
Respiratory System PhysiologyDr. Amjed Hassan lecture 2
1
Compliance of the Respiratory System
Lung Compliance
Lung compliance expresses the dispensability of the lungs, that is, how
easily the lungs expand when trans-pulmonary pressure increases. It is
expressed by the following equation:
C = ΔV/ΔP
where
C = lung compliance
ΔV = increase in lung volume (mL)
ΔP = increase in trans-pulmonary pressure (mm Hg).
– Compliance is inversely related to stiffness.
– Compliance is inversely related to the elastic recoil, or elastance, of the
lung. Recoil causes the lungs to return to their previous volume when
stretching ceases following an increase in trans-pulmonary pressure. It is
mediated by surface tension in the alveoli and by elastic fibers in the
lung connective tissue.
Compliance of the Lung–Chest Wall Combination
Because the lungs and chest wall expand and contract together, the
overall compliance of the respiratory system is that of the lung–chest wall
combination. The compliance of the lung–chest wall combination is
lower than the compliance of the lungs alone or chest wall alone.
– The compliance of the lung–chest wall combination varies with lung
volume. Compliance is highest at the normal resting volume (functional
residual capacity [FRC]) and decreases at both very low and very high
volumes.
– At low volumes, compression of the chest wall reduces compliance.
– At high volumes, the increased stretch of elastic tissues in the lung
parenchyma causes the lungs to get stiffer (less compliant). High trans-

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
2
pulmonary pressure is required to drive this increase in volume, but it is
not responsible for the decrease in compliance.
Changes in lung compliance in disease states
– Lung compliance is decreased in pulmonary fibrosis because the
interstitium surrounding the alveoli becomes infiltrated with inelastic
collagen.
– Lung compliance is increased in emphysema because many small
alveoli are replaced by fewer but larger coalesced air spaces that have less
elastic recoil.
Surface Tension in the Alveoli
Surface tension is due to the cohesive forces between water molecules at
the air–water interface in the alveoli of lungs. It acts to contract the
alveoli and is a major contributor to the force of elastic recoil of the lung.
If there were no opposing force, surface tension would cause the alveoli
to collapse (atelectasis).
However, the collapsing force is opposed by trans-pulmonary pressure,
which is always positive, allowing the alveoli to remain open.
According to the law of Laplace, the trans-pulmonary pressure P (in
dynes/cm2) required to prevent collapse of an alveolus is directly
proportional to surface tension T (in dynes/cm), and inversely
proportional to alveolar radius r (in cm), as expressed by
P = 2T/r
– All alveoli in a given region of the lungs have about the same trans-
pulmonary pressure. If they all had the same surface tension, the Laplace

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
3
relationship predicts that the smaller alveoli would collapse and force
their volume into larger alveoli. However, surface tension is reduced by
pulmonary surfactant, and the reduction is greater in small alveoli than in
larger ones because small alveoli concentrate the surfactant. Thus, the
increased tendency to collapse because of small radius is just balanced by
a greater reduction in surface tension.
Surfactant
Surfactant is a complex substance, consisting of proteins and
phospholipids (mainly dipalmitoyl lecithin), that is produced in type II
pneumocytes. It lines alveoli and lowers surface tension by the same
mechanism as detergents and soaps (i.e., it coats the water surface and
reduces cohesive
interactions between water molecules).
As an extension of its role in lowering surface tension, surfactant also
produces the following effects:
– It increases compliance at all lung volumes, which allows for easier
lung inflation and greatly decreases the work of breathing.
– It reduces the otherwise highly negative pressure in the interstitial
space, which reduces the rate of filtration from pulmonary capillaries.
This assists in maintaining lungs without excessive water.
Failure of surfactant production and/or excessive surfactant breakdown
occurs in neonatal respiratory distress syndrome (RDS).
Effect of Alveolar Radius on the Pressure Caused by Surface
Tension. Note from the preceding formula that the pressure generated as a
result of surface tension in the alveoli is inversely affected by the radius
of the alveolus, which means that the smaller the alveolus, the greater the
alveolar pressure caused by the surface tension. Thus, when the alveoli

