
Chemical Equilibrium
Chapter 14
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
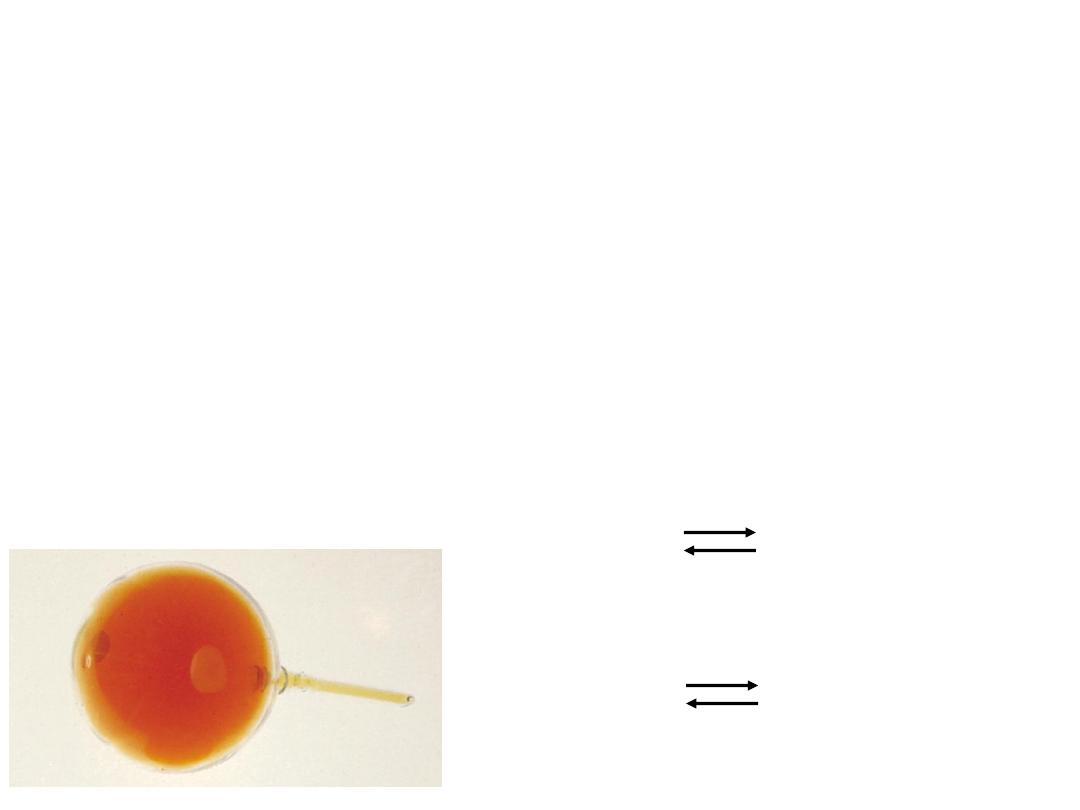
Equilibrium is a state in which there are no observable
changes as time goes by.
Chemical equilibrium is achieved when:
•
the rates of the forward and reverse reactions are equal and
•
the concentrations of the reactants and products remain
constant
Physical equilibrium
H
2
O
(l)
Chemical equilibrium
N
2
O
4
(g)
14.1
H
2
O
(g)
2NO
2
(g)
N
2
O
4
(g)
2NO
2
(g)
Start with NO
2
Start with N
2
O
4
Start with NO
2
& N
2
O
4
equilibrium
equilibrium
equilibrium
14.1
14.1
constant
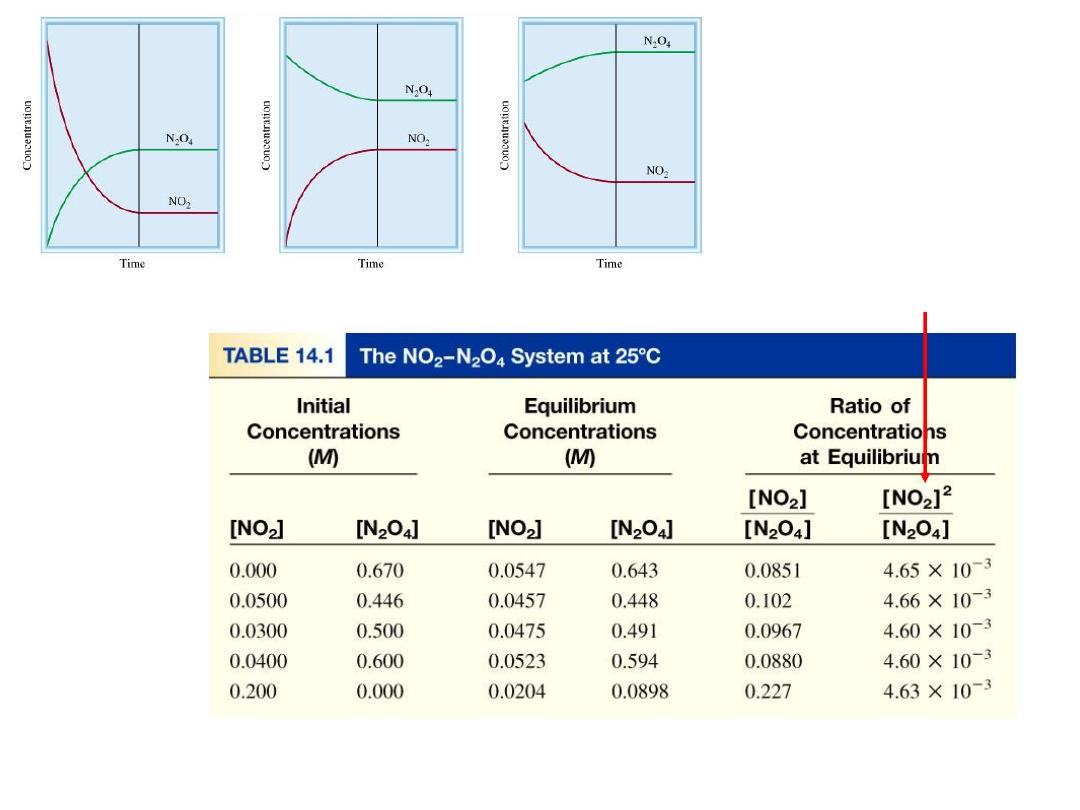
N
2
O
4
(g)
2NO
2
(g)
= 4.63 x 10
-3
K =
[NO
2
]
2
[N
2
O
4
]
aA + bB cC + dD
K =
[C]
c
[D]
d
[A]
a
[B]
b
Law of Mass Action
14.1
