present > 40 years should undergo colonoscopy to exclude colorectal cancer.Major gastrointestinal bleeding of unknown cause
In some who present with major GI bleeding, upper endoscopy and colonoscopy fail to reveal a diagnosis.When severe life-threatening bleeding continues,
urgent CT mesenteric angiography is indicated.
This will usually identify the site if the bleeding > 1 mL/min and then formal angiographic embolisation can often stop the bleeding. If angiography is negative or bleeding is less severe, push or double balloon enteroscopy can visualise the small intestine and treat the bleeding source. Wireless capsule endoscopy is often used. When all else fails, laparotomy with on-table endoscopy is indicated.
Chronic occult gastrointestinal bleeding
Occult means that blood or its breakdown products are present in the stool but cannot be seen by the naked eye. Occult bleeding may reach 200 mL per day and cause iron deficiency anaemia. Any cause of GI bleeding may be responsible but the most important is colorectal cancer, particularly caecum, which may produce no GI symptoms.
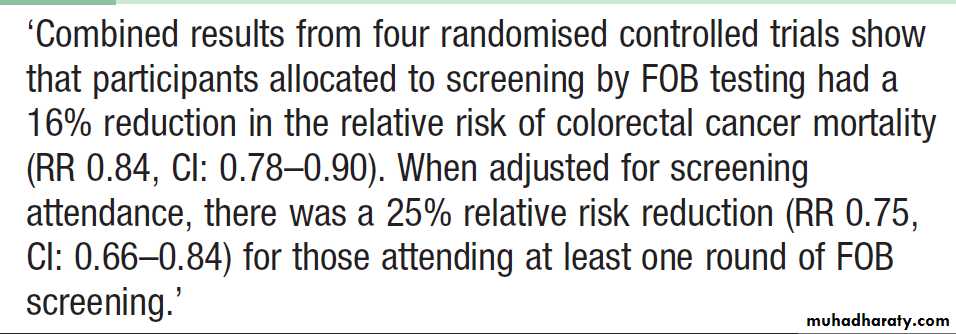
In clinical practice, investigation of the upper and lower GIT should be considered whenever a patient presents with unexplained iron deficiency anaemia. Testing the stool for the presence of blood is unnecessary and should not influence whether or not the GIT is imaged because bleeding from tumours is often intermittent and a negative faecal occult blood (FOB) test does not exclude the diagnosis. The only value of FOB testing is as a means of population screening for colonic neoplasia in asymptomatic individuals .
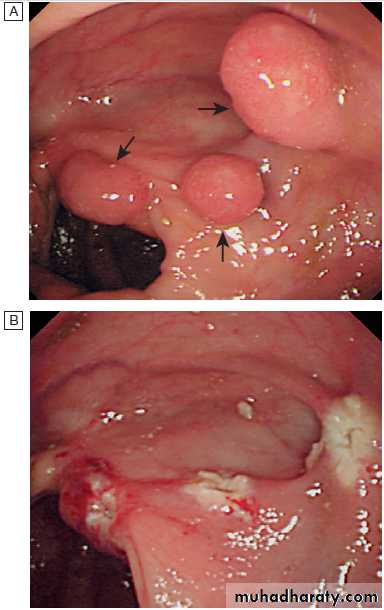
Jejunal angiodysplastic lesion seen at enteroscopy in a patient with recurrent obscure bleeding.
Diarrhoea
The passage of > 200 g of stool daily. The most severe symptom in many is urgency of defecation, and faecal incontinence is a common event.Acute diarrhoea
This is extremely common and is usually due to faecal–
oral transmission of bacteria or their toxins, viruses or
Parasites. Infective is usually short-lived <10 days .
A variety of drugs, antibiotics, cytotoxic, PPIs and NSAIDs, may be responsible.
Chronic or relapsing diarrhoea
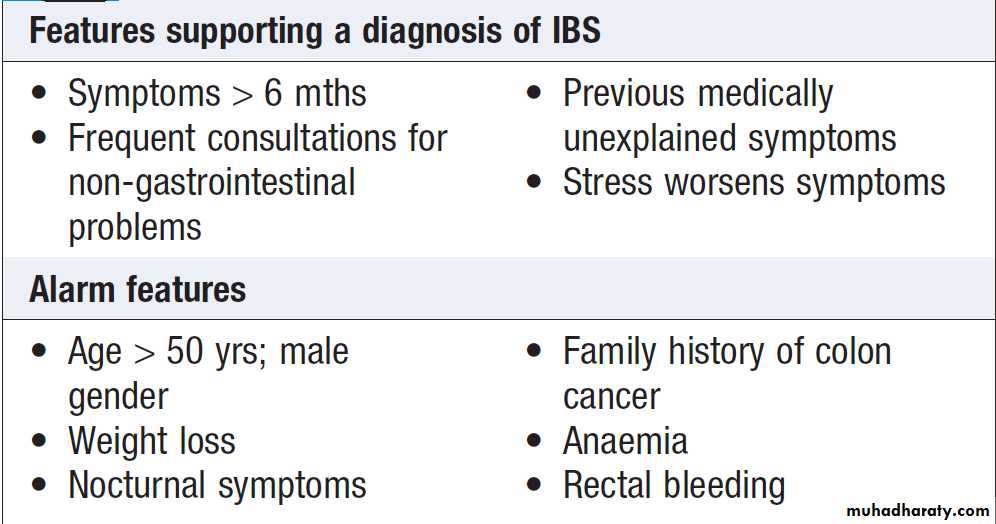
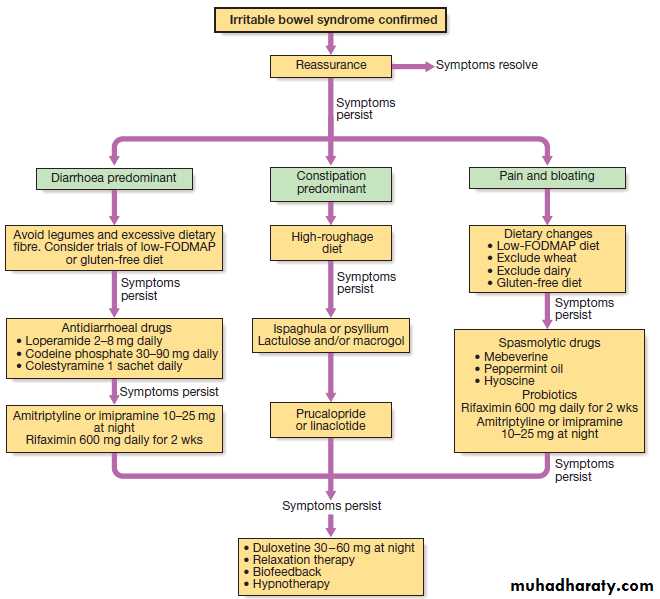
The most common is irritable bowel syndrome which can present with increased frequency of defecation and loose, watery or pellety stools. Diarrhoea rarely occurs at night and is most severe before and after breakfast.
At other times, the patient is constipated and there are other characteristic symptoms of IBS . The stool often contains mucus but never blood, 24-hour stool volume is < 200 g.
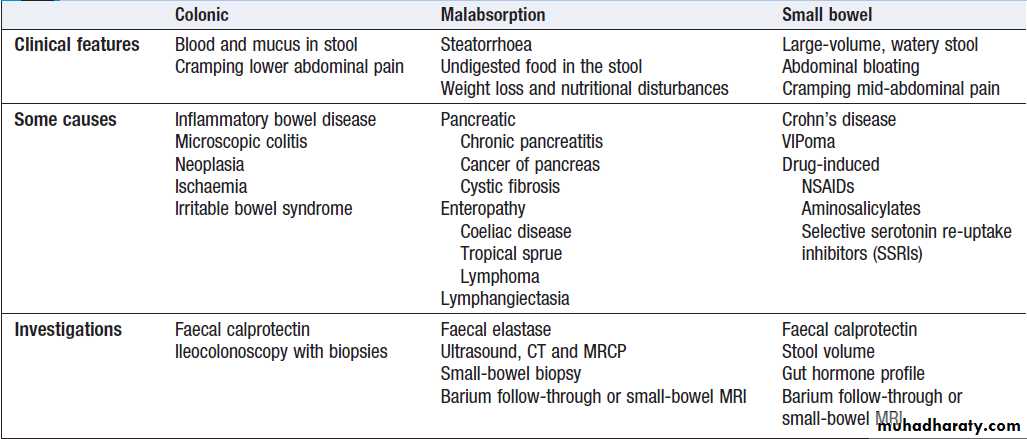
Chronic diarrhoea can be categorised as being due to
disease of the colon or small bowel, or to malabsorption.
Clinical presentation, examination of the stool, routine blood tests and imaging reveal a diagnosis in many cases.
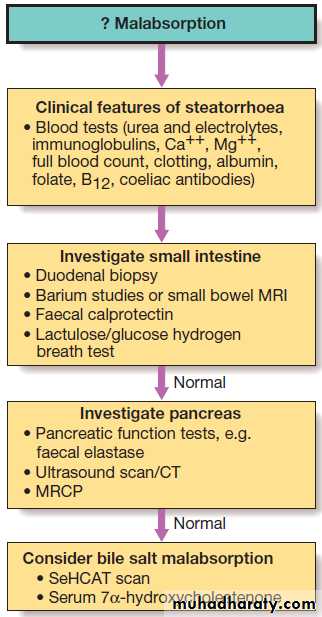
Malabsorption
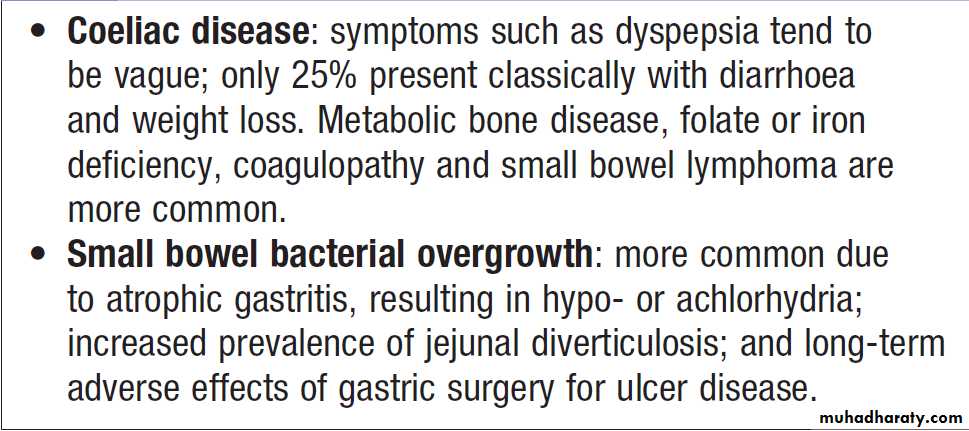
Diarrhoea and weight loss in patients with a normal dietis likely to be caused by malabsorption. A few
patients have apparently normal bowel habit but diarrhoea is usual and may be watery and voluminous.
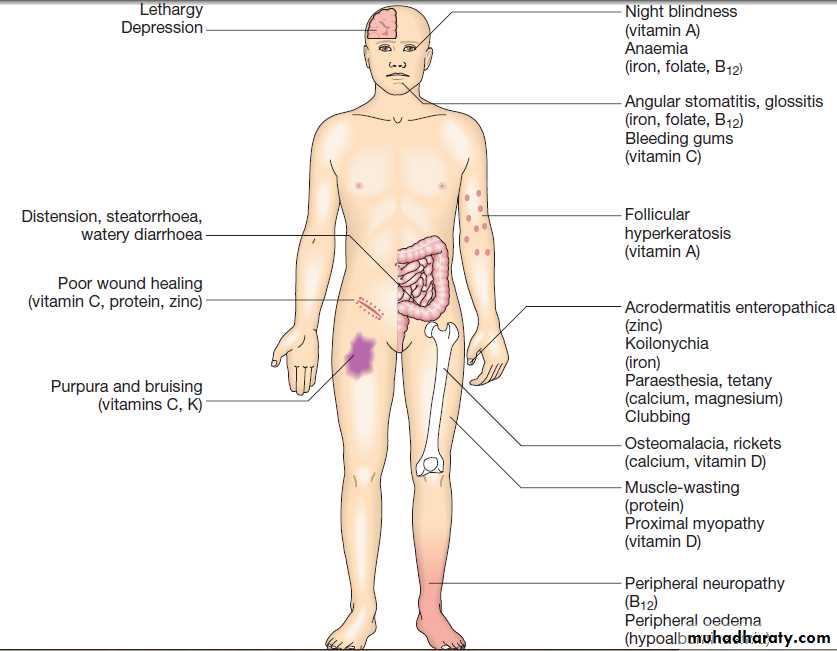
Bulky, pale and offensive stools which float in the toilet
(steatorrhoea) signify fat malabsorption. Abdominal distension, borborygmi, cramps, weight loss and undigested food in the stool may be present. Some patients
complain only of malaise and lethargy. In others symptoms related to deficiencies of specific vitamins, trace elements and minerals may occur.
Pathophysiology
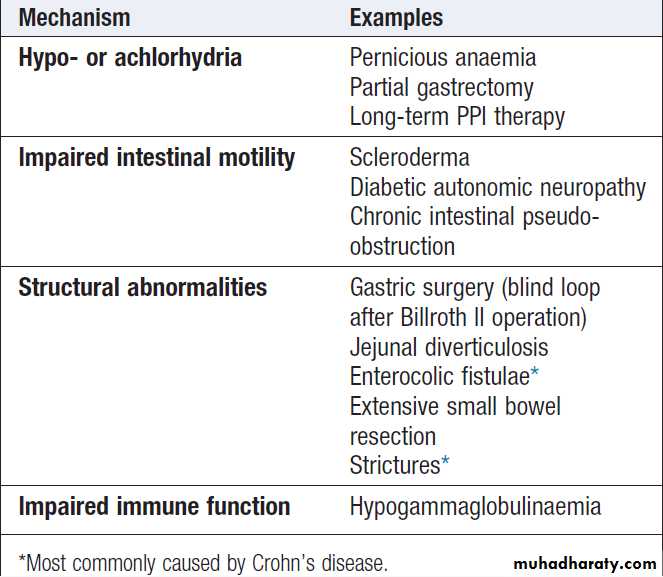
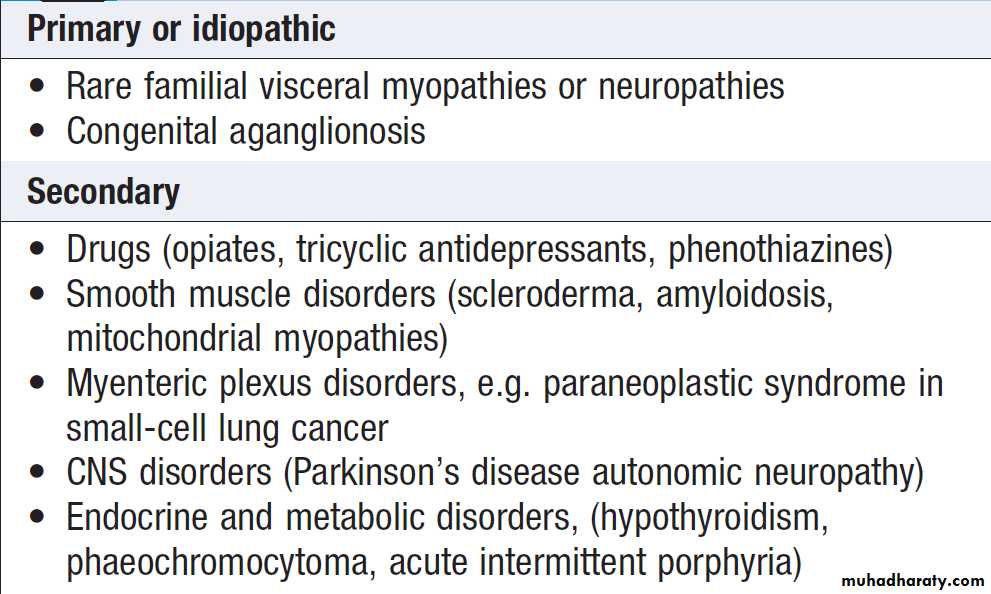
Malabsorption results from :
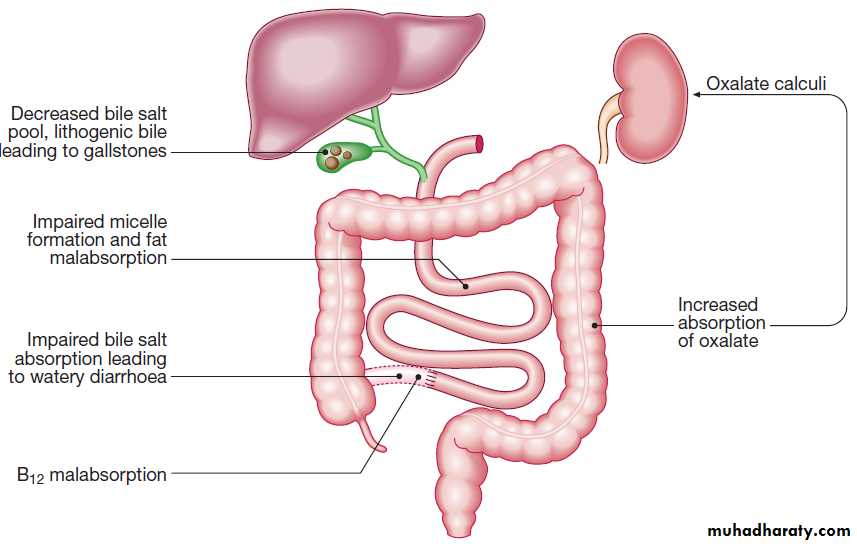
1. Intraluminal maldigestion occurs when deficiency of bile or pancreatic enzymes. Fat and protein malabsorption results, also occur with small bowel bacterial overgrowth.
2. Mucosal malabsorption results from small bowel resection or damage, diminishing the surface area for absorption and depleting brush border enzyme activity.
3. ‘Post-mucosal’ lymphatic obstruction prevents the uptake and transport of absorbed lipids into lymphatics. Increased pressure in these vessels results in leakage into the intestinal lumen, leading to protein-losing enteropathy.
Investigations
Both to confirm the presence and the underlying cause.
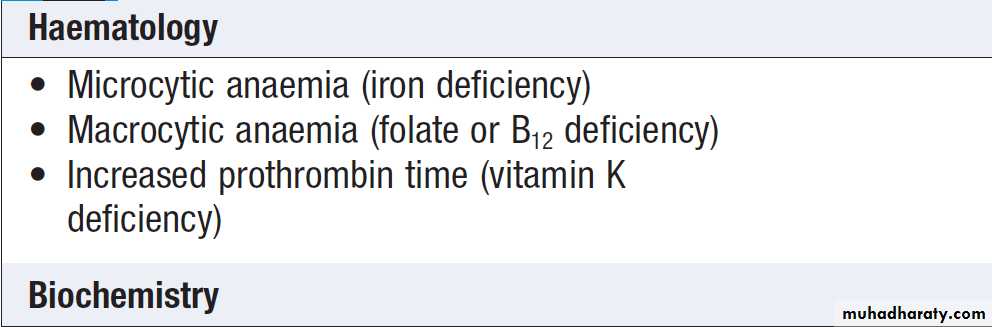
Blood tests may show one or more of the abnormalities.
Investigation for suspected malabsorption
Routine blood test abnormalities in malabsorptionPossible physical consequences of malabsorption
Weight lossWeight loss may be physiological, due to dieting, exercise, starvation, or the decreased nutritional intake
which accompanies old age. Weight loss of more than
3 kg over 6 months is significant and often indicates the
presence of an underlying disease. Hospital and general
practice weight records may be valuable in confirming
that weight loss has occurred, as may reweighing
patients at intervals; sometimes weight is regained or
stabilises in those with no obvious cause.
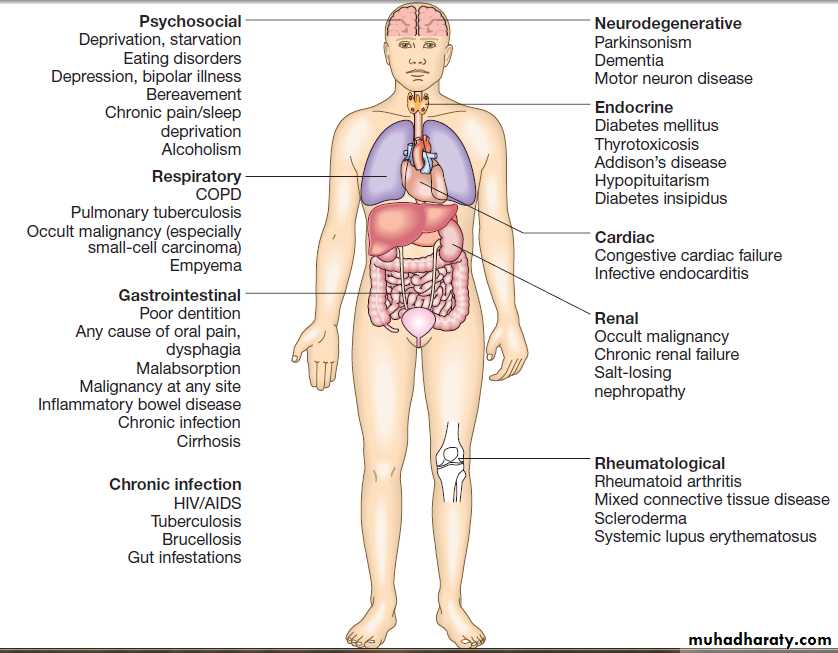
Pathological weight loss can be due to psychiatric illness, systemic disease, gastrointestinal causes or advanced disease of many organ systems .
Physiological
Weight loss can occur in the absence of serious disease in healthy individuals who have changes in physical activity or social circumstances. It may be difficult to be sure of this diagnosis in older patients, when the dietary history may be unreliable, and professional help from a dietitian is often valuable under these circumstances.
Psychiatric illness
Features of anorexia nervosa , bulimia and affective disorders may only be apparent
after formal psychiatric input. Alcoholic patients lose weight as a consequence of self-neglect and poor dietary intake. Depression may cause weight loss.
Systemic diseases
Chronic infections, including tuberculosis ,recurrent urinary or chest infections, and a range of parasitic and protozoan infections ,should be considered. A history of foreign travel, high-risk activities and specific features, such as fever, night sweats, rigors, productive cough and dysuria, must be sought. Promiscuous sexual activity and drug misuse suggestHIV-related illness .
Weight loss is a late feature of disseminated malignancy, but by the time the patient presents, other features of cancer are often present.
Chronic inflammatory diseases, such as rheumatoid, and polymyalgia rheumatica ,are associated with weight loss.
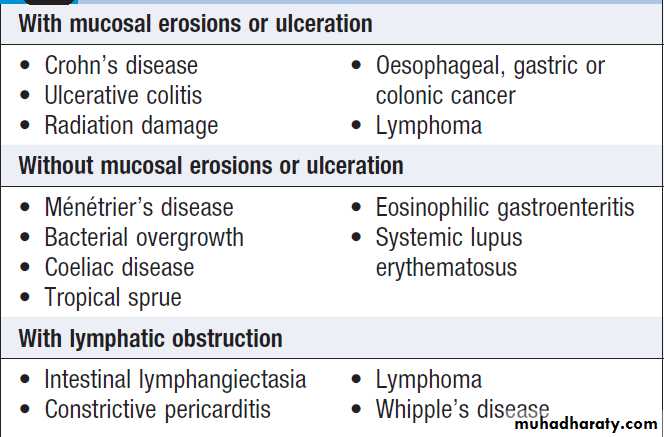
Gastrointestinal disease
Almost any disease of the gastrointestinal tract can cause weight loss. Dysphagia and gastric outflow obstruction cause weight loss by reducing food intake. Malignancy at any site may cause weight loss by mechanical obstruction, anorexia or cytokine-mediated systemic effects. Malabsorption from pancreatic diseases or small bowel causes may lead to profound weight loss with specific nutritional deficiencies .Inflammatory diseases, such as Crohn’s disease or ulcerative colitis ,cause anorexia, fear of eating and loss of protein, blood and nutrients from the gut.
Metabolic disorders and miscellaneous causes
Weight loss may occur in association with metabolic disorders, end-stage respiratory and cardiac disease.
Investigations
urinalysis for sugar, protein and blood; blood tests, including liver function tests, random blood glucose and thyroid function tests; CRP and ESR (may be raised in unsuspected infections, such as tuberculosis, connective tissue disorders and malignancy); and faecal calprotectin. Sometimes invasive tests, such as bone marrow aspiration or liver biopsy, may be necessary to identify conditions like cryptic miliary tuberculosis .Rarely, abdominal and pelvic imaging by CT may be necessary, but before embarking on invasive or very costly investigations, it is always worth revisiting the patient’s history and reweighing at intervals.Some important causes of weight loss
Some easily overlooked causes of
unexplained weight lossConstipation
Constipation is defined as infrequent passage of hard stools. Patients may also complain of straining, a sensation of incomplete evacuation and either perianal or abdominal discomfort.Clinical assessment and management
The onset, duration and characteristics are important; for example, a neonatal onset suggests Hirschsprung’s disease, while a recent change in bowel activity in middle age should raise the suspicion of organic disorders, such as colonic carcinoma. The presence of rectal bleeding, pain and weight loss is important, as are excessive straining, symptoms suggestive of irritable bowel ,a history of childhood constipation and emotional distress.
Careful examination , search for general medical disorders, neurological disorders, especially spinal cord lesions, should be sought. Perineal inspection and rectal examination are essential and may reveal abnormalities of the pelvic floor (abnormal descent, impaired sensation), anal canal or rectum (masses, faecal impaction, prolapse). It is neither possible nor appropriate to investigate every person with constipation. Most respond to increased fluid intake, dietary fibre supplementation, exercise and the judicious use of laxatives.
Middle-aged or elderly with a short history or worrying symptoms (rectal bleeding, pain or weight loss) must be investigated promptly, by barium enema or colonoscopy.
Initial visit
Digital rectal examination, proctoscopy,sigmoidoscopy biochemistry, i serum calcium and thyroid function tests, and a full blood count should be done. If these are normal,a 1-month trial of dietary fibre and/or laxatives is justified.
Next visit
If symptoms persist, barium enema or CT colonography is indicated.
Further investigation
If no cause is found and disabling symptoms are present, then investigation of dysmotility may be necessary. The problem may be one of infrequent desire to defecate (‘slow transit’) or may result from neuromuscular incoordination and excessive straining (‘functional obstructive defecation’). Intestinal marker, anorectal manometry, electrophysiological studies and MR proctography can all be used.