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
4
have half the normal radius (50 instead of 100 micrometers), the
pressures noted earlier are doubled. This is especially significant in small
premature babies, many of whom have alveoli with radii less than one
quarter that of an adult person.
Further, surfactant does not normally begin to be secreted into the alveoli
until between the sixth and seventh months of gestation, and in some
cases, even later than that. Therefore, many premature babies have little
or no surfactant in the alveoli when they are born, and their lungs have an
extreme tendency to collapse, sometimes as great as six to eight times
that in a normal adult person. This causes the condition called respiratory
distress syndrome of the newborn. It is fatal if not treated with strong
measures, especially properly applied continuous positive pressure
breathing.
The work of inspiration can be divided into three fractions: (1) that
required to expand the lungs against the lung and chest elastic forces,
called compliance work or elastic work; (2) that required to overcome the
viscosity
of the lung and chest wall structures, called tissue resistance work; and
(3) that required to overcome airway resistance to movement of air into
the lungs, called airway resistance work.
Energy Required for Respiration. During normal quiet respiration, only 3
to 5 per cent of the total energy expended by the body is required for
pulmonary ventilation. But during heavy exercise, the amount of energy
required can increase as much as 50-fold, especially if the person has any
degree of increased airway resistance or decreased pulmonary
compliance. Therefore, one of the major limitations on the intensity of
exercise that can be performed is the person’s ability to provide enough
muscle energy for the respiratory process alone.

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
5
Airflow through the Bronchial Tree
Airflow through the bronchial tree obeys the same principles as blood
flow through blood vessels except that the viscosity of air is much lower
than that of blood. Airflow is related to the driving pressure and the
resistance to flow by
Q = ΔP/R
where Q is airflow (mL/min), ΔP is pressure gradient between the
mouth/nose and alveoli (cm H2O),
and R is airway resistance (cm H2O/mL/min).
– Airflow is directly proportional to the pressure difference between the
mouth/nose and the alveoli and inversely proportional to airway
resistance.
Airway Resistance
Resistance is derived from Poiseuille’s equation as expressed by
R = 8ηL/πr4
where R is airway resistance, r is radius of the airway (cm), η is viscosity
of air, and L is length of the airway.
– Like the circulatory system, the length of the bronchial tree is relatively
constant, as is the viscosity of inspired air. Therefore, any changes in
resistance to airflow are mainly due to changes in the radius of the
airways. Because resistance is inversely proportional to the airway radius
to the fourth power, small changes in diameter cause large changes in
resistance.
– The large airways offer little resistance to airflow. The small airways
individually have high resistance, but their enormous number in parallel
reduces their combined resistance to a small value. Therefore, the sites of
highest resistance in the bronchial tree are normally in the medium
airways.

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
6
Regulation of Airway Resistance. Airway resistance is primarily
regulated by modulation of airway radius by the parasympathetic and
sympathetic nervous systems.
– Parasympathetic nervous system: Vagal stimulation releases
acetylcholine that acts on muscarinic (M3) receptors in the lungs, leading
to bronchoconstriction. This increases the resistance to airflow.
– Sympathetic nervous system: Postganglionic sympathetic nerves release
norepinephrine that act on β2 receptors, leading to broncho-dilation. This
decreases the resistance to airflow
Lung Volumes and Capacities
– Lung volumes are a way to functionally divide volumes of air that
occur during different phases of the breathing cycle (Fig. 12.5). They are
all measured by spirometry, except for residual volume.
They vary with height, sex, and age.
– Lung capacities are the sums of two or more lung volumes.
– Tidal, inspiratory, and expiratory reserve volumes and inspirational and
vital capacities are used in basic pulmonary function tests.
Lung Volumes
– Tidal volume (TV) is the volume of air that moves in or out of the
lungs during one normal, resting inspiration or expiration.
– Inspiratory reserve volume (IRV) is the volume of air that can be
inspired beyond a normal inspiration.
– Expiratory reserve volume (ERV) is the volume of air that can be
expired beyond a normal expiration.
– Residual volume (RV) is the volume of air left in the lungs and
airways after maximal expiration.