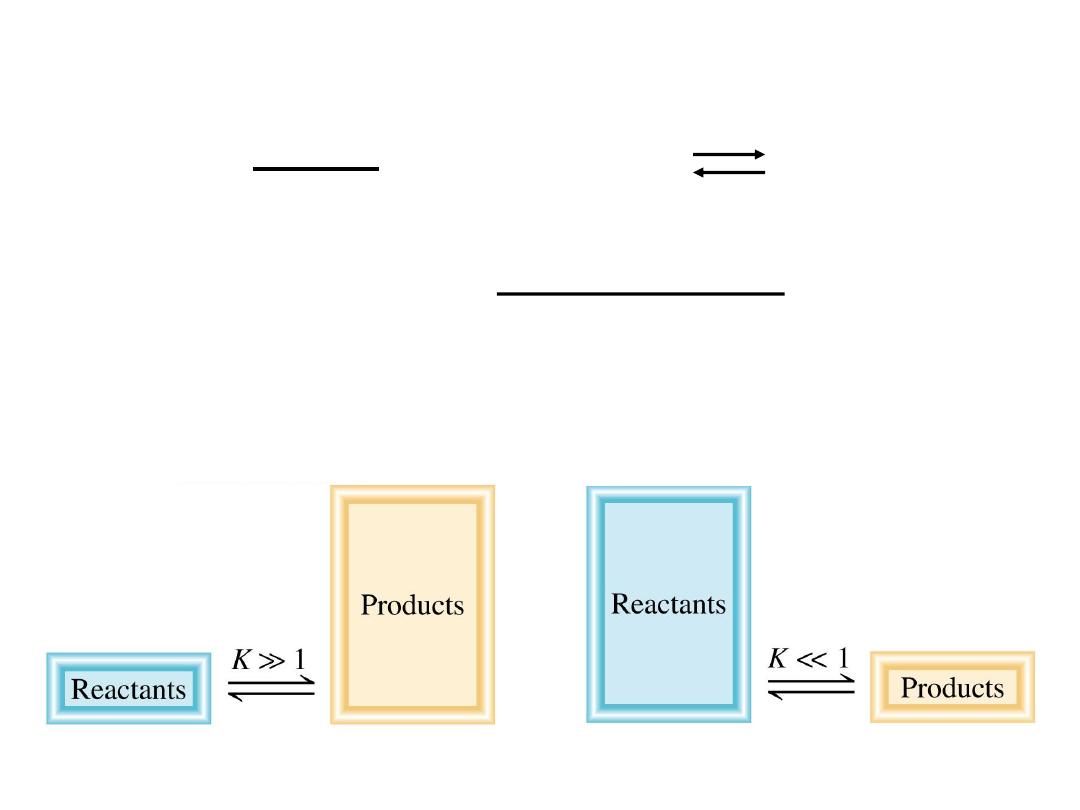
K >> 1
K << 1
Lie to the right
Favor products
Lie to the left
Favor reactants
Equilibrium Will
K =
[C]
c
[D]
d
[A]
a
[B]
b
aA + bB cC + dD
14.1
Homogenous equilibrium applies to reactions in which all
reacting species are in the same phase.
N
2
O
4
(g)
2NO
2
(g)
K
c
=
[NO
2
]
2
[N
2
O
4
]
K
p
=
NO
2
P
2
N
2
O
4
P
aA
(g)
+ bB
(g)
cC
(g)
+ dD
(g)
14.2
K
p
= K
c
(RT)
D
n
Dn = moles of gaseous products – moles of gaseous reactants
= (c + d)
– (a + b)
In most cases
K
c
K
p
Homogeneous Equilibrium
CH
3
COOH
(aq)
+ H
2
O
(l)
CH
3
COO
-
(aq)
+ H
3
O
+
(aq)
K
c
=
‘
[CH
3
COO
-
][H
3
O
+
]
[CH
3
COOH][H
2
O]
[H
2
O] = constant
K
c
=
[CH
3
COO
-
][H
3
O
+
]
[CH
3
COOH]
= K
c
[H
2
O]
‘
General practice not to include units for the
equilibrium constant.