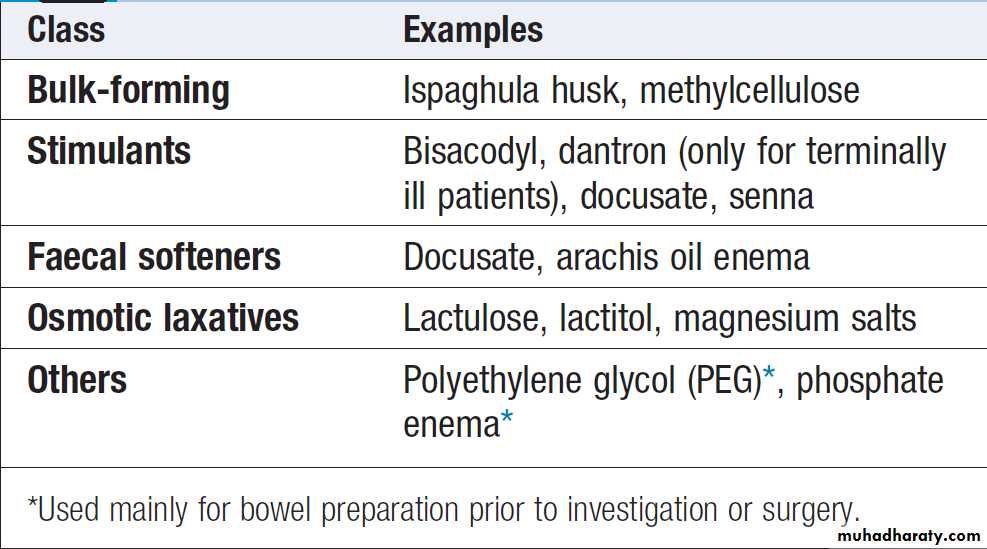
Causes of constipation
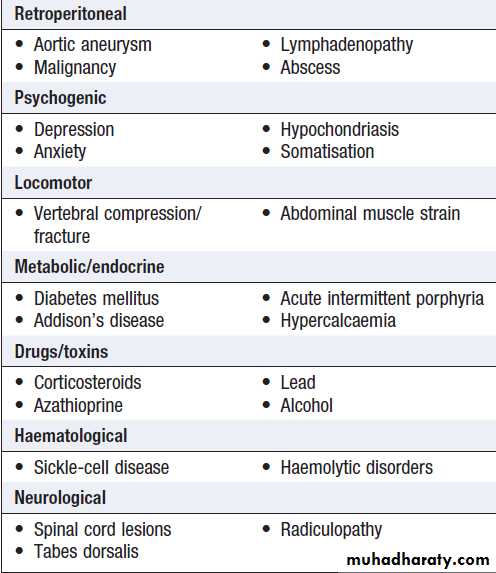
Abdominal pain
• Visceral. Gut organs are insensitive to stimuli suchas burning and cutting but are sensitive to distension, contraction, twisting and stretching. Pain from unpaired structures is usually but not always felt in the midline.
• Parietal. parietal peritoneum is innervated by somatic nerves, and its involvement by inflammation, infection or neoplasia causes sharp, well- localised.
• Referred pain. (For example, gallbladder pain is
referred to the back or shoulder tip.)
• Psychogenic. Cultural, emotional and psychosocial factors influence everyone’s experience of pain.
In some, no organic cause can be found despite investigation, and psychogenic causes (depression or somatisation disorder) may be responsible .
The acute abdomen
This accounts for approximately 50% of all urgentadmissions to general surgical units.
• Inflammation. Pain develops gradually, over several hrs.
It is initially rather diffuse until the parietal peritoneum is involved, when it becomes localised. Movement exacerbates the pain; rigidity and guarding occur.
• Perforation. When a viscus perforates, pain starts
abruptly; it is severe and leads to generalised peritonitis.
• Obstruction. Pain is colicky, with spasms which cause the patient to writhe around and double up.
Initial clinical assessment
If there are signs of peritonitis (guarding and rebound tenderness with rigidity), the patient should be resuscitated with oxygen, intravenous fluids and antibiotics.
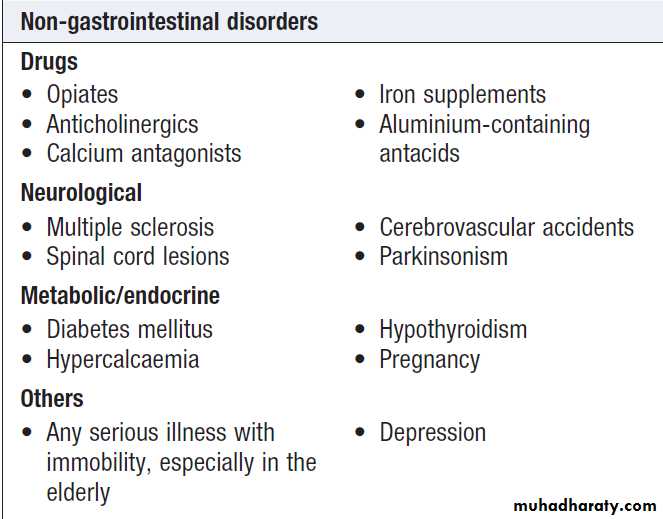
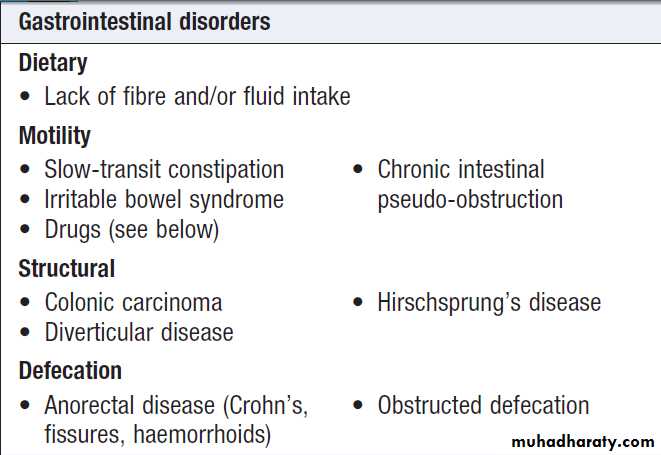
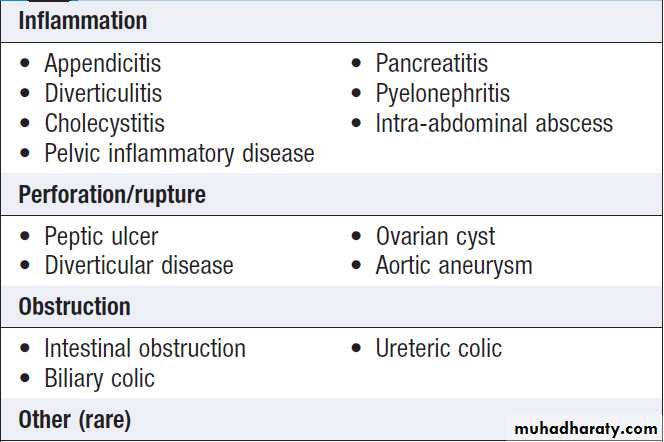
Causes of acute abdominal pain
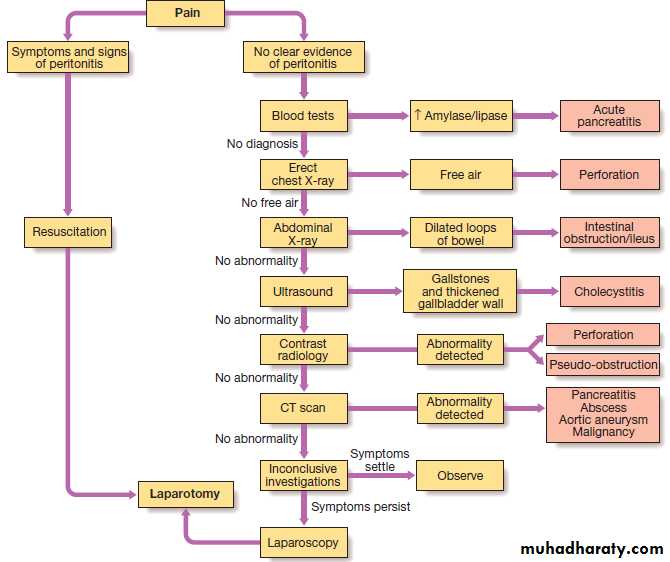
Management of acute abdominal pain:
an algorithmInvestigations
Full blood count, urea and electrolytes, amylase, leucocytosis. An erect chest X-ray may show air under the diaphragm, suggestive of perforation, and a plain abdominal film may show evidence of obstruction or ileus. An abdominal US may help if gallstones or renal stones are suspected also useful in the detection of free fluid and any possible intra-abdominal abscess. Contrast studies, by either mouth or anus, are useful in the further evaluation of intestinal obstruction, and essential in the differentiation of pseudo obstruction from mechanical large-bowel obstruction. Other investigations include CT(pancreatitis, retroperitoneal collections or masses, aortic aneurysm) and angiography (mesenteric ischaemia).
Diagnostic laparotomy should be considered when the diagnosis has not been revealed by other investigations.
All patients must be re-assessed (every 2–4 hours) so that any change in condition that might alter both the suspected diagnosis and clinical decision.
Management
The general approach is to close perforations, treat
inflammatory conditions with antibiotics or resection,
and relieve obstructions. The speed of intervention and the necessity for surgery depend on the organ that is involved and on a number of other factors, of which the presence or absence of peritonitis is the most important.
A treatment summary of some of the more common
surgical conditions follows.
Acute appendicitis
Treated by early surgery, since there is a risk of perforation and recurrent attacks with nonoperative treatment, can be removed through a conventional right iliac fossa incision or by laparoscopic techniques.
Acute cholecystitis
This can be successfully treated non-operatively but the high risk of recurrence and the low morbidity of surgery have made early laparoscopic cholecystectomy the treatment of choice.
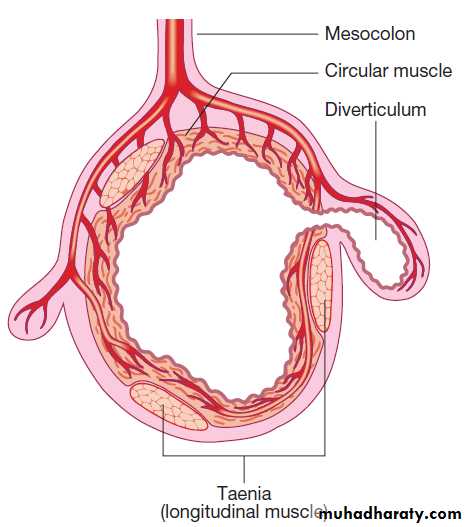
Acute diverticulitis
Conservative therapy is standard but if perforation has occurred, resection is advisable. Depending on peritoneal contamination and the state of the patient, primary anastomosis is preferable to a Hartmann’s procedure (oversew of rectal stump and end colostomy).
Small bowel obstruction
If the cause is obvious and surgery inevitable (such aswith a strangulated hernia), an early operation is appropriate. If the suspected cause is adhesions from previous surgery, only those patients who do not resolve within the first 48 hours or who develop signs of strangulation (colicky pain becomes constant, peritonitis, tachycardia, fever, leucocytosis) should have surgery.
Large bowel obstruction
Pseudo-obstruction should be treated non-operatively.
Some patients benefit from colonoscopic decompression, but mechanical obstruction merits resection, usually with a primary anastomosis. Differentiation between the two is made by water-soluble contrast enema.
Perforated peptic ulcer
Surgical closure of the perforation is standard practice but some patients without generalised peritonitis can be treated non-operatively once a water-soluble contrast has confirmed spontaneous sealing of the perforation. Adequate and aggressive resuscitation with IV fluids, antibiotics and analgesia is mandatory before surgery.Chronic or recurrent abdominal pain
It is essential to take a detailed history, paying particular attention to features of the pain and any associated symptoms .
Note should be made of the patient’s general demeanour, mood and emotional state, signs of weight loss, fever, jaundice or anaemia.
If a thorough abdominal and rectal examination is normal, a careful search should be made for evidence of disease affecting other structures, particularly the vertebral column, spinal cord, lungs and cardiovascular system.
Investigations will depend on the clinical features
elicited during the history and examination:
• Endoscopy and US for epigastric pain, dyspepsia and symptoms suggestive of gallbladder disease
• Colonoscopy is indicated for patients with altered bowel habit, rectal bleeding or features of
obstruction suggesting colonic disease.
• CT or MR angiography , considered when pain is provoked by food in a patient with widespread atherosclerosis, may indicate mesenteric ischaemia.
• Persistent symptoms require exclusion of colonic or
small bowel disease. Blood tests, faecal calprotectin and sigmoidoscopy) are sufficient in the absence of rectal bleeding, weight loss and abnormal physical findings.
• US , CT and faecal elastase for patients with upper abdominal pain radiating to the back. A history of alcohol misuse, weight loss and diarrhoea suggests chronic pancreatitis or pancreatic cancer.
• Recurrent attacks of pain in the loins radiating to
the flanks with urinary symptoms should prompt
investigation for renal or ureteric stones by
abdominal X-ray, ultrasound and CT urography.
• A past history of psychiatric disturbance, repeated negative investigations or vague symptoms which do not fit any disease or organ pattern suggest a psychological origin for the pain .
Acute abdominal pain in old age
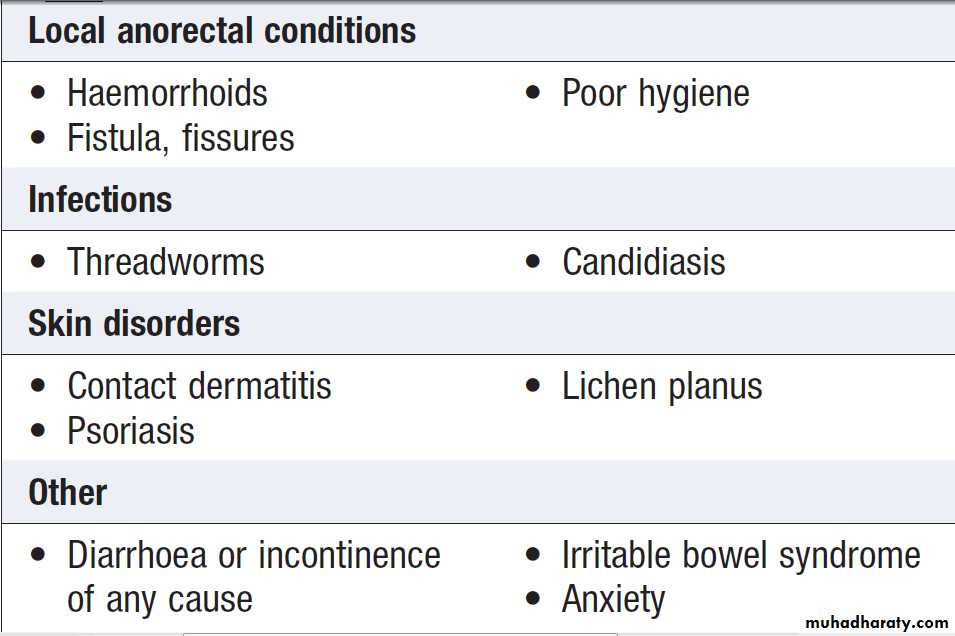
Extra-intestinal causes of chronic or
recurrent abdominal painHow to assess abdominal pain
Constant abdominal painPatients with chronic pain that is constant or nearly always present usually have features to suggest the underlying diagnosis. In a minority, no cause will be found, despite thorough investigation, leading to the diagnosis of ‘chronic functional abdominal pain’. In these patients, there appears to be abnormal CNS processing of normal visceral afferent sensory input, psychosocial factors are often , and the most important tasks are to provide symptom control, if not relief, and to minimise the effects of the pain on social, personal and occupational life. Patients are best managed in specialised pain clinics where, in addition to psychological support, appropriate use of drugs, including tricyclic antidepressants, gabapentin or pregabalin, ketamine and opioids, may be necessary.
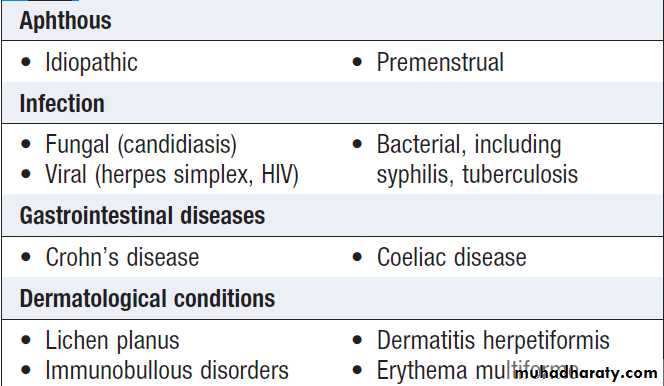
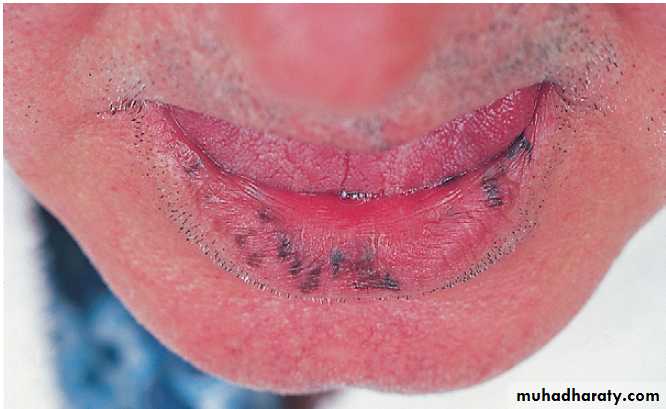
Aphthous ulceration
Superficial and painful; in any part of the mouth. Recurrent up to 30% ,common in women prior to menstruation. The cause is unknown, severe cases other causes must be considered .Occasionally, biopsy is necessary.Candidiasis
The yeast Candida albicans is a normal mouth commensal
may proliferate to cause thrush. This occurs in babies, debilitated, corticosteroid or antibiotic, diabetic and immunosuppressed. White patches are seen on the tongue and buccal mucosa. Odynophagia or dysphagia suggests pharyngeal and oesophageal candidiasis.
Oral thrush treated with nystatin or amphotericin suspensions or lozenges. Resistant cases or immunosuppressed may require oral fluconazole
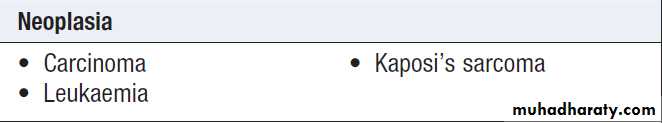
Causes of oral ulceration
Causes of oral ulceration'cont'd
Oral cancer
Squamous carcinoma of the oral cavity is common .The mortality rate is around 50%, largely as a result of late diagnosis. Poor diet, alcohol excess and smoking or tobacco chewing are the traditional risk factors but high-risk, oncogenic strains of human papillomavirus (HPV-16 and HPV-18) have been identified as responsible for much of the recent increase in incidence, especially in cases affecting the base of tongue, soft palate and tonsils. Patients with suspicious lesions , all possible sources of local trauma or infection treated and should be reviewed after 2 weeks, with biopsy if the lesion persists. Small cancers can be resected but extensive surgery, with neck dissection to remove involved lymph nodes, may be necessary.
Some patients can be treated with radical radiotherapy alone, and sometimes given after surgery to treat microscopic residual disease. Some tumours may be amenable to photodynamic therapy (PDT).
Symptoms and signs of oral cancer
Parotitis
Due to viral or bacterial infection. Mumps causes a self-limiting acute parotitis . Bacterial usually occurs as a complication of major surgery, a consequence of dehydration and poor oral hygiene. Patients present with painful parotid swelling and can be complicated by abscess formation. Broad-spectrum antibiotics are required, surgical drainage is necessary for abscesses.
• Infection : Mumps. Bacterial (post-operative)
• Calculi
• Sjِgren’s syndrome
• Sarcoidosis
• Tumours
Benign: pleomorphic adenoma (95% of cases) Intermediate: mucoepidermoid tumour Malignant: carcinoma
Causes of salivary gland swelling
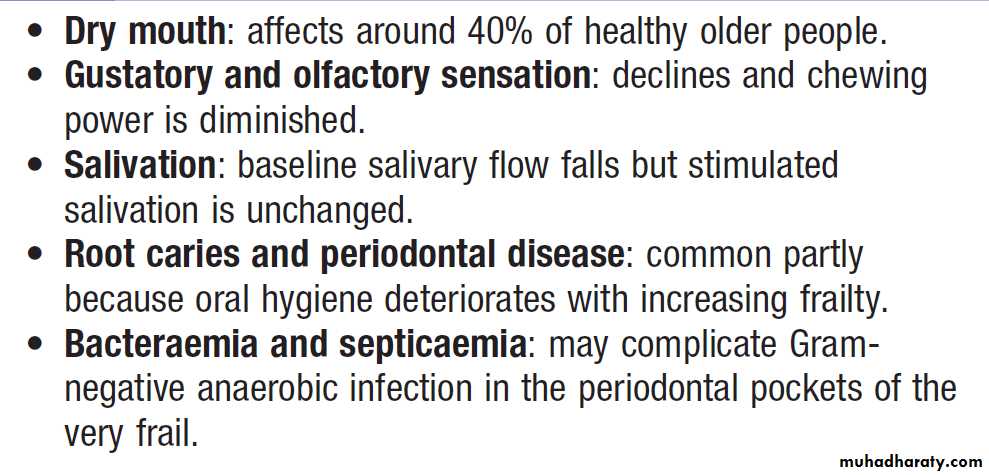
Oral health in old age
Factors associated with the development of
gastro-esophagealreflux disease.
Dietary fat, chocolate, alcohol and coffee relax the lower oesophageal sphincter and may provoke symptoms.
Gastro-oesophageal reflux disease (GERD)
Affects approximately 30% of general population. Pathophysiology
Occasional episodes are common in healthy individuals. Reflux is normally followed by oesophageal peristaltic waves which efficiently clear the gullet, alkaline saliva neutralises residual acid, and symptoms do not occur. Gastro- oesophageal reflux disease (GERD)
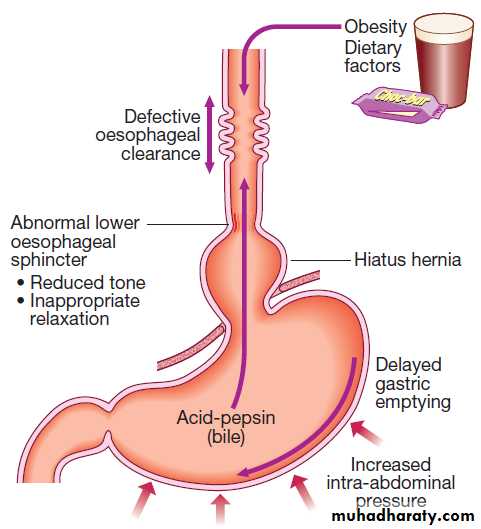
develops when the oesophageal mucosa is exposed to gastroduodenal contents for prolonged periods of time, resulting in symptoms and, in a proportion of cases, oesophagitis. Several factors are known to
be involved in the development of GERD .
Abnormalities of the lower oesophageal sphincter
The lower sphincter is tonically contracted under normal circumstances, relaxing only during swallowing.Some patients with GERD have reduced lower oesophageal sphincter tone, permitting reflux when intra-abdominal pressure rises. In others, basal sphincter tone is normal but reflux occurs in response to frequent episodes of inappropriate sphincter relaxation.
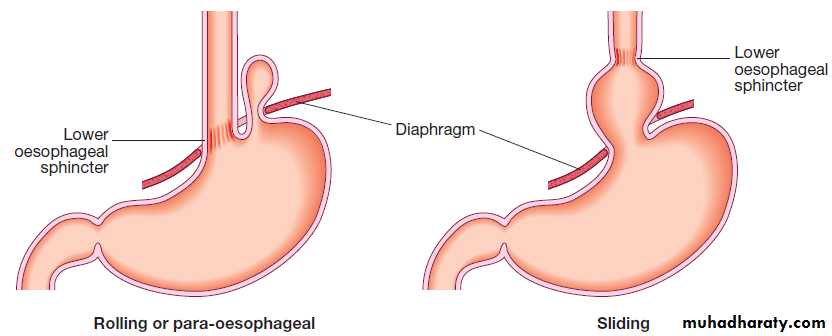
Hiatus hernia
Causes reflux because the pressure gradient between the abdominal and thoracic cavities, which normally pinches the hiatus, is lost. In addition, the oblique angle between the cardia and oesophagus disappears. Almost all who develop oesophagitis, Barrett’s oesophagus or strictures have a hiatus hernia.
Delayed oesophageal clearance
Defective peristaltic activity found in patients who have oesophagitis. It is a primary abnormality, since it persists after oesophagitis has been healed by acid-suppressing drug therapy. Poor clearance increase acid exposure time.Gastric contents
Gastric acid is important oesophageal irritant and there is a close relationship between acid exposure time and symptoms. Pepsin and bile also contribute to mucosal injury.
Defective gastric emptying
In patients with GERD. The reason is unknown.
Increased intra-abdominal pressure
Pregnancy and obesity are established predisposing
causes. Weight loss may improve symptoms.
Dietary and environmental factors
Dietary fat, chocolate, alcohol and coffee relax the lower oesophageal sphincter and may provoke symptoms.
Patient factors
Visceral sensitivity and patient vigilance play a role in
determining symptom severity.
Clinical features
The major symptoms are heartburn and regurgitation,
often provoked by bending, straining or lying down.
‘Waterbrash’, which is salivation due to reflex salivary
gland stimulation as acid enters the gullet, is often
present. The patient is often overweight. Some are woken at night by choking as refluxed fluid irritates the larynx. Others develop odynophagia or dysphagia.
Atypical chest pain which may be severe and can mimic angina, and may be due to reflux-induced oesophageal spasm. Others include hoarseness (‘acid laryngitis’), recurrent chest infections, chronic cough and asthma.
Complications of GERD
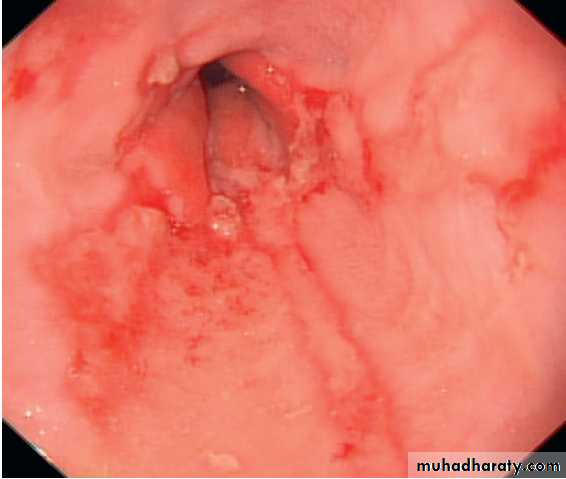
1.Oesophagitis
Mild redness to severe, bleeding ulceration with stricture formation, are recognised, although appearances may be completely normal . There is a poor correlation between
symptoms and histological and endoscopic findings.
2. Barrett’s oesophagus
pre-malignant, the normal squamous lining of the lower oesophagus is replaced by columnar mucosa (columnar lined oesophagus( CLO)) that may contain areas of intestinal metaplasia .
It is an adaptive response to chronic GERD and is found in 10% undergoing gastroscopy for reflux symptoms. The condition is often asymptomatic until discovered with oesophageal cancer. The relative risk of oesophageal cancer is 40–120-fold increased but the absolute risk is low (0.1–0.5% per year). The epidemiology and aetiology
of CLO are poorly understood. It is more common in men, obese and those >50 years of age. It is weakly associated with smoking but not alcohol intake. The risk of cancer seems to relate to the severity and duration rather than the presence of CLO per se and it has been suggested that duodenogastrooesophageal reflux of bile, pancreatic enzymes and pepsin, as well as gastric acid, may be important in pathogenesis.
Diagnosis. This requires multiple systematic biopsies to
detect intestinal metaplasia and/or dysplasia.
Management.
