Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
1
Cholesterol Metabolism
The word cholesterol is derived from Greek words, chole =bile; steros = solid;
ol = alcohol. Almost all nucleated cells(including arterial walls) can synthesis
cholesterol.
Cholesterol is present in tissues and in plasma either as free
cholesterol or as a storage form, combined with along-chain fatty acid as
cholesteryl ester. In plasma,both forms are transported in lipoproteins .
Functions of Cholesterol
1. Cell membranes: Cholesterol is a component of membranes and has a
modulating effect on the fluid state of the membrane.
2. Nerve conduction: Cholesterol has an insulating effect on nerve fibers.
3. Bile acids and bile salts are derived from cholesterol. Bile salts are
important for fat absorption.
4. Steroid hormones: Glucocorticoids, androgens and estrogens are from
cholesterol.
5. Vitamin D3 is from 7-dehydro-cholesterol.
6. Esterification: The OH group of cholesterol is esterified to fatty acids to
form cholesterol esters. This esterification occurs in the body by transfer
of a PUFA moiety by lecithin cholesterol acyl transferase
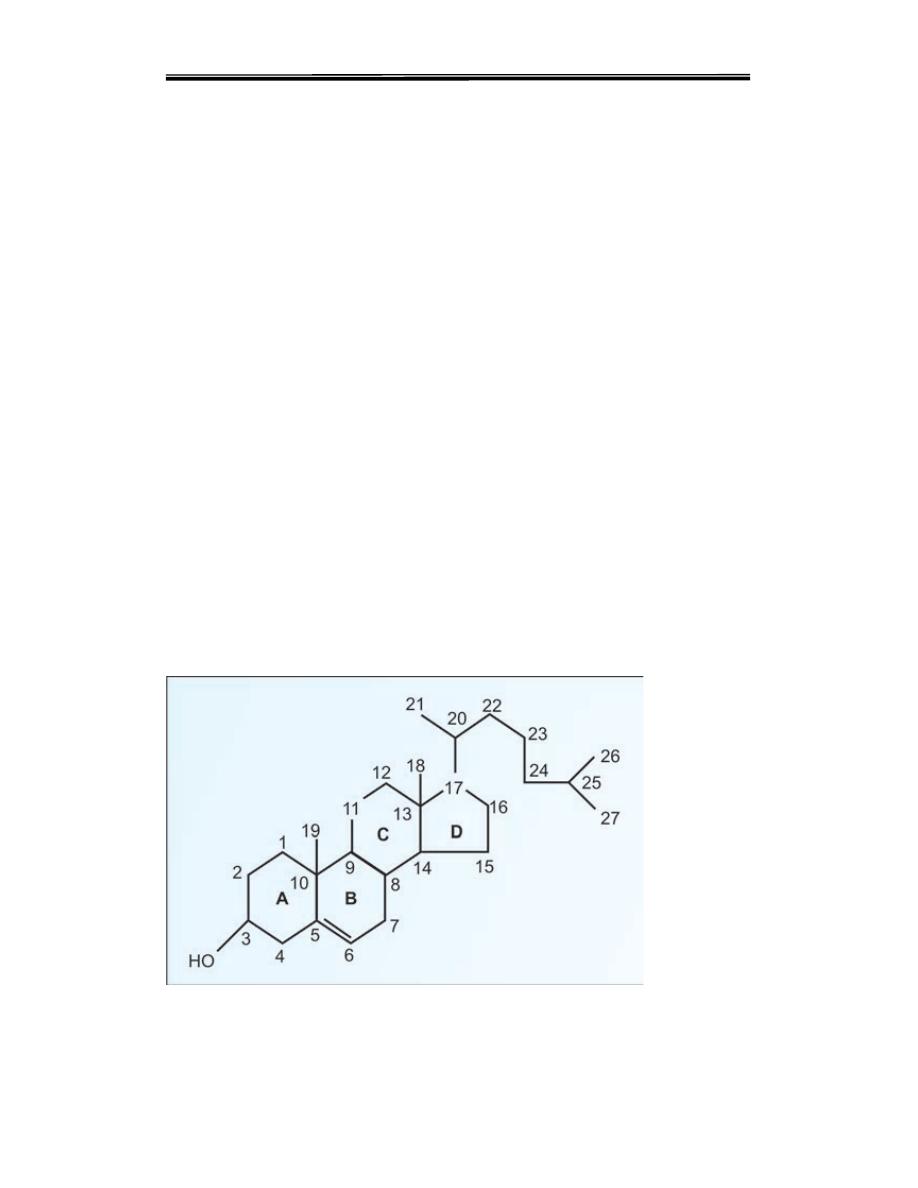
Structure of cholesterol

Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
2
Biosynthesis of Cholesterol
The synthesis and utilization of cholesterol must be tightly regulated in order
to prevent over-accumulation and abnormal deposition within the body. Of
particular importance clinically is the abnormal deposition of cholesterol and
cholesterol-rich lipoproteins in the coronary arteries. Such deposition,
eventually leading to atherosclerosis, is the leading contributory factor in
diseases of the coronary arteries.