14.2
The equilibrium concentrations for the reaction between
carbon monoxide and molecular chlorine to form COCl
2
(g)
at 74
0
C are [CO] = 0.012 M, [Cl
2
] = 0.054 M, and [COCl
2
] =
0.14 M. Calculate the equilibrium constants K
c
and K
p
.
CO
(g)
+ Cl
2
(g)
COCl
2
(g)
K
c
=
[COCl
2
]
[CO][Cl
2
]
=
0.14
0.012 x 0.054
= 220
K
p
= K
c
(RT)
D
n
D
n = 1
– 2 = -1
R = 0.0821
T = 273 + 74 = 347 K
K
p
= 220 x (0.0821 x 347)
-1
= 7.7
14.2
The equilibrium constant K
p
for the reaction
is 158 at 1000K. What is the equilibrium pressure of O
2
if
the P
NO
= 0.400 atm and P
NO
= 0.270 atm?
2
2NO
2
(g) 2NO (g) + O
2
(g)
14.2
K
p
=
2
P
NO
P
O
2
P
NO
2
2
P
O2
= K
p
P
NO
2
2
P
NO
2
P
O2
= 158 x (0.400)
2
/(0.270)
2
= 347 atm
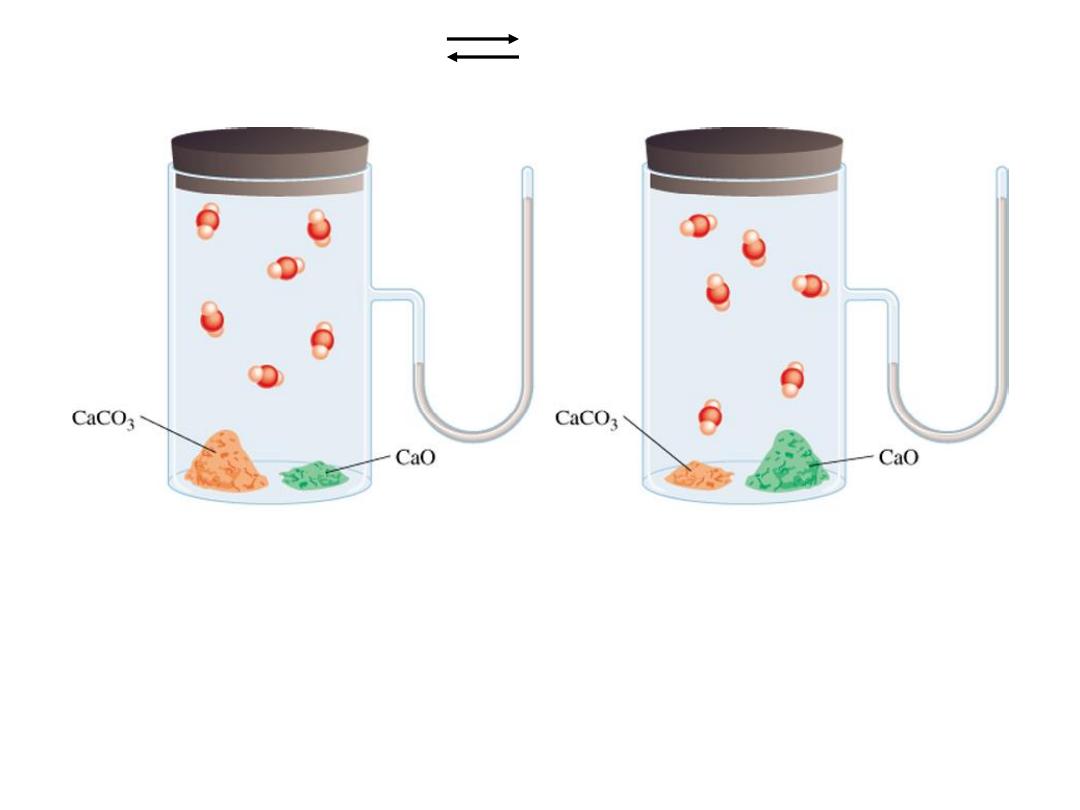
Heterogenous equilibrium applies to reactions in which
reactants and products are in different phases.
CaCO
3
(s)
CaO
(s)
+ CO
2
(g)
K
c
=
‘
[CaO][CO
2
]
[CaCO
3
]
[CaCO
3
] = constant
[CaO] = constant
K
c
= [CO
2
] = K
c
x
‘
[CaCO
3
]
[CaO]
K
p
= P
CO2
The concentration of solids and pure liquids are not
included in the expression for the equilibrium constant.
14.2
P
CO 2
= K
p
CaCO
3
(s)
CaO
(s)
+ CO
2
(g)
P
CO 2
does not depend on the amount of CaCO
3
or CaO
14.2

Consider the following equilibrium at 295 K:
The partial pressure of each gas is 0.265 atm. Calculate
K
p
and K
c
for the reaction?
