
Dr.Amjed H.Abbas Respiratory physiology lec.4
1
Clinical notes related to hemoglobin–oxygen dissociation Curve
Fetal hemoglobin (Hb F) has a slightly higher affinity for O2 than does the adult
form (Hb A) because it binds 2,3-diphosphoglycerate (2,3-DPG) less avidly. This
facilitates the uptake of O2 by the fetus at the relatively low values of PO2 that exist
in the placenta.
Methemoglobinemia is a condition in which there are higher levels of
methemoglobin in the blood than normal. Methemoglobin is formed when the heme
moiety contains iron in the ferric state (Fe 3+) rather than the ferrous state (Fe 2+).
This may occur when red blood cells are exposed to exogenous oxidizing drugs and
their metabolites. Methemoglobin does not bind O2, so people with this condition will
show signs of hypoxia, including shortness of breath (dyspnea), dizziness, cyanosis,
fatigue, and mental changes.
Treatment includes the administration of methylene blue, a substance that is able to
reduce iron in hemoglobin to its normal, oxygen carrying
state, and supplemental O2
Shifts in the Hemoglobin–Oxygen Dissociation Curve
The affinity of hemoglobin for O2 is somewhat dependent on conditions.
A decrease in affinity is called a “shift to the right” of the hemoglobin–
O2 dissociation curve, whereas an increase in affinity is called a “shift to
the left” .
– A right shift facilitates the unloading of O2 in peripheral tissues.
Conditions favoring a shift to the right (high temperature, low pH, and
high PCO2) exist in exercising muscle. Unloading more O2 raises local
PO2 and drives O2 diffusion into nearby tissues. In muscle, this benefits
oxidative metabolism. A shift to the right also occurs when there is
increased levels of 2,3-DPG in the blood at high altitude.
Figure shows shift to the right
Dr.Amjed H.Abbas Respiratory physiology lec.4
2
Shift to the left:
This occurs in the lungs when the affinity of Hb for oxygen increases. So
there will be loading of O2 from the lungs.
Factors that cause shift to the left:
1.Decrease in PCO2 and increases in pH:
When tissue metabolism decrease. CO2 production and H+ conc.
decreases. Thus, when O2 demand decreases, O2 unloading to tissue
decreases.
2.Decreases in temperature:
When tissue metabolism decreases, less heat is produced and less O2 is
unloaded to the tissues.
3.Decrease in 2,3-DPG concentration:
This reflects decreased tissue metabolism, causing a left shift of the curve
and less oxygen to be unloaded in the tissues.
Effect of Carbon Monoxide (CO)
CO combines Hb at the same point as does O2, and can displace O2 from
hemoglobin.CO binds with about 250 times as much as O2. PCO greater than 40
mmHg can be lethal. Administering 100 percent (pure) oxygen is the usual treatment
for carbon monoxide poisoning as it speeds up the separation of carbon monoxide
from hemoglobin.
Carbon Dioxide Transport in Blood
CO2 is mainly transported in blood in the form of bicarbonate (HCO3−)
(70%). It is also transported as dissolved CO2 (10%) or
carbaminohemoglobin (20%).
Less than 10 percent of the total quantity of carbon dioxide carried in the
blood is eliminated during passage through the lungs. Complete
elimination would lead to large changes in acidity between arterial and
ains in the pulmonary
venous blood. Furthermore, blood normally rem
Dissolved
bound to Hb
HCO3-

Dr.Amjed H.Abbas Respiratory physiology lec.4
3
carbon
capillaries less than a second, an insufficient time to eliminate all
dioxide.
Carbon dioxide transport:
1. Dissolved in the plasma (10%)
About 10% of the total CO2 in the blood is transported as a dissolved
form in the plasma.
Solubility of CO2= 0.03ml CO2/100ml blood/mmHg.
Dissolved CO2 in arterial blood = 40mmHg x0.03mlCO2/100ml
blood/mmHg= 1.2ml/100ml blood.
2. Carbamino-hemoglobin (30%)
CO2 binds to terminal aminogroups on proteins (eg. Hb and plasma
proteins such as albumin). When CO2 is bound to Hb, it is called
carbaminohemoglobin, which accounts for about 30% of the total CO2.