Cholesterol synthesis in microsomal (endoplasmic reticulum) and cytosol
fraction of the cell is responsible for cholesterol synthesis from the two-carbon
acetate group of acetyl-CoA. A little more than half the cholesterol of the body
arises by synthesis (about 700 mg/d), and the remainder is provided by the
average diet.
The acetyl-CoA utilized for cholesterol biosynthesis is derived from an
oxidation reaction (e.g., fatty acids or pyruvate) in the mitochondria and is
transported to the cytoplasm by the same process as that described for
fatty
acid synthesis
.. All the reduction reactions of cholesterol biosynthesis use
NADPH as a cofactor
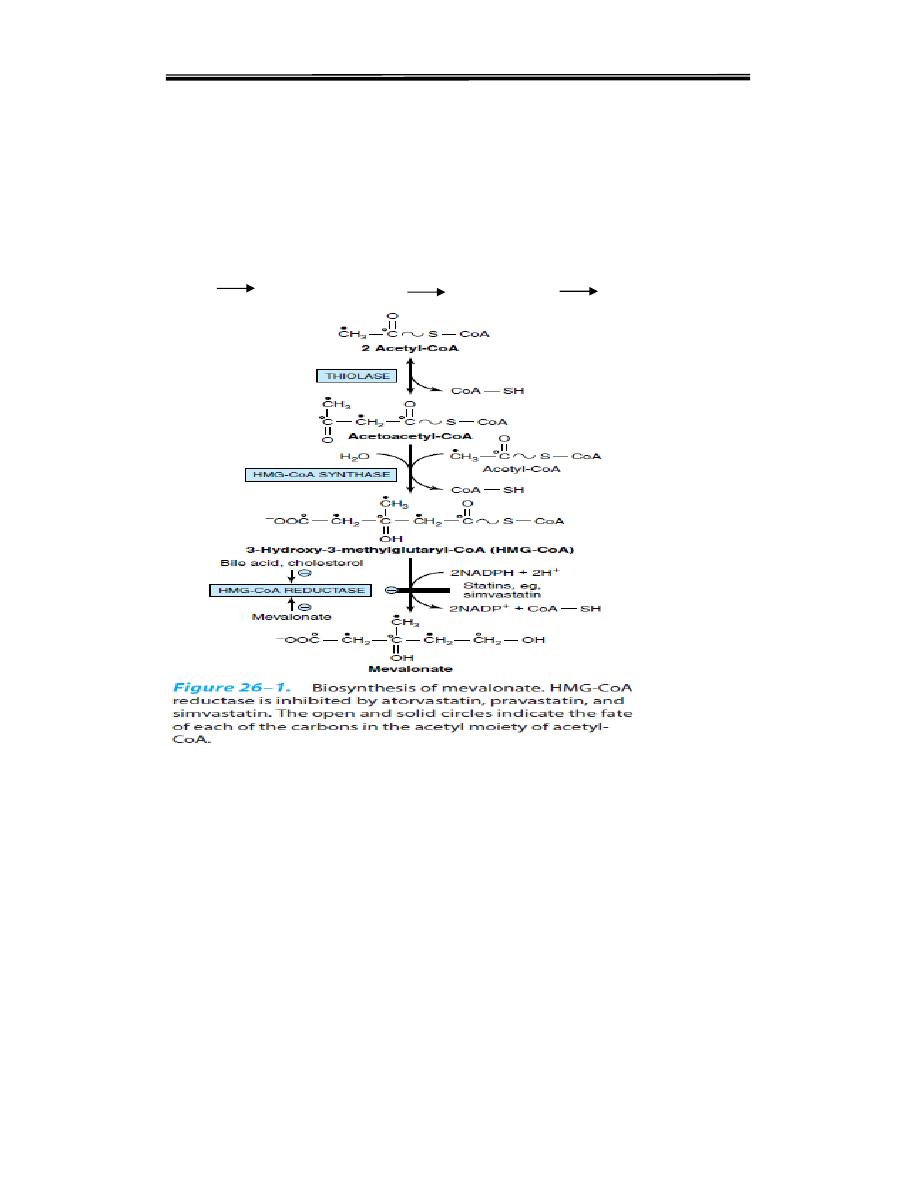
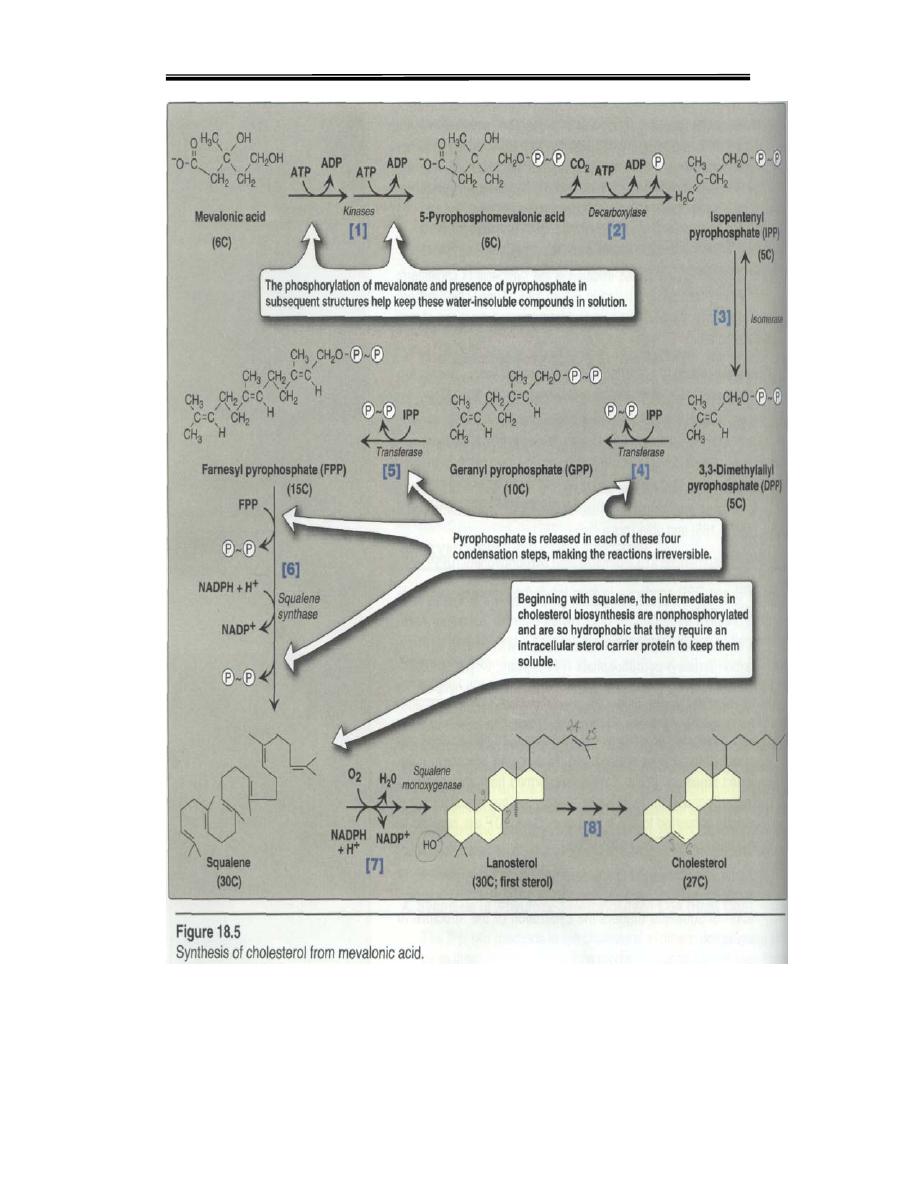
The process of cholesterol synthesis has five major steps:
1. Acetyl-CoAs are converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA)
2. HMG-CoA is converted to mevalonate
3. Mevalonate is converted to the isoprene based molecule, isopentenyl
pyrophosphate (IPP), with the concomitant loss of CO
2
4. IPP is converted to squalene
5. Squalene is converted to cholesterol.
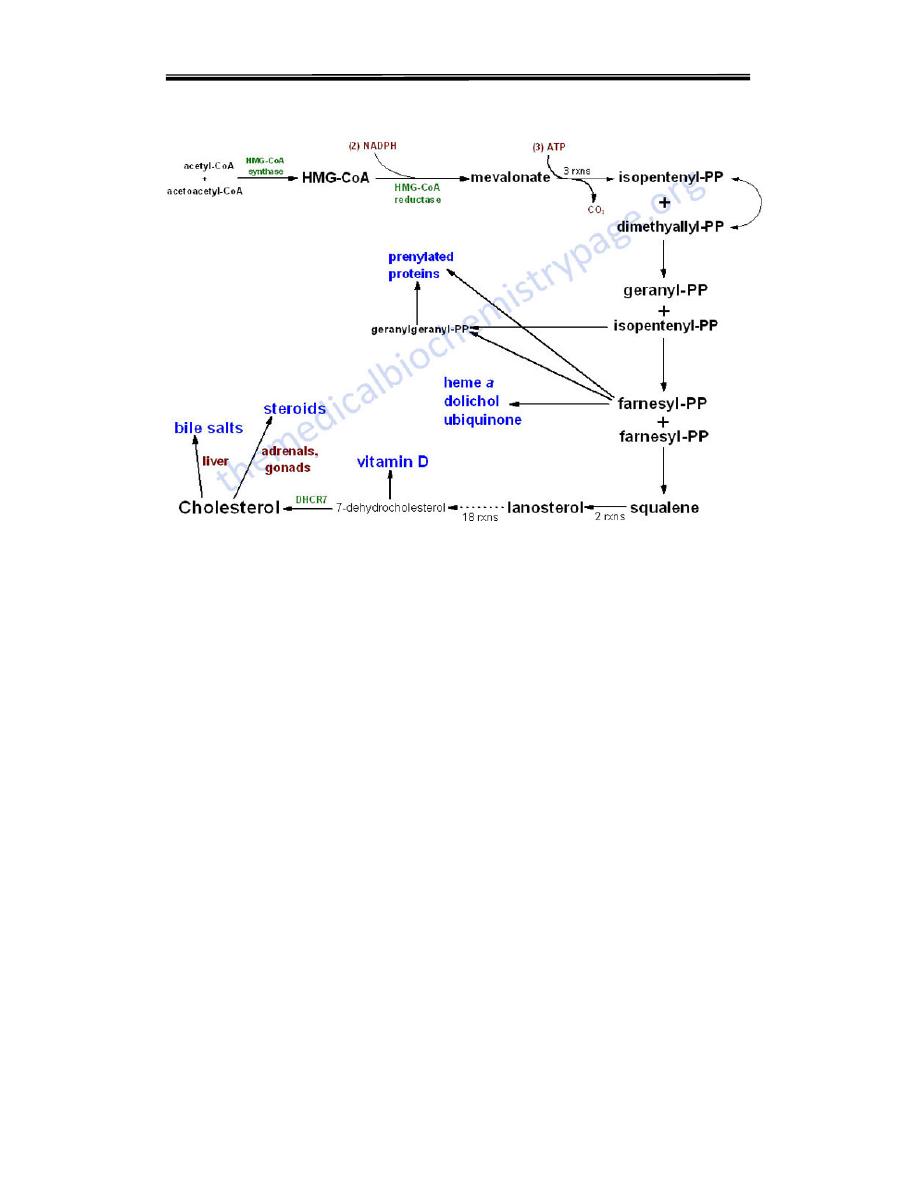
Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
3
Pathway of cholesterol biosynthesis. Synthesis begins with the transport of acetyl-CoA
from the mitochondrion to the cytosol. The rate limiting step occurs at the 3-hydroxy-3-
methylglutaryl-CoA (HMG-CoA) reducatase, HMGR catalyzed step. The
phosphorylation reactions are required to solubilize the isoprene intermediates in the
pathway.