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
7
Table 12.1 contains the normal approximate lung volumes and expresses
them as a percentage of total lung capacity (TLC).
Lung Capacities
– Inspirational capacity (IC) is the maximum volume of air that can be
inspired with a deep breath following a normal expiration. It is the sum of
TV and IRV.
– Functional residual capacity (FRC) is the volume of the lungs after
passive expiration with relaxed respiratory muscles. It is the sum of ERV
and RV.
– Vital capacity (VC) or forced vital capacity (FVC): is the maximum
volume of air that can be expired in one breath after deep inspiration. It is
the sum of TV, IRV, and ERV.
– Total lung capacity (TLC) is the total volume of air that can be
contained in the lungs and airways after a deep inspiration. It is the sum
of all four lung volumes: TV, IRV, ERV, and RV.
Note: TLC and FRC cannot be measured by spirometry because residual
volume is needed for their calculation.
Table 12.2 contains the normal lung capacity volumes.
Forced Expiratory Volume (FEV1) is the volume of air that can be
forcibly expired in the first second following a deep breath
It is usually > 70% of the FVC (FEV1/FVC > 70%).
– In obstructive lung disease (e.g., asthma and COPD), FEV1
is reduced proportionally more than FVC; therefore, FEV1 /FVC < 70%.
– In restrictive lung disease (e.g., fibrosis), both FEV1 and FVC are
reduced. This means that FEV1 /FVC is normal or increased.
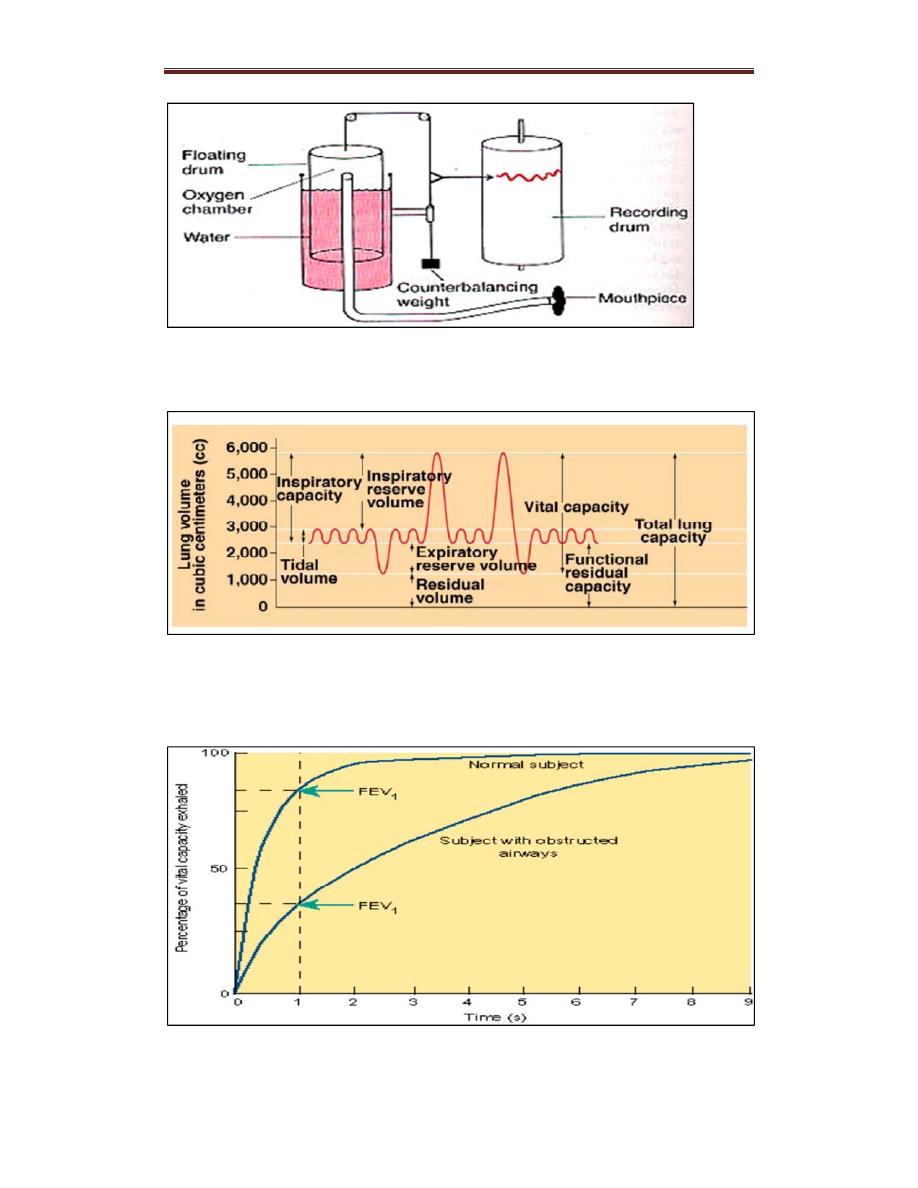
Respiratory System PhysiologyDr. Amjed Hassan lecture 2
8
Figure: old spirometry technique
Figure: lung volumes and capacities
Dead Space

