Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
1
Conversion of Amino Acids to Specialized Products
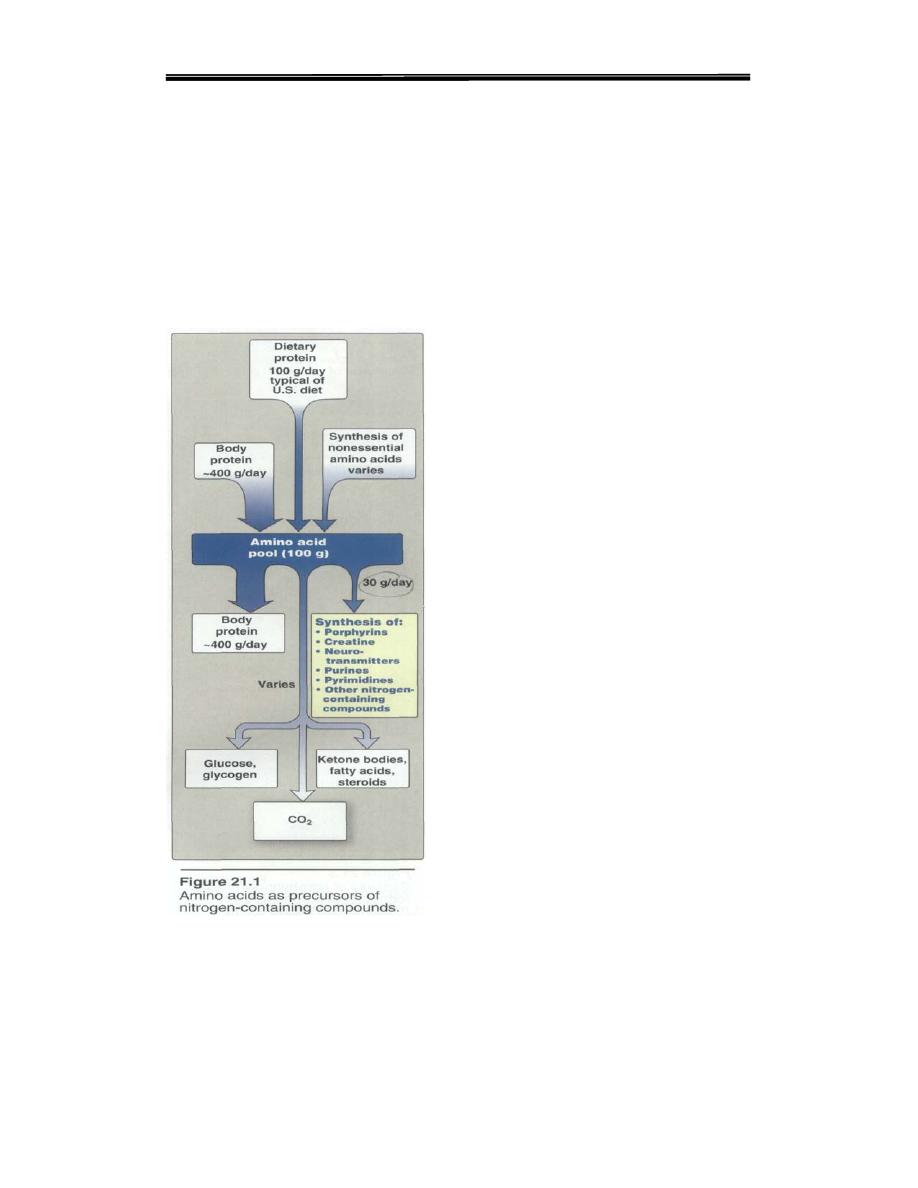
In addition to serving as building blocks for proteins, amino acids are
precursors of many nitrogen-containing compounds that have important
physiologic functions (Figure 21.1). These molecules include
porphyrins,
neurotransmitters,
hormones,
purines, and pyrimidines
Specialized Products of Amino Acids
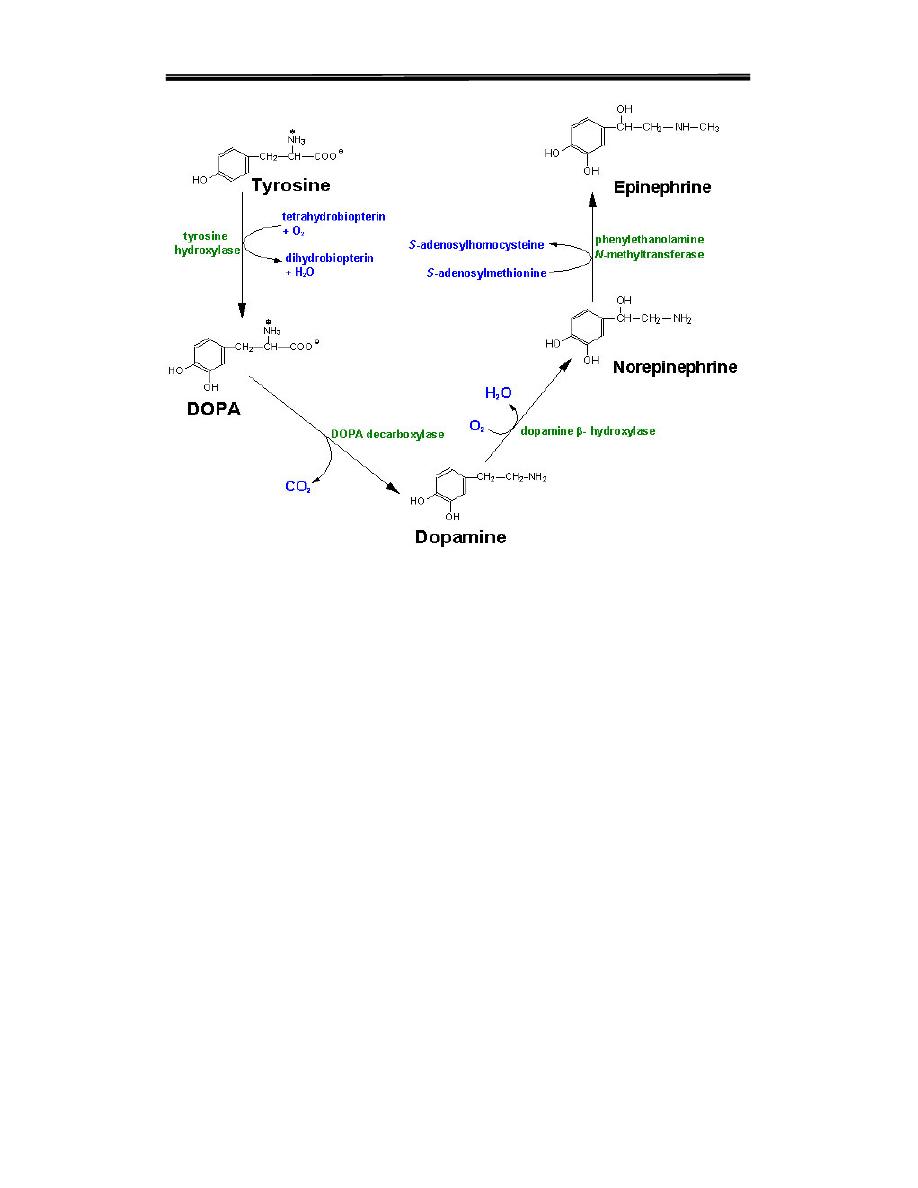
A. Tyrosine precursor of Catecholamines
Tyrosine gives rise to a family of catecholamines that include Dopamine,
Norepinephrine and epinephrine.
Dopamine, norepinephrine (noradrenaline)and epinephrin (adrenaline) are
biologically active amines and are collectively called as Catecholeamines
Dopamine and norepinephrine functions as a neurotransmitters

Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
2
Outside the nervous system, norepinephrine and its methylated derivative,
epinephrine regulates carbohydrate and lipid metabolism
They are released from storage vehicles in the adrenal medulla in
response to stress fright, exercise, cold, and low levels of blood glucose
(
They increase the degradation of glycogen and triglycerides, as well as
increase blood pressure and the output of heart
Dopamine
Dopamine act as neural transmission
Dopamine levels are abnormally low in a particular region of the brain of
patients with Parkinson’s disease,This disease is associated with tremor
of arm occasional muscle cramping.