NH
4
HS
(s)
NH
3
(g)
+ H
2
S
(g)
K
p
= P
NH
3
H
2
S
P
= 0.265 x 0.265 = 0.0702
K
p
= K
c
(RT)
D
n
K
c
= K
p
(RT)
-
D
n
D
n = 2
– 0 = 2
T = 295 K
K
c
= 0.0702 x (0.0821 x 295)
-2
= 1.20 x 10
-4
14.2
A + B C + D
C + D E + F
A + B E + F
K
c
=
‘
[C][D]
[A][B]
K
c
=
‘‘
[E][F]
[C][D]
[E][F]
[A][B]
K
c
=
K
c
‘
K
c
‘‘
K
c
K
c
=
K
c
‘‘
K
c
‘
x
If a reaction can be expressed as the sum of
two or more reactions, the equilibrium
constant for the overall reaction is given by
the product of the equilibrium constants of
the individual reactions.
14.2
N
2
O
4
(g)
2NO
2
(g)
= 4.63 x 10
-3
K =
[NO
2
]
2
[N
2
O
4
]
2NO
2
(g)
N
2
O
4
(g)
K =
[N
2
O
4
]
[NO
2
]
2
‘
=
1
K
= 216
When the equation for a reversible reaction
is written in the opposite direction, the
equilibrium constant becomes the reciprocal
of the original equilibrium constant.
14.2

Writing Equilibrium Constant Expressions
1. The concentrations of the reacting species in the
condensed phase are expressed in M. In the gaseous
phase, the concentrations can be expressed in M or in atm.
2. The concentrations of pure solids, pure liquids and solvents
do not appear in the equilibrium constant expressions.
3. The equilibrium constant is a dimensionless quantity.
4. In quoting a value for the equilibrium constant, you must
specify the balanced equation and the temperature.
5. If a reaction can be expressed as a sum of two or more
reactions, the equilibrium constant for the overall reaction is
given by the product of the equilibrium constants of the
individual reactions.
14.2
14.3
Chemical Kinetics and Chemical Equilibrium
A + 2B AB
2
k
f
k
r
rate
f
= k
f
[A][B]
2
rate
r
= k
r
[AB
2
]
Equilibrium
rate
f
= rate
r
k
f
[A][B]
2
= k
r
[AB
2
]
k
f
k
r
[AB
2
]
[A][B]
2
= K
c
=
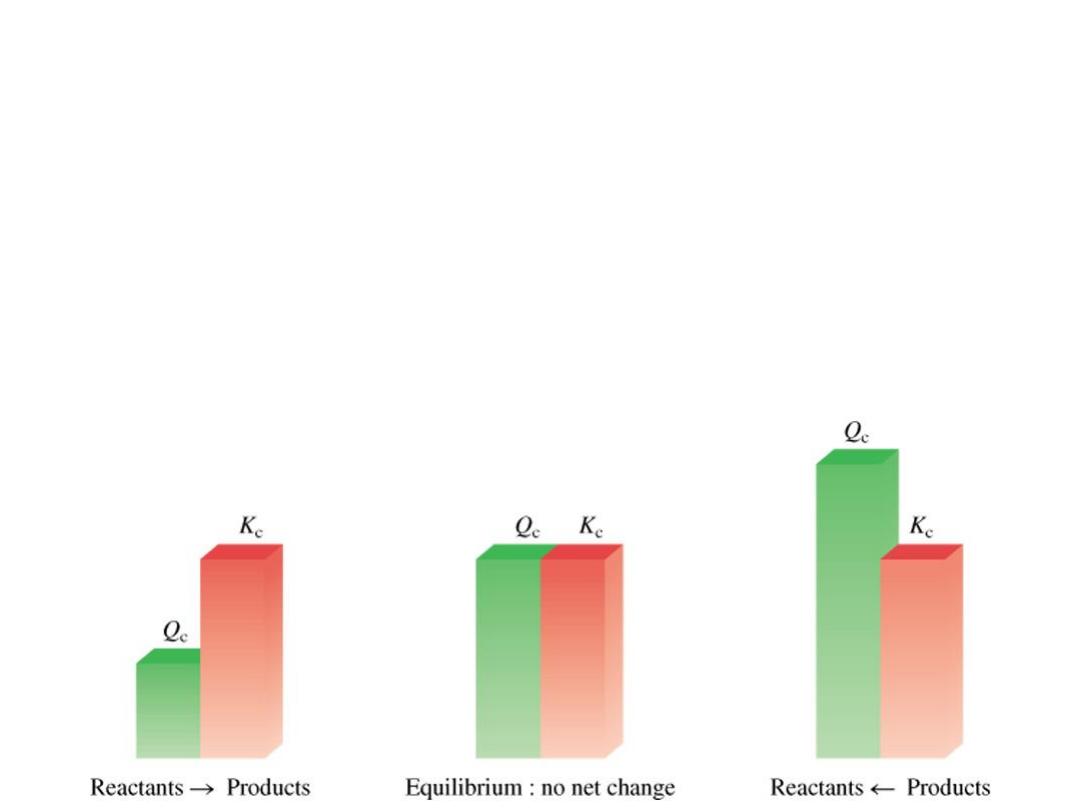
The reaction quotient (Q
c
) is calculated by substituting the
initial concentrations of the reactants and products into the
equilibrium constant (K
c
) expression.