Steps of synthesis are as follow
Step 1: Condensation
The acetyl CoA is provided by the ATP-citrate lyase reaction as in the case of
fatty acid synthesis. Two molecules of acetyl CoA condense to form acetoacetyl
CoA catalysed by cytoplasmic acetoacetyl CoA synthase
Step 2: Production of HMG CoA
A third molecule of acetyl CoA condenses with acetoacetyl CoA to form beta-
hydroxy beta-methyl glutaryl CoA (HMG CoA). The enzyme is HMG CoA
synthase. HMG CoA is present in both cytosol and mitochondria of liver.
The mitochondrial pool is used for ketogenesis whereas the cytosolic
fraction is utilized for cholesterol synthesis
Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
4
Step 3: The Committed Step
The reduction of HMG CoA to mevalonate is catalysed by HMG CoA
reductase. It is a microsomal (endoplasmic reticulum) enzyme. It uses 2
molecules of NADPH Steps 1 and 2 are shared with ketogenic pathway; but
step 3 is the first
reaction that is unique to the cholesterol biosynthetic pathway. It is the rate-
limiting step.
Step 4: Production of 5 Carbon Unit
i. Mevalonate is successively phosphorylated to phospho-mevalonate, to
pyrophosphomevalonate, then to 3-phospho-5-pyrophosphomevalonate.
ii. This then undergoes decarboxylation to give isopentenyl
pyrophosphate, a 5 carbon unit ,the over all reaction including the following
HMG CoA is reduced to Mevalonate by a reductase.
• Mevalonate undergoes three times Phosphorylation, in the presence of 3
ATPs and various kinases.The product is 3- phosphor-5 pyrophospho
mevalonate.
• Dephosphorylation, decarboxylation converts it to Isopentenyl
pyrophosphate.
• It is isomerised to dimethyl allyl pyrophosphate by isomeras.
• Isopentenyl pyrophosphate and dimethyl allyl pyrophosphate form Geranyl