Neither potent acid suppression nor antireflux surgery stops progression of CLO, and treatment is only indicated for symptoms of reflux or complications, such as stricture. Endoscopic therapies, radiofrequency ablation or photodynamic therapy, can induce regression but, at present, are used only for those with dysplasia or intramucosal cancer. Regular endoscopic surveillance can detect dysplasia at an early stage and may improve survival but, because most CLO is undetected until cancer develops, surveillance strategies are unlikely to influence the overall mortality rate of oesophageal cancer.
Surveillance is expensive and cost-effectiveness studies have been conflicting, but it is currently recommended that patients with CLO without dysplasia should undergo endoscopy at 3–5-yearly intervals and those with lowgrade dysplasia at 6–12-monthly intervals.
For those with high-grade dysplasia (HGD) or intramucosal carcinoma, the treatment options are either
oesophagectomy or endoscopic therapy with a combination
of endoscopic resection (ER) of any visibly abnormal
areas and radiofrequency ablation (RFA) of the
remaining Barrett’s mucosa as an ‘organ-preserving’
alternative to surgery.
3. Anaemia
Iron deficiency anaemia can occur as a consequence ofoccult blood loss from long-standing oesophagitis. Most
patients have a large hiatus hernia and bleeding can
stem from subtle erosions in the neck of the sac (‘Cameron lesions’). Nevertheless, hiatus hernia is very common and other causes, particularly colorectal cancer, must be considered, even when endoscopy reveals oesophagitis.
4. Benign oesophageal stricture
Fibrous strictures can develop as a consequence of longstanding oesophagitis. The typical presentation is with dysphagia that is worse for solids than for liquids.
A history of heartburn is common but not invariable; many elderly patients presenting with strictures have no preceding heartburn. Diagnosis is by endoscopy,
Biopsies taken to exclude malignancy. Endoscopic balloon dilatation is helpful. Subsequently, long-term therapy with a PPI drug at full dose should be started. The patient should be advised to chew food thoroughly, and it is important to ensure adequate dentition.
5. Gastric volvulus
Occasionally, a massive intrathoracic hiatus hernia may
twist upon itself, leading to a gastric volvulus. This gives rise to complete oesophageal or gastric obstruction and
presents with severe chest pain, vomiting and dysphagia. The diagnosis is made by chest X-ray (air bubble in the chest) and barium swallow. Most cases spontaneously resolve but recurrence is common, and surgery is usually advised after the acute episode has been treated by nasogastric decompression.
Investigations
Young patients who present with typical symptoms ofgastro-oesophageal reflux, without worrying features such as dysphagia, weight loss or anaemia, can be treated empirically without investigation. Investigation is advisable if patients present over the age of 50–55 years, if symptoms are atypical or if a complication is suspected. Endoscopy is the investigation of choice. This is performed to exclude other upper GIT diseases that can mimic GERD and to identify complications. A normal endoscopy in a patient with compatible symptoms should not preclude treatment for gastro-oesophageal reflux disease.
Twenty-four-hour pH monitoring is indicated if the
diagnosis is unclear or surgical intervention is underconsideration. This involves tethering a slim catheter
with a terminal radiotelemetry pH-sensitive probe
above the gastro-oesophageal junction. The intraluminal
pH is recorded whilst the patient undergoes normal
activities, and episodes of symptoms are noted and
related to pH. A pH of less than 4 for more than 6–7%
of the study time is diagnostic of reflux disease. In a few
patients with difficult reflux, impedance testing can
detect weakly acidic or alkaline reflux that is not revealed by standard pH testing.
Management
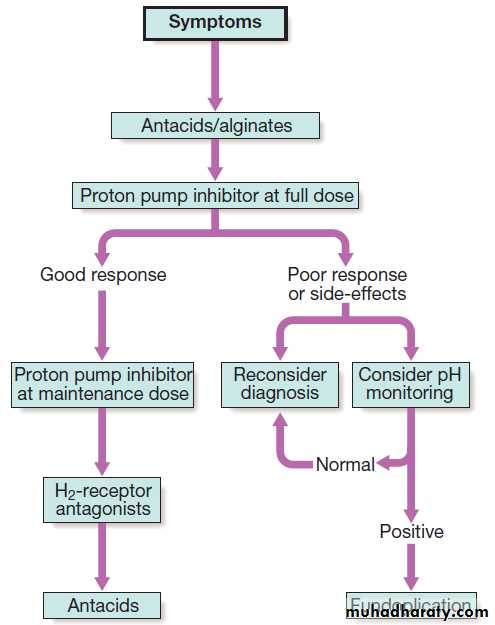
A treatment algorithm for gastro- oesophageal reflux is outlined in Figure . Lifestyle advice, including
weight loss, avoidance of dietary items that the patient finds worsen symptoms, elevation of the bed head in those who experience nocturnal symptoms, avoidance of late meals and giving up smoking, should be recommended.
Patients who fail to respond to these measures
should be offered PPIs, which are usually effective
in resolving symptoms and healing oesophagitis.
Recurrence of symptoms is common when therapy is
stopped and some patients require life-long treatment
at the lowest acceptable dose.
Long-term PPI is associated with reduced absorption of iron, B12 and magnesium, and a small increased risk of osteoporosis and fractures , also predispose to infections with Salmonella, Campylobacter and Clostridium difficile.
Increases the risk of Helicobacter-associated progression of gastric mucosal atrophy and H. pylori eradication is advised in patients requiring PPIs for >1 year.
When dysmotility features are prominent, domperidone can be helpful.
There is no evidence that H. pylori eradication has any
therapeutic value.
Proprietary antacids and alginates can also provide symptomatic benefit. H2-receptor antagonist drugs also help symptoms without healing oesophagitis.
Patients who fail to respond to medical therapy, those who are unwilling to take long-term PPIs and those whose major symptom is severe regurgitation should be considered for laparoscopic anti-reflux surgery .
Although heartburn and regurgitation are alleviated in most patients, a small minority develop complications, such as inability to vomit and abdominal bloating (‘gas-bloat’ syndrome’).
Types of hiatus hernia.
Important features of hiatus hernia
The association between gastro- oesophageal reflux disease and asthma
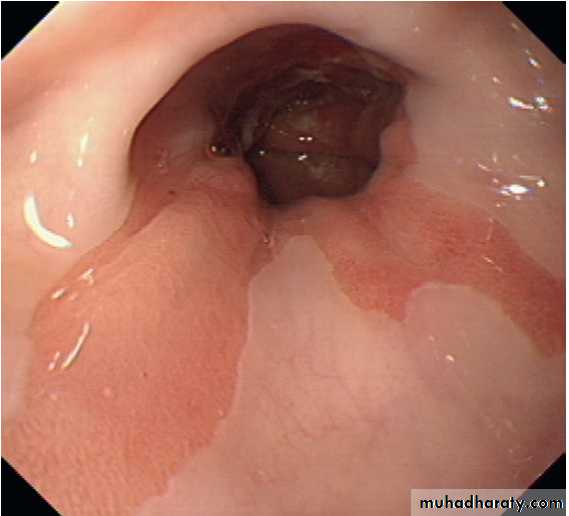
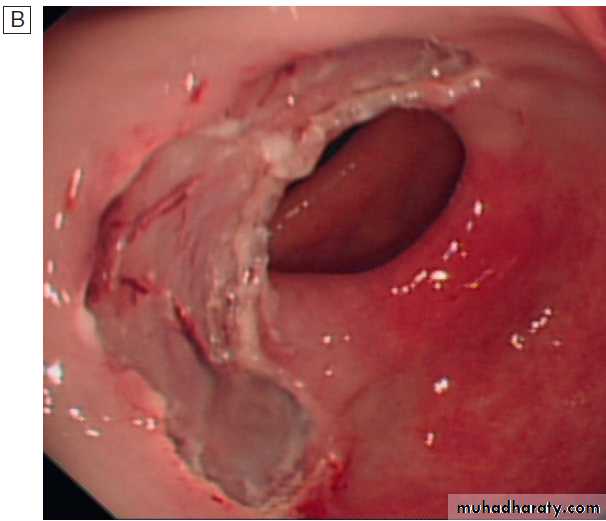
Severe reflux oesophagitis.
There is near-circumferentialsuperficial ulceration and inflammation extending up the gullet.
Barrett’s oesophagus.
Tongues of pink columnar mucosa are seen extending upwards above the oesophago-gastric junction.Treatment of gastro- oesophageal reflux disease:
a ‘step-down’ approach.
Gastro- oesophageal reflux disease in old age
Other causes of oesophagitisInfection
Oesophageal candidiasis occurs in debilitated patients
and those taking broad-spectrum antibiotics or cytotoxic
drugs. It is a particular problem in patients with HIV.
Corrosives
Suicide attempt by strong household bleach or battery
acid is followed by painful burns of the mouth and
pharynx and by extensive erosive oesophagitis. This may
be complicated by oesophageal perforation with mediastinitis and by stricture formation. At the time of presentation, treatment is conservative, analgesia and nutritional support; vomiting and endoscopy should be avoided because of the high risk of perforation.
After the acute phase, a barium swallow should
be performed to demonstrate the extent of stricture formation. Endoscopic dilatation is usually necessary but it
is difficult and hazardous because strictures are often
long, tortuous and easily perforated.
Drugs
Potassium supplements and NSAIDs may cause
oesophageal ulcers when the tablets are trapped above
an oesophageal stricture. Liquid preparations of these
drugs should be used in such patients. Bisphosphonates
cause oesophageal ulceration and should be used with
caution in patients with known oesophageal disorders.
Motility disorders
Pharyngeal pouchThis occurs because of incoordination of swallowing
within the pharynx, which leads to herniation through
the cricopharyngeus muscle and formation of a pouch.
It is rare, affecting 1 in 100 000 people; it usually develops in middle life but can arise at any age. Many patients have no symptoms, but regurgitation, halitosis and dysphagia can be present. Some notice gurgling in the throat after swallowing.
The investigation of choice is a barium swallow, which demonstrates the pouch and reveals incoordination of swallowing, often with pulmonary aspiration. Endoscopy may be hazardous,
since the instrument may enter and perforate the pouch. Surgical myotomy (‘diverticulotomy’), with or without resection of the pouch, is indicated in symptomatic patients.
Achalasia of the oesophagus
Pathophysiology
Achalasia is characterised by:
• a hypertonic lower oesophageal sphincter, which
fails to relax in response to the swallowing wave
• failure of propagated oesophageal contraction,
leading to progressive dilatation of the gullet.
The cause is unknown. Defective release of nitric
oxide by inhibitory neurons in the lower oesophageal sphincter has been reported, and there is degeneration
of ganglion cells within the sphincter and the body of
the oesophagus.
Infection with Trypanosoma cruzi in Chagas’ disease causes a syndrome that is clinically indistinguishable from achalasia.
Clinical features
Dysphagia, develops slowly, is initially intermittent, and is worse for solids and eased by drinking liquids, and by standing and moving around after eating. Heartburn does not occur because the closed oesophageal sphincter prevents GER. Some experience episodes of chest pain due to oesophageal spasm. As the disease progresses, dysphagia worsens, the oesophagus empties poorly and nocturnal pulmonary aspiration develops. Achalasia predisposes to squamous carcinoma of the oesophagus. InvestigationsEndoscopy should always be carried out because carcinoma of the cardia can mimic the presentation and radiological and manometric features of achalasia (‘pseudo-achalasia’).
A barium swallow shows tapered narrowing of the lower oesophagus and, in late disease, the oesophageal body is dilated, aperistaltic and food-filled . Manometry confirms the high pressure, non-relaxing lower oesophageal sphincter with poor contractility of the oesophageal body .
Management
Endoscopic
Forceful pneumatic dilatation using a 30–35-mm diameter fluoroscopically positioned balloon disrupts the sphincter and improves symptoms in 80%. Some require more than one dilatation but those needing frequent dilatation are best treated surgically. Endoscopically directed injection of botulinum toxin into the lower sphincter induces clinical remission but relapse is common.
Surgical
Surgical myotomy (Heller’s operation), performed either laparoscopically or as an open operation, is effective but is more invasive than endoscopic dilatation. Both pneumatic dilatation and myotomy may be complicated by gastro-oesophageal reflux, and this can lead to severe oesophagitis because oesophageal clearance is so poor.
For this reason, Heller’s myotomy is accompanied by a
partial fundoplication anti-reflux procedure. PPI therapy is often necessary after surgery. Recently, a complex endoscopic technique has been developed in specialist centres (peroral endoscopic myotomy, POEM).
Secondary causes of oesophageal dysmotility
In systemic sclerosis or CREST syndrome, the muscle of the oesophagus is replaced by fibrous tissue, which causes failure of peristalsis leading to heartburn and dysphagia.Oesophagitis is often severe, and benign fibrous strictures occur. These patients require long-term therapy with PPIs.
Dermatomyositis, rheumatoid arthritis and myasthenia gravis may also cause dysphagia.
Benign oesophageal stricture
Is usually a consequence of gastro-oesophageal reflux disease and occurs most often in elderly patients who have poor oesophageal clearance. Rings, due to submucosal fibrosis, are found at the oesophago-gastric junction (‘Schatzki ring’) and cause intermittent dysphagia, often starting in middle age. A post-cricoid web is a rare complication of iron deficiency anaemia (Paterson–Kelly or Plummer–Vinson syndrome), and may be complicated by the development of squamous carcinoma.Benign strictures can be treated by endoscopic dilatation, in which wire guided bougies or balloons are used to disrupt the fibrous tissue of the stricture.
Causes of oesophageal stricture
Tumours of the oesophagusBenign tumours
The most common is a leiomyoma. This is usually
asymptomatic but may cause bleeding or dysphagia.
Carcinoma of the oesophagus
Squamous oesophageal cancer is relatively rare in Caucasians (4 : 100 000) but is more common in Iran, parts of Africa and China (200 : 100 000). Squamous cancer can occur in any part of the oesophagus, and almost all tumours in the upper oesophagus are squamous cancers.
Adenocarcinomas typically arise in the lower third from Barrett’s esophagus or from the cardia of the stomach. Despite modern treatment, the overall 5-year survival of patients presenting with oesophageal cancer is only 13%.
Squamous carcinoma: aetiological factors
Clinical featuresMost patients have a history of progressive, painless
dysphagia for solid foods. Others present acutely
because of food bolus obstruction. In late stages, weight loss is often extreme; chest pain or hoarseness suggests mediastinal invasion. Fistulation between the oesophagus and the trachea or bronchial tree leads to coughing after swallowing, pneumonia and pleural effusion.
Physical signs may be absent but, even at initial presentation, cachexia, cervical lymphadenopathy or other evidence of metastatic spread is common.
Investigations
The investigation of choice is upper endoscopy with biopsy. A barium swallow demonstrates the site and length of the stricture but adds little useful information. Once a diagnosis has been made, investigations should be performed to stage the tumour and define operability. Thoracic and abdominal CT, often combined with positron emission tomography (CT-PET), should be carried out. Invasion of the aorta, major airways or coeliac axis usually precludes surgery, but patients with resectable disease on imaging should undergo EUS to determine the depth of penetration of the tumour into the wall and to detect regional lymph node involvement .Management
The treatment of choice is surgery if the patient presentsat a point at which resection is possible. Patients with
tumours that have extended beyond the wall of the
oesophagus (T3) or which have lymph node involvement
(N1) have a 5-year survival of around 10%.
Overall survival following ‘potentially curative’ surgery (all macroscopic tumour removed) is about 30% at 5 years, but recent studies have suggested that this can be improved by neoadjuvant chemotherapy. Although squamous carcinomas are radiosensitive, radiotherapy alone is associated with a 5-year survival of only 5%, but combined chemoradiotherapy for these tumours can achieve 5-year survival rates of 25–30%.
Approximately 70% of patients have extensive
disease at presentation; in these, treatment is palliative
and should focus on relief of dysphagia and pain. Endoscopic laser therapy or self-expanding metallic stents can be used to improve swallowing.
Palliative radiotherapy may induce shrinkage of both squamous cancers and adenocarcinomas but symptomatic response may be slow.
Quality of life can be improved by nutritional support and appropriate analgesia.
Perforation of the oesophagus
The most common cause is endoscopic perforation complicating dilatation or intubation. Malignant, corrosive or post-radiotherapy strictures are more likely to be perforated than peptic strictures.A perforated peptic stricture is managed conservatively using broad spectrum antibiotics and parenteral nutrition; most cases heal within days.
Malignant, caustic and radiotherapy stricture perforations require resection or stenting.
Spontaneous oesophageal perforation (‘Boerhaave’s
syndrome’) results from forceful vomiting and retching.Severe chest pain and shock occur as oesophago-gastric
contents enter the mediastinum and thoracic cavity.
Subcutaneous emphysema, pleural effusions and pneumothorax develop.
The diagnosis can be made using a water-soluble contrast swallow but, in difficult cases, both CT and careful endoscopy (usually in an intubated patient) may be required.
Treatment is surgical.
Delay in diagnosis is a key factor In the high mortality associated with this condition.
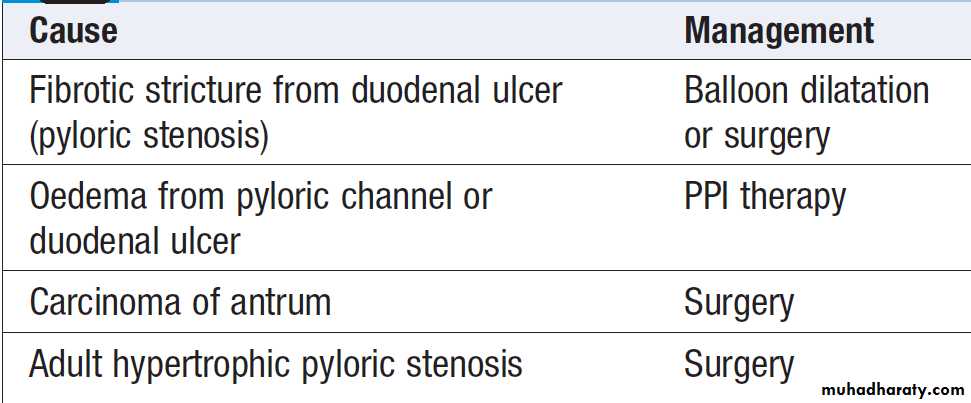
DISEASES OF THE STOMACH AND DUODENUM
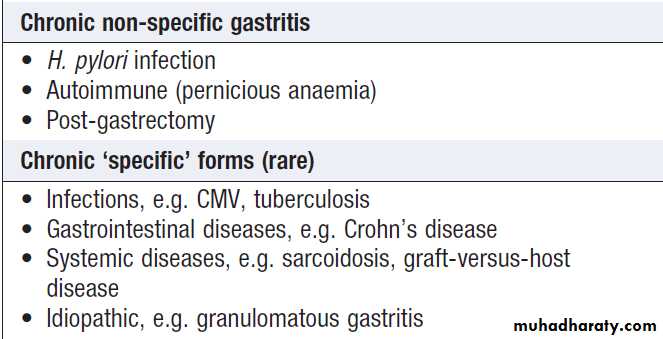
GastritisGastritis is a histological diagnosis, although it can
sometimes be recognised at endoscopy.
Acute gastritis
Often erosive and haemorrhagic. Neutrophils are the predominant in the superficial epithelium. Many result from aspirin or NSAID ingestion . Often produces no symptoms but may cause dyspepsia, anorexia, nausea , vomiting, haematemesis or melaena. Many cases resolve quickly; in others, endoscopy and biopsy may be necessary to exclude peptic ulcer or cancer. Treatment should be directed at the underlying cause. Short-term symptomatic therapy with antacids, and PPIs, prokinetics (domperidone) or antiemetics (metoclopramide) may be necessary.
Chronic gastritis due to Helicobacter pylori infection
This is the most common cause of chronic gastritis. The predominant inflammatory cells are lymphocytes and plasma cells. Most patients are asymptomatic and do not require treatment, but patients with dyspepsia may benefit from H. pylori eradication.
Autoimmune chronic gastritis
This involves the body of the stomach but spares the
antrum; it results from autoimmune damage to parietal cells. The histological features are diffuse chronic
inflammation, atrophy and loss of fundic glands,
intestinal metaplasia and sometimes hyperplasia of
enterochromaffin-like (ECL) cells. Circulating antibodies
to parietal cell and intrinsic factor may be present.
In some patients, the degree of gastric atrophy is severe,
and loss of intrinsic factor secretion leads to perniciousanaemia .The gastritis itself is usually asymptomatic.
Some patients have evidence of other organspecific
autoimmunity, particularly thyroid disease. Long-term, there is a two- to threefold increase in the risk of gastric cancer . Ménétrier’s disease
In this rare condition, the gastric pits are elongated and tortuous, with replacement of the parietal and chief cells by mucus-secreting cells. The cause is unknown but there is excessive production of TGF-α. As a result, the mucosal folds of the body and fundus are greatly enlarged.
Most patients are hypochlorhydric.
Whilst some have upper Gl symptoms, majority present in middle or old age with protein losing enteropathy due to exudation from the gastric mucosa.
Endoscopy shows enlarged, nodular and coarse folds, although biopsies may not be deep enough to show all the histological features.
Treatment with antisecretory drugs, such as PPIs with or without octreotide, may reduce protein loss and H. pylori eradication may be effective, but unresponsive patients require partial gastrectomy.
Common causes of gastritis
Peptic ulcer disease
The term ‘peptic ulcer’ refers to an ulcer in the loweroesophagus, stomach or duodenum, in the jejunum after
surgical anastomosis to the stomach or, rarely, in the
ileum adjacent to a Meckel’s diverticulum. Ulcers in the
stomach or duodenum may be acute or chronic; both
penetrate the muscularis mucosae but the acute ulcer
shows no evidence of fibrosis. Erosions do not penetrate
the muscularis mucosae. Peptic ulcer disease is a chronic condition with spontaneous relapses and remissions lasting for decades, if not for life.
The most common presentation is with recurrent abdominal pain which has three notable characteristics: localisation to the epigastrium, relationship to food and episodic occurrence.
Gastric and duodenal ulcer
The prevalence of peptic ulcer (0.1–0.2%) is decreasingin many Western communities as a result of widespread
use of Helicobacter pylori eradication. The male-to-female
ratio for duodenal ulcer varies from 5 : 1 to 2 : 1,
whilst that for gastric ulcer is 2 : 1 or less. Chronic gastric
ulcer is usually single; 90% are situated on the lesser
curve within the antrum or at the junction between body
and antral mucosa. Chronic duodenal ulcer usually
occurs in the first part and 50% are on the anterior wall. Gastric and duodenal ulcers coexist in 10% of patients and more than one peptic ulcer is found in 10–15%.
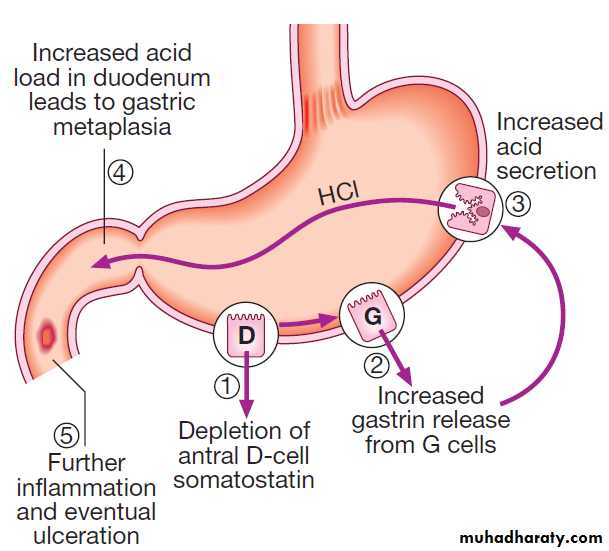
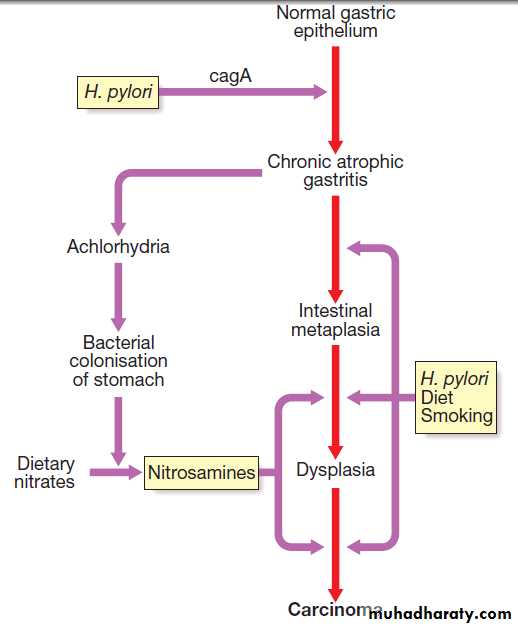
Sequence of events in the pathophysiology of
duodenal ulceration.Consequences of H. pylori infection
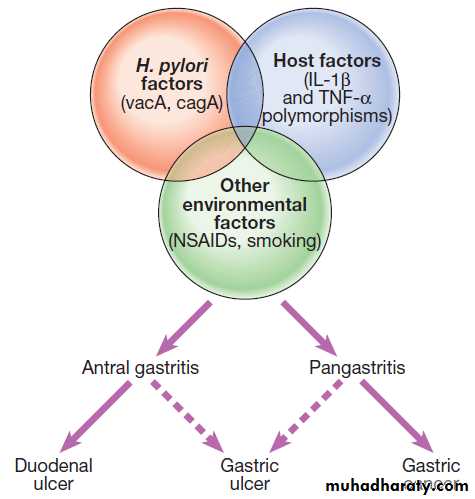
PathophysiologyH. pylori
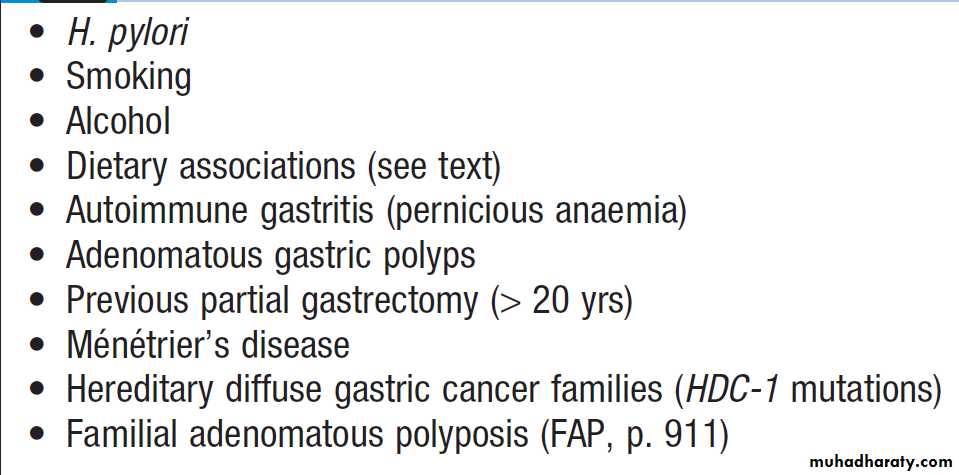
Peptic ulceration is strongly associated with H. pylori
infection. The prevalence in developed nations rises
with age, and in the UK approximately 50% over the age of 50 years are infected. In the developing world, infection is more common, affecting up to 90% of adults. These infections are probably acquired in childhood by person-to-person contact. The vast majority of colonised people remain healthy and asymptomatic. Around 90% of duodenal ulcer patients and 70% of gastric ulcer patients are infected with H. pylori. The remaining 30% of gastric ulcers are caused by NSAIDs.