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
9
Dead space is volume within the bronchial tree that is ventilated but does
not participate in gas exchange.
– Anatomical dead space is the volume of the conducting airways
(pharynx, trachea, and bronchi) that do not contain alveoli and therefore
cannot participate in gas exchange. It is ~150 to 200 mL.
– Physiological dead space is the total volume of the bronchial tree that is
ventilated but does not participate in gas exchange.
Fig. 12.6 Volume exhaled versus time during a forced exhalation.
The total volume exhaled is the forced vital capacity (FVC), and the
volume exhaled in the first second is the forced expiratory volume
(FEV1).
– In healthy lungs, physiological dead space is approximately equal to
anatomical dead space.
However, physiological dead space may be increased in lung diseases
where there are mismatches between ventilation (V) and perfusion
(pulmonary blood flow [Q]).
– Physiological dead space can be calculated using Bohr’s equation.
This calculation assumes that the partial pressure of CO2
(Paco2) in the alveoli is the same as that in systemic arterial blood.
Ventilation Rate
Minute ventilation refers to the total ventilation per minute. It is
expressed as
Minute ventilation = TV × breaths/min
Alveolar ventilation refers to ventilation of alveoli that participate in gas
exchange per minute. It is
expressed as Alveolar ventilation = (TV – physiological dead space) ×
breaths/min.
Distribution of Pulmonary Blood Flow

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
10
When a person is upright, the force of gravity affects the distribution of
pulmonary blood flow within the lungs (but not the total amount of blood
flow) because vascular pressures progressively fall at locations above the
heart. This distribution of blood flow is described in terms of “zones” of
the lung.
Zone 1: Lung Apex. If pulmonary artery pressure is not high enough to
support the column of blood
from the right ventricle all the way to the apices of the lungs, the
uppermost blood vessels collapse, and there is no flow in this region. This
does not normally occur in healthy lungs but may occur if right
ventricular pressure is extremely low (e.g., due to hemorrhage). Also, if
alveolar pressure is
increased to the point where it exceeds vascular pressure, blood vessels
collapse (e.g., due to positive pressure ventilation).
Zone 2: Middle of the Lung. In zone 2, blood flow is intermittent.
Pulmonary artery pressure drives blood flow at its peak during systole,
but not during the rest of the cardiac cycle.
Zone 3: Lung Base. Zone 3 has no gravitational impediment to blood
flow because regions located below the heart always have vascular
pressures greater than alveolar pressure. Therefore, blood flow
is continuous.
Gas Exchange and Transport