PP(10C).
• Geranyl PP and one more molecule of Isopentenyl PP→ Farnesyl PP(15C).
• Two of Farnesyl PP join to form Squalene (30C).
1. Squalene undergoes cyclization, loses three carbon atoms,aquire a double
bond,forms cholesterol
.
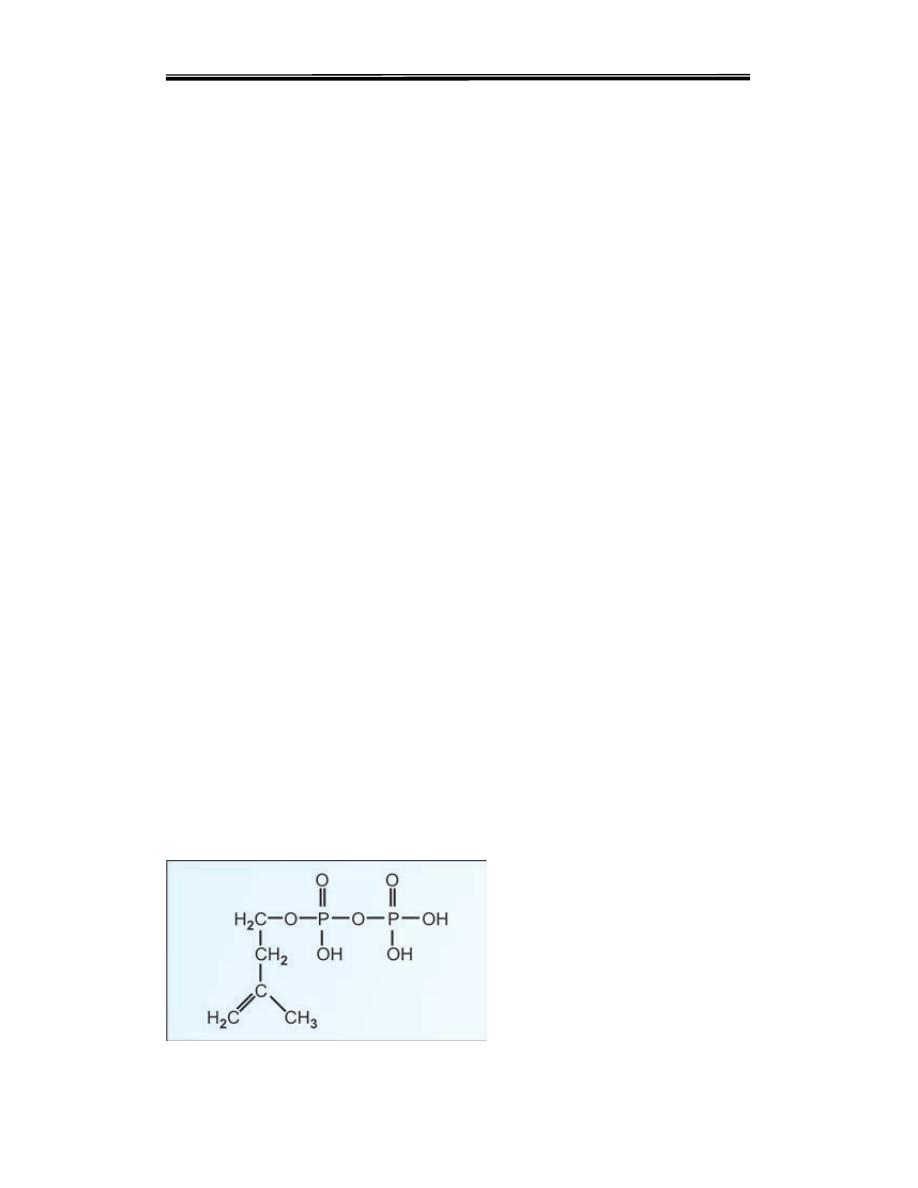
Isopentenyl pyrophosphate; 5 carbon unit
Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
5
Step 5: Condensation of 5-Carbon Units
Thus, 6 numbers of 5- carbon units are condensed to form a 30 carbon
compound, Squalene. In summary
5C + 5C 10C; 10C+5C 15C; 15C+15C 30C
Regulating Cholesterol Synthesis
Normal healthy adults synthesize cholesterol at a rate of approximately 1g/day
and consume approximately 0.3g/day. A relatively constant level of cholesterol
in the blood (150–200 mg/dL) is maintained primarily by controlling the level of
de novo
synthesis. The level of cholesterol
.
synthesis is regulated in part by the
dietary intake of cholesterol.
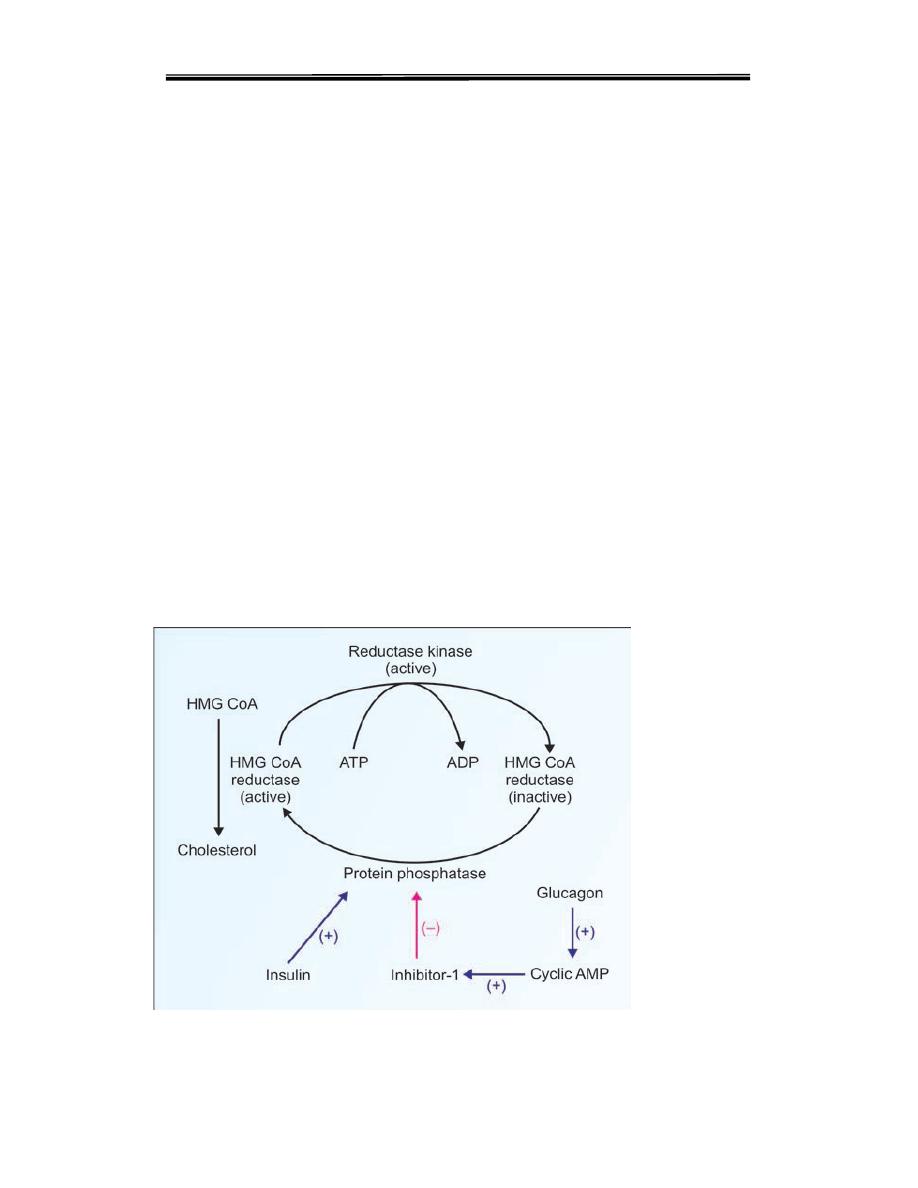
Regulation of cholesterol synthesis is exerted near
the beginning of the pathway, at the HMG-CoA reductase step
The enzyme is
controlled by four distinct mechanisms:
Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
6
1-feed-back inhibition, HMG-CoA reductase in liver is inhibited by mevalonate,
the immediate product of the pathway, and by cholesterol, the main product.
2- control of gene expressionand
Regulation at transcription: The
regulatory enzyme is HMG CoA reductase. Long-term regulation involves
regulation of transcription of the gene for HMG CoA reductase. When
sufficient cholesterol is present in the cell, transcription of the gene for HMG
CoA reductase is suppressed,