H. pylori is Gram-negative and spiral, and has multiple
flagella at one end, which make it motile, allowingit to burrow and live beneath the mucus layer adherent
to the epithelial surface. It uses an adhesin molecule
(BabA) to bind to the Lewis b antigen on epithelial cells.
Here the surface pH is close to neutral and any acidity
is buffered by the organism’s production of the enzyme
urease. This produces ammonia from urea and raises the
pH around the bacterium and between its two cell membrane layers. H. pylori exclusively colonises gastric-type epithelium and is only found in the duodenum in association with patches of gastric metaplasia. It causes chronic gastritis by provoking a local inflammatory response in the underlying epithelium .
In most people, H. pylori causes localised antral gastritis
associated with depletion of somatostatin (from D
cells) and increased gastrin release from G cells. The
subsequent hypergastrinaemia stimulates increased acid
production by parietal cells but, in the majority of cases,
this has no clinical consequences. In a minority of
patients, this effect is exaggerated, leading to duodenal
ulceration . In 1% of infected people, H. pylori
causes a pangastritis, leading to gastric atrophy and
hypochlorhydria. This allows other bacteria to proliferate
within the stomach; these produce mutagenic nitrites
from dietary nitrates, predisposing to the development
of gastric cancer .
The effects of H. pylori are more complex in gastric ulcer patients compared to those with duodenal ulcers. The ulcer probably arises because of impaired mucosal defence resulting from a combination of H. pylori infection, NSAIDs and smoking, rather than excess acid.
NSAIDs
Treatment with NSAIDs is associated with peptic ulcers
due to impairment of mucosal defences.
Smoking
Smoking confers an increased risk of gastric ulcer and,
to a lesser extent, duodenal ulcer. Once the ulcer has
formed, it is more likely to cause complications and less
likely to heal if the patient continues to smoke.
Clinical features
Peptic ulcer disease is a chronic condition with spontaneous relapses and remissions lasting for decades, if not for life.The most common presentation is with recurrent
abdominal pain which has three notable characteristics:
localisation to the epigastrium, relationship to food and
episodic occurrence. Occasional vomiting occurs in
about 40% of ulcer subjects; persistent daily vomiting
suggests gastric outlet obstruction. In one-third, the
history is less characteristic, especially in elderly people
or those taking NSAIDs. In them, pain may be absent or
so slight that it is experienced only as a vague sense of
epigastric unease. Occasionally, the only symptoms are
anorexia and nausea, or early satiety after meals .
In some patients, the ulcer is completely ‘silent’, presenting
for the first time with anaemia from chronic undetectedblood loss, as an abrupt haematemesis or perforation; in others, there is recurrent acute bleeding without ulcer pain. The history is therefore a poor predictor of the presence of an ulcer.
Investigations
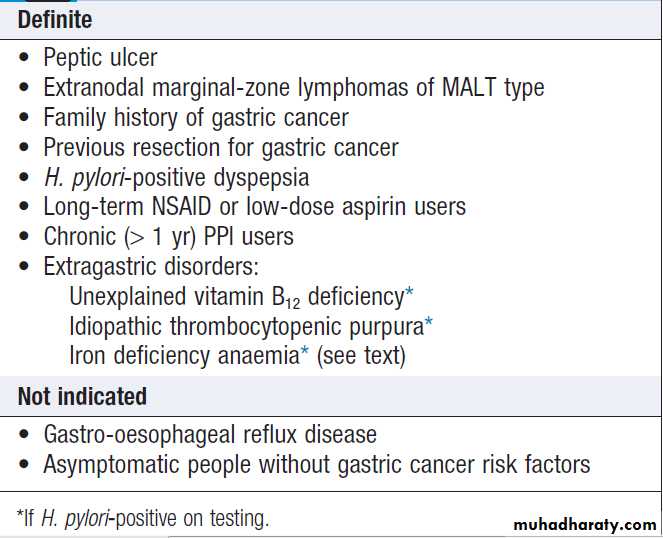
Endoscopy is the preferred investigation. Gastric ulcers
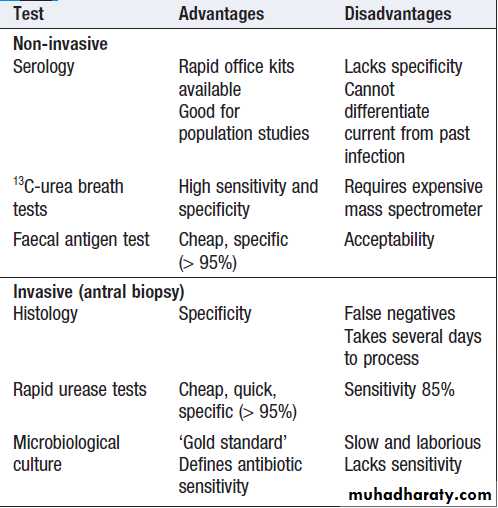
may occasionally be malignant and must always be biopsied and followed up to ensure healing. Patients should be tested for H. pylori infection. Some are invasive and require endoscopy; others are noninvasive. They vary in sensitivity and specificity. Breath or faecal antigen tests are best because of accuracy, simplicity and non-invasiveness.
Management
The aims of management are to relieve symptoms, induce healing and prevent recurrence. H. pylori eradication is the cornerstone of therapy for peptic ulcers, as this will successfully prevent relapse and eliminate the need for long-term therapy in the majority of patients.H. pylori eradication
All patients with proven ulcers who are H. pylori-positive
should be offered eradication as primary therapy. Treatment is based upon a PPI taken simultaneously with two antibiotics (from amoxicillin, clarithromycin and metronidazole) for 7 days . High-dose, twice-daily
PPI therapy increases efficacy of treatment, as does
extending treatment to 10–14 days.
Success is achieved in 80–90% of patients, although compliance, side-effects and antibiotic resistance influence this. Resistance to amoxicillin is rare but rates of metronidazole resistance reach 40% in some countries and, recently, rates of clarithromycin resistance of 20–40% have appeared.
Where the latter exceed 15–20%, a quadruple therapy regimen, consisting of omeprazole (or another PPI), bismuth subcitrate, metronidazole and tetracycline (OBMT) for 10–14 days, is recommended.
In areas of low clarithromycin resistance, this regimen should also be offered as second-line therapy to those who remain infected after initial therapy, once compliance has been checked.
For those who are still colonised after two treatments,
the choice lies between a third attempt guided bysensitivity testing, rescue therapy (levofloxacin, PPI and clarithromycin) or long-term acid suppression.
H. pylori and NSAIDs are independent risk factors for
ulcer disease and patients requiring long-term NSAID
therapy should first undergo eradication therapy to
reduce ulcer risk. Subsequent co-prescription of a PPI
along with the NSAID is advised but is not always necessary for patients being given low-dose aspirin, in
whom the risk of ulcer complications is lower. Eradication of the infection has proven benefits in several extragastric disorders, including unexplained B12 deficiency and iron deficiency anaemia, once sources of gastrointestinal bleeding have been looked for and excluded.
Platelet counts improve and may normalise after eradication therapy in patients with idiopathic thrombocytopenic purpura , the mechanism is unclear.
General measures
Cigarette smoking, aspirin and NSAIDs should be
avoided. Alcohol in moderation is not harmful and no
special dietary advice is required.
Maintenance treatment
Continuous maintenance treatment should not be necessary
after successful H. pylori eradication.
For the minority who do require it, the lowest effective dose of PPI should be used.
Indications for H. pylori eradication
Methods for the diagnosis of
Helicobacter pylori infectionH. pylori eradication and peptic ulcer healing
Common side-effects of H. pylori eradication therapy
Surgical treatmentSurgery is now rarely required for peptic ulcer disease
but it is needed in some cases .
The operation of choice for a chronic non-healing
gastric ulcer is partial gastrectomy, preferably with a
Billroth I anastomosis, in which the ulcer itself and the
ulcer-bearing area of the stomach are resected. The
reason for this is to exclude an underlying cancer.
In emergency, ‘under-running’ the ulcer for bleeding
or ‘oversewing’ (patch repair) for perforation is all that
is required, in addition to taking a biopsy.
For giant duodenal ulcers, partial gastrectomy using a ‘Polya’ or Billroth II reconstruction may be required.
Indications for surgery in peptic ulcer
Complications of gastric resection or vagotomyUp to 50% experience long-term adverse
effects. In most cases, these are minor, but in 10% they
significantly impair quality of life.
Dumping. Rapid gastric emptying leads to distension
of the proximal small intestine as the hypertonic contents draw fluid into the lumen. This leads to abdominal discomfort and diarrhoea after eating. Autonomic reflexes release a range of gastrointestinal hormones that provoke vasomotor features, such as flushing, palpitations, sweating, tachycardia and hypotension. Patients should therefore avoid large meals with high carbohydrate content.
Chemical (bile reflux) gastropathy.
Duodenogastric bile reflux leads to chronic gastropathy. Treatment with aluminium-containing antacids or sucralfate may be effective. A few patients require revisional surgery with creation of a Roux en Y loop to prevent bile reflux. Diarrhoea and maldigestion.Diarrhoea may develop after any peptic ulcer operation and usually occurs 1–2 hours after eating. Poor mixing of food in the stomach, with rapid emptying, inadequate mixing with pancreaticobiliary secretions, rapid transit and bacterial overgrowth, may lead to malabsorption. Diarrhoea often responds to small, dry meals with a reduced intake of refined carbohydrates. Antidiarrhoeal drugs, such as codeine phosphate (15–30 mg 4–6 times daily) or loperamide (2 mg after each loose stool), are helpful.
Weight loss. Most patients lose weight shortly after
surgery and 30–40% are unable to regain all the weightthat is lost. The usual cause is reduced intake because of
a small gastric remnant, but diarrhoea and mild steatorrhoea also contribute.
Anaemia. Anaemia is common many years after subtotal
gastrectomy. Iron deficiency is the most common
cause; folic acid and B12 deficiency are much less frequent.
Inadequate dietary intake of iron and folate, lack
of acid and intrinsic factor secretion, mild chronic lowgrade blood loss from the gastric remnant and recurrent ulceration are responsible.
Metabolic bone disease. Both osteoporosis and osteomalacia can occur as a consequence of calcium and vitamin D malabsorption.
Gastric cancer. An increased risk of gastric cancer has
been reported from several epidemiological studies.
Surgery itself is an independent risk factor for late development of malignancy in the gastric remnant but the risk is higher in those with hypochlorhydria, duodenogastric reflux of bile, smoking and H. pylori infection. Although the relative risk is increased, the absolute risk of cancer remains low and endoscopic surveillance is not indicated following gastric surgery.
Complications of peptic ulcer disease
PerforationWhen perforation occurs, the contents of the stomach escape into the peritoneal cavity, leading to peritonitis.
This is more common in duodenal than in gastric ulcers, and is usually found with ulcers on the anterior wall.
About one-quarter of all perforations occur in acute ulcers and NSAIDs are often incriminated. Perforation can be the first sign of ulcer. The most striking symptom is sudden, severe pain; its distribution follows the spread of the gastric contents over the peritoneum.
The pain initially develops in the upper abdomen and rapidly becomes generalised; shoulder tip pain is caused by irritation of the diaphragm.
The pain is accompanied by shallow respiration due to limitation of diaphragmatic movements and by shock. The
abdomen is held immobile and there is generalised
‘board-like’ rigidity. Bowel sounds are absent and liver
dullness to percussion decreases due to the presence of
gas under the diaphragm. After some hours, symptoms
may improve, although abdominal rigidity remains.
Later, the patient’s condition deteriorates as general
peritonitis develops. In at least 50%, an erect chest X-ray shows free air beneath the diaphragm. If not, a water-soluble contrast swallow will confirm leakage s. After resuscitation, the acute perforation should be treated surgically, either by simple closure or by converting the perforation into a pyloroplasty if it is large.
On rare occasions, a ‘Polya’ partial gastrectomy is required. Following surgery, H. pylori should be treated (if present) and NSAIDs avoided. Perforation carries a mortality of 25%, reflecting the advanced age and significant comorbidity of the population that are affected. Gastric outlet obstruction
The most common is an ulcer in the region of the pylorus. The presentation is with nausea, vomiting and abdominal distension. Large quantities of gastric content are often vomited, and food eaten 24 hours or more previously may be recognised. Physical examination may show evidence of wasting and dehydration. A succussion splash may be elicited 4 hours or more after the last meal or drink.
Visible gastric peristalsis is diagnostic of gastric outlet obstruction. Loss of gastric contents leads to dehydration with low serum chloride and potassium, and raised serum bicarbonate and urea concentrations. This results in enhanced renal absorption of Na+ in exchange for H+ and paradoxical aciduria. Endoscopy should be
performed after the stomach has been emptied by a wide-bore nasogastric tube. Intravenous correction of dehydration is undertaken and, in severe cases, at least
4 L of isotonic saline and 80 mmol of potassium may be necessary during the first 24 hours.
In some patients, PPI drugs heal ulcers, relieve pyloric oedema and overcome the need for surgery.
Endoscopic balloon dilatation of benign stenoses may be possible in some patients, but in others
partial gastrectomy is necessary, although this is best done after a 7-day period of nasogastric aspiration, which enables the stomach to return to normal size. A gastroenterostomy is an alternative operation but, unless this is accompanied by vagotomy, patients will require long-term PPI therapy to prevent stomal ulceration.
Bleeding. Discussed
Differential diagnosis and management of
gastric outlet obstructionPeptic ulcer disease in old age
Zollinger–Ellison syndrome
This is a rare disorder characterised by the triad ofsevere peptic ulceration, gastric acid hypersecretion and a non-β cell islet tumour of the pancreas (‘gastrinoma’).
It probably accounts for about 0.1% of all cases of duodenal ulceration. The syndrome occurs in either sex at any age, it is most common between 30-50 years of age. Pathophysiology
The tumour secretes gastrin, which stimulates acid
secretion to its maximal capacity and increases the parietal cell mass three- to sixfold. The acid output may be so great that it reaches the upper small intestine, reducing the luminal pH to 2 or less. Pancreatic lipase is inactivated and bile acids are precipitated.
Diarrhoea and steatorrhoea result. Around 90% of tumours occur in the pancreatic head or proximal duodenal wall. At least half are multiple, and tumour size can vary from 1 mm to 20 cm. Approximately one-half to two-thirds are malignant but are often slow-growing. Adenomas of the parathyroid and pituitary (multiple endocrine neoplasia, MEN type 1) are present in 20–60% of patients.
Clinical features
The presentation is with severe and often multiple
peptic ulcers in unusual sites, such as the post-bulbar duodenum, jejunum or oesophagus. There is a poor response to standard ulcer therapy. The history is usually short; bleeding and perforations are common. Diarrhoea is seen in one-third and can be the presenting feature.
Investigations
Hypersecretion of acid under basal conditions, withlittle increase following pentagastrin, may be confirmed by gastric aspiration.
Serum gastrin levels are grossly elevated (10- to 1000-fold).
Injection of the hormone secretin normally causes no change or a slight decrease in circulating gastrin concentrations, but in Zollinger–Ellison syndrome produces a paradoxical and dramatic increase in gastrin.
Tumour localisation is best achieved by EUS and radio-labelled somatostatin receptor scintigraphy.
Management
Some 30% of small and single tumours can be localised and resected but many tumours are multifocal. Some patients present with metastatic disease and, in these circumstances, surgery is inappropriate. In the majority of these patients, continuous therapy with omeprazole or other PPIs can be successful in healing ulcers and alleviating diarrhoea, although double the normal dose is required.
The synthetic somatostatin analogue, octreotide,
given by subcutaneous injection, reduces gastrin
secretion and is of value. Overall 5-year survival is
60–75% and all patients should be monitored for the
later development of other manifestations of MEN 1.
Functional disorders
Functional dyspepsiaChronic dyspepsia in the absence of organic disease. Other commonly reported symptoms include early satiety, fullness, bloating and nausea.‘Ulcer-like’ and ‘dysmotility-type’ subgroups are often reported, but there is overlap between these and irritable bowel syndrome.
Pathophysiology
The cause is poorly understood but probably a spectrum of mucosal, motility and psychiatric disorders.
Clinical features
Patients are usually young (< 40 years) and women are
affected twice as commonly as men. Abdominal discomfort is associated with ‘dyspeptic symptoms, nausea, satiety and bloating after meals.
Morning symptoms are characteristic and pain or nausea may occur on waking. Direct enquiry may elicit symptoms suggestive of irritable bowel syndrome. Peptic ulcer disease must be considered, whilst in older subjects intra-abdominal malignancy is a prime concern. There are no diagnostic signs, apart perhaps from inappropriate tenderness on abdominal palpation. Symptoms may appear disproportionate to clinical well-being and there is no weight loss. Patients often appear anxious. A drug history should be taken.
Pregnancy should be ruled out in young women
before radiological studies are undertaken.
Alcohol misuse should be suspected when early morning nausea and retching are prominent.
Investigations
The history will often suggest the diagnosis. All patients should be checked for H. pylori infection and patients over the age of 55 years should undergo endoscopy to exclude mucosal disease. While an ultrasound may detect gallstones, these are rarely responsible for dyspeptic symptoms. Management
The most important elements are explanation and reassurance. Possible psychological factors should be
explored and explained. Idiosyncratic and restrictive diets are of little benefit, but smaller portions and fat restriction may help. Up to 10% benefit from H. pylori eradication and should be offered to infected patients. Eradication also removes a major risk factor for gastric cancer but at the cost of a small risk of side effects and worsening symptoms of underlying GERD.
Drug treatment is not especially successful but merits trial. Antacids, such as hydrotalcite, are sometimes helpful. Prokinetic drugs, such as metoclopramide (10 mg 3 times daily) or domperidone (10–20 mg 3 times daily), may be given before meals if nausea, vomiting or bloating is prominent.
Metoclopramide may induce extrapyramidal side effects, including tardive dyskinesia in young subjects.
H2-receptor antagonist drugs may be tried if night pain or heartburn is troublesome. Low-dose tricyclic agents, such as amitriptyline, are of value in up to two-thirds. Symptoms that can be associated with an identifiable cause of stress.
Some patients have major psychological disorders that result in persistent or recurrent symptoms and need behavioural or other formal psychotherapy .
Functional causes of vomiting
Psychogenic retching or vomiting may arise in anxiety.It typically occurs on wakening or immediately after
breakfast and only rarely later in the day. The disorder
is probably a reaction to facing up to the worries of
everyday life; in the young, it can be due to school
phobia. Early morning vomiting also occurs in pregnancy,
alcohol misuse and depression, there is little or no weight loss. The cause is unknown.In all patients, it is essential to exclude other common causes .Tranquillisers and antiemetic drugs (metoclopramide 10 mg 3 times daily, domperidone 10 mg 3 times daily, prochlorperazine 5–10 mg 3 times daily) have only a secondary place in management.
Antidepressants in full dose may be effective .
Gastroparesis
Defective gastric emptying without mechanical obstruction of the stomach or duodenum can occur as a primary event, due to inherited or acquired disorders of the gastric pacemaker, or can be secondary to disorders of autonomic nerves (diabetic neuropathy) or the gastroduodenal musculature (systemic sclerosis,myotonic dystrophies and amyloidosis). Drugs such as
opiates, calcium channel antagonists and those with
anticholinergic activity (tricyclics, phenothiazines) can
also cause gastroparesis. Early satiety and recurrent
vomiting are the major symptoms; abdominal fullness
and a succussion splash may be present on examination.
Treatment is based upon small, frequent, low-fat meals and the use of metoclopramide and domperidone.
In severe cases, nutritional failure can occur and long-term jejunostomy feeding or total parenteral nutrition is required.
Surgical insertion of a gastric neurostimulator
has been successful in some cases, especially those complicating diabetic autonomic neuropathy.
Tumours of the stomach
Gastric carcinomaIs the fourth leading cause of cancer death worldwide, but there is marked geographical variation in incidence. It is most common in China, Japan, Korea (incidence 40/100 000 males). In most countries, the incidence is 50% lower in women. In both sexes, it rises sharply after 50 years of age. Studies of Japanese migrants to the USA have revealed a much lower incidence in second generation migrants, confirming the importance of environmental factors.
The overall prognosis is poor, with < 30% surviving 5 years, and the best hope for improved survival lies in efficient detection at an earlier stage.
Pathophysiology
Infection with H. pylori plays a key pathogenic role. It isassociated with chronic atrophic gastritis, gastric mucosal atrophy and with gastric cancer . H. pylori infection may contribute to the occurrence of gastric cancer in 60–70% of cases. Although the majority of H. pylori-infected have normal or increased acid secretion, a few become hypo- or achlorhydric and these people are thought to be at greatest risk. Chronic inflammation with generation of reactive oxygen species and depletion of the normally abundant antioxidant ascorbic acid are also important. There is strong evidence that H. pylori eradication, especially if achieved before irreversible pre-neoplastic changes have developed, reduces the risk of cancer development in high-risk populations and is cost-effective.
Diets rich in salted, smoked or pickled foods and the consumption of nitrites and nitrates may increase cancer risk. Carcinogenic N- nitroso-compounds are formed from nitrates by the action of nitrite-reducing bacteria which colonise the achlorhydric stomach. Diets lacking fresh fruit and vegetables, as well as vitamins C and A, may also contribute. No predominant genetic abnormality has been identified, although cancer risk is increased two- to threefold in first-degree relatives of patients, and links with blood group A have been reported.
Rarely, may be inherited in an autosomal dominant in association with mutations of the E-cadherin (CDH1) gene.
Gastric carcinogenesis: a possible mechanism
Risk factors for gastric cancer
Virtually all tumours are adenocarcinomas arisingfrom mucus-secreting cells in the base of the gastric
crypts. Most develop upon a background of chronic
atrophic gastritis with intestinal metaplasia and dysplasia. Cancers are either ‘intestinal’, arising from areas of intestinal metaplasia with histological features reminiscent of intestinal epithelium, or ‘diffuse’, arising from normal gastric mucosa. Intestinal carcinomas are more common and arise against a background of chronic
mucosal injury. Diffuse cancers tend to be poorly differentiated and occur in younger patients. In the developing world, 50% of gastric cancers develop in the
antrum; 20–30% occur in the gastric body, often on
the greater curve; and 20% are found in the cardia.
In Western populations, however, proximal gastric
tumours are becoming more common than those arisingin the body and distal stomach. This change in disease
pattern may be a reflection of changes in lifestyle or the
decreasing prevalence of H. pylori in the West.
Diffuse submucosal infiltration by a scirrhous cancer (linitis plastica) is uncommon. Early gastric cancer is defined as cancer confined to the mucosa or submucosa .
It is more often recognised in Japan, where widespread screening is practised. Some cases can be cured by endoscopic mucosal or submucosal resection .
The majority of patients (> 80%) in the West, however, present with advanced gastric cancer.
Clinical features
Early gastric cancer is usually asymptomatic but may be discovered during endoscopy for investigation of dyspepsia. Two-thirds of patients with advanced cancers
have weight loss and 50% have ulcer-like pain. Anorexia and nausea occur in one-third, while early satiety, haematemesis, melaena and dyspepsia alone are less common. Dysphagia occurs in tumours of the gastric cardia which obstruct the gastro-oesophageal junction.
Anaemia from occult bleeding is also common. Examination may reveal no abnormalities but signs of weight loss, anaemia and a palpable epigastric mass are not infrequent.
Jaundice or ascites signify metastatic spread.
Occasionally, tumour spread occurs to the supraclavicular lymph nodes (Troisier’s sign), umbilicus (Sister Joseph’s nodule) or ovaries (Krukenberg tumour).Paraneoplastic phenomena,
such as acanthosis nigricans, thrombophlebitis (Trousseau’s sign) and dermatomyositis, occur rarely.
Metastases
arise most commonly in the
liver, lungs, peritoneum and bone marrow.
Investigations
Upper gastrointestinal endoscopy is the investigationof choice and should be performed promptly in any dyspeptic patient with ‘alarm features’ (see Box). Multiple biopsies from the edge and base of a gastric ulcer are required. Barium meal is a poor alternative since any abnormalities must be followed by endoscopy and biopsy. Once the diagnosis is made, further imaging is necessary for staging and assessment of resectability.
CT will provide evidence of intraabdominal spread or liver metastases.
laparoscopy with peritoneal washings is required to determine whether the tumour is resectable, as it is the only modality that will reliably detect peritoneal spread.
Management
SurgeryResection offers the only hope of cure, can be achieved in 90% with early cancer. For the majority of patients with locally advanced disease, total gastrectomy with lymphadenectomy is the operation of choice, preserving the spleen if possible. Proximal tumours involving the GE-junction also require a distal oesophagectomy. Small,
distally sited tumours can be managed by a partial gastrectomy with lymphadenectomy and either a Billroth I
or a Roux en Y reconstruction.
More extensive lymph node resection may increase survival rates but carries greater morbidity.
Even for those who cannot be cured, palliative resection may be necessary when patients present with bleeding or gastric outflow obstruction.
Following surgery, recurrence is much more likely if
serosal penetration has occurred, although complete
removal of all macroscopic tumour combined with
lymphadenectomy will achieve a 50–60% 5-year survival.
Recent evidence suggests that perioperative
chemotherapy with epirubicin, cisplatin and fluorouracil
(ECF) improves survival rates.
Palliative treatment
In patients with inoperable tumours, survival can beimproved and palliation of symptoms achieved with
chemotherapy using 5-fluorouracil and cisplatin, ECF or
other platinum and taxane-based regimens. The biological agent trastuzumab may benefit some patients whose tumours over-express HER2 .
Endoscopic laser ablation for control of dysphagia or
recurrent bleeding benefits some patients. Carcinomas
at the cardia or pylorus may require endoscopic dilatation or insertion of expandable metallic stents for relief of dysphagia or vomiting.
A nasogastric tube may offer temporary relief of vomiting due to gastric outlet obstruction .
Gastric carcinoma.
A Endoscopic image of a small superficial pre-pyloric cancer (arrows).
Gastric carcinoma.
B Appearance after endoscopicmucosal resection (EMR). The tumour has been completely removed.
Chemotherapy for advanced gastric cancer
How to insert
a nasogastric tubeGastric lymphoma
This is a rare tumour accounting for < 5% of allgastric malignancies. The stomach is, however, the most
common site for extranodal non-Hodgkin lymphoma
and 60% of all primary GI lymphomas occur at this site. Lymphoid tissue is not found in the normal stomach but lymphoid aggregates develop in the presence of H. pylori infection. Indeed, H. pylori infection is closely associated with the development of a lowgrade
lymphoma (classified as extranodal marginalzone lymphomas of MALT type). EUS plays an important role in staging these lesions by accurately defining the depth of invasion into the gastric wall. The clinical presentation is similar to that of gastric cancer, and endoscopically the tumour appears as a polypoid or ulcerating mass.
While initial treatment of low-grade lesions confined to the superficial layers of the gastric wall consists of H. pylori eradication and close observation, 25% contain t(11 : 18) chromosomal translocations.
In these cases, additional radiotherapy or chemotherapy is usually necessary. High-grade B-cell lymphomas should be treated by a combination ofrituximab, chemotherapy, surgery and radiotherapy. The choice depends on the site and extent of tumour, the presence of comorbid illnesses, and other factors, such as symptoms of bleeding and gastric outflow obstruction. The prognosis depends on the stage at diagnosis. Features predicting a favourable prognosis are stage I or II disease, small resectable tumours, tumours with lowgrade histology, and age < 60.
Other tumours of the stomach
Hyperplastic polyps are common and of no consequence. Adenomatous polyps are rare but have malignant potential and should be removed. Gastric carcinoid seen inthe fundus and body in patients with long-standing pernicious anaemia. These benign tumours arise from ECL and are often multiple but rarely invasive. Unlike carcinoid tumours arising elsewhere in the GI tract, they usually run a benign course. However, large (> 2 cm) carcinoids may metastasise and should be removed.