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
11
Partial Pressures
In a gas mixture, each gas species exerts a pressure, the partial pressure of
that gas. The sum of the partial pressures of the gases in a mixture equals
the total gas pressure.
Partial pressure for an individual gas = the fraction of that gas in the gas
mixture × total gas pressure Calculation of Partial Pressure of Oxygen
(Po2) in Dry Inspired Air O2 comprises 21% of air; total gas pressure =
760 mm Hg (at sea level)
At high altitude, the Po2 is reduced because barometric pressure is lower.
Correction of Po2 for the Presence of Water Vapor
Dry air entering the lungs becomes completely saturated with water as air
passes through moist airways. This displaces some of the other gases and
slightly reduces their partial pressures.
Partial pressure of water vapor (PH2o) is 47 mm Hg at body temperature.
Total pressure of gases other than water = 760 mm Hg − 47 mm Hg
= 713 mm Hg
Therefore, the Po2 in warm, humidified inspired air is
Gas Exchange
Diffusion of Gases
O2 and carbon dioxide (CO2 ) diffuse between alveolar gas and
pulmonary capillary blood according to standard physical principles
– The total amount moved per unit of time is proportional to the area
available for diffusion and to the difference in partial pressure between
alveolar gas and pulmonary capillary blood, and inversely proportional to
the thickness of the diffusion barrier.
– Gas will diffuse from the alveoli (higher partial pressures) to the
pulmonary capillaries (lower partial pressures) until they equilibrate and
no partial pressure gradient exists. As a result, blood entering the
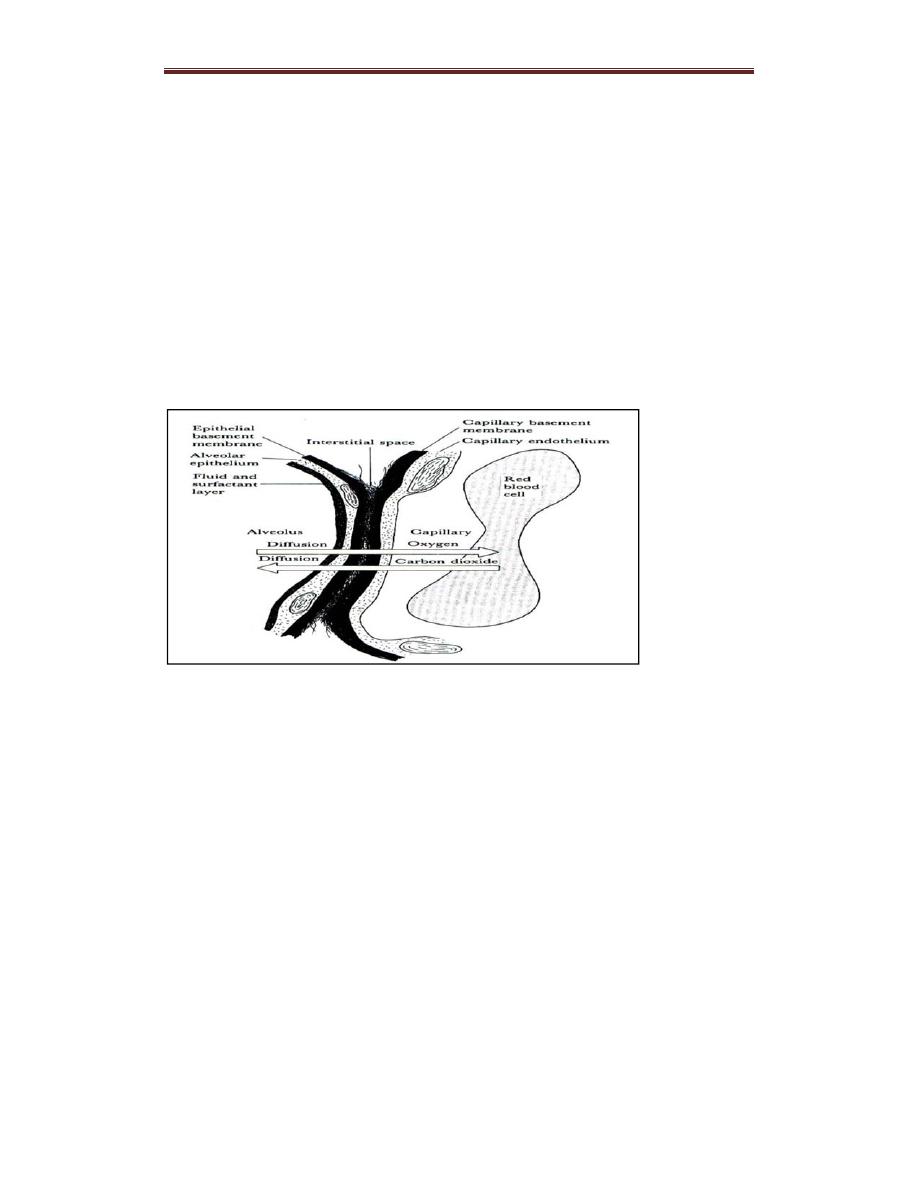
Respiratory System PhysiologyDr. Amjed Hassan lecture 2
12
pulmonary veins from the pulmonary capillaries has virtually the same
partial
pressures as gases in the alveoli.
– The diffusion barrier, composed of alveolar epithelial cells (type I
pneumocytes) and capillary endothelial cells, is very thin, which ensures
that the diffusion distance between alveolar gas and pulmonary capillary
blood is very short. This allows blood in the pulmonary capillaries to
equilibrate with alveolar gas during the short time (< 1 sec) that the blood
is in the capillaries.
Figure . Ultra structure of the respiratory membrane where diffusion
occurs.
Partial Pressure Changes of Oxygen and Carbon Dioxide
Following Gas Exchange
Partial Pressure Changes of Oxygen
– The Po2 of humidified inspired air is 150 mm Hg.
– The Po2 of alveolar air is 100 mm Hg. This is due to the diffusion of
O2
from alveolar air into pulmonary capillary blood.