and cellular synthesis of cholesterol is decreased. When cholesterol in diet is
low, synthesis is
3. Hormonal regulation Regulation of HMGR through covalent modification
occurs as a result of phosphorylation and dephosphorylation HMG CoA
reductase
activity is controlled covalently through the actions of a protein
kinase
and a phosphoprotein phosphatase The phosphorylated form of the
enzyme is inactive, whereas the dephosphorylated form is active.
Insulin or thyroid hormone increases HMG-CoA reductase activity,whereas
glucagon or glucocorticoids decrease it.

Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
7
4-Inhibition by drugs: The statin drugs, including simvastatin, lovastatin,
and are structural analogs of HMG CoA, and are reversible, competitive
inhibitors of HMG CoA reductase They are used to decrease plasma
cholesterol levels in patients with hypercholesterolemia.
1
Cholesterol Pool and Cholesterol Metabolism
The total body cholesterol content varies from 130-150 grams. LDL (low
density lipoprotein) transports cholesterol from the liver to the peripheral
tissues and HDL (high density lipoprotein) transports cholesterol from tissues
to liver. Cells of extrahepatic tissues take up cholesterol from LDL. The free
cholesterol released within the cell has the following fates:
1. Incorporated into cell membranes.
2. Metabolised to steroid hormones, especially in adrenal cortex and gonads.
3. Esterified with saturated fatty acids and stored in the cell. The enzyme
ACAT (acyl cholesterol acyl transferase) helps in this reaction.
4. Esterified with poly-unsaturated fatty acids (PUFA) by the action of LCAT
(lecithin cholesterol acyl transferase) and incorporated into HDL, transported
and finally excreted through liver.