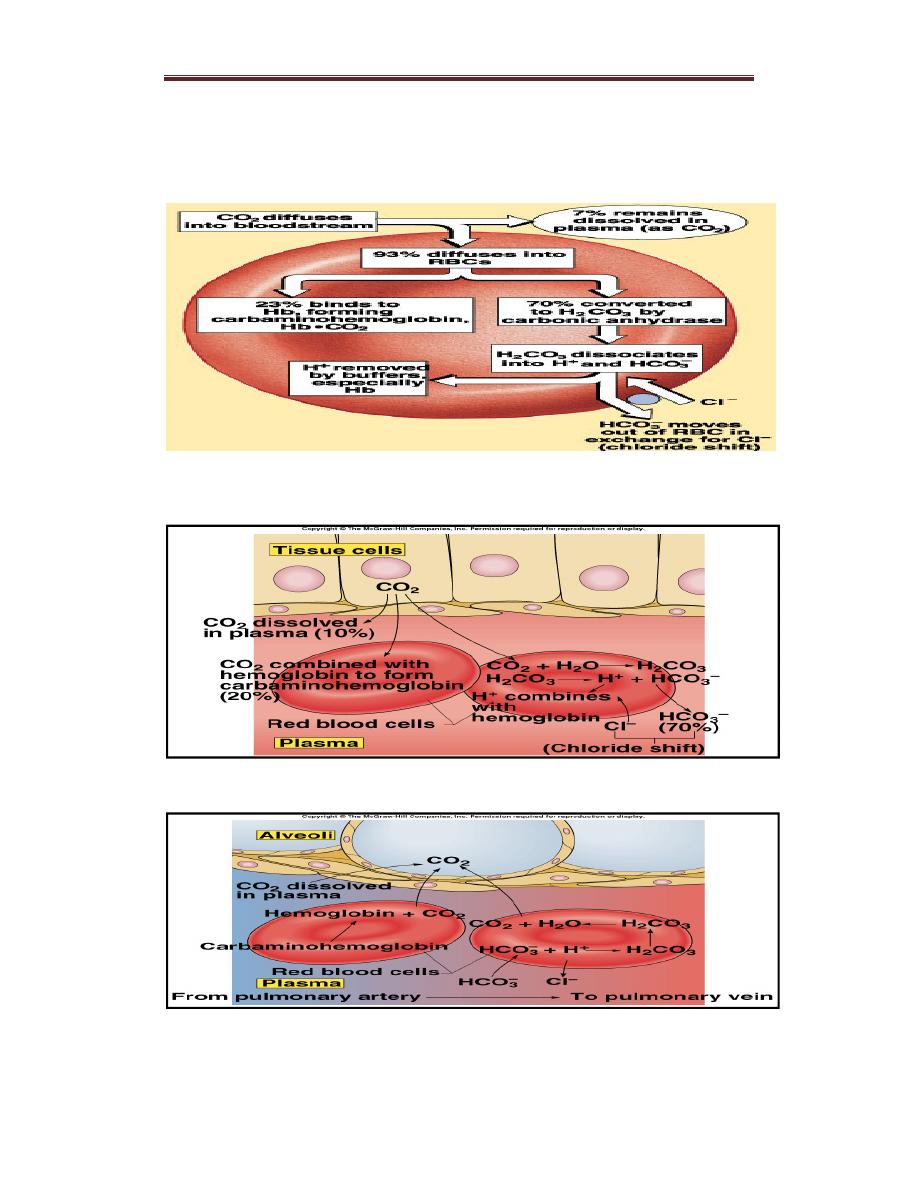
3.Transport of Carbon Dioxide as Bicarbonate
CO2 is produced by tissues as a result of aerobic respiration. It freely
diffuses into red blood cells and is then transported in blood by the
following steps:– CO2 combines with H2O within red blood cells to form
carbonic acid (H2CO3). This is catalyzed by carbonic anhydrase. H2CO3
dissociates into H+ and HCO3−.
– Much of the HCO3− moves into the plasma in exchange for Cl−(the
“chloride shift”). Plasma, as well as the red blood cells, is therefore a
vehicle for the transportation of CO2 to the lungs.
– The H+ produced is largely buffered by hemoglobin. Removal of O2
from the blood by uptake into tissues increases the amount of CO2
that can be converted to HCO3− because deoxygenated hemoglobin is a
better buffer than oxygenated hemoglobin. In addition, deoxygenated
hemoglobin binds more carbamino-CO2 than oxygenated hemoglobin.
– In the lungs, the reverse of the process described above occurs: HCO3
Dr.Amjed H.Abbas Respiratory physiology lec.4
4
− enters red blood cells in exchange for Cl−, and HCO3− binds to H+
to form H2CO3. H2CO3 then breaks down to form CO2 and H2O, and
CO2 is expired.
Figure :CO2 Transport and Cl- Movement
Transport of CO2 from tissues
Figure: Transport of CO2 to the alveoli