Rarely, small nodules of ectopic pancreatic exocrine
tissue are found. These ‘pancreatic rests’ may be mistaken for gastric neoplasms and usually cause no symptoms.
EUS is the most useful investigation.
DISEASES OF THE SMALL INTESTINE
Disorders causing malabsorptionCoeliac disease
Is an inflammatory disorder of the small bowel occurring in genetically susceptible individuals, which results from intolerance to wheat gluten and similar proteins found in rye, barley and, to a lesser extent, oats. It can result in malabsorption and responds to a gluten-free diet. The prevalence in the UK is approximately 1%, although 50% of these people are asymptomatic .These include both undiagnosed ‘silent’ cases of the disease and cases of ‘latent’ coeliac disease – genetically susceptible people who may later develop clinical coeliac disease.
Pathophysiology
The precise mechanism of mucosal damage is unclear but immunological responses to gluten play a key role.Tissue transglutaminase (tTG) is now recognised as the autoantigen for anti-endomysial antibodies.
Clinical features
Coeliac disease can present at any age. In infancy, it
occurs after weaning on to cereals and typically presents
with diarrhoea, malabsorption and failure to thrive. In older children, it may present with non-specific features,
such as delayed growth. Features of malnutrition are
found on examination and mild abdominal distension
may be present. Affected children have growth and
pubertal delay, leading to short stature in adulthood.
In adults, the disease usually presents during the
third or fourth decade and females are affected twice as often as males. The presentation is highly variable,
depending on the severity and extent of small bowel
involvement. Some have florid malabsorption, while
others develop non-specific symptoms, such as tiredness, weight loss, folate deficiency or iron deficiency anaemia. Other presentations include oral ulceration, dyspepsia and bloating.
Unrecognised coeliac disease is associated with mild under-nutrition and osteoporosis.
Coeliac disease is associated with other human leucocyte
antigen (HLA)-linked autoimmune disorders.
Pathophysiology of coeliac disease
Pathophysiology of coeliac disease. After being taken up by epithelial cells, gluten peptides are deamidated by the enzyme tissue transglutaminase in the subepithelial layer. They are then able to fit the antigen-binding motif on human leucocyte antigen (HLA)-DQ2-positive antigen-presenting cells. Recognition by CD4+ T cells triggers a Th1 immune response with generation of pro-inflammatory cytokines – interleukin-1 (IL-1), interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α). Lymphocytes infiltrate the lamina propria, and increased intra-epithelial lymphocytes (IEL), crypt hyperplasia and villous atrophy ensue.Disease associations of coeliac disease
InvestigationsThese are performed to confirm the diagnosis and to look for consequences of malabsorption.
Duodenal biopsy
Endoscopic small bowel biopsy is the gold standard. The histological features are usually characteristic but other causes of villous atrophy should be considered .
Sometimes the villi appear normal but there are excess numbers of intra-epithelial lymphocytes.
Antibodies
Anti-endomysial antibodies of the IgA class are detectable by immunofluorescence in most untreated . They are sensitive (85–95%) and specific (99%), except in very young infants.IgG antibodies, however, must be analysed in patients with coexisting IgA deficiency.
The tTG assay is easier to perform, semi-quantitative and more accurate in patients with IgA deficiency. These constitute a valuable screening tool in patients with diarrhoea but are not a substitute for small bowel biopsy; they usually become negative with successful treatment.
Haematology and biochemistry
A full blood count may show microcytic or macrocytic
anaemia from iron or folate deficiency and features of
hyposplenism (target cells, spherocytes and Howell–Jolly bodies). Biochemical tests may reveal reduced concentrations of calcium, magnesium, total protein, albumin or vitamin D.
Other investigations
Measurement of bone density should be considered to
look for evidence of osteoporosis, especially in older
patients and post-menopausal women.
Important causes of subtotal villous atrophy
Jejunal mucosa.
A Normal.B Subtotal villous atrophy in coeliac disease. There is blunting of villi (B), crypt hyperplasia (H) and inflammatory infiltration of the lamina propria (I).
Management
The aims are to correct existing deficiencies of iron,folate, calcium and/or vitamin D, and to commence a life-long gluten-free diet. This requires the exclusion of wheat, rye, barley and initially oats, although oats may be re-introduced safely in most patients after 6–12 months. Initially, frequent dietary counselling is required to make sure the diet is being observed, as the most common reason for failure to improve with dietary treatment is accidental or unrecognised gluten ingestion.
Mineral and vitamin supplements are also given when indicated but are seldom necessary when a strict gluten free diet is adhered to.
Patients should be followed up with assessment of symptoms, weight and nutritional status, and measurement of tTG or anti- endomysial antibodies. Repeat small
bowel biopsies are not required routinely but should be
considered in patients whose symptoms fail to improve
and in whom antibody levels remain high. Dietary compliance should be carefully assessed.
If the diet is satisfactory, then other conditions,
such as pancreatic insufficiency or microscopic colitis,
should be sought, as should complications, such as ulcerative jejunitis or enteropathy associated T-cell lymphoma. There remain a small number who fail to respond adequately to a gluten-free diet, and require therapy with corticosteroids or immunosuppressive drugs.
Complications
A twofold-increased risk of malignancy, particularly ofenteropathy-associated T-cell lymphoma, small bowel
carcinoma and squamous carcinoma of the oesophagus, has been reported.
A few patients develop ulcerative jejuno-ileitis. This
may present with fever, pain, obstruction or perforation.
Treatment is difficult. Corticosteroids are used
with mixed success and some patients require surgical
resection and parenteral nutrition. The course is often
progressive and relentless.
Osteoporosis and osteomalacia may occur in patients
with long-standing, poorly controlled coeliac disease.
Dermatitis herpetiformis
This is characterised by crops of intensely itchy blisters
over the elbows, knees, back and buttocks .
Immunofluorescence shows granular or linear IgA
deposition at the dermo-epidermal junction. Almost all
patients have partial villous atrophy on duodenal
biopsy, identical to that seen in coeliac disease, even
though they usually have no gastrointestinal symptoms.
In contrast, fewer than 10% of coeliac patients have evidence of dermatitis herpetiformis, although both disorders are associated with the same histocompatibility
antigen groups. The rash usually responds to a gluten-free diet but some patients require additional treatment with dapsone (100–150 mg daily).
Tropical sprue
Tropical sprue is defined as chronic, progressive malabsorption in a patient in or from the tropics, associated with abnormalities of small intestinal structure and function. The disease occurs mainly in the West Indies and in India, Malaysia and Indonesia.Pathophysiology
The epidemiological pattern and occasional epidemics suggest that an infective agent may be involved.
Although no single bacterium has been isolated, the condition often begins after an acute diarrhoeal illness.
Small bowel bacterial overgrowth with Escherichia coli, Enterobacter and Klebsiella is frequently seen. The changes closely resemble those of coeliac.
Clinical features
There is diarrhoea, abdominal distension, anorexia,fatigue and weight loss. In visitors to the tropics, the
onset of severe diarrhoea may be sudden and accompanied by fever. When the disorder becomes chronic, the features of megaloblastic anaemia (folic acid malabsorption) and other deficiencies, including ankle oedema, glossitis and stomatitis, are common.
Remissions and relapses may occur. The differential diagnosis in the indigenous tropical population is an infective cause of diarrhoea. The important differential diagnosis in visitors to the tropics is giardiasis .
Management
Tetracycline (250 mg 4 times daily for 28 days) is the treatment of choice and brings about long-term remission or cure.
In most patients, pharmacological doses of
folic acid (5 mg daily) improve symptoms and jejunal morphology.
In some cases, treatment must be prolonged before improvement occurs, and occasionally
patients must leave the tropics.
Small bowel bacterial overgrowth
(‘blind loop syndrome’)The normal duodenum and jejunum contain fewer than
104/mL organisms, which are usually derived from saliva.
The count of coliform organisms never exceeds 103/ mL.
In bacterial overgrowth, there may be 108–1010/mL organisms, most of which are normally found only in the
colon. Disorders which impair the normal physiological
mechanisms controlling bacterial proliferation in the
intestine predispose to bacterial overgrowth (Box).
The most important are loss of gastric acidity, impaired
intestinal motility and structural abnormalities which
allow colonic bacteria to gain access to the small intestine.
Pathophysiology
Bacterial overgrowth can occur in patients with smallbowel diverticuli. Another cause is diabetic autonomic
neuropathy ,which reduces small bowel motility
and affects enterocyte secretion. In scleroderma, bacterial overgrowth arises because the circular and longitudinal layers of the intestinal muscle are fibrosed and motility is abnormal.
In idiopathic hypogammaglobulinaemia, bacterial overgrowth occurs because the IgA and IgM levels in serum and jejunal secretions are reduced. Chronic diarrhoea and malabsorption occur because of bacterial overgrowth and recurrent gastrointestinal infections (particularly giardiasis).
Clinical features
The patient presents with watery diarrhoea and/or steatorrhoea, with anaemia due to B12 deficiency. These arise because of deconjugation of bile acids, which impairs micelle formation, and because of bacterial utilisation of vitamin B12. There may also be symptoms from the underlying intestinal cause.
Investigations
The diagnosis of blind loops or fistulae can often be made by barium follow-through or small bowel enema.
Endoscopic duodenal biopsies are useful in excluding coeliac disease. Jejunal contents for bacteriological examination can also be aspirated, requires anaerobic and aerobic culture techniques.
Bacterial overgrowth can also be diagnosed non-invasively using hydrogen breath tests. This simple, non-radioactive test involves serial measurement of breath samples for hydrogen after oral ingestion of 50 g glucose or lactulose. If bacteria are present within the small bowel, they rapidly metabolise the glucose, causing an early rise in exhaled hydrogen, in advance of that normally resulting from metabolism by colonic flora. Biochemical analysis may reveal low serum levels of vitamin B12 with normal or elevated folate levels because the bacteria produce folic acid.
Hypogammaglobulinaemia can be diagnosed by measurement of serum immunoglobulins and by intestinal biopsy, which shows reduced or absent plasma cells and nodular lymphoid hyperplasia.
Management
The underlying cause of small bowel bacterial overgrowthshould be addressed, where possible.
Tetracycline
(250 mg 4 times daily for 7 days) is the treatment of choice, although up to 50% of patients do not respond adequately. Metronidazole (400 mg 3 times daily) or ciprofloxacin (250 mg twice daily) is an alternative.
Some patients require up to 4 weeks of treatment
and, in a few, continuous rotating courses of antibiotics
are necessary. Intramuscular vitamin B12 supplementation
may be needed in chronic cases.
Patients with motility disorders, such as diabetes and scleroderma, can sometimes benefit from antidiarrhoeal drugs (diphenoxylate (5 mg 3 times daily orally) or loperamide (2 mg 4–6 times daily) orally).
Giardiasis should be controlled in patients with hypogammaglobulinaemia using metronidazole
or tinidazole, but if symptoms fail to respond
adequately, IG infusions may be required.
Causes of small bowel bacterial
overgrowthWhipple’s disease
This rare condition is characterised by infiltration of small intestinal mucosa by ‘foamy’ macrophages, which stain positive with periodic acid–Schiff (PAS) reagent.The disease is a multisystem one and almost any organ can be affected, sometimes long before gastrointestinal involvement becomes apparent.
Pathophysiology
Whipple’s disease is caused by infection with the Gram positive bacillus Tropheryma whipplei, which becomes resident within macrophages in the bowel mucosa.
Villi are widened and flattened, containing densely packed macrophages in the lamina propria, which obstruct lymphatic drainage and cause fat malabsorption.
Clinical features of Whipple’s disease
Clinical featuresMiddle-aged men are most frequently affected. Low-grade fever is common and most patients have joint symptoms, often as the first manifestation. Occasionally, neurological manifestations may predominate.
Diagnosis
This is made by the characteristic features on small
bowel biopsy, with characterisation of the bacillus by
polymerase chain reaction (PCR).
Management
Often fatal if untreated but responds well, at least initially, to IV ceftriaxone (2 g daily for 2 weeks), followed by oral co- trimoxazole for at least 1 year. Symptoms usually resolve quickly and biopsy changes revert to normal in a few weeks. relapse occurs in
up to one-third, often within the CNS; in this case, the same therapy is repeated or with doxycycline and hydroxychloroquine is necessary.
Malabsorption in old age
Ileal resectionMalabsorption can occur as a complication of small
bowel resection. The most common scenario is in patients
with Crohn’s disease who have undergone ileal resection,
leading to malabsorption of vitamin B12 and bile
salts . Unabsorbed bile salts pass into the colon, stimulating water and electrolyte secretion and
causing diarrhoea. If hepatic synthesis of new bile salts
cannot keep pace with faecal losses, fat malabsorption
occurs. Another consequence is the formation of lithogenic
bile, leading to gallstones. Renal calculi, rich in
oxalate, develop. Normally, oxalate in the colon is bound
to and precipitated by calcium. Unabsorbed bile salts
preferentially bind calcium, leaving oxalate to be
absorbed, with development of urinary oxalate calculi.
Patients have urgent watery diarrhoea or mild steatorrhoea.
Contrast studies and tests of B12 and bile acid absorption are useful investigations. Parenteral vitamin B12 is necessary. Diarrhoea usually responds to colestyramine, a resin which binds bile salts in the intestinal lumen. Aluminium hydroxide can be used as an alternative.Consequences of ileal resection
Short bowel syndrome
Defined as malabsorption resulting from extensive small intestinal resection or disease, it usually results from extensive surgery for Crohn’s or mesenteric infarction. Irrespective of the underlying cause, three main types ofpatient are seen:
• jejunal resection with an intact ileum and colon
• jejunal and ileal resection with an intact colon
• jejunal and ileal resection and colectomy (jejunostomy).
Pathophysiology: Patients who undergo a jejunal resection but who have an intact ileum and colon are rarely encountered and seldom require nutritional support. Those who have undergone both jejunal and ileal resection have in common the fact that they have lost a large amount of surface area for digestion and absorption.
Digestion and absorption are normally completed within the first 100 cm of jejunum, and enteral feeding is usually still possible if this amount of small intestine remains. The proximal small bowel normally reabsorbs most of the 8 L of fluid it receives daily, and patients with a high jejunostomy are at great risk of hypovolaemia, dehydration and electrolyte losses. The presence of some or all of the colon can markedly improve these losses by increased water reabsorption. The presence of an intact ileocaecal valve ameliorates the clinical picture by slowing small intestinal transit and reducing bacterial overgrowth.
Aetiology of short bowel syndrome
Effects of
a short bowelClinical features
Severely affected have large volumes of jejunostomy fluid losses or, if the colon is preserved, diarrhoea and steatorrhoea. Dehydration and signs of hypovolaemia are common, as are weight loss, loss of muscle bulk and malnutrition. Some remain in satisfactory but precarious fluid balance until a minor intercurrent illness or intestinal upset occurs, when they can become rapidly dehydrated. Management
In the immediate post-operative period, total parenteral
nutrition (TPN) should be started and PPI therapy given
to reduce gastric secretions. Enteral feeding should be
cautiously introduced after 1–2 weeks under careful
supervision and slowly increased as tolerated.
For patients with a jejunostomy, parenteral saline is likely to be necessary if less than 100 cm of jejunum remains. If less than 75 cm of small bowel remains, TPN is also needed.
The principles of long-term management are:
• Detailed nutritional assessments at regular intervals.
• Monitoring of fluid and electrolyte balance. Patients
can usually be taught how to do this for themselves.
A readily available supply of oral rehydration solution is useful for intercurrent illness.
• Adequate calorie and protein intake. Fats are a
good energy source and should be taken as tolerated. Medium-chain triglyceride supplements are given because they are more easily absorbed.
• Use of oral/enteral rather than parenteral nutrition.
• Replacement of vitamin B12, calcium, vitamin D,magnesium, zinc and folic acid.
• Loperamide (2–4 mg 4 times daily) or codeine
phosphate (30 mg 4–6 times daily) for diarrhoea.
Octreotide (50–200 μg 2–3 times daily by subcutaneous
injection) reduces Gl secretions and is useful in patients who are unable to maintain positive fluid balance.
Despite these measures, some require
long-term home TPN for survival and this is best
managed in specialist centres.
Small bowel transplantation is an option but rejection and graft-versus-host disease remain significant hurdles.
Chronic complications of intestinal irradiation
Motility disordersChronic intestinal pseudo-obstruction
Small intestinal motility is disordered in conditions which affect the smooth muscle or nerves of the intestine. Many cases are ‘primary’ (idiopathic), while others are ‘secondary’ to a variety of disorders or drugs .
Clinical features
There are recurrent episodes of nausea, vomiting,
abdominal discomfort and distension, often worse after
food. Alternating constipation and diarrhoea occur
and weight loss results from malabsorption (due to bacterial overgrowth) and fear of eating. There may also be dysphagia, bladder dysfunction. Some develop severe abdominal pain for reasons that are poorly understood.
Causes of chronic intestinal pseudo-obstruction
InvestigationsThe diagnosis is often delayed. Plain X-rays show distended loops of bowel and air–fluid levels, but barium studies demonstrate no mechanical obstruction.
Laparotomy is sometimes required to exclude obstruction and to obtain full-thickness biopsies of the intestine. Examination of biopsy material using specialised techniques, such as electron microscopy, and immunohistochemistry can diagnose the many rare diseases of enteric smooth muscle and nerves that can cause this syndrome. Management
Often difficult. Underlying causes should be addressed and further surgery avoided. Metoclopramide or domperidone may enhance motility, antibiotics for bacterial overgrowth. Nutritional and psychological support.
Miscellaneous disorders of the small intestine
Protein-losing enteropathy
This term is used when there is excessive loss of protein
into the gut lumen, sufficient to cause hypoproteinaemia.
Protein-losing enteropathy occurs in many gut
disorders but is most common in those in which ulceration
occurs (Box). In other disorders, protein loss
can result from increased mucosal permeability or
obstruction of intestinal lymphatic vessels.
Patients present with peripheral oedema and hypoproteinaemia in the presence of normal liver function and without proteinuria.
The diagnosis can be confirmed by measurement of faecal clearance of α1-antitrypsin or 51Cr-labelled albumin after intravenous injection. Other investigations should be performed to determine the underlying cause.
Treatment is that of the underlying disorder, nutritional support and measures to control peripheral oedema.
Causes of protein-losing enteropathy
Intestinal lymphangiectasiaThis may be primary, resulting from congenital malunion
of lymphatics, or secondary to lymphatic obstruction
due to lymphoma, filariasis or constrictive pericarditis. Impaired drainage of intestinal lymphatic vessels leads to discharge of protein and fat-rich lymph into the gastrointestinal lumen. The condition presents with peripheral lymphoedema, pleural effusions or chylous ascites, and steatorrhoea. Investigations reveal
hypoalbuminaemia, lymphocytopenia and reduced
serum immunoglobulin concentrations. The diagnosis
can be made by CT scanning and by enteroscopy and
jejunal biopsy, which shows greatly dilated lacteals.
Treatment consists of a low-fat diet with medium-chain
triglyceride supplements.
Ulceration of the small intestine
Small bowel ulcers are uncommon and are either idiopathic or secondary to underlying intestinal disorders .Ulcers are more common in the ileum, and cause bleeding, perforation, stricture formation or obstruction. Barium studies and enteroscopy confirm the diagnosis.
Causes of small intestinal ulcers
NSAID-associated small intestinal toxicityThese drugs cause a spectrum of small intestinal lesions
ranging from erosions and ulcers to mucosal webs, strictures and, rarely, a condition known as ‘diaphragm
disease’, in which intense submucosal fibrosis results in
circumferential stricturing. The condition can present
with pain, obstruction, bleeding or anaemia, and may
mimic Crohn’s disease, carcinoma or lymphoma. Enteroscopy or capsule endoscopy can reveal the diagnosis but sometimes this is only discovered at laparotomy.
Meckel’s diverticulum
This is the most common congenital anomaly of thegastrointestinal tract and occurs in 0.3–3% of people,
but the vast majority of affected individuals are asymptomatic throughout life. Usually occurs within 100 cm of the ileocaecal valve and is up to 5 cm long. Approximately 50% contain ectopic gastric mucosa; rarely, colonic, pancreatic or endometrial tissue is present.
Complications most commonly occur in the first 2 years
of life but are occasionally seen in young adults.
Bleeding can result from ulceration of ileal mucosa
adjacent to the ectopic parietal cells and presents as
recurrent melaena or altered blood per rectum
The diagnosis can be made by scanning the abdomen using a gamma counter following an intravenous injection of 99mTc-pertechnate, which is concentrated by ectopic parietal cells.
Other complications include intestinal obstruction, diverticulitis, intussusception and perforation.
Intervention is unnecessary unless complications occur.
Adverse food reactionsAre common and are subdivided into food intolerance and food allergy, the former being much more common. In food intolerance, there is an adverse reaction to food which is not immune-mediated and results from pharmacological (histamine, tyramine or monosodium glutamate), metabolic (lactase deficiency) or other mechanisms (toxins or chemical contaminants in food).
Lactose intolerance
Human milk contains around 200 mmol/L (68 g/L) of lactose, which is normally digested to glucose and galactose by the brush border enzyme lactase prior to absorption. In most populations, enterocyte lactase activity declines throughout childhood. The enzyme is deficient in up to 90% of adult Africans, Asians and South Americans, but only 5% of northern Europeans.
In cases of genetically determined (primary) lactase deficiency, jejunal morphology is normal. ‘Secondary’ lactase deficiency occurs as a consequence of disorders which damage the jejunal mucosa, such as coeliac disease and viral gastroenteritis. Unhydrolysed lactose enters the colon, where bacterial fermentation produces volatile short-chain fatty acids, hydrogen and carbon dioxide.
Clinical features
In most people, lactase deficiency is asymptomatic. However, some complain of colicky pain, abdominal distension, increased flatus, borborygmi and diarrhoea after ingesting milk or milk products. IBS may be suspected but the correct diagnosis is suggested by clinical improvement on lactose withdrawal.
The lactose hydrogen breath test is a useful
non-invasive confirmatory investigation. Dietary exclusion of lactose is recommended, although most sufferers are able to tolerate small amounts of milk without symptoms. Addition of commercial lactase preparations to milk has been effective in some studies but is costly.Intolerance of other sugars
‘Osmotic’ diarrhoea can be caused by sorbitol, an unabsorbable carbohydrate which is used as an artificial sweetener.
Fructose contained within fruit juices may also cause diarrhoea if it is consumed in greater quantities than can be absorbed.
Food allergy
Are immune-mediated disorders, most commonly due to type I hypersensitivity reactions with production of IgE antibodies, although type IV delayed hypersensitivity reactions are also seen. The most common culprits are peanuts, milk, eggs, soya and shellfish.Clinical manifestations
occur immediately on exposure and range from trivial to life-threatening or even fatal anaphylaxis. The common oral allergy syndrome results from contact with benzoic acid in certain fresh fruit juices, leading to urticaria and angioedema. Fatal reactions to trace amounts of peanuts are well documented.
The diagnosis is difficult. Skin prick tests and measurements of antigen-specific IgE antibodies in serum have limited predictive value. Treatment
patient education and awareness, antihistamines or sodium cromoglicate. Anaphylaxis should be treated as a medical emergency with resuscitation, airway support and IV adrenaline.
Infections of the small intestine
Travellers’ diarrhoea, giardiasis and amoebiasis. Tuberculosis. Cryptosporidiosis
Abdominal tuberculosis
Results from human M. tuberculosis,which is swallowed after coughing. Many patients have no pulmonary symptoms and a normal chest X-ray. The area most commonly affected is the ileocaecal region. The presentation and radiological findings may be very similar to those of Crohn’s disease. Abdominal pain can be acute or of several months’ duration, but diarrhoea is less common in TB than in Crohn. Low-grade fever is common. Like Crohn, tuberculosis can affect any part of the GIT, and perianal disease with fistula is recognised. Peritoneal tuberculosis may result in peritonitis with exudative ascites, associated with abdominal pain and fever. Granulomatous hepatitis occurs.
Diagnosis
Elevated ESR; a raised serum alkaline phosphatase suggests hepatic involvement. Histological confirmation should be sought by endoscopy, laparoscopy or liver biopsy.Caseation of granulomas is not always seen and acid and alcohol-fast bacteria are often scanty. Culture may be helpful but may take 6 weeks and diagnosis is now possible on biopsy specimens using PCR-based techniques.Management
When the presentation is very suggestive of abdominal TB, chemotherapy with four drugs – isoniazid, rifampicin, pyrazinamide and ethambutol should be commenced, even if bacteriological or histological proof is lacking.
Tumours of the small intestine
The small intestine is rarely affected by neoplasia, andfewer than 5% of all gastrointestinal tumours occur at
this site.
Benign tumours
The most common are adenomas, GIST, lipomas and
hamartomas. Adenomas found in the periampullary region and are usually asymptomatic, although occult bleeding or obstruction due to intussusception may occur. Transformation to adenocarcinoma is rare. Multiple adenomas are common in the duodenum of patients with familial adenomatous polyposis (FAP), who merit regular endoscopic surveillance. Hamartomatous polyps with no malignant potential occur in Peutz– Jeghers syndrome.
Malignant tumours
These are rare and include, in decreasing order of frequency, adenocarcinoma, carcinoid tumour, malignantGIST and lymphoma. The majority occur in middle age.
Kaposi’s sarcoma may arise in patients with AIDS.
Adenocarcinomas
Occur with increased frequency in patients with FAP, coeliac disease and Peutz– Jeghers syndrome. The non-specific presentation and rarity of these lesions often lead to delay in diagnosis. Barium follow-through examination or small bowel enema studies demonstrate most lesions of this type. Enteroscopy, capsule endoscopy, mesenteric angiography and CT also play a role in investigation. Treatment is by surgical resection.
Neuro-endocrine tumours
Also known as carcinoid tumours, derived from enterochromaffin cells and are most common in the appendix. Localised spread and the potential for metastasis to the liver increase with primary lesions > 2 cm in diameter. They also occur in the rectum and appendix.
Overall, these tumours are less aggressive and their growth is usually slow. Symptoms depend on the location of the and whether or not it is producing hormones or vasoactive
peptides. The term ‘carcinoid syndrome’ refers to the systemic symptoms that result when secretory products of the neoplastic enterochromaffin cells reach the systemic circulation . When produced by the primary tumour, they are usually metabolised in the liver and do not reach the systemic circulation.
The syndrome is therefore only seen with hepatic metastases.
Management
The treatment is surgical resection. Surgical removal of the primary tumour and hepatic metastases may be of value, as reduction of tumour mass (cytoreductive surgery) improves symptoms.Hepatic artery embolisation is another option,
since it can retard growth of hepatic deposits. Octreotide (200 μg 3 times daily by subcutaneous injection) can be used to reduce release of 5-HT, bradykinin and peptide
hormones by the tumour. Cytotoxic chemotherapy with
streptozotocin and 5-fluorouracil has limited benefits
and is reserved as second-line therapy after other therapies have failed.
Clinical features of neuro-endocrine tumours*
LymphomaNon-Hodgkin lymphoma may involve the gastrointestinal
tract as part of more generalised disease or
may rarely arise in the gut, the small intestine being
most commonly affected. Lymphomas occur with
increased frequency in patients with coeliac disease,
HIV-AIDS and other immunodeficiency states. Most are
of B-cell origin, although lymphoma associated with
coeliac disease is derived from T cells (enteropathyassociated T-cell lymphoma).