IF
•
Q
c
> K
c
system proceeds from right to left to reach equilibrium
•
Q
c
= K
c
the system is at equilibrium
•
Q
c
< K
c
system proceeds from left to right to reach equilibrium
14.4

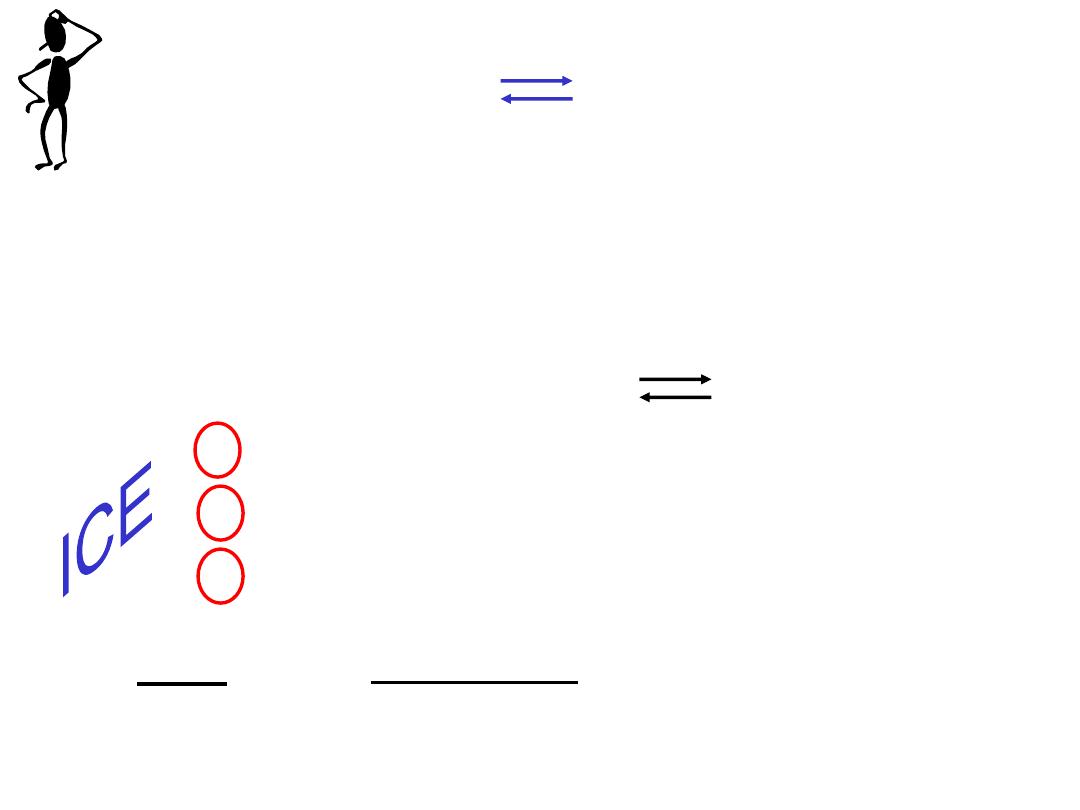
Calculating Equilibrium Concentrations
1. Express the equilibrium concentrations of all species in
terms of the initial concentrations and a single unknown x,
which represents the change in concentration.
2. Write the equilibrium constant expression in terms of the
equilibrium concentrations. Knowing the value of the
equilibrium constant, solve for x.
3. Having solved for x, calculate the equilibrium
concentrations of all species.
14.4
At 1280
0
C the equilibrium constant (K
c
) for the reaction
Is 1.1 x 10
-3
. If the initial concentrations are [Br
2
] = 0.063
M and [Br] = 0.012 M, calculate the concentrations of these
species at equilibrium.
Br
2
(g) 2Br (g)
Br
2
(g) 2Br (g)
Let x be the change in concentration of Br
2
Initial (M)