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
13
– The Po2 of systemic arterial blood is 95 mm Hg. It is almost the same
as the Po2 of alveolar air because the partial pressure of pulmonary
capillary blood equilibrates with alveolar air. However, ~2% of the
cardiac output bypasses the pulmonary circulation, which accounts for the
slight discrepancy in partial pressures.
– The Po2 of venous blood is 40 mm Hg because O2 has diffused from
arterial blood into the tissues.
Partial Pressure Changes of Carbon Dioxide
– The Pco2 of humidified inspired air is almost zero.
– The Pco2 of alveolar air is 40 mm Hg because CO2 from venous blood
entering the pulmonary capillaries diffuses into alveolar air.
– The Pco2 of systemic arterial blood is 40 mm Hg because pulmonary
capillary blood equilibrates with alveolar air.
– The Pco2 of venous blood is 46 mm Hg. It is higher than systemic
arterial blood due to the diffusion of CO2 from the tissues into venous
blood following cellular respiration.
Ventilation and Perfusion Ratios for Optimum Gas Exchange
Ventilation/perfusion ratio is the ratio of alveolar ventilation V to
perfusion (pulmonary blood flow)Q.
– In healthy lungs, the V/Q ratio is close to 1:1, resulting in optimum gas
pressures and oxygenation in systemic arterial blood.
Distribution of V/Q Ratios
There are regional differences in alveolar ventilation and blood flow in
the upright individual.

Respiratory System PhysiologyDr. Amjed Hassan lecture 2
14
– Alveolar ventilation is higher at the base of the lungs than the apices
because the base is more compliant and changes more in volume during
each breathing cycle.
– Blood flow is very low at the apex of the lung and very high at the base
due to the effects of gravity.
The differences in regional blood flow are greater than the differences in
regional ventilation. This creates different V/Q ratios at various levels of
the lung. Typical values are as follows:
– Apex V/Q is ~3:1.
– Middle of lungs (heart level) V/Q is ~1:1.
– Base of lungs V/Q is ~1:2.