Colicky abdominal pain, obstruction and weight loss
are the presenting features, and perforation is also occasionally seen. Malabsorption is a feature of diffuse bowel involvement and hepatosplenomegaly is rare.
‘B’ symptoms (fever, weight loss, night sweats).
DISEASES OF THE PANCREAS
Acute pancreatitis
Accounts for 3% of all cases of abdominal pain admitted. It is a potentially serious with an overall mortality of 10%. 80% are mild and have a favourable outcome. About 98% of deaths from pancreatitis occur in the 20% of patients with severe disease and about one-third of these occur within the first week, usually from multi-organ failure. After this time, the majority of deaths result from sepsis, especially that complicating infected necrosis. Patients who are predicted to have severe and those with necrosis or other complications should be managed in a specialist centre with an intensive therapy unit and multidisciplinary hepatobiliary specialists.
Pathophysiology
Acute pancreatitis occurs as a consequence of prematureintracellular trypsinogen activation, releasing proteases
which digest the pancreas and surrounding tissue.
Triggers for this are many, including alcohol, gallstones and pancreatic duct obstruction .
There is simultaneous activation of nuclear factor kappa B (NFκB), leading to mitochondrial dysfunction, autophagy and a vigorous inflammatory response. The normal pancreas has only a poorly developed capsule, and adjacent structures, including the common bile duct, duodenum, splenic vein and transverse colon, are commonly involved in the inflammatory process.
The severity is dependent upon the balance between the activity of proteolytic enzymes and antiproteolytic factors.
The latter comprise an intracellular pancreatic trypsin inhibitor protein and circulating β2-macroglobulin, α1-antitrypsin and Cl-esterase inhibitors. Acute pancreatitis is often self-limiting, but in some patients with severe disease, local complications, such as necrosis, pseudocyst or abscess, occur, as well as systemic complications that lead to multi-organ failure.
Clinical features
The typical presentation is severe, constant upper abdominal pain, of increasing intensity over 15–60 minutes, which radiates to the back.
Nausea and vomiting are common.
There is marked epigastric tenderness, but in the early stages (and in contrast to a perforated peptic ulcer),
guarding and rebound tenderness are absent because
the inflammation is principally retroperitoneal.
Bowel sounds become quiet or absent as paralytic ileus develops. In severe cases, the patient becomes hypoxic and develops hypovolaemic shock with oliguria.
Discoloration of the flanks (Grey Turner’s sign) or the periumbilical region (Cullen’s sign) is a feature of severe pancreatitis with haemorrhage.
The differential diagnosis, a perforated viscus, acute cholecystitis and myocardial infarction. A collection of fluid and debris may develop in the lesser sac, following inflammatory rupture of the pancreatic duct; this is known as a pancreatic fluid collection.
It is initially contained within a poorly defined, fragile wall of granulation tissue, which matures over a 6-week period to form a fibrous capsule . Such ‘pseudocysts’ are common and usually asymptomatic, resolving as the pancreatitis recovers. Pseudocysts greater than 6 cm in diameter seldom disappear spontaneously and can cause constant abdominal pain and compress or erode surrounding structures, including blood vessels, to form pseudoaneurysms. Large pseudocysts can be detected clinically as a palpable abdominal mass. Pancreatic ascites occurs when fluid leaks from a disrupted pancreatic duct into the peritoneal cavity.
Leakage into the thoracic cavity can result in a pleural
effusion or a pleuro-pancreatic fistula.
Causes of acute pancreatitis
Pathophysiology of acute pancreatitis.
Glasgow criteria for prognosis in acute pancreatitis*
Features that predict severe pancreatitis
Investigations
Raised serum amylase or lipase concentrations and ultrasound or CT evidence of pancreatic swelling. Plain X-rays should be taken to exclude other diagnoses, such as perforation or obstruction, and to identify pulmonary complications. Amylase is efficiently excreted by the kidneys, and concentrations may have returned to normal if measured 24–48 hours after the onset of pancreatitis. A persistently elevated serum amylase concentration suggests pseudocyst formation.Peritoneal amylase concentrations are massively elevated in pancreatic ascites. Serum amylase concentrations
are also elevated in intestinal ischaemia, perforated peptic ulcer and ruptured ovarian cyst, whilst the salivary isoenzyme of amylase is elevated in parotitis.
If available, serum lipase measurements are preferable to amylase, as they have greater diagnostic accuracy for acute pancreatitis.
Ultrasound scanning can confirm the diagnosis,
although in the earlier stages the gland may not be
grossly swollen. The ultrasound scan is also useful
because it may show gallstones, biliary obstruction or
pseudocyst formation.
Contrast-enhanced pancreatic CT 6–10 days after
admission can be useful in assessing viability of the pancreas if persisting organ failure, sepsis or clinical deterioration is present, since these features may indicate that pancreatic necrosis has occurred.
Necrotising pancreatitis is associated with decreased pancreatic enhancement on CT, following IV injection of contrast material. The presence of gas within necrotic material suggests infection and impending abscess formation, in which case percutaneous aspiration of material for bacterial culture should be carried out and appropriate antibiotics prescribed. Involvement of the colon, blood vessels and other adjacent structures by the inflammatory process is best seen by CT. Certain investigations stratify the severity of acute pancreatitis and have important prognostic value at the time of presentation . In addition, serial assessment of C-reactive protein (CRP) is a useful indicator of progress. A peak CRP > 210 mg/L in the first 4 days predicts severe acute pancreatitis with 80% accuracy. It is worth noting that the serum amylase concentration has no prognostic value.
Complications of acute pancreatitis
Complications of acute pancreatitis'cont'd
Complications of acute pancreatitis'cont'd
CT showing large pancreatic pseudocyst (C)
compressing the stomach (S). The pancreas is atrophic and calcified (arrows).Management
Management comprises several related steps:• establishing the diagnosis and disease severity
• early resuscitation, according to whether the disease
is mild or severe
• detection and treatment of complications
• treating the underlying cause.
Opiate should be given to treat pain hypovolaemia should be corrected. All severe cases should be managed
in a high-dependency or intensive care unit. A central
venous line and urinary catheter should be established
to monitor patients. Oxygen should be given to hypoxic patients, and those who develop systemic inflammatory response syndrome (SIRS) may require ventilatory support.
Hyperglycaemia should be corrected using insulin, but it is not usually necessary to correct hypocalcaemia by intravenous calcium injection, unless tetany occurs.
Nasogastric aspiration is only required if paralytic
ileus is present. Enteral feeding, if tolerated, should be
started at an early stage in severe pancreatitis because they are in a severely catabolic state and need nutritional support . Enteral feeding decreases endotoxaemia and so may reduce systemic complications. Nasogastric feeding is just as effective as the nasojejunal route. Prophylaxis of thromboembolism with subcutaneous low-molecular-weight heparin is also advisable. The use of prophylactic, broad-spectrum intravenous antibiotics, such as imipenem or cefuroxime, to prevent infection of pancreatic necrosis is of unproven benefit but they are often given empirically.
Patients who present with cholangitis or jaundice
in association with severe acute pancreatitis shouldundergo urgent ERCP to diagnose and treat choledocholithiasis.
In less severe cases of gallstone pancreatitis, biliary imaging (using MRCP or EUS) can be carried out after the acute phase has resolved. If the liver function tests return to normal and US has not demonstrated a dilated biliary tree, laparoscopic cholecystectomy with an on-table cholangiogram is appropriate because any common bile duct stones have probably passed. When the operative cholangiogram detects residual common bile duct stones, these should be removed by laparoscopic exploration of the duct or by post-operative ERCP.
Cholecystectomy should be undertaken within 2 weeks of resolution of pancreatitis to prevent further potentially fatal attacks. Patients with necrotising pancreatitis or abscess require urgent endoscopic or minimally invasive retroperitoneal pancreatic (MIRP) necrosectomy to debride all cavities of necrotic material. Pseudocysts can be treated by drainage into the stomach, duodenum or jejunum. This is usually performed after an interval of at least 6 weeks, once a pseudocapsule has matured, by surgical or endoscopic cystogastrostomy.
Enteral versus total parenteral nutritional (TPN) support in acute pancreatitis
Chronic pancreatitisChronic pancreatitis is a chronic inflammatory disease characterised by fibrosis and destruction of exocrine pancreatic tissue. Diabetes mellitus occurs in advanced cases because the islets of Langerhans are involved.
Pathophysiology
Around 80% of cases in Western countries result from alcohol misuse. In southern India, severe chronic calcific pancreatitis occurs in non-alcoholics, possibly as a result
of malnutrition and cassava consumption.
Clinical features
predominantly affects middle-aged alcoholic men. Almost all present with abdominal pain. In 50%, this occurs as episodes of ‘acute pancreatitis’, although each attack results in a degree of permanent pancreatic damage.
Relentless, slowly progressive chronic pain without acute exacerbations affects 35%, whilst the remainder have no pain but present with diarrhoea. Pain is due to a combination of increased pressure within the pancreatic ducts and direct involvement of peripancreatic nerves by the inflammatory process. Pain may be relieved by leaning forwards or by alcohol. Approximately one fifth of patients chronically consume opiate analgesics.
Weight loss is common and results from a combination
of anorexia, avoidance of food because of post-prandial
pain, malabsorption and/or diabetes.
Steatorrhoea occurs when > 90% of the exocrine tissue has been destroyed; protein malabsorption only develops in the most advanced cases.
Overall, 30% of patients are diabetic, but this figure rises to 70% in those with chronic calcific pancreatitis. Physical examination reveals a thin, malnourished patient with epigastric tenderness. Skin pigmentation over the abdomen and back is common and results from chronic use of a hot water bottle (erythema ab igne). Many patients have features of other alcohol- and smoking-related diseases.
Investigations
Investigations are carried out to:
• make a diagnosis of chronic pancreatitis
• define pancreatic function
• demonstrate anatomical abnormalities prior to surgical intervention.
Pathophysiology of chronic pancreatitis.
Pathophysiology of chronic pancreatitisAlcohol and other risk factors may trigger acute pancreatitis (AP) through multiple mechanisms. The first (or ‘sentinel’) episode of acute pancreatitis initiates an inflammatory response involving T-helper cells. Ongoing exposure to alcohol drives further inflammation but this is modified by regulatory T cells with subsequent fibrosis, via activation of pancreatic stellate cells. A cycle of inflammation and fibrosis ensues, with development of chronic pancreatitis. Alcohol is the most relevant risk factor, as it is involved at multiple steps.
Causes of chronic pancreatitis*
Investigations in chronic pancreatitis
ManagementAlcohol misuse
Alcohol avoidance is crucial in halting progression of
the disease and reducing pain.
Pain relief
A range of analgesic drugs, particularly NSAIDs, are
valuable, but the severe and unremitting nature of the
pain often leads to opiate use with the risk of addiction.
Oral pancreatic enzyme supplements suppress pancreatic secretion and their regular use reduces analgesic consumption in some patients. Patients who are abstinent
from alcohol and who have severe chronic pain which is resistant to conservative measures should be considered for surgical or endoscopic pancreatic therapy .
Coeliac plexus neurolysis sometimes produces long-lasting pain relief, although relapse occurs in the majority. In some, MRCP does not show a surgically or endoscopically correctable abnormality and, in these patients, the only surgical approach is total pancreatectomy. Unfortunately, even after this operation, some patients continue to experience pain. Moreover, the procedure causes diabetes, which may be difficult to control, with a high risk of hypoglycaemia (since both insulin and glucagon release are absent), and significant morbidity and mortality.
Malabsorption
This is treated by dietary fat restriction (with supplementary medium-chain triglyceride therapy in malnourished patients) and oral pancreatic enzyme supplements. A PPI is added to optimise duodenal pH for pancreatic enzyme activity.
Management of complications
Surgical or endoscopic therapy may be necessary for themanagement of pseudocysts, pancreatic ascites, common
bile duct or duodenal stricture and the consequences of
portal hypertension. Many patients with chronic pancreatitis also require treatment for other alcohol- and
smoking-related diseases and for the consequences of
self-neglect and malnutrition.
Autoimmune pancreatitis (AIP)
Is a form of chronic pancreatitis that can mimic cancer but which responds to corticosteroids. It is characterised by abdominal pain, weight loss or obstructive jaundice, without acute attacks of pancreatitis. Blood tests reveal increased serum IgG or IgG4, and the presence of other autoantibodies.Imaging shows a diffusely enlarged pancreas,
narrowing of the pancreatic duct and stricturing of the
lower bile duct. AIP may occur alone or with other
autoimmune disorders, such as Sjِgren’s syndrome,
primary sclerosing cholangitis (PSC) or inflammatory
bowel disease. The response to steroids is usually excellent
but some patients require azathioprine.
Intervention in chronic pancreatitis
Complications of chronic pancreatitis
Congenital abnormalities affecting the pancreasPancreas divisum
This is due to failure of the primitive dorsal and ventral ducts to fuse during embryonic development of the pancreas. As a consequence, most of the pancreatic drainage occurs through the smaller accessory ampulla rather than through the major ampulla, occurs in 7–10% of the normal population and is usually asymptomatic, but some patients develop acute pancreatitis, chronic pancreatitis or atypical abdominal pain.
Annular pancreas
In this congenital anomaly, the pancreas encircles the second/third part of the duodenum, leading to gastric outlet obstruction. Associated with malrotation of the intestine, atresias and cardiac anomalies.
Cystic fibrosis
Pancreatic insufficiency and meconium ileus. Peptic ulcer and hepatobiliary disease may also occur. Pancreatic secretions are protein- and mucus-rich. The resultant viscous juice forms plugs which obstruct the pancreatic ductules, leading to progressive destruction of acinar cells. Steatorrhoea is universal and the large volume bulky stools predispose to rectal prolapse. Malnutrition is compounded by the metabolic demands of respiratory failure and by diabetes, which develops in 40% of patients by adolescence. Most patients now survive well into adulthood and heart/lung transplantation can further prolong life. Nutritional counselling and supervision are important to ensure intake of high-energy foods, providing 120–150% of the recommended intake for normal subjectsFats are an important calorie source and, despite the presence of steatorrhoea, fat intake should not be restricted. Supplementary fat-soluble vitamins are also necessary. High-dose oral pancreatic enzymes are required, to control steatorrhoea and stool frequency.
A PPI aids fat digestion by producing an optimal duodenal pH. Diabetic patients usually require insulin injections.
Meconium ileus
Mucus-rich plugs within intestinal contents can obstruct the small or large intestine. Meconium ileus is treated by the mucolytic agent N- acetylcysteine, given orally, by
Gastrografin enema or by gut lavage using polyethylene glycol. In resistant cases, surgical resection may be necessary.
Tumours of the pancreas
Pancreatic carcinoma affects 10–15 per 100 000 in Western populations, rising to 100 per 100 000 in those over the age of 70. Men are affected twice as often as women. The disease is associated with increasing age, smoking and chronic pancreatitis. Between 5 and 10% of patients have a genetic predisposition (hereditary pancreatitis, MEN, hereditary non-polyposis colon cancer (HNPCC) and familial atypical mole multiple melanoma syndrome (FAMMM).
Overall survival is only 3–5%, with median survival of 6–10 months for those with locally advanced disease and 3–5 months if metastases are present.
Pathophysiology
Some 90% of pancreatic neoplasms are adenocarcinomaswhich arise from the pancreatic ducts. These tumours
involve local structures and metastasise to regional
lymph nodes at an early stage. Most patients have
advanced disease at the time of presentation.
Clinical features
Many patients are asymptomatic until an advanced , when they present with central abdominal pain, weight loss and obstructive jaundice .The pain results from invasion of the coeliac plexus and is characteristically incessant and gnawing. It often radiates from the upper abdomen through to the back and may be eased a little by bending forwards, patients lose weight and many are cachectic.
Around 60% of tumours arise from the head of the pancreas, and involvement of the common bile duct results in the development of obstructive jaundice, often with severe pruritus.
A few patients present with diarrhoea, vomiting from duodenal obstruction, diabetes mellitus, recurrent venous thrombosis, acute pancreatitis or depression. Physical examination reveals clear evidence of weight loss. An abdominal mass due to the tumour itself, a palpable gallbladder or hepatic metastasis is commonly found.
A palpable gallbladder in a jaundiced patient is usually the consequence of distal biliary obstruction by
a pancreatic cancer (Courvoisier’s sign).
Features of pancreatic cancer
Investigations
The diagnosis is usually made by US and contrast-enhanced CT . Diagnosis in non jaundiced patients is often delayed because presenting symptoms are relatively non-specific. Fit patients with small localised tumours should undergo staging to define operability. EUS or laparoscopy with laparoscopic ultrasound will define tumour size, involvement of blood vessels and metastatic spread. MRCP and ERCP are sensitive methods of diagnosing pancreatic cancer and are valuable when the diagnosis is in doubt, although differentiation between cancer and localised chronic pancreatitis can be difficult. The main role of ERCP is to insert a stent into the common bile duct to relieve obstructive jaundice in inoperable.Management
Surgical resection is the only method of effecting cure, and 5-year survival in patients undergoing a complete resection is around 12%. Recent trials have demonstrated improved survival (21–29%) with adjuvant chemotherapy using gemcitabine. Unfortunately, only 10–15% of tumours are resectable for cure, since most are locally advanced at the time of diagnosis. For the great majority of patients, treatment is palliative. Chemotherapy with FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan and oxaliplatin) improves median survival to 11 months. Pain relief can be achieved using analgesics but, in some patients, coeliac plexus neurolysis may be required.Jaundice can be relieved by choledochojejunostomy in fit patients, whereas percutaneous or endoscopic stenting is preferable in the elderly and those with very advanced disease.
Ampullary or periampullary adenocarcinomas are rare neoplasms which arise from the ampulla of Vater or adjacent duodenum.
They are often polypoid and may ulcerate; they frequently infiltrate the duodenum but behave less aggressively than
pancreatic adenocarcinoma. Around 25% of patients
undergoing resection of ampullary or periampullary
tumours survive for 5 years, in contrast to patients with
pancreatic ductal cancer.
Cystic neoplasms of the pancreas are increasingly
being seen with widespread use of CT. These are aheterogeneous group; serous cystadenomas rarely, if
ever, become malignant and do not require surgery.
Mucinous cysts occur more often in women, are usually
in the pancreatic tail and display a spectrum of behaviour from benign to frankly malignant. Aspiration of the cyst contents for cytology and measurement of CEA and amylase concentrations in fluid obtained at EUS can help determine whether alesion is mucinous or not. In fit patients, all mucinous lesions should be resected.
Pancreatic neuro-endocrine tumours
These can occur in association with parathyroid and
pituitary adenomas (MEN 1). The majority of endocrine tumours are non-secretory and, although malignant, grow slowly and metastasise late. Other tumours secrete hormones and present because of their
endocrine effects .Neuro-endocrine pancreatic tumours may be single, but are frequently multifocal and arise from other clusters of neuroendocrine cells derived from neural crest tissues. They are localised by CT and endoscopic ultrasound. 111In-labelled DTPA is very sensitive in the diagnosis of glucagonoma.
Pancreatic neuro-endocrine tumours
INFLAMMATORY BOWEL DISEASE (IBD)Ulcerative colitis and Crohn’s disease are chronic , pursue a protracted relapsing and remitting course, extending over years. The diseases have many similarities and it is sometimes impossible to differentiate between them.
A crucial distinction is that ulcerative colitis only involves the colon, while Crohn’s disease can involve any part of the GIT from mouth to anus. The incidence of IBD varies widely between populations. There was a dramatic increase in the incidence of Crohn’s disease in the Western world, starting in the second half of the last century and coinciding with the introduction of a more ‘hygienic’ environment with the advent of domestic
refrigeration and the widespread use of antibiotics.
The developing world has seen similar patterns, as these
countries adopt an increasingly Westernised lifestyle. In the West, incidence of UC is stable at 10–20 per 100 000, with a prevalence of 100–200 per 100 000, while the incidence of Crohn’s is increasing and is now 5–10 per 100 000, with a prevalence of 50–100 per 100 000. Both commonly start in the second and third decades of life, with a second smaller incidence peak in the seventh. Life expectancy in patients with IBD is similar to that of the general population. Although many patients require surgery and admission to hospital for other reasons, with substantial associated morbidity, the majority have an excellent work record and pursue a normal life.Comparison of ulcerative colitis and Crohn’s disease
Pathophysiology
IBD has both environmental and genetic components, and evidence from genome wide association studies suggests that genetic variants that predispose to Crohn’s disease may have undergone positive selection by protecting against infectious diseases, including tuberculosis (Box). It is thought that IBD develops because these genetically susceptible individuals mount an abnormal inflammatory response to environmental triggers, such as intestinal bacteria. This leads to inflammation of the intestine with release of inflammatory mediators, including TNF, IL-12 and IL-23, which cause tissue damage . In both diseases, the intestinal wall is infiltrated with acute and chronic inflammatory cell.Pathophysiology :Ulcerative colitis
Inflammation invariably involves the rectum (proctitis)and spreads proximally in a continuous manner to
involve the entire colon in some cases (pancolitis). In
long-standing pancolitis, the bowel can become shortened
and post-inflammatory ‘pseudopolyps’ develop;
these are normal or hypertrophied residual mucosa
within areas of atrophy. The inflammatory process is
limited to the mucosa .Both acute and chronic
inflammatory cells infiltrate the lamina propria and the
crypts (‘cryptitis’). Crypt abscesses are typical. Goblet
cells lose their mucus and, in long-standing cases, glands
become distorted. Dysplasia, characterised by heaping of cells within crypts, nuclear atypia and increased
mitotic rate, may herald development of colon cancer.
Pathophysiology : Crohn’s disease
The sites most commonly involved are, in order of frequency, the terminal ileum and right side of colon, colon alone, terminal ileum alone, ileum and jejunum. The entire wall of the bowel is oedematous and thickened, and there are deep ulcers which often appear as linear fissures; thus the mucosa between them is described as ‘cobblestone’. These may penetrate through the bowel wall to initiate abscesses or fistulae involving the bowel, bladder, uterus, vagina and skin of the perineum. The mesenteric lymph nodes are enlarged and the mesentery is thickened. Crohn’s disease has a patchy distribution, interrupted by islands of normal mucosa. On histological examination, the bowel wall is thickened with a chronic inflammatory infiltrate throughout all layers .Factors associated with the development
of inflammatory bowel diseasePathogenesis of inflammatory bowel disease
(1) Bacterial antigens are taken up by specialised M cells, pass between leaky epithelial cells or enter the lamina propria through ulcerated mucosa.(2) After processing, they are presented to type 1 T-helper cells by antigen-presenting cells (APC) in the lamina propria.
(3) T-cell activation and differentiation results in a Th1 T cell-mediated cytokine response
(4) with secretion of cytokines, including interferon-gamma (IFN-γ). Further amplification of T cells perpetuates the inflammatory process with activation of non-immune cells and release of other important cytokines, including interleukin (IL)-12, IL-23, IL-1 IL-6 and tumour necrosis factor (TNF). These pathways occur in all normal individuals exposed to an inflammatory insult and this is self-limiting in healthy subjects. In genetically predisposed persons, dysregulation of innate immunity may trigger inflammatory bowel disease.
Histology of ulcerative colitis There is surface ulceration and inflammation is confined to the mucosa with excess inflammatory cells in the lamina propria, loss of goblet cells, and crypt abscesses (arrows). (SM = submucosa)
Histology of Crohn’s disease.
A Inflammation is ‘transmural’; there is fissuring ulceration (arrow), with inflammation extending into the submucosa (SM).
B At higher power, a characteristic non- caseating granuloma is seen
Common patterns of disease distribution in inflammatory bowel disease
Clinical features : Ulcerative colitisThe cardinal symptoms are rectal bleeding with passage of mucus and bloody diarrhoea. The first attack is usually the most severe , followed by relapses and remissions. Emotional stress, intercurrent infection, gastroenteritis, antibiotics or NSAID may all provoke a relapse. Proctitis causes rectal bleeding and mucus discharge, tenesmus. Some pass frequent, small volume fluid stools, while others pass pellety stools due to constipation upstream of the inflamed rectum. Constitutional symptoms do not occur. Left-sided and extensive colitis causes bloody diarrhoea with mucus, often with abdominal cramps. In severe cases, anorexia, malaise, weight loss and abdominal pain occur, and the patient is toxic, with fever, tachycardia and signs of peritoneal inflammation .
Clinical features : Crohn’s disease
The major symptoms are abdominal pain, diarrhoea andweight loss. Ileal Crohn’s disease may cause subacute or even acute intestinal obstruction.
The pain is often associated with diarrhoea, which is
usually watery and does not contain blood or mucus.
Almost all patients lose weight because they avoid food,
since eating provokes pain. Weight loss may also be due
to malabsorption, and some patients present with features
of fat, protein or vitamin deficiencies. Crohn’s colitis presents in an identical manner to ulcerative colitis, but rectal sparing and the presence of perianal disease are features which favour a diagnosis of Crohn’s disease.
Many patients present with symptoms of both small bowel and colonic disease. A few patients present with isolated perianal disease, vomiting from jejunal strictures or severe oral ulceration.
Physical examination often reveals evidence of
weight loss, anaemia with glossitis and angular stomatitis. There is abdominal tenderness, most marked
over the inflamed area. An abdominal mass may be palpable and is due to matted loops of thickened bowel or an intra-abdominal abscess. Perianal skin tags, fissures
or fistulae are found in at least 50% of patients.
Barium follow-through showing terminal ileal Crohn’s disease.
A long stricture is present (arrow A), and more proximally thereis ulceration with characteristic ‘rose thorn’ ulcers (arrow B).
Differential diagnosis
The most important issue is to distinguish the first attackof acute colitis from infection. In general, diarrhoea
lasting longer than 10 days in Western countries is
unlikely to be the result of infection, whereas a history
of foreign travel, antibiotic exposure (pseudomembranous
colitis) or homosexual contact increases the possibility
of infection, which should be excluded by the
appropriate investigations (see below). The diagnosis of
Crohn’s disease is usually more straightforward and is
made on the basis of imaging and clinical presentation,
but in atypical cases, biopsy or surgical resection is necessary to exclude other diseases .
Conditions which can mimic ulcerative or
Crohn’s colitisDifferential diagnosis of small bowel
Crohn’s diseaseComplications
Life-threatening colonic inflammationThis can occur in both ulcerative colitis and Crohn’s
colitis. In the most extreme cases, the colon dilates (toxic
megacolon) and bacterial toxins pass freely across the
diseased mucosa into the portal and then systemic circulation. Box .
An abdominal X-ray should be taken daily because, when the transverse colon is dilated to > 6 cm , there is a high risk of colonic perforation, although this complication can also occur in the absence of toxic megacolon.
Haemorrhage
Haemorrhage due to erosion of a major artery is rare but can occur in both conditions.
Fistulae
These are specific to Crohn’s disease. Enteroenteric fistulae can cause diarrhoea and malabsorption due to
blind loop syndrome. Enterovesical fistulation causes
recurrent urinary infections and pneumaturia. An
enterovaginal fistula causes a faeculent vaginal discharge. Fistulation from the bowel may also cause perianal or ischiorectal abscesses, fissures and fistulae.
Cancer .
The risk of dysplasia and cancer increases with the duration and extent of colonic inflammation. Thus long-standing, extensive colitis are at highest risk. Oral mesalazine reduces the risk of dysplasia and neoplasia in ulcerative colitis. Azathioprine also seems to reduce the risk of colorectal cancer in ulcerative colitis and Crohn’s colitis.