Change (M)
Equilibrium (M)
0.063
0.012
-x
+2x
0.063 - x
0.012 + 2x
[Br]
2
[Br
2
]
K
c
=
K
c
=
(0.012 + 2x)
2
0.063 - x
= 1.1 x 10
-3
Solve for x
14.4
K
c
=
(0.012 + 2x)
2
0.063 - x
= 1.1 x 10
-3
4x
2
+ 0.048x + 0.000144 = 0.0000693
– 0.0011x
4x
2
+ 0.0491x + 0.0000747 = 0
ax
2
+ bx + c =0
-b
± b
2
– 4ac
2a
x =
Br
2
(g) 2Br (g)
Initial (M)
Change (M)
Equilibrium (M)
0.063
0.012
-x
+2x
0.063 - x
0.012 + 2x
x = -0.00178
x = -0.0105
At equilibrium, [Br] = 0.012 + 2x = -0.009 M or 0.00844 M
At equilibrium, [Br
2
] = 0.062
– x = 0.0648 M
14.4
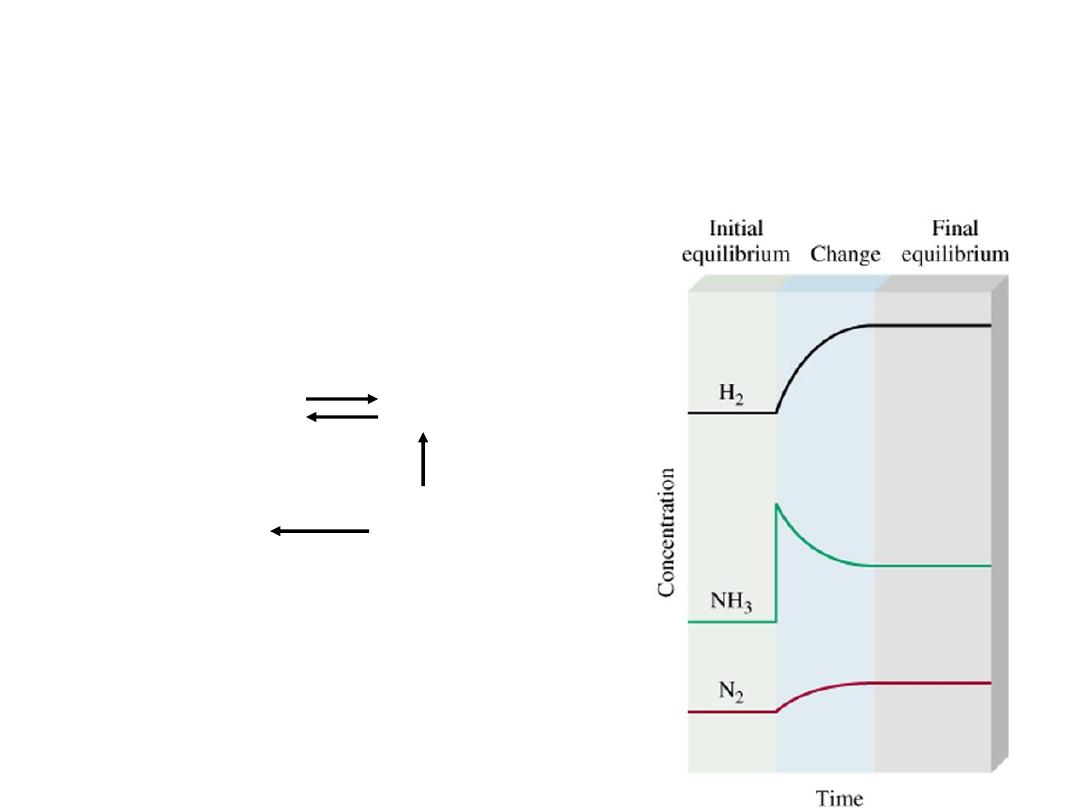
If an external stress is applied to a system at equilibrium, the
system adjusts in such a way that the stress is partially offset
as the system reaches a new equilibrium position.
Le Ch
âtelier’s Principle
• Changes in Concentration
N
2
(g)
+ 3H
2
(g)
2NH
3
(g)
Add
NH
3
Equilibrium
shifts left to
offset stress
14.5
Le Ch
âtelier’s Principle
• Changes in Concentration continued
Change