Epinephrine
Epinephrine, also known as adrenaline is the principal hormone
governing the fight or flight response to various stimuli. In addition it
stimulates glycogenolysis (breakdown of glycogen),
and a variety of physiological event, such as increasing depth and
frequency of heartbeats.
Norepinephrine (nor-adrenaline)
It is a precursor of epinephrine. It causes greater constriction of the blood
vessels of muscles, as a result of which the arterial pressure is raised. It
acts as a neurotransmitter between sympathetic synthesis of
catecholamines in nervous system and smooth muscles.
2. Synthesis of catecholamines:
1-Tyrosine is hydroxylated by tyrosine hydroxylase (rate limiting
step in the pathway) to form form3,4-dihydroxy- phenylalanine DOPA
2-DOPA is decarboxylated by DOPA decarboxylase (pyridoxal
phosphate requiring enzyme to form dopamine
(
3-Dopamine is then hydroxylated by Dopamine β-hydroxylase to give
norepinephrine
.
4-Epinephrine is formed by N-methylation reaction using S-
adenosylmethionine as a methyl donor
Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
3
Synthesis of catecholamines:
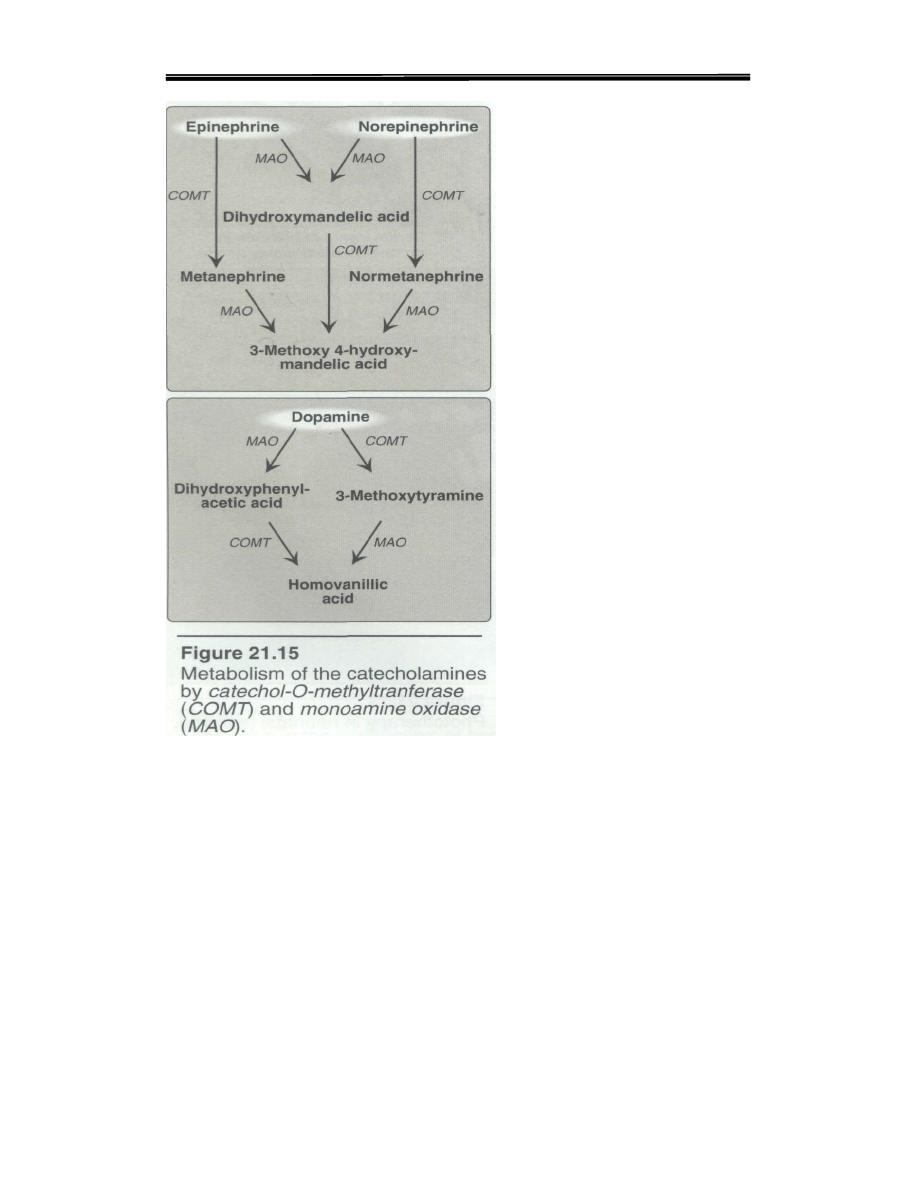
3. Degradation of catecholamines: The catecholamines are inactivated
by oxidative deamination catalyzed by monoamine oxidase (MAO), and
by O-methylation carried out by catechol-O-methyl transferase
{COMT,Figure 21.15). The two reactions can occur in either order. The
aldehyde products of the MAO reaction are oxidized to the corresponding
acids. The metabolic products of these reactions are Vanillylmandelic
metanephrine and normetanephrine.
Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
4
Melanin
-Pigment that occurs in several tissues, e.g. in eye, skin, and hair
-Synthesized from tyrosine in the epidermis by melaocytes
catalyzed by the copper-containing enzyme tyrosine hydroxylase (also
called tyrosinase,. Function is to protect tissues from sun-light
-
B.
Glycine
Glycine is used for heme, purine and creatine synthesis
1- a carbon and nitrogen atoms of glycine are used for synthesis of
porphyrine, prosthetic group of heme. Porphyrins are cyclic compounds
that readily bind metal ions—usually Fe2+ or Fe3+.The most prevalent
metalloporphyrin in humans is heme
Heme is the prosthetic group for hemoglobin, myoglobin, the
cytochromes.

Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
5
Porphyrins are synthesized from glycine and succinyl coenzyme A
Glycine and succiny CoA condense to form5-aminolevulinic acid(ALA)
reaction catalyzed by ALA synthase This reaction requires pyridoxal
phosphate as a coenzyme, ands the rate-controlling step of hepatic
porphyrin biosynthesis and by series of reaction lead to synthesis of
heme from Porphyrins
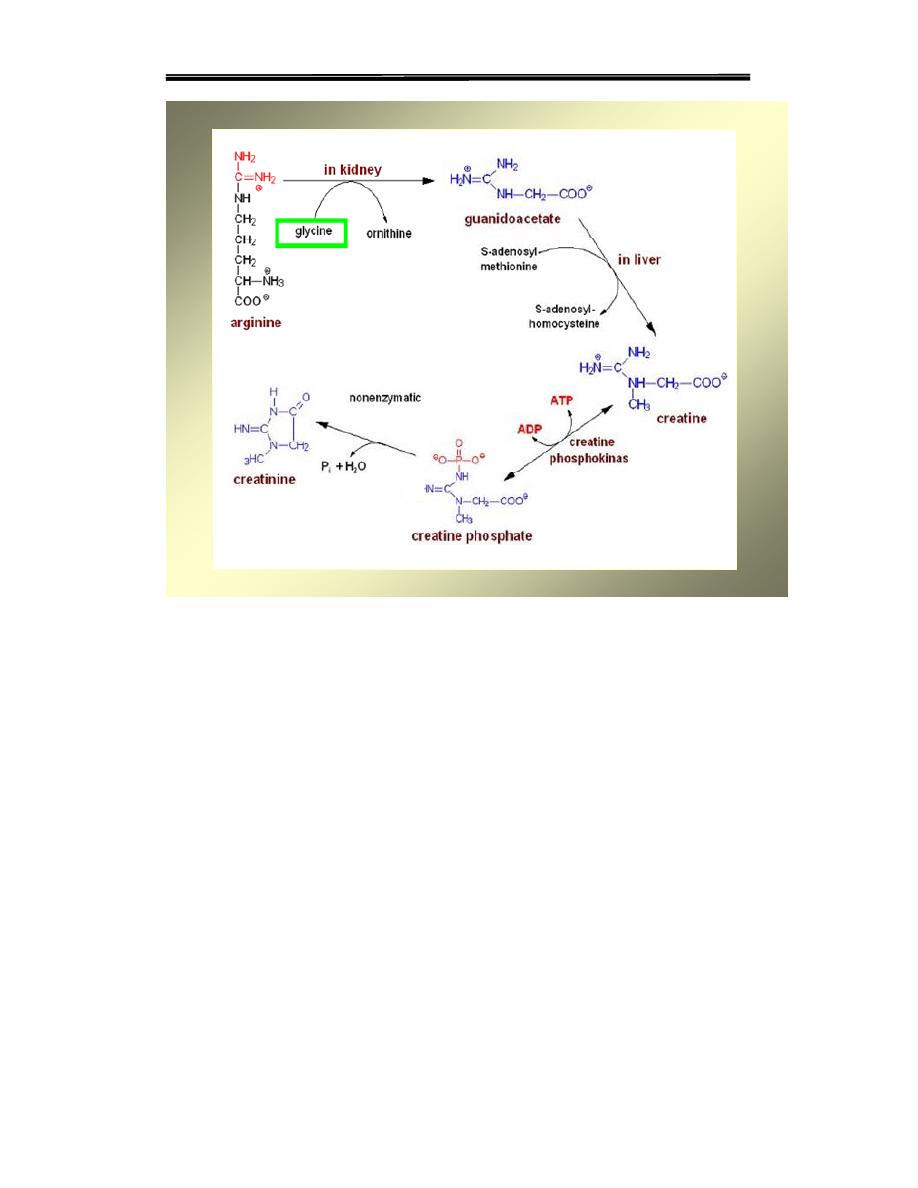
2- Creatine and Creatine Phosphate
-Found in muscle
-High energy compound that can donate phosphate group to ADP to form
ATP
Synthesized from glycine, and the guanidino group of arginine -
plus a methyl group from S-adenosylmethionine
-Creatine is reversibly phosphorylated to creatine phosphate by creatine
kinase using ATP as a phosphate donor
--Creatine phosphate serves as a reserve of high-energy phosphates that
can be used tomaintain ATP level
-Levels of creatine kinase in plasma is an indicator of tissue damage and
is used in the diagnosis of myocardial infarction
Synthesis of creatine
Synthesized from glycine, and the guanidino group of arginine,
plus a methyl group from S-adenosylmethionine.
-reversibly phosphorylated to creatine phosphate by
creatine kinase using ATP as a phosphate donor.
Degradation
Both creatine and creatine phosphate cyclize to form
creatinine which is then excreted in the urine The amount of creatinine
excreted is proportional to the total creatine phosphate content of the
body, and thus can be used to estimate muscle mass. When muscle mass
decreases for any reason (for example, from paralysis or muscular
dystrophy), the creatinine content of the urine falls.
In addition, any rise in blood creatinine is a sensitive indicator of kidney
malfunction ,because creatinine is normally rapidly removed from the
blood and excreted.
hi level of creatinine excretion (clearance rate)
is a measure of renal function.
Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
6
Take over
http://www.indstate.edu/thcme/mwking/aminoacidderivatives.html
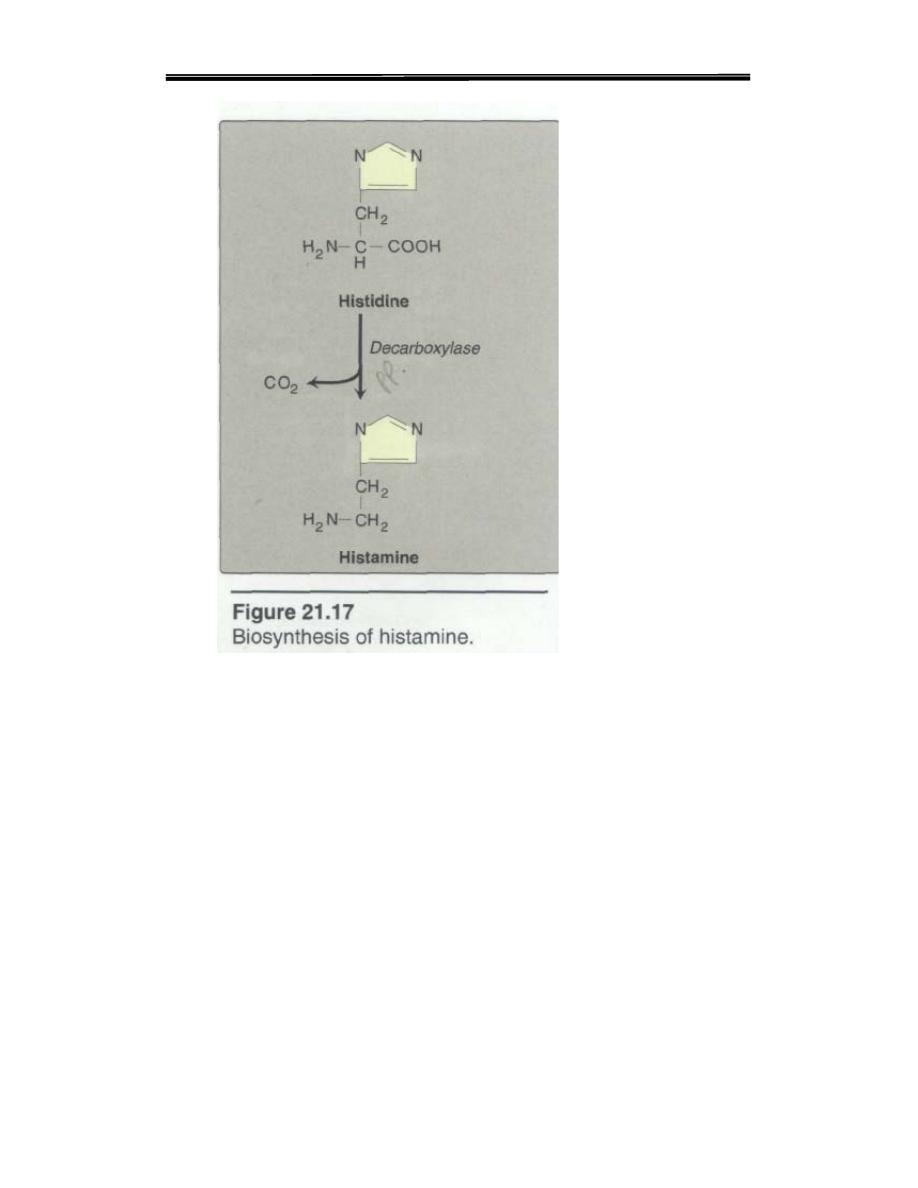
C. Histamine
Histamine is a chemical messenger that mediates a wide range of
cellular responses, including allergic and inflammatory reactions,
gastric acid secretion, and possibly neurotransmission in parts of the
brain. A powerful vasodilator, histamine is formed by decarboxylation
of histidine in a reaction requiring pyridoxal phosphate(Figure
21.17). is secreted by mast cells as a result of allergic reactions or
trauma.
Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
7
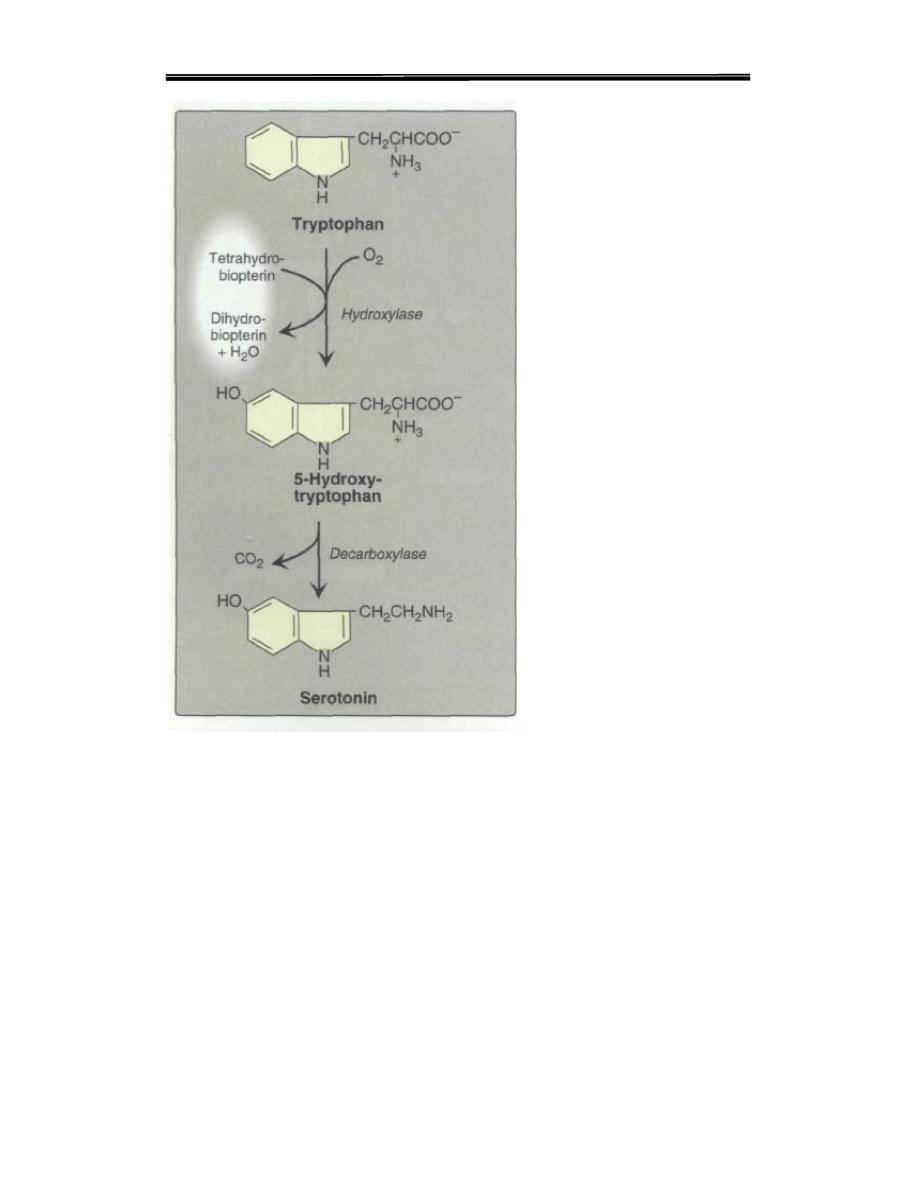
D.Serotonin
Serotonin, also called 5-hydroxytryptamine, is synthesized and stored
at several sites in the body (Figure 21.18). By far the largest amount of
serotonin is found in cells of the intestinal mucosa .Smaller amounts
occur in platelets and in the central nervous system. Serotonin is
synthesized from tryptophan, which is hydroxylate in a reaction
analogous to that catalyzed by phenylalaninehydroxylase. The product,
5-hydroxytryptophan, is decarboxylated to serotonin. Serotonin has
multiple physiologic roles, including pain perception, affective
disorders, and regulation of sleep, temperature,and blood pressure.
Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
8
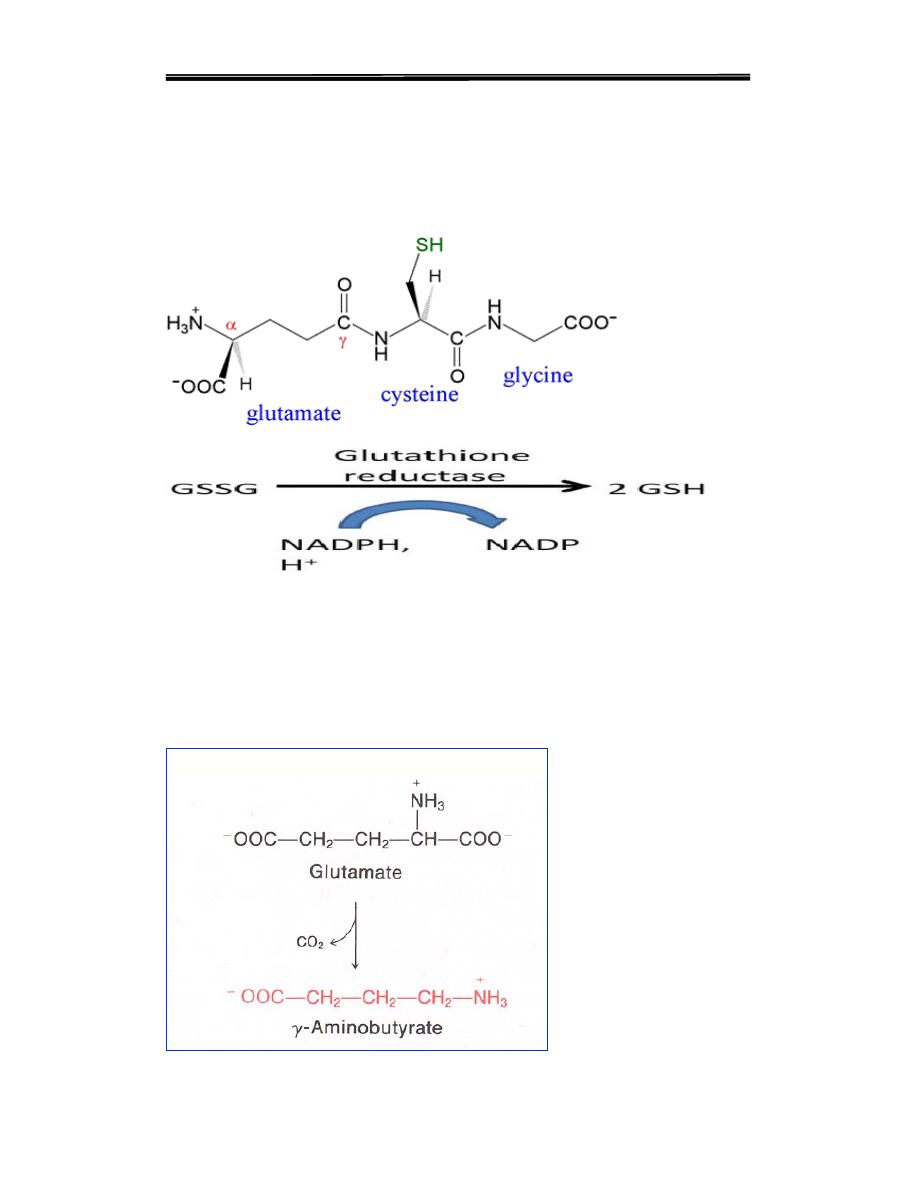
Glutathione
Glutathione are tripeptides consisting of glutamate, cysteine and glycine.
Glutathione serves as a reductant; is conjugated to drugs to make
them more water soluble (detoxification).
Reduces peroxides formed during oxygen transport. Glutathione is
master Antioxidants are reducing agents that limit free radical activity
and limit the damage from reactive oxygen species The resulting
oxidized form of GSH consists of two molecules disulfide bonded
together (abbreviated GSSG).
Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
9
Is involved in amino acid transport across cell membranes (the g-glutamyl
cycle).
Serves as a cofactor for some enzymatic reactions and as an aid in
the rearrangement of protein disulfide bonds.
GSH redused form of Glutathione
GSSGoxidized form of Glutathione
γ-aminobutyric acid (GABA)
Inhibitory neurotransmitter (CNS).
Directly regulates muscle tone.
Its lack leads to convulsions, epilepsia.
Involved in mechanism of memory.

Clinical biochemistry second stage lecture 6 Dr.Thana Alsewedy
10
Nitric Oxide NO
• Nitric oxide (NO) is produced by vascular endothelium and smooth
muscle, cardiac muscle, and many other cell types. serves many
important functions as Vasodilation
• Inhibition of platelet adhesion to the vascular endothelium (anti-
thrombotic)
The substrate for NO is L-arginine that is transported into the cell.