This protective effect probably extends to any treatment that results in sustained healing of the colonic mucosa. The cumulative risk for dysplasia in UC may be as high as 20% after 30 years but is probably lower for Crohn’s colitis. The risk is particularly high in patients who have concomitant primary sclerosing cholangitis for unknown reasons. Patients with long-standing colitis are therefore entered into surveillance programmes beginning 10 years after diagnosis. Targeted biopsies of areas that show abnormalities on staining with indigo carmine or methylene blue increase the chance of detecting dysplasia and this technique (pancolonic chromo-endoscopy) has replaced colonoscopy with random biopsies taken every 10 cm in screening for malignancy.
The procedure allows patients to be stratified into high-, medium- or low-risk groups to determine the interval between surveillance procedures. If high-grade dysplasia is found, panproctocolectomy is usually recommended because of the high risk of colon cancer.
Extra-intestinal complications
Are common in IBD and may dominate the clinical picture. Some of these occur during relapse of intestinal disease; others appear unrelated to intestinal disease activity (Fig). Investigations
To confirm the diagnosis, define distribution and activity, and identify complications. Full blood count may show anaemia resulting from bleeding or malabsorption of iron, folic acid or vitamin B12.
Serum albumin concentration falls as a consequence of protein-losing enteropathy, inflammatory disease or poor nutrition. The ESR and CRP are elevated in exacerbations and abscess formation.
Faecal calproctectin has a high sensitivity for detecting inflammation and may be elevated, even when the CRP is normal. It is particularly useful in distinguishing IBD from IBS, and for subsequent monitoring of disease activity.
Bacteriology
At initial presentation, stool microscopy, culture and
examination for Clostridium difficile toxin or for ova and
cysts, blood cultures and serological tests should be performed. During acute flares necessitating hospital admission, three separate stool samples should be sent for
bacteriology to maximise sensitivity.
In Crohn’s disease, patchy inflammation, with discrete, deep ulcers, strictures and perianal disease (fissures, fistulae and skin tags), is typically observed, often with rectal sparing.
In established disease, colonoscopy may show active
inflammation with pseudopolyps or a complicating carcinoma. Biopsies should be taken from each anatomical
segment (terminal ileum, right colon, transverse colon,
left colon and rectum) to confirm the diagnosis and
define disease extent, and also to seek dysplasia in
patients with long-standing colitis.
In Crohn’s disease, wireless capsule endoscopy is useful in the identification of small bowel inflammation but should be avoided in the presence of strictures.
Endoscopy
Patients who present with diarrhoea plus raised inflammatory markers or alarm features, such as weight loss, rectal bleeding and anaemia, should undergo ileocolonoscopy.Flexible sigmoidoscopy is occasionally performed
to make a diagnosis, especially during acute
severe presentations when ileocolonoscopy may confer
an unacceptable risk; ileocolonoscopy should still be
performed at a later date, however, in order to evaluate
disease extent.
In US , there is loss of vascular pattern, granularity, friability and contact bleeding, with or without ulceration .
Enteroscopy may be required to make a histological diagnosis of small bowel Crohn’s disease, when the inflamed segment is out of reach of standard endoscopes. All children and most adults with
Crohn’s disease should have upper gastrointestinal
endoscopy and biopsy to complete their staging. Not
only is upper gastrointestinal Crohn’s disease relatively
common in this group, but it may help to make a definitive diagnosis in patients who otherwise appear to have non-specific colonic inflammation.
Radiology
Barium enema is a less sensitive investigation than
colonoscopy in patients with colitis and, where colonoscopy
is incomplete, a CT colonogram is preferred.
Small bowel imaging is essential to complete staging of
Crohn’s disease. Traditional contrast imaging by barium
follow-through demonstrates affected areas of the bowel
as narrowed and ulcerated, often with multiple strictures.
This has now largely been replaced
by MRI enterography, which does not involve exposure
to radiation and is a sensitive way of detecting extraintestinal manifestations and of assessing pelvic and perineal involvement.
A plain abdominal X-ray is essential in the management of patients who present with severe active disease. Dilatation of the colon , mucosal oedema
(thumb-printing) or evidence of perforation may be
found. In small bowel Crohn’s disease, there may be
evidence of intestinal obstruction or displacement of
bowel loops by a mass. Ultrasound is a very powerful
tool to detect small bowel inflammation and stricture
formation, but it is rather operator-dependent. The role
of CT is limited to screening for complications, such as
perforation or abscess formation, in the acutely unwell.
Sigmoidoscopic view of moderately active ulcerative colitis. Mucosa is erythematous and friable with contact bleeding.
Submucosal blood vessels are no longer visible.
Assessment of disease severity in
ulcerative colitisSystemic complications of inflammatory bowel disease
Plain abdominal X-ray showing a grossly dilated colon
due to severe ulcerative colitis. There is also marked mucosal oedemaand ‘thumb-printing’ (arrows).
Management
Although medical therapy plays an important role, optimal management depends on establishing a multidisciplinary team-based approach involving physicians, surgeons, radiologists, nurse specialists and dietitians. Both ulcerative colitis and Crohn’s disease are life-long conditions and have important psychosocial implications; specialist nurses, counsellors and patient support groups have key roles in education, reassurance.
The key aims of medical therapy are to:
• treat acute attacks (induce remission)
• prevent relapses (maintain remission)
• prevent bowel damage
• detect dysplasia and prevent carcinoma
• select appropriate patients for surgery.
Ulcerative colitis
Active proctitis. Most patients with ulcerative proctitisrespond to a 1 g mesalazine suppository but some will
additionally require oral 5-aminosalicylate (5-ASA)
therapy. Topical corticosteroids are less effective and
are reserved for patients who are intolerant of topical
mesalazine. Patients with resistant disease may
require treatment with systemic corticosteroids and
immunosuppressants.
Active left-sided or extensive ulcerative colitis.
In mild to moderately active cases, the combination of a once daily oral and a topical 5-ASA preparation (‘top and tail approach’) is usually effective.
The topical preparation (1 g foam or liquid enema) is typically withdrawn after 1 month. The oral 5-ASA is continued long-term to prevent relapse and minimise the risk of dysplasia. In patients who do not respond to this approach within 2–4 weeks, oral prednisolone (40 mg daily, tapered by 5 mg/week over an 8-week total course) is indicated.
Corticosteroids should never be used for maintenance
therapy. At the first signs of corticosteroid resistance
or in patients who require high corticosteroid doses to maintain control, immunosuppressive therapy with a thiopurine should be introduced.
Severe ulcerative colitis. Patients who fail to respond to maximal oral therapy and those who present with acute severe colitis (meeting the Truelove– Witts criteria) are best managed in hospital and should be monitored jointly by a physician and surgeon:
• clinically: for the presence of abdominal pain,
temperature, pulse rate, stool blood and frequency
• by laboratory testing: haemoglobin, white cell
count, albumin, electrolytes, ESR and CRP
• radiologically: for colonic dilatation on plain X-rays.
All patients should be given supportive treatment with IV fluids to correct dehydration, and enteral nutritional support should be provided for malnourished patients .
IV corticosteroids (methylprednisolone 60 mg or hydrocortisone 400 mg/day) should be given by IV infusion or bolus injection.
Topical and oral aminosalicylates have no role to play in the acute severe attack. Response to therapy is judged over the first 3 days. Patients who do not respond promptly to corticosteroids should be considered for medical rescue therapy with ciclosporin (IV infusion or oral) or infliximab (5 mg/kg), which, in approximately 60% of cases, can avoid the need for urgent colectomy. Patients who develop colonic dilatation (> 6 cm),
those whose clinical and laboratory measurements deteriorate and those who do not respond after 7–10 days’ maximal medical treatment usually require urgent colectomy. Subtotal colectomy can also be performed laparoscopically, given sufficient local expertise.
Maintenance of remission.
Life-long maintenancetherapy is recommended for all patients with left-sided or extensive disease but is not necessary in those with proctitis (although 20% of these patients will develop proximal ‘extension’ over the lifetime of their disease). Once-daily oral 5-aminosalicylates are the preferred first-line agents.
Sulfasalazine has a higher incidence of side-effects but is equally effective and can be considered in patients with coexistent arthropathy.
Patients who frequently relapse despite aminosalicylate drugs should be treated with thiopurines.
Medical management of fulminant ulcerative colitis
Crohn’s diseasePrinciples of treatment.
Crohn’s disease is a progressive condition which may result in stricture or fistula formation if suboptimally treated. It is therefore important to agree long-term treatment goals with the patient; these are to induce remission and then maintain corticosteroid-free remission with a normal quality of life. Treatment should focus on monitoring the patient carefully for disease activity and complications , and ensuring that mucosal healing is achieved.
Induction of remission. Corticosteroids remain the
mainstay of treatment for active Crohn’s disease. The
drug of first choice in patents with ileal disease is budesonide, since it undergoes 90% first-pass metabolism in the liver and has very little systemic toxicity.
A typical regimen is 9 mg once daily for 6 weeks, with a gradual reduction in dose over the subsequent 2 weeks when therapy is stopped. If there is no response to budesonide within 2 weeks, the patient should be switched to prednisolone, which has greater potency. This is typically given in a dose of 40 mg daily, reducing by 5 mg/week over 8 weeks, at which point treatment is stopped. Oral prednisolone in the above dose regimen is the treatment of choice for inducing remission in colonic crohn’s disease.
Calcium and vitamin D supplements should be co-prescribed in patients who are on corticosteroids, to try to compensate for their inhibitory effect on intestinal calcium absorption.
As an alternative to corticosteroid therapy, enteral nutrition with either an elemental (amino acids) or polymeric (liquid protein) diet may induce remission.
Both types of diet are equally effective but the
polymeric one is more palatable when taken by mouth.
It is particularly effective in children, in whom equal efficacy to corticosteroids has been demonstrated, and in extensive ileal disease in adults. As well as resting the gut and providing excellent nutritional support, it also has a direct anti-inflammatory effect. It is an effective bridge to urgent staging investigations at first presentation and can be given by mouth or by nasogastric tube.
With sufficient explanation, encouragement and motivation, most patients will tolerate it well.
Some patients with severe colonic disease require
admission to hospital for intravenous corticosteroids. In severe ileal or panenteric disease, induction therapy
with an anti-TNF agent is appropriate, provided that
acute perforating complications, such as abscess, have
not occurred. Both infliximab and adalimumab are
licensed for use in the UK. Randomised trials have demonstrated that combination therapy with an anti-TNF antibody and a thiopurine is the most effective strategy for inducing and maintaining remission in luminal Crohn’s patients. This strategy is more effective than anti-TNF monotherapy, which, in turn, is more effective than thiopurine monotherapy.
Following induction of remission, a substantial proportion of patients (20–30%) remain well without the requirement for maintenance therapy. Patients with evidence of persistently active disease require further treatment (see below).
Maintenance therapy. Immunosuppressive treatment
with thiopurines (azathioprine and mercaptopurine)
forms the core of maintenance therapy, but methotrexate
is also effective and can be given once weekly, either
orally or by subcutaneous injection. Women of childbearing potential who are prescribed methotrexate must use a robust contraceptive method, since it is teratogenic. Combination therapy ,immunosuppressant and an anti-TNF antibody is the most effective strategy but costs are high and there is an increased risk of serious adverse effects.
Careful monitoring of disease activity. Cigarette smokers should be strongly counselled to stop smoking at every possible opportunity. Those that do not manage to stop smoking fare much worse, with increased rates of relapse and surgical intervention. Fistulae and perianal disease. Fistulae may develop in relation to active Crohn’s disease and are often associated with sepsis. The first step is to define the site by imaging (usually MRI of the pelvis). Surgical exploration and drain abscesses. Seton sutures can be inserted through fistula tracts to ensure adequate drainage and to prevent future sepsis. Corticosteroids are ineffective. For simple perianal disease, metronidazole and/or ciprofloxacin are first-line therapies.
Drugs used in the treatment of inflammatory bowel disease
How to give anti-TNF therapy in
inflammatory bowel diseaseBiological therapy in the management of
inflammatory bowel diseaseSurgical treatment
Ulcerative colitisUp to 60% of patients with extensive ulcerative colitis
eventually require surgery. The indications are listed in
Box. Impaired quality of life, with impact upon
occupation and on social and family life, is the most
important of these. Surgery involves removal of the
entire colon and rectum, and cures the patient. One-third
of those with pancolitis undergo colectomy within
5 years of diagnosis. Before surgery, patients must be
counselled by doctors, stoma nurses and patients who
have undergone similar surgery. The choice of procedure
is either panproctocolectomy with ileostomy, or
proctocolectomy with ileal–anal pouch anastomosis.
Indications for surgery in ulcerative colitis
Crohn’s diseaseThe indications for surgery are similar to those for ulcerative colitis.
Operations are often necessary to deal with fistulae, abscesses and perianal disease, and may also be
required to relieve small or large bowel obstruction. In
contrast to ulcerative colitis, surgery is not curative and
disease recurrence is the rule. The only method that
has consistently been shown to reduce post-operative
recurrence is smoking cessation. Antibiotics are effective
in the short term only. It is common to undertake colonoscopy
6 months after surgery to inspect and biopsy the
anastomosis and neo-terminal ileum. Patients with
endoscopic recurrence are then prescribed thiopurines.
Surgery should be as conservative as possible in
order to minimise loss of viable intestine and to avoidcreation of a short bowel syndrome. Obstructing or fistulating small bowel disease may require resection of
affected tissue. Patients who have localised segments of
Crohn’s colitis may be managed by segmental resection
and/or multiple stricturoplasties, in which the stricture
is not resected but instead incised in its longitudinal axis
and sutured transversely. Others who have extensive
colitis require total colectomy but ileal–anal pouch formation should be avoided because of the high risk of
recurrence within the pouch and subsequent fistulae,
abscess formation and pouch failure.
Historical datasets show that around 80% of Crohn’s patients undergo surgery at some stage, and 70% of these require more than one operation during their lifetime.
Clinical recurrence following resectional surgery is
present in 50% of all cases at 10 years. Emerging data demonstrate that aggressive medical therapy, coupled with intense monitoring, probably reduces the requirement for surgery substantially.
Monitoring of inflammatory bowel disease
IBD in special circumstancesChildhood
Chronic ill health in childhood or adolescent IBD may
result in growth failure, metabolic bone disease and
delayed puberty. Loss of schooling and social contact, as
well as frequent hospitalisation, can have important psychosocial consequences. Treatment is similar to that
described for adults and may require corticosteroids,
immunosuppressive drugs, biological agents and surgery. Monitoring of height, weight and sexual development is crucial. Children with IBD should be managed by specialised paediatric gastroenterologists and transitioned to adult care in dedicated clinics .
Inflammatory bowel disease in
adolescence
Pregnancy
A women’s ability to become pregnant is adverselyaffected by active IBD. Pre-conceptual counselling
should focus on optimising disease control. During
pregnancy, the rule of thirds applies – roughly one-third
of women improve, one-third get worse and one third
remain stable with active disease. In the post-partum
period, these changes sometimes reverse spontaneously.
Drug therapy, including aminosalicylates, corticosteroids
and azathioprine, can be safely continued throughout
pregnancy but methotrexate must be avoided, both
during pregnancy and if the patient is trying to conceive
Anti-TNF agents are transmitted through the placenta (but not breast milk) and are usually omitted during the last trimester.
Pregnancy and inflammatory bowel disease
Pregnancy and inflammatory bowel Disease 'cont'd
Metabolic bone diseasePatients with IBD are prone to developing osteoporosis due to effects of chronic inflammation, corticosteroids, weight loss, malnutrition and malabsorption. Osteomalacia can also occur in Crohn’s disease that is complicated by malabsorption, but is less common than
osteoporosis. The risk of osteoporosis increases with age and with the dose and duration of corticosteroid therapy.
IRRITABLE BOWEL SYNDROME (IBS)
Characterised by recurrent abdominal pain in association with abnormal defecation in the absence of a structural abnormality of the gut. About 10–15% of the population are affected at some time but only 10% of these consult their doctors because of symptoms. Nevertheless, IBS is the most common cause of gastrointestinal referral.Young women are affected 2–3 times more often
than men. Coexisting conditions, such as non-ulcer dyspepsia, chronic fatigue syndrome, dysmenorrhoea and fibromyalgia, are common. Between 5 and 10% of
patients have a history of physical or sexual abuse.
Pathophysiology
The cause of IBS is incompletely understood but biopsychosocial factors are thought to play an importantrole, along with luminal factors, such as diet and the gut
microbiota, as discussed below.
Behavioural and psychosocial factors
Most patients seen in general practice do not have psychological problems but about 50% of patients referred to hospital have a psychiatric illness, such as anxiety, depression, somatisation and neurosis. Panic attacks are also common. Acute psychological stress and overt psychiatric disease are known to alter visceral perception and gastrointestinal motility. There is an increased prevalence of abnormal illness behaviour, with frequent consultations for minor symptoms and reduced coping ability. These factors contribute to but do not cause IBS.
Physiological factors
There is some evidence that IBS may be a serotoninergic (5-HT) disorder, as evidenced by relatively excessive release of 5-HT in diarrhoea-predominant IBS (D-IBS) and relative deficiency with constipation-predominant IBS (C-IBS). Accordingly, 5-HT3 receptor antagonists are effective in D-IBS, while 5-HT4 agonists improve bowel function in C-IBS.
There is some evidence that IBS may represent a state of low-grade gut inflammation or immune activation, not detectable by tests, with raised numbers of mucosal mast cells, which sensitise enteric neurons by releasing histamine and tryptase.
Some patients respond positively to mast cell stabilisers, such as ketotifen, which supports a pathogenic role of mast cells in at least some patients. Immune activation may
be associated with altered CNS processing of visceral
pain signals. This is more common in women and in
D-IBS, and may be triggered by a prior episode of gastroenteritis with Salmonella or Campylobacter species.
Luminal factors
Both quantitative and qualitative alterations in intestinal bacterial contents (the gut microbiota) have been reported. Small intestinal bacterial overgrowth (SIBO) may be present in some patients and lead to symptoms. This ‘gut dysbiosis’ may explain the response to probiotics or the non-absorbable antibiotic rifaximin ,reported in trials.
Dietary factors are also important. Some patients have chemical food intolerances (not allergy) to poorly absorbed, short-chain carbohydrates (lactose, fructose and sorbitol, among others), collectively known as FODMAPs (fermentable oligo-, di- and monosaccharides, and polyols). Their fermentation in the colon leads
to bloating, pain, wind and altered bowel habit.
Noncoeliac gluten sensitivity (negative coeliac serology and normal duodenal biopsies) seems to be present in some IBS patients, while others may be intolerant of chemicals such as salicylates or benzoates, in certain foods.
Clinical features
The most common presentation is that of recurrent abdominal discomfort .This is usually colicky or cramping in nature, felt in the lower abdomen and relieved by defecation. Abdominal bloating worsens throughout the day; the cause is unknown but it is not due to excessive intestinal gas.
The bowel habit is variable. Most patients alternate between episodes of diarrhoea and constipation, but it is useful to classify patients as having predominantly constipation or predominantly diarrhoea. Those with constipation tend to pass infrequent pellety stools, in association with abdominal pain or proctalgia.
Those with diarrhoea have frequent defecation but produce low-volume stools and rarely have nocturnal symptoms. Passage of mucus is common but rectal bleeding does not occur. Patients do not lose weight and are constitutionally well. Physical examination is generally unremarkable, with the exception of variable tenderness to palpation.
Rome III criteria for diagnosis of IBS
Diagnosis
Can be made confidently in most patients using the Rome criteria combined with the absence of alarm symptoms, without resorting to complicated tests (Box).Full blood count and faecal calprotectin, with or without sigmoidoscopy, are normal in IBS. Colonoscopy should be undertaken in older patients (over 40 years of age) to exclude colorectal cancer. Endoscopic examination is also required in patients who report rectal bleeding to exclude colon cancer and IBD. Diarrhoea predominant patients justify investigations to exclude coeliac disease ,microscopic colitis , lactose intolerance , bile acid malabsorption , thyrotoxicosis and parasitic infection.
Supporting diagnostic features and alarm features in IBS
ManagementThe most important steps are to make a positive diagnosis and reassure the patient. Many patients are concerned that they have developed cancer, and a cycle of anxiety leading to colonic symptoms, which further heighten anxiety, can be broken by explanation that symptoms are not due to a serious underlying disease but instead are the result of behavioural, psychosocial, physiological and luminal factors described above. In patients who fail to respond to reassurance, treatment is traditionally tailored to the predominant symptoms . Up to 20% may benefit from a wheat-free diet, some may respond to lactose exclusion, and excess intake of caffeine or artificial sweeteners, such as sorbitol, should be addressed.
A more restrictive, ‘low-FODMAP’ diet, supervised by a dietitian, with gradual re-introduction of different food, may help some, as may a trial of a gluten-free diet.
Probiotics,
in capsule form, can be effective if taken for several
months, although the optimum combination of bacterial
strains and dose have yet to be clarified. Patients with intractable symptoms sometimes benefit from several months of therapy with a tricyclic antidepressant, such as amitriptyline or imipramine (10–25 mg orally at night). Side-effects include dry mouth and drowsiness but these are usually mild and the drug is generally well tolerated, although patients with features of somatisation tolerate the drug poorly and lower doses should be used.
It may act by reducing visceral sensation and by altering gastrointestinal motility. The 5-HT4 agonist prucalopride, the guanylate cyclase-C receptor agonist linaclotide, and chloride channel activators, such as lubiprostone, can be effective in constipation predominant IBS.
Trials of anti-inflammatory agents, such as ketotifen
or mesalazine, and the antibiotic rifaximin may be considered in some with difficult symptoms.
Psychological interventions, such as cognitive behavioural
therapy, relaxation and gut-directed hypnotherapy,
should be reserved for the most difficult cases.
Most patients have a relapsing and remitting course.
Exacerbations often follow stressful life events, occupational dissatisfaction and difficulties with interpersonal relationships.
Management of irritable bowel syndrome. (FODMAP = fermentable oligo-, di- and monosaccharides, and polyols)
Complementary and alternative therapies for IBS
*Some evidence for benefit existsISCHAEMIC GUT INJURY
Usually the result of arterial occlusion.Acute small bowel ischaemia
An embolus from the heart or aorta to the superior mesenteric artery is responsible for 40–50%, thrombosis of underlying atheromatous disease for approximately 25%, and non-occlusive ischaemia due to hypotension complicating myocardial infarction, heart failure, arrhythmias or sudden blood loss for approximately 25%. Vasculitis and venous occlusion are rare causes. The clinical spectrum ranges from transient alteration of bowel function to transmural haemorrhagic necrosis and gangrene. Almost all develop abdominal pain that is more impressive than the physical findings.
In the early stages, the only physical signs may be a silent, distended abdomen or diminished bowel sounds, peritonitis only developing later. If treatment is instituted early, embolectomy and vascular reconstruction may salvage some small bowel.
In these rare cases, a ‘second look’ laparotomy should be undertaken 24 hours later and further necrotic bowel resected. Leucocytosis, metabolic acidosis, hyperphosphataemia and hyperamylasaemia are typical. Plain abdominal X-rays show ‘thumb-printing’ due to mucosal oedema. Mesenteric or CT angiography reveals an occluded or narrowed major artery with spasm of arterial arcades, although most patients undergo laparotomy on the basis of a clinical diagnosis without angiography. Resuscitation, management of cardiac disease and IV antibiotic therapy, followed by laparotomy, are key steps.
In patients at high surgical risk, thrombolysis
may sometimes be effective. The results of therapydepend on early intervention; patients treated late have
a 75% mortality rate. Survivors often have nutritional
failure from short bowel syndrome and require
intensive nutritional support, including home parenteral
nutrition and anticoagulation.
Small bowel transplantation is promising in selected patients. Patients with mesenteric venous thrombosis also require surgery if there are signs of peritonitis but are otherwise treated with anticoagulation. Investigations for underlying prothrombotic disorders should be performed.
Acute colonic ischaemia
The splenic flexure and descending colon have little collateral circulation and lie in ‘watershed’ areas of arterial supply. The spectrum of injury ranges from reversible colopathy to transient colitis, colonic stricture, gangrene and fulminant pancolitis. Arterial thromboembolism is usually responsible but colonic ischaemia can also follow severe hypotension, colonic volvulus, strangulated hernia, systemic vasculitis or hypercoagulable states. Ischaemia of the descending and sigmoid colon is also a complication of abdominal aortic aneurysm surgery (where the inferior mesenteric artery is ligated).
The patient is usually elderly and presents with sudden onset of cramping, left-sided, lower abdominal pain and rectal bleeding.
Symptoms usually resolve spontaneously over 24–48 hours and healing occurs in 2 weeks.
Some may develop a fibrous stricture or segment of colitis. A minority develop gangrene and peritonitis. The diagnosis is established by colonoscopy within 48 hours of presentation; otherwise, mucosal ulceration may have resolved. Resection is required for peritonitis.Chronic mesenteric ischaemia
This results from atherosclerotic stenosis of the coeliac axis, superior mesenteric and inferior mesenteric artery.At least two of the three vessels must be affected for symptoms to develop. The typical presentation is with dull but severe mid- or upper abdominal pain developing about 30 minutes after eating. Weight loss is common because the patient is reluctant to eat, and some experience diarrhoea. Physical examination shows evidence of generalised arterial disease. An abdominal bruit is sometimes audible.
The diagnosis is made by mesenteric angiography.
Treatment is by vascular reconstruction or percutaneous angioplasty, if the patient’s clinical condition permits.
DISORDERS OF THE COLON AND RECTUM
Tumours of the colon and rectumPolyps and polyposis syndromes
Polyps may be neoplastic or non-neoplastic. The latter
include hamartomas, metaplastic (‘hyperplastic’) polyps
and inflammatory polyps. These have no malignant
potential. Polyps may be single or multiple and vary
from a few millimetres to several centimetres in size.
Colorectal adenomas are extremely common in the
Western world , the prevalence rises with age; 50%
of people over 60 years of age have adenomas, and in
half of these the polyps are multiple. They are more
common in the rectum and distal colon and are either
pedunculated or sessile.
Histologically, they are classified as either tubular, villous or tubulovillous, according to the glandular architecture.
Nearly all forms of colorectal carcinoma develop from adenomatous polyps, although not all polyps carry the same degree of risk. Features associated with a higher risk of subsequent malignancy are listed in Box
Adenomas are usually asymptomatic and discovered incidentally. Occasionally, they cause bleeding and anaemia. Villous adenomas can secrete large amounts of mucus, causing diarrhoea and hypokalaemia.
Risk factors for malignant change in colonic polyps
Discovery of a polyp at sigmoidoscopy is an indicationfor colonoscopy because proximal polyps are
present in 40–50% of such patients. Colonoscopic
polypectomy should be carried out wherever possible,
as this considerably reduces subsequent colorectal
cancer risk (Fig.). Very large or sessile polyps can
sometimes be removed safely by endoscopic mucosal
resection (EMR) but many require surgery. Once all
polyps have been removed, surveillance colonoscopy
should be undertaken at 3–5-year intervals, as new
polyps develop in 50% of patients. Patients over 75 years
of age do not require repeated colonoscopies, as their
subsequent lifetime cancer risk is low.
Between 10 and 20% of polyps show histological evidence of malignancy. When cancer cells are found
within 2 mm of the resection margin of the polyp,
when the polyp cancer is poorly differentiated or when
lymphatic invasion is present, segmental colonic resection
is recommended because residual tumour or lymphatic
spread (in up to 10%) may be present. Malignant
polyps without these features can be followed up by
surveillance colonoscopy.