Shifts the Equilibrium
Increase concentration of product(s)
left
Decrease concentration of product(s)
right
Decrease concentration of reactant(s)
Increase concentration of reactant(s)
right
left
14.5
aA + bB cC + dD
Add
Add
Remove
Remove
Le Ch
âtelier’s Principle
• Changes in Volume and Pressure
A
(g)
+ B
(g)
C
(g)
Change
Shifts the Equilibrium
Increase pressure
Side with fewest moles of gas
Decrease pressure
Side with most moles of gas
Decrease volume
Increase volume
Side with most moles of gas
Side with fewest moles of gas
14.5
Le Ch
âtelier’s Principle
• Changes in Temperature
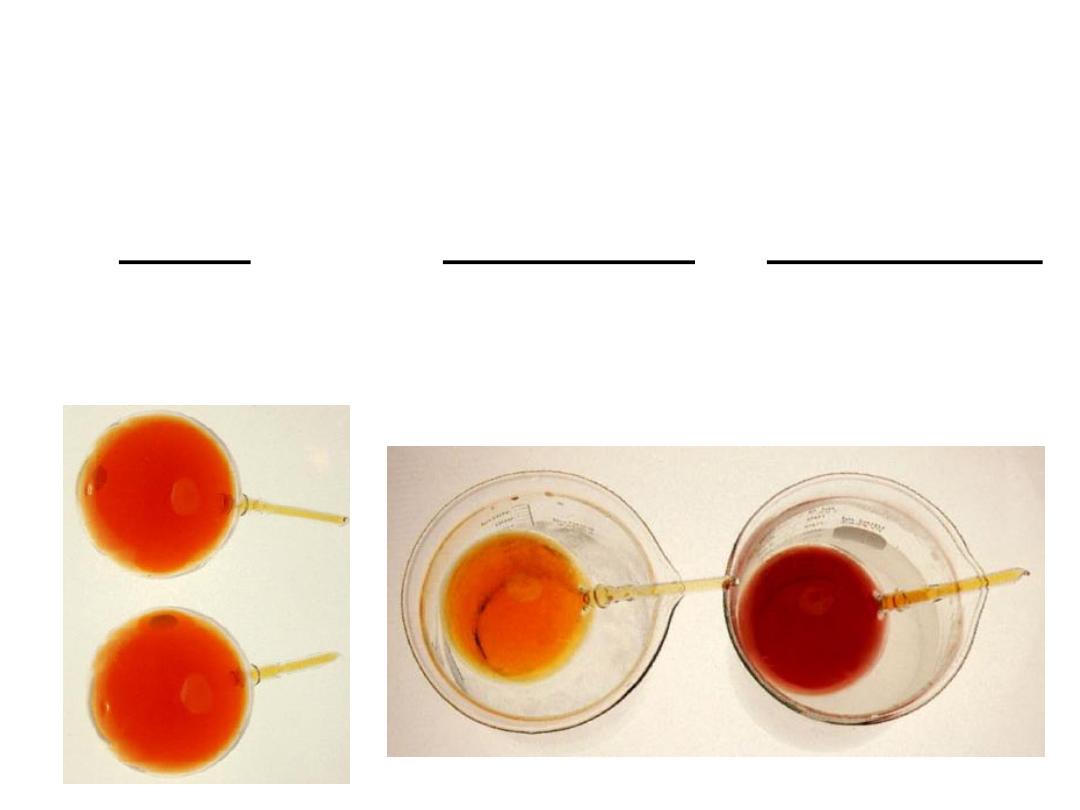
Change
Exothermic Rx
Increase temperature
K decreases
Decrease temperature
K increases
Endothermic Rx
K increases
K decreases
14.5
colder
hotter
uncatalyzed
catalyzed
14.5
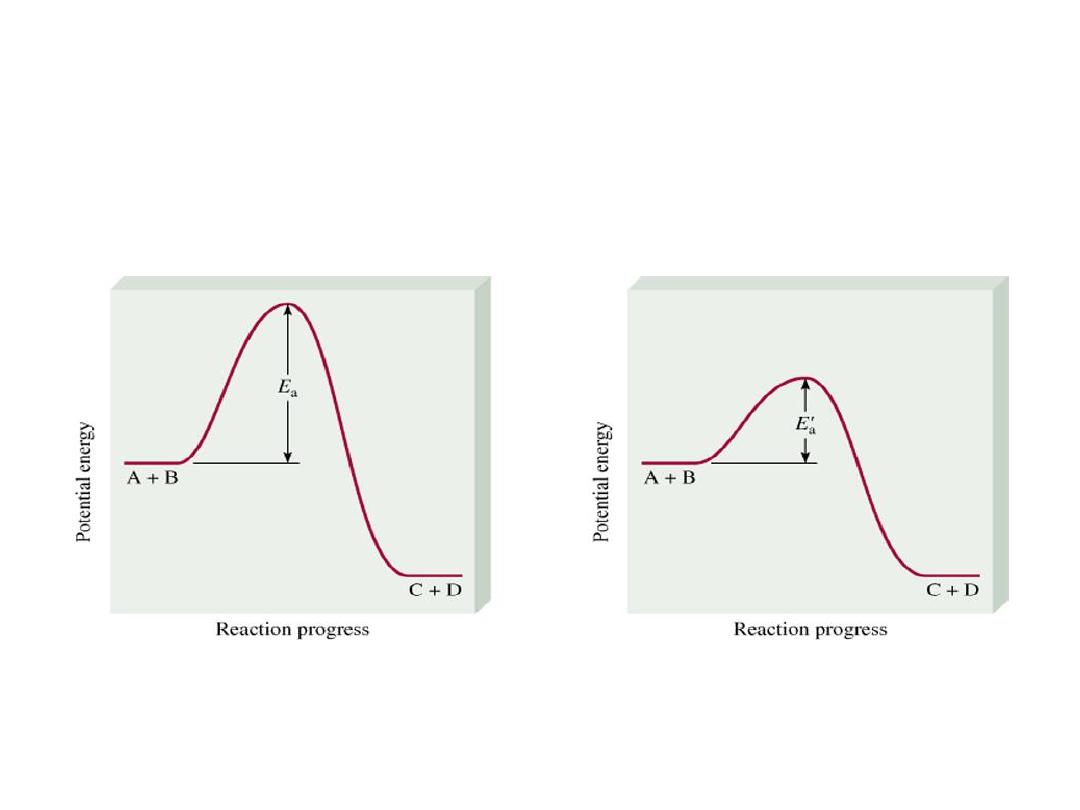
Catalyst lowers E
a
for both forward and reverse reactions.
Catalyst does not change equilibrium constant or shift equilibrium.