DEGRADATION OF CHOLESTEROL
The ring structure of cholesterol cannot be metabolized and in humans. Rather,
the intact sterol nucleus is eliminated from the body by conversion to bile acids
and bile salts,
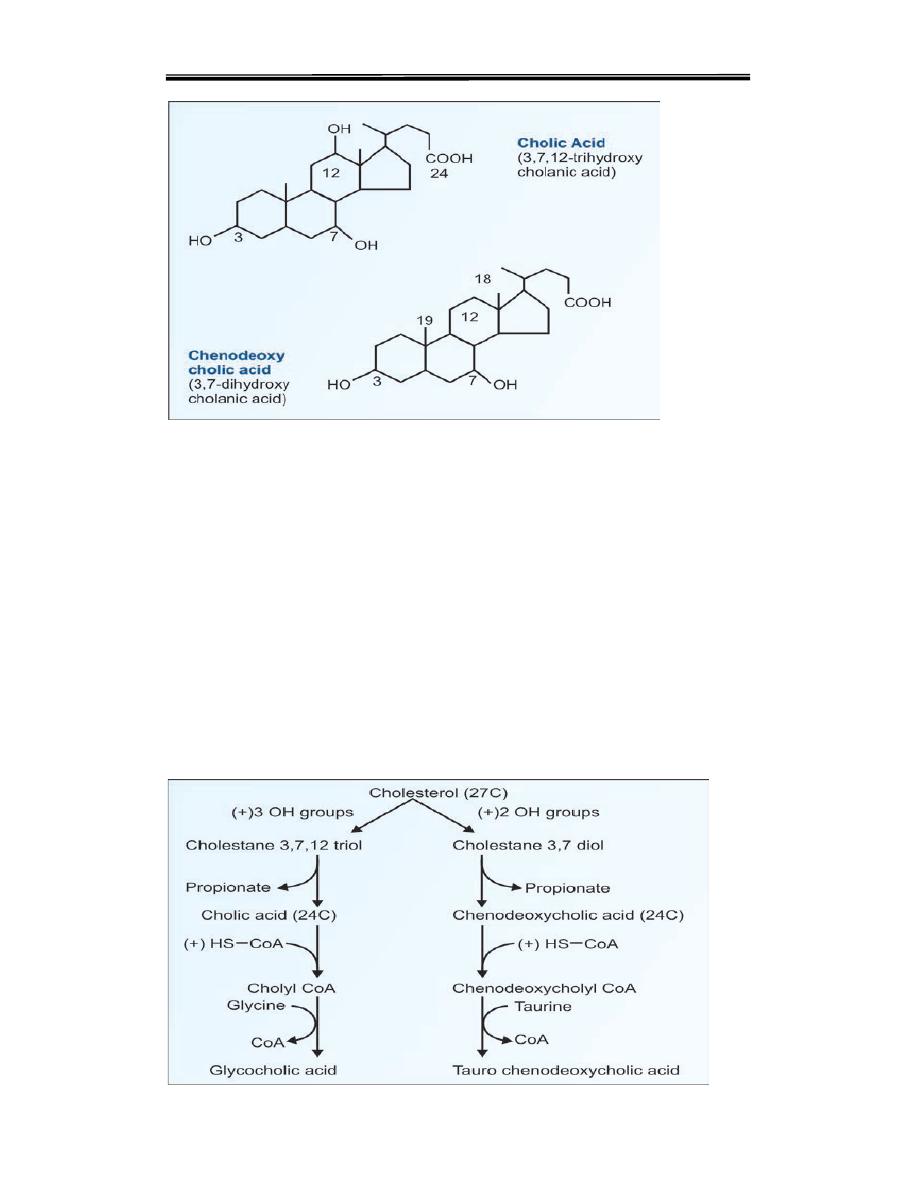
FORMATION OF BILE ACIDS
Bile acids are synthesised in the liver from cholesterol. The contain 24 carbon
atoms hydroxyl group at position 7. The reactions for synthesis of bile acids are
summarized below:
1. Cholesterol hydroxylated at 3/7/12 positions The first and rate-limiting step
is the introduction of this hydroxyl group by the enzyme 7-alpha-hydroxylase
2. Removal of 3-carbon unit, to make it 24 C
3. Conjugation with glycine
4. Secretion into intestinal canal
5. In the intestine, deconjugation and removal of hydroxyl groups.
Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
8
Formation of Bile Salts
The primary bile acids are now conjugated with either glycine or taurine to
form bile acids. They are glyco-cholic acid, tauro cholic acid, glyco
chenodeoxycholic acid and tauro chenodeoxycholic
acid (Fig. 12.20). The major conjugated bile acid is glycocholic acid.
Conjugation adds more polar groups and increases the efficiency of bile acids
as surfactants. The conjugated bile acids are excreted through the bile. In the
bile they exist as bile salts (sodium or potassium salts of conjugated
bile acids)

Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
9
Functions of Bile
1. The alkaline pH of the bile serves to neutralise the acidity of the gastric
juice.
2. The bile salts are efficient surfactants and detergents.
3. Bile is the only route of excretion for bilirubin, the end product of heme
catabolism.
4. It serves to excrete cholesterol, thus regulating the body cholesterol pool.
5. Bile serves as the medium of excretion for several drugs, which are
detoxified by the liver.
Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
10

Clinical biochemistry second stage lipid lecture 2 Dr.Thana Alsewedy
11