Polyposis syndromes are classified by histopathology.
It should be noted that, while the hamartomatous polyps in Peutz–Jeghers syndrome and juvenile polyposis are not themselves neoplastic, these disorders are associated with an increased risk of malignancy of the breast, colon, ovary and thyroid.
Familial adenomatous polyposis
Familial adenomatous polyposis (FAP) is an uncommonautosomal dominant disorder affecting 1 in 13 000 of the population and accounting for 1% of all colorectal
cancers. It results from germline mutation of the tumour
suppressor APC gene, followed by acquired mutation of
the remaining allele .The APC gene is large and
over 1400 different mutations have been reported, but
most are loss-of-function mutations resulting in a truncated APC protein. This protein normally binds to and sequesters β-catenin but is unable to do so when mutated, allowing β-catenin to translocate to the nucleus, where it up-regulates the expression of many genes.
Around 20% of cases arise as new mutations and have no family history. Hundreds to thousands of adenomatous colonic polyps develop in 80% of patients by age 15 (Fig.), with symptoms such as rectal bleeding beginning a few years later. In those affected, cancer will develop within 10–15 years of the appearance of adenomas and 90% of patients will develop colorectal cancer by the age of 50 years.
Despite surveillance, approximately 1 in 4 patients with FAP have cancer by the time they undergo colectomy.
A second gene involved in base excision repair (MutY
homolog, MUTYH) has been identified which may give
rise to colonic polyposis. MUTYH displays autosomal
recessive inheritance and leads to tens to hundreds
of polyps and proximal colon cancer. This variant is
referred to as MUTYH-associated polyposis (MAP).
Non-neoplastic cystic fundic gland polyps occur in
the stomach but adenomatous polyps also occur uncommonly.
Duodenal adenomas occur in over 90% and are
most common around the ampulla of Vater. Malignant
transformation to adenocarcinoma occurs in 10% and
is the leading cause of death in those who have had
prophylactic colectomy. Many extra-intestinal features
are also seen in FAP .
Extra-intestinal features of familial adenomatous polyposis
Adenomatous colonic polyps.
A Before colonoscopicpolypectomy (arrows show polyps).
B After polypectomy.
Familial adenomatous polyposis.
There are hundreds ofadenomatous polyps throughout the colon.
Peutz–Jeghers syndrome
Multiple hamartomatous polyps occur in the small intestine and colon, as well as melanin pigmentation of the lips, mouth and digits .Most cases are asymptomatic, although chronic bleeding, anaemia or intussusception can occur. There is a significant risk of small bowel or colonic adenocarcinoma and of cancer of the pancreas, lung, ovary, breast and endometrium. It is anautosomal dominantly inherited disorder, most commonly
resulting from truncating mutations in a serine–
threonine kinase gene on chromosome 19p (STK11).
Diagnosis requires two of the three following features:
• small bowel polyposis
• mucocutaneous pigmentation
• a family history suggesting autosomal dominant inheritance.
Peutz– Jeghers syndrome.Typical lip pigmentation
The diagnosis can be made by genetic testing but thismay be inconclusive, since mutations in genes other than
STK11 can cause the disorder. Affected people should
undergo regular upper endoscopy, colonoscopy, and
small bowel and pancreatic imaging. Polyps greater
than 1 cm in size should be removed. Testicular examination is essential for men, while women should
undergo pelvic examination, cervical smears and regular
mammography. Asymptomatic relatives of affected
patients should also undergo screening.
Juvenile polyposis
In juvenile polyposis (JPS), tens to hundreds of mucus filledhamartomatous polyps are found in the colorectum.
One-third of cases are inherited in an autosomal
dominant manner and up to 20% develop colorectal
cancer before the age of 40. The criteria for diagnosis are:
• ten or more colonic juvenile polyps
• juvenile polyps elsewhere in the gut, or
• any polyps in those with a family history.
Germline mutations in the SMAD4 gene are often
found, as are PTEN mutations. Colonoscopy with
polypectomy should be performed every 1–3 years and
colectomy considered for extensive involvement.
Colorectal cancer
Is the second most common cancer in men. Although relatively rare in the developing world, colorectal cancer is the second most common internal malignancy and the second leading cause of cancer deaths in Western.
Pathophysiology
Both environmental and genetic factors are important Environmental
factors account for 70% of all ‘sporadic’ colorectal cancers. This figure is based on the wide geographical variation in incidence and the decrease in risk seen in migrants who move from high- to low-risk countries.
Dietary factors are most important . Box.
Colorectal cancer development results from the accumulation of multiple genetic mutations arising from two major pathways: chromosomal instability and microsatellite instability .
• Chromosomal instability. Mutations or deletions of
portions of chromosomes arise, with loss of heterozygosity (LOH) and inactivation of specific tumour suppressor genes. In LOH, one allele of a gene is deleted but gene inactivation only occurs when a subsequent unrelated mutation affects the other allele.
• Microsatellite instability. This involves germline mutations in one of six genes encoding enzymes involved in repairing errors that occur normally during DNA replication (DNA mismatch repair); these genes are designated hMSH2, hMSH6, hMLH1, hMLH3, hPMS1 and hPMS2.
About 5–10% of colon cancers are caused by HNPCC.
Pedigrees with this disorder have an autosomal dominant mode of inheritance and a positive family history of colon cancer occurring at a young age. The lifetime risk in affected individuals is 80%, with a mean age at cancer development of 45 years. In contrast to sporadic colon cancer, two-thirds of tumours occur proximally.The diagnostic criteria are listed in Box In a subset of patients, there is also an increased incidence of cancers of the endometrium, ovary, urinary tract, stomach, pancreas, small intestine and CNS, related to inheritance of different mismatch repair gene mutations.
Those who fulfil the criteria for HNPCC should be referred for pedigree assessment, genetic testing (see above) and colonoscopy.
These should begin around 25 years of age or
5–10 years earlier than the youngest case of cancer in
the family. Colonoscopy needs to be repeated every
1–2 years but, even then, interval cancers can still occur.
A family history of colorectal cancer can be obtained
in 20% of patients who do not fulfil the criteria for
HNPCC. In these families, the lifetime risk of developing
colon cancer is 1 in 12 and 1 in 6, respectively, when
one or two first-degree relatives are affected.
The risk is even higher if relatives were affected at an early age. The genes responsible for these cases are unknown, however.
Most tumours arise from malignant transformation of a
benign adenomatous polyp.
> 65% occur in the rectosigmoid and a further 15% recur in the caecum or ascending colon. Synchronous tumours are present in 2–5% of patients. Spread occurs through the bowel wall.
Rectal cancers may invade the pelvic viscera and side
walls. Lymphatic invasion is common at presentation, as is spread through both portal and systemic circulations
to reach the liver and, less commonly, the lungs.
Tumour stage at diagnosis is the most important determinant of prognosis .
Dietary risk factors for colorectal cancer
Non-dietary risk factors for colorectal cancerMedical conditions
• Colorectal adenomas
• Long-standing extensive ulcerative colitis or Crohn’s colitis , especially if associated with primary sclerosing cholangitis (PSC)
• Ureterosigmoidostomy
• Acromegaly
• Pelvic radiotherapy
Others
• Obesity and sedentary lifestyle – may be related to diet
• Smoking (relative risk 1.5–3.0)
• Alcohol (weak association)
• Cholecystectomy (effect of bile acids in right colon)
• Type 2 diabetes (hyperinsulinaemia)
Use of aspirin or NSAIDs (COX-2 inhibition) and perhaps
statins associated with reduced risk
Pathogenesis of colorectal cancer (CRC).
(FAP = familialadenomatous polyposis; HNPCC = hereditary non-polyposis colon cancer;
JPS = juvenile polyposis syndrome; MAP = MUTYH-associated polyposis;
PJS = Peutz– Jeghers syndrome)
Modified Amsterdam criteria* for
hereditary non-polyposis colon cancerThe multistep origin of cancer: molecular events implicated in colorectal carcinogenesis. (GTP = guanine triphosphate)
Modified Dukes classification and survival in colorectal cancer
Clinical featuresSymptoms vary depending on the site. In tumours of the left colon, fresh rectal bleeding is common and obstruction occurs early. Tumours of the right colon present with anaemia from occult bleeding or with altered bowel habit, but obstruction is a late. Colicky lower abdominal pain is present in two third and rectal bleeding occurs in 50%.
A minority present with obstruction or perforation, leading to peritonitis, abscess or fistula formation. Carcinoma of the rectum usually causes early bleeding, mucus discharge or a feeling of incomplete emptying. Between 10 and 20% present with iron deficiency anaemia or weight loss. On examination, there may be a palpable mass, signs of anaemia or hepatomegaly from metastases. Low rectal tumours may be palpable on digital examination.
Investigations
Colonoscopy is the investigation of choice because. Furthermore, lesions can be biopsied and polyps removed.Patients in whom colonoscopy is incomplete and those who are at high risk of complications can be investigated by
CT colonography (virtual colonoscopy). This is a sensitive and non-invasive technique for diagnosing tumours and polyps of more than 6 mm diameter. When the diagnosis of colon cancer has been made, CT of chest,abdomen and pelvis as a staging investigations. Pelvic MRI or endoanal ultrasound should be used for local staging of rectal cancer. Measurement of serum CEA levels are of limited
value in diagnosis, since values are normal in many
patients, but CEA testing can be helpful during follow-up to monitor for the prescience of recurrence.
Management :
SurgeryAll patients should be discussed at a multidisciplinary team meeting. Those with locally advanced rectal cancer should be offered neoadjuvant radiotherapy or chemoradiotherapy to increase the subsequent chance of a complete (R0) surgical resection. A 1-week course of
radiotherapy just prior to surgery reduces the risk of local recurrence in operable rectal cancer. The tumour should be removed, along with adequate resection margins and pericolic lymph nodes. Continuity should be restored by direct anastomosis, wherever possible.
Carcinomas within 2 cm of the anal verge may require
abdominoperineal resection and formation of a colostomy.All patients should be counselled pre-operatively
about the possible need for a stoma. Total mesorectal
excision (TME) reduces recurrence rates and increases
survival in rectal cancer. Metastatic disease confined to
liver or lung should be considered for resection, as this
can be potentially curative if there is truly no disease at
other sites. Post-operatively, patients should undergo
colonoscopy after 6–12 months and then at 5 years to
search for local recurrence or development of new
lesions, which occur in 6% of cases.
Adjuvant therapy
About 30–40% of patients have lymph node involvement
at presentation (see Fig.) and are, therefore, at risk of recurrence. Most recurrences are within 3 years
of diagnosis and affect the liver, lung, distant lymph
nodes and peritoneum. Adjuvant chemotherapy with
5-fluorouracil/folinic acid or capecitabine, preferably in
combination with oxaliplatin, can reduce the risk of
recurrence in patients with Dukes stage C cancers and
some high-risk Dukes B cancers. Post-operative radiotherapy reduces the risk of local recurrence in rectal cancer if operative resection margins are involved.
Palliation of advanced disease
Surgical resection of the primary tumour is appropriatefor some patients with metastases to treat obstruction,
bleeding or pain. Palliative chemotherapy with 5-
fluorouracil/ folinic acid, capecitabine, oxaliplatin or irinotecan improves survival. Patients with advanced
metastatic disease may be treated with monoclonal antibodies using bevacizumab or cetuximab, either alone or together with chemotherapy.
Pelvic radiotherapy is sometimes useful for distressing rectal symptoms such as pain, bleeding or severe tenesmus.
Endoscopic laser therapy or insertion of an expandable metal stent can be used to relieve obstruction .
Prevention and screening
Secondary prevention aims to detect and remove lesionsat an early or pre-malignant stage.
• Population-based screening of people over the age
of 50 years by regular FOB testing reduces mortality.
• Colonoscopy remains the gold standard but is
expensive and carries risks; many countries lack the
resources to offer this form of screening.
• Flexible sigmoidoscopy is an alternative option and has been shown to reduce overall mortality by approximately 35% (70% for cases arising in the rectosigmoid). It is recommended
in the USA every 5 years in all persons > age of 50.
• Screening for high-risk patients by molecular genetic analysis is an exciting prospect but is not yet available.
Faecal occult blood (FOB) testing and
colon cancer mortalityDiverticulosis
Diverticula are acquired and are most common in the sigmoid and descending colon of middle-aged people.Asymptomatic diverticula (diverticulosis) are present in over 50% of people above the age of 70 years. Symptomatic diverticular disease supervenes in 10–25% of cases, while complicated diverticulosis (acute diverticulitis, pericolic abscess, bleeding, perforation or stricture) is uncommon.
Pathophysiology
A life-long refined diet with a relative deficiency of fibre is widely thought to be responsible and the condition is rare in populations with a high dietary fibre intake, such as in Asia, where it more often affects the right side.
It is postulated that small-volume stools require high intracolonic pressures for propulsion and this leads to herniation of mucosa between the taeniae coli . Diverticula consist of protrusions of mucosa covered by peritoneum.
There is commonly hypertrophy of the circular muscle coat. Inflammation is thought to result from impaction of diverticula with faecoliths. This may resolve spontaneously or progress to cause haemorrhage, perforation, local abscess formation, fistula and peritonitis. Repeated attacks of inflammation lead to thickening of the bowel wall, narrowing of the lumen and eventual obstruction.
The human colon in diverticulosis. The colonic wall is weak between the taeniae. The blood vessels that supply the colon pierce the circular muscle and weaken it further by forming tunnels. Diverticula usually emerge through these points of least resistance
Clinical features
Symptoms are usually the result of associated constipation or spasm. Colicky pain is suprapubic or felt in the left iliac fossa. The sigmoid colon may be palpable and, in attacks of diverticulitis, there is local tenderness, guarding, rigidity (‘left-sided appendicitis’) and sometimes a palpable mass. During these episodes, there may be diarrhoea, rectal bleeding or fever. The differential diagnosis includes colorectal cancer, ischaemic colitis, inflammatory bowel disease and infection. Diverticular disease may be complicated by perforation, pericolic abscess, fistula formation (usually colovesical) or acute rectal bleeding.These complications are more common in patients who take NSAIDs or aspirin.
After one attack of diverticulitis, the recurrence rate is around 3% per year.
Over 10–30 years, perforation, obstruction or bleeding may occur, each affecting 5% of patients.
Investigations
Investigations are usually performed to exclude colorectal neoplasia. Barium enema can be used to confirm the presence of diverticula , strictures and fistulae. If barium studies are performed, flexible sigmoidoscopy is also necessary to exclude a coexisting neoplasm, which is easily missed radiologically. In severe diverticulosis, colonoscopy requires expertise and carries a risk of perforation. CT is used to assess complications, such as perforation or pericolic abscess.Management
Diverticular disease that is asymptomatic and discovered coincidentally requires no treatment. Constipation can be relieved by a high-fibre diet, with or without a bulking laxative (ispaghula husk, 1–2 sachets daily), taken with plenty of fluids. Stimulant laxatives (see Box) should be avoided. Antispasmodics may sometimes help. Acute attacks of diverticulitis should be treated with 7 days of metronidazole (400 mg 3 times daily orally), along with co-amoxiclav (500/125 mg 4 times daily). Severe cases require intravenous fluids, intravenous antibiotics, analgesia and nasogastric suction, but randomised trials show no benefit from acute resection compared to conservative management, and emergency surgery is reserved for severe haemorrhage or perforation.Percutaneous drainage of acute paracolic abscesses can be effective and avoids the need for emergency surgery.
Patients who have repeated attacks of obstruction should undergo elective surgery once the acute episode has settled, in order to resect the affected segment of bowel with restoration of continuity by primary anastomosis.
Constipation and disorders of defecation
Simple constipationSimple constipation is extremely common and does not signify underlying organic disease. It usually responds to increased dietary fibre or the use of bulking agents; an adequate fluid intake is also essential. Many types of
laxative are available, listed in Box.
Severe idiopathic constipation
Occurs almost exclusively in young women and often begins in childhood or adolescence. The cause is unknown but some have ‘slow transit’ with reduced motor activity in the colon. Others have ‘obstructed defecation’, resulting from inappropriate contraction of the external anal sphincter and puborectalis muscle (anismus).
The condition is often resistant to treatment. Bulking agents may exacerbate symptoms but prokinetic
agents or balanced solutions of polyethylene glycol ‘3350’ benefit some patients with slow transit.
Glycerol suppositories and biofeedback techniques are
used for those with obstructed defecation. Others benefit
from agents such as prucalopride or linaclotide. Rarely,
subtotal colectomy may be necessary as a last resort.
Laxatives
Faecal impaction
In faecal impaction, a large, hard mass of stool fills therectum. This tends to occur in disabled, immobile or
institutionalised patients, especially the frail elderly or
those with dementia. Constipating drugs, autonomic
neuropathy and painful anal conditions also contribute.
Megacolon, intestinal obstruction and urinary tract
infections may supervene. Perforation and bleeding
from pressure-induced ulceration are occasionally seen. Treatment involves adequate hydration and careful digital disimpaction after softening the impacted stool with arachis oil enemas. Stimulants should be avoided.
Constipation in old age
Melanosis coli and laxative misuse syndromesLong-term consumption of stimulant laxatives leads to accumulation of lipofuscin pigment in macrophages in the lamina propria. This imparts a brown discoloration to the colonic mucosa, often described as resembling ‘tiger skin’. The condition is benign and resolves when the laxatives are stopped. Prolonged laxative use may rarely result in megacolon or ‘cathartic colon’, in which
barium enema demonstrates a featureless mucosa, loss of haustra and shortening of the bowel. Surreptitious laxative misuse is a psychiatric condition seen in young women, some of whom have a history of bulimia or anorexia nervosa . They complain of
refractory watery diarrhoea. Laxative use is usually denied and may continue, even when patients are undergoing investigation. Screening of urine for laxatives may reveal the diagnosis.
Hirschsprung’s disease
This disease is characterised by constipation and colonic
dilatation (megacolon) due to congenital absence of ganglion cells in the large intestine. The incidence is approximately 1 : 5000. About one-third of patients have a positive family history and, in these families, the disease is inherited in an autosomal dominant manner with incomplete penetrance. About 50% of familial cases and 15% of sporadic cases have mutations affecting the RET proto-oncogene, which is also implicated in multiple
endocrine neoplasia type 2 (MEN 2) .
Unlike MEN 2, which is caused by activating RET mutations,
Hirschprung’s disease is caused by loss-of-function mutations.
Although RET is the most important susceptibility gene, some patients with RET mutations do not develop clinical disease, and mutations in other genes have been identified which interact to cause the disease.
All of the genes are implicated in Hirschprung’s disease are involved in the regulation of enteric neurogenesis, and the mutations cause failure of migration of neuroblasts into the gut wall during embryogenesis. Ganglion cells are absent from nerve plexuses, most commonly in a short segment of the rectum and/or sigmoid colon. As
a result, the internal anal sphincter fails to relax. Constipation, abdominal distension and vomiting usually develop immediately after birth but a few cases do not present until childhood or adolescence.
The rectum is empty on digital examination.
Barium enema shows a small rectum and colonic dilatation above the narrowed segment.Full-thickness biopsies are required to demonstrate nerve plexuses and confirm the absence of ganglion cells. Histochemical stains for acetylcholinesterase are also used. Anorectal manometry demonstrates failure of the rectum to relax with balloon distension. Treatment involves resection of the affected segment.
Acquired megacolon
This may develop in childhood as a result of voluntarywithholding of stool during toilet training. In such cases,
it presents after the first year of life and is distinguished
from Hirschsprung’s disease by the urge to defecate and
the presence of stool in the rectum. It usually responds
to osmotic laxatives.
In adults, acquired megacolon has several causes. It
is seen in depressed or demented patients, either as part
of the condition or as a side-effect of antidepressant
drugs. Prolonged misuse of stimulant laxatives may
cause degeneration of the myenteric plexus, while interruption of sensory or motor innervation may be responsible in a number of neurological disorders.
Patients taking large doses of opioid analgesics can develop a megacolon: so-called ‘narcotic bowel syndrome’. Scleroderma and hypothyroidism are other recognised causes.
Most patients can be managed conservatively by
treatment of the underlying cause, high-residue diets,
laxatives and the judicious use of enemas. Prokinetics
are helpful in a minority of patients.
Subtotal colectomy is a last resort for the most severely affected patients.
Acute colonic pseudo-obstruction (Ogilvie’s syndrome)
Has many causes and is characterised bysudden onset of painless, massive enlargement of the proximal colon; there are no features of mechanical obstruction. Bowel sounds are normal or high-pitched, rather than absent. Left untreated, it may progress to perforation, peritonitis and death. Abdominal X-rays show colonic dilatation with air extending to the rectum.
Caecal diameter greater than10 cm is associated with a high risk of perforation.
Single-contrast or water-soluble barium enemas demonstrate the absence of mechanical obstruction.
Management consists of treating the underlying disorder
and correcting any biochemical abnormalities.
The anticholinesterase, neostigmine, is effective in
enhancing parasympathetic activity and gut motility.
Decompression, either with a rectal tube or by colonoscopy, may be effective but needs to be repeated until the condition resolves. In severe cases, surgical or
fluoroscopic defunctioning caecostomy is necessary.
Causes of acute colonic pseudo-obstruction
Anorectal disordersFaecal incontinence
Common causes of incontinence are listed in Box. High-risk patients include frail older people, women after childbirth and those with severe neurological/spinal disorders, learning difficulties or cognitive impairment.
Patients are often embarrassed to admit incontinence
and may complain only of ‘diarrhoea’. A careful history
and examination, especially of the anorectum and
perineum, may help to establish the underlying cause.
Endoanal ultrasound is valuable for defining the integrity
of the anal sphincters, while anorectal physiology
and MR proctography are also useful investigations.
Management
This is often very difficult. Underlying disorders shouldbe treated and diarrhoea managed with loperamide,
diphenoxylate or codeine phosphate. Attention must be
paid to a proper diet and adequate fluid intake. Pelvic
floor exercises, biofeedback and bowel retraining techniques help some patients, and those with confirmed
anal sphincter defects may benefit from sphincter repair
operations. Where sphincter repair is not appropriate, a trial of sacral nerve stimulation is undertaken with a
view to insertion of a permanent stimulator but, if
unsuccessful, creation of a neo-sphincter may be possible, by graciloplasty or by an artificial anal sphincter retract spontaneously.
Causes of faecal incontinence
Third-degree piles are those which require manual replacement after prolapsing.Bright red rectal bleeding occurs after defecation.
Other symptoms include pain, pruritus ani and mucus
discharge. Treatment involves measures to prevent constipation and straining. Band ligation is effective for
many, but a minority of patients require haemorrhoidectomy, which is usually curative. Recently, haemorrhoidal artery ligation operation (HALO) procedures have been developed and may replace surgery. HALO involves using Doppler ultrasound to identify all the arteries feeding the haemorrhoids and ligating them.
Pruritus ani
This is common, most of which result in contamination of the perianal skin with faecal contents. Itching may be severe. When no underlying cause is found, all local barrier ointments and creams must be stopped. Good personal hygiene is essential, with careful washing after defecation. The perineal area must be kept dry and clean. Bulk-forming laxatives may reduce faecal soiling.Causes of pruritus ani
Solitary rectal ulcer syndromeThis is most common in young adults and occurs on the anterior rectal wall. It is thought to result from localised chronic trauma and/or ischaemia associated with disordered puborectalis function and mucosal prolapse. The ulcer is seen at sigmoidoscopy and biopsies show a characteristic accumulation of collagen.
Symptoms include minor bleeding and mucus per rectum, tenesmus and perineal pain. Treatment is often difficult but avoidance of straining at defecation is important and treatment of constipation may help. Marked mucosal prolapse is treated surgically.
Anal fissure
In this common problem, traumatic or ischaemic damage to the anal mucosa results in a superficial mucosal tear, most commonly in the midline posteriorly. Spasm of the internal anal sphincter exacerbates the condition. Severe pain occurs on defecation and there may be minor bleeding, mucus discharge and pruritus.
The skin may be indurated and an oedematous skin tag, or ‘sentinel pile’, adjacent to the fissure is common.
Avoidance of constipation with bulk-forming laxatives and increased fluid intake is important. Relaxation of the internal sphincter is normally mediated by nitric oxide, and 0.2% glyceryl trinitrate, which donates nitric oxide and improves mucosal blood flow, is effective in 60–80% of patients. Diltiazem cream (2%) can be used as an alternative. Resistant cases may respond to injection of botulinum toxin into the internal anal sphincter to
induce relaxation. Manual dilatation under anaesthesia
leads to long-term incontinence and should not be considered.
The majority of cases can be treated without surgery, but where these measures fail, healing can be achieved surgically by lateral internal anal sphincterotomy or advancement anoplasty.
Anorectal abscesses and fistulae
Perianal abscesses develop between the internal andexternal anal sphincters and may point at the perianal
skin. Ischiorectal abscesses occur lateral to the sphincters
in the ischiorectal fossa. They usually result from infection
of anal glands by normal intestinal bacteria. Crohn’s
disease is sometimes responsible.
Patients complain of extreme perianal pain, fever
and/or discharge of pus. Spontaneous rupture may lead
to the development of fistulae. These may be superficial
or track through the anal sphincters to reach the rectum.
Abscesses are drained surgically and superficial fistulae
are laid open with care to avoid sphincter damage.
Diseases of the peritoneal cavity
PeritonitisSurgical peritonitis occurs as the result of a ruptured
viscus . Peritonitis may also complicate ascites in chronic liver disease (spontaneous bacterial peritonitis) or may occur in children in the absence of ascites, due to infection with Streptococcus pneumoniae or β-haemolytic streptococci.
Chlamydial peritonitis is a complication of pelvic
inflammatory disease .The patient presents with
right upper quadrant pain, pyrexia and a hepatic rub
(the Fitz-Hugh–Curtis syndrome). Tuberculosis may
cause peritonitis and ascites .
Tumours
The most common is secondary adenocarcinoma from
the ovary or gastrointestinal tract. Mesothelioma is a
rare tumour complicating asbestos exposure. It presents
as a diffuse abdominal mass, due to omental infiltration,
and with ascites. The prognosis is extremely poor.
Other disorders
Endometriosis
Ectopic endometrial tissue can become embedded on the serosal aspect of the intestine, most frequently in the sigmoid and rectum.
The overlying mucosa is usually intact.
Cyclical engorgement and inflammation result in
pain, bleeding, diarrhoea, constipation, and adhesionsor obstruction. Low backache is frequent. The onset is
usually between 20 and 45 years and is more common
in nulliparous women. Bimanual examination may
reveal tender nodules in the pouch of Douglas. Endoscopic studies only reveal the diagnosis if carried out during menstruation, when a bluish mass with intact
overlying mucosa is apparent. In some patients,
laparoscopy is required. Treatment options include
laparoscopic diathermy and hormonal therapy with progestogens (e.g. norethisterone), gonadotrophin-releasing hormone analogues or danazol.
Pneumatosis cystoides intestinalis
In this rare condition, multiple gas-filled submucosal
cysts line the colonic and small bowel walls.
The cause is unknown, but the condition may be seen in patients with chronic cardiac or pulmonary disease, pyloric obstruction, scleroderma or dermatomyositis. Most
patients are asymptomatic, although there may be
abdominal cramp, diarrhoea, tenesmus, rectal bleeding
and mucus discharge. The cysts are recognised on sigmoidoscopy, plain abdominal X-rays or barium enema.
Therapies reported to be effective include prolonged
high-flow oxygen, elemental diets and antibiotics.