• Adding a Catalyst
• does not change K
• does not shift the position of an equilibrium system
• system will reach equilibrium sooner
Le Ch
âtelier’s Principle

Chemistry In Action
Life at High Altitudes and Hemoglobin Production
K
c
=
[HbO
2
]
[Hb][O
2
]
Hb (aq) + O
2
(aq) HbO
2
(aq)
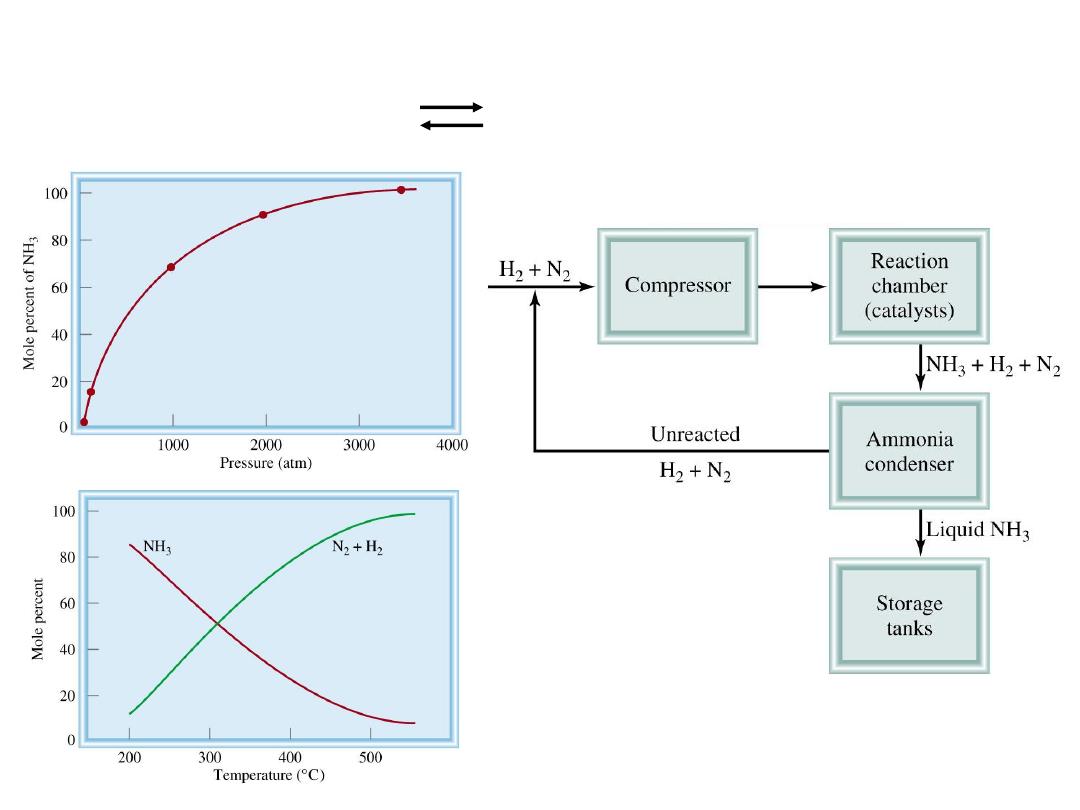
Chemistry In Action: The Haber Process
N
2
(g) + 3H
2
(g) 2NH
3
(g)
DH
0
= -92.6 kJ/mol
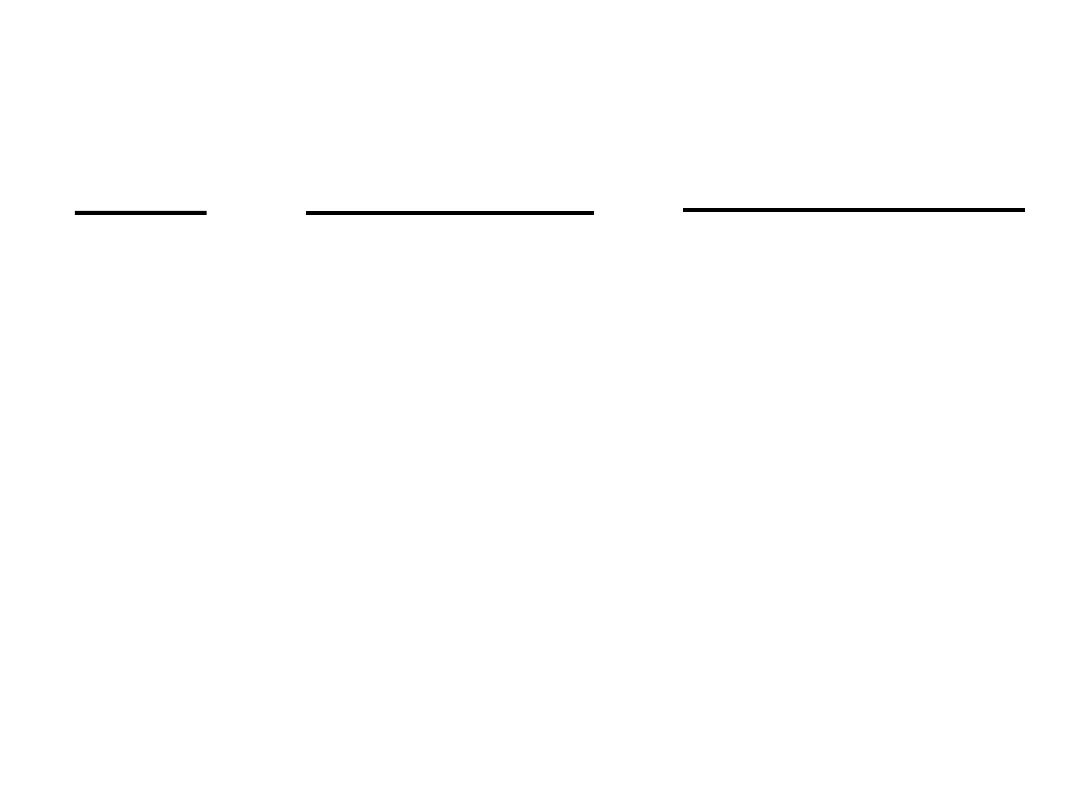
Le Ch
âtelier’s Principle
Change
Shift Equilibrium
Change Equilibrium
Constant
Concentration
yes
no
Pressure
yes
no
Volume
yes
no
Temperature
yes
yes
Catalyst
no
no
14.5